Download July Through June Calendar 2019-2020 Ascending
Start Preamble Start Printed Page 39104
AGENCY:
Centers for Medicare & Medicaid Services (CMS), Health and Human Services (HHS).
ACTION:
Proposed rule.
SUMMARY:
This major proposed rule addresses: Changes to the physician fee schedule (PFS); other changes to Medicare Part B payment policies to ensure that payment systems are updated to reflect changes in medical practice, relative value of services, and changes in the statute; Medicare Shared Savings Program requirements; updates to the Quality Payment Program; Medicare coverage of opioid use disorder services furnished by opioid treatment programs; updates to certain Medicare provider enrollment policies; requirements for prepayment and post-payment medical review activities; requirement for electronic prescribing for controlled substances for a covered Part D drug under a prescription drug plan, or a Medicare Advantage Prescription Drug (MA-PD) plan; updates to the Medicare Ground Ambulance Data Collection System; changes to the Medicare Diabetes Prevention Program (MDPP) expanded model; and amendments to the physician self-referral law regulations.
DATES:
To be assured consideration, comments must be received at one of the addresses provided below, no later than 5 p.m. on September 13, 2021.
ADDRESSES:
In commenting, please refer to file code CMS-1751-P. Comments, including mass comment submissions, must be submitted in one of the following three ways (please choose only one of the ways listed):
1. Electronically. You may submit electronic comments on this regulation to http://www.regulations.gov. Follow the "Submit a comment" instructions.
2. By regular mail. You may mail written comments to the following address ONLY: Centers for Medicare & Medicaid Services, Department of Health and Human Services, Attention: CMS-1751-P, P.O. Box 8016, Baltimore, MD 21244-8016.
Please allow sufficient time for mailed comments to be received before the close of the comment period.
3. By express or overnight mail. You may send written comments to the following address ONLY: Centers for Medicare & Medicaid Services, Department of Health and Human Services, Attention: CMS-1751-P, Mail Stop C4-26-05, 7500 Security Boulevard, Baltimore, MD 21244-1850.
Start Further Info
FOR FURTHER INFORMATION CONTACT:
DivisionofPractitionerServices@cms.hhs.gov, for any issues not identified below.
Michael Soracoe, (410) 786-6312, for issues related to practice expense, work RVUs, conversion factor, and PFS specialty-specific impacts.
Larry Chan, (410) 786-6864, for issues related to potentially misvalued services under the PFS.
Donta Henson, (410) 786-1947, Patrick Sartini, (410) 786-9252, and Larry Chan, (410) 786-6864, for issues related to telehealth services and other services involving communications technology.
Julie Adams, (410) 786-8932, for issues related to payment for anesthesia services.
Sarah Leipnik, (410) 786-3933, for issues related to split (or shared) services.
Christiane LaBonte, (410) 786-7237, for issues related to indirect practice expense, PFS payment for critical care services, and PFS payment for teaching physician services.
DivisionofPractitionerServices@cms.hhs.gov, for issues related to payment for vaccine administration services.
Regina Walker-Wren, (410) 786-9160, for issues related to billing for services of physician assistants.
Pamela West, (410) 786-2302, for issues related to PFS payment for therapy services, medical nutrition therapy services, and services of registered dieticians and nutrition professionals.
Liane Grayson, (410) 786-6583, and Donta Henson, (410) 786-1947, for issues related to coinsurance for certain colorectal cancer screening services.
Lisa Parker, (410) 786-4949, for issues related to RHCs and FQHCs.
Laura Kennedy, (410) 786-3377, for issues related to drugs payable under Part B.
Heather Hostetler, (410) 786-4515, and Elizabeth Truong, 410-786-6005, for issues related to removal of select national coverage determinations.
Sarah Fulton, (410) 786-2749, for issues related to Appropriate Use Criteria for Advanced Diagnostic Imaging (AUC); and Pulmonary Rehabilitation, Cardiac Rehabilitation and Intensive Cardiac Rehabilitation.
Rachel Katonak, (410) 786-8564, for issues related to Medical Nutrition Therapy.
Fiona Larbi, (410) 786-7224, for issues related to the Medicare Shared Savings Program (Shared Savings Program) Quality performance standard and quality reporting requirements.
Janae James, (410) 786-0801, or Elizabeth November, (410) 786-4518, or SharedSavingsProgram@cms.hhs.gov, for issues related to Shared Savings Program beneficiary assignment, repayment mechanism requirements, and benchmarking methodology.
Naseem Tarmohamed, (410) 786-0814, or SharedSavingsProgram@cms.hhs.gov, for inquiries related to Shared Savings Program application, compliance and beneficiary notification requirements.
Amy Gruber, AmbulanceDataCollection@cms.hhs.gov, for issues related to the Medicare Ground Ambulance Data Collection System.
Juliana Tiongson, (410) 786-0342, for issues related to the Medicare Diabetes Prevention Program (MDPP).
Laura Ashbaugh, (410) 786-1113, for issues related to Clinical Laboratory Fee Schedule: Laboratory Specimen Collection and Travel Allowance and Use of Electronic Travel Logs.
Frank Whelan, (410) 786-1302, for issues related to Medicare provider enrollment regulation updates.
Thomas J. Kessler, (410) 786-1991, for issues related to provider and supplier prepayment and post-payment medical review requirements.
Lindsey Baldwin, (410) 786-1694, and Michele Franklin, (410) 786-9226, for issues related to Medicare coverage of opioid use disorder treatment services furnished by opioid treatment programs.
Lisa O. Wilson, (410) 786-8852, or Meredith Larson, (410) 786-7923, for inquiries related to the physician self-referral law.
Joella Roland, (410) 786-7638, for issues related to requirement for electronic prescribing for controlled substances for a covered Part D drug under a prescription drug plan or an MA-PD plan.
Kathleen Ott, (410) 786-4246, for issues related to open payments. Start Printed Page 39105
Molly MacHarris, (410) 786-4461, for inquiries related to Merit-based Incentive Payment System (MIPS).
Brittany LaCouture, (410) 786-0481, for inquiries related to Alternative Payment Models (APMs).
End Further Info End Preamble
SUPPLEMENTARY INFORMATION:
Inspection of Public Comments: All comments received before the close of the comment period are available for viewing by the public, including any personally identifiable or confidential business information that is included in a comment. We post all comments received before the close of the comment period on the following website as soon as possible after they have been received: http://www.regulations.gov. Follow the search instructions on that website to view public comments. CMS will not post on Regulations.gov public comments that make threats to individuals or institutions or suggest that the individual will take actions to harm the individual. CMS continues to encourage individuals not to submit duplicative comments. We will post acceptable comments from multiple unique commenters even if the content is identical or nearly identical to other comments.
Addenda Available Only Through the Internet on the CMS Website: The PFS Addenda along with other supporting documents and tables referenced in this proposed rule are available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html. Click on the link on the left side of the screen titled, "PFS Federal Regulations Notices" for a chronological list of PFS Federal Register and other related documents. For the CY 2022 PFS proposed rule, refer to item CMS-1751-P. Readers with questions related to accessing any of the Addenda or other supporting documents referenced in this proposed rule and posted on the CMS website identified above should contact DivisionofPractitionerServices@cms.hhs.gov.
CPT (Current Procedural Terminology) Copyright Notice: Throughout this proposed rule, we use CPT codes and descriptions to refer to a variety of services. We note that CPT codes and descriptions are copyright 2020 American Medical Association. All Rights Reserved. CPT is a registered trademark of the American Medical Association (AMA). Applicable Federal Acquisition Regulations (FAR) and Defense Federal Acquisition Regulations (DFAR) apply.
I. Executive Summary
This major proposed rule proposes to revise payment polices under the Medicare PFS and makes other policy changes, including proposals to implement certain provisions of the Consolidated Appropriations Act, 2021 (CAA, 2021) (Pub. L. 116-260, December 27, 2020), Bipartisan Budget Act of 2018 (BBA of 2018) (Pub. L. 115-123, February 9, 2018) and the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act (the SUPPORT Act) (Pub. L. 115-271, October 24, 2018), related to Medicare Part B payment. In addition, this major proposed rule includes proposals regarding other Medicare payment policies described in sections III. and IV.
A. Summary of the Major Provisions
The statute requires us to establish payments under the PFS, based on national uniform relative value units (RVUs) that account for the relative resources used in furnishing a service. The statute requires that RVUs be established for three categories of resources: Work, practice expense (PE), and malpractice (MP) expense. In addition, the statute requires that we establish each year by regulation the payment amounts for physicians' services paid under the PFS, including geographic adjustments to reflect the variations in the costs of furnishing services in different geographic areas.
In this major proposed rule, we are proposing to establish RVUs for CY 2022 for the PFS to ensure that our payment systems are updated to reflect changes in medical practice and the relative value of services, as well as changes in the statute. This proposed rule also includes discussions and provisions regarding several other Medicare Part B payment policies.
Specifically, this proposed rule addresses:
- Practice Expense RVUs (section II.B.)
- Potentially Misvalued Services Under the PFS (section II.C.)
- Telehealth and Other Services Involving Communications Technology (section II.D.)
- Valuation of Specific Codes (section II.E.)
- Evaluation and Management Visits (section II.F.)
- Billing for Physician Assistant Services (section II.G.)
- Therapy Services (section II.H.)
- Changes to Beneficiary Coinsurance for Additional Procedures Furnished During the Same Clinical Encounter as Certain Colorectal Cancer Screening Tests (section II.I.)
- Vaccine Administration Services (section II.J.)
- Payment for Medical Nutrition Therapy Services and Related Services (section II.K.)
- Rural Health Clinics (RHCs) and Federally Qualified Health Centers (FQHCs) (sections III.A., III.B., and III.C.)
- Requiring Certain Manufacturers to Report Drug Pricing Information for Part B and Determination of ASP for Certain Self-administered Drug Products (sections III.D.1. and 2.)
- Medicare Part B Drug Payment for Drugs Approved under Section 505(b)(2) of the Federal Food, Drug, & Cosmetic Act (section III.E.)
- Appropriate Use Criteria for Advanced Diagnostic Imaging (section III.F.)
- Removal of Select National Coverage Determinations (section III.G.)
- Pulmonary Rehabilitation, Cardiac Rehabilitation and Intensive Cardiac Rehabilitation (section III.H.)
- Medical Nutrition Therapy (section III.I.)
- Medicare Shared Savings Program (section III.J.)
- Medicare Ground Ambulance Data Collection System (section III.K.)
- Medicare Diabetes Prevention Program (MDPP) (section III.L.)
- Clinical Laboratory Fee Schedule: Laboratory Specimen Collection and Travel Allowance for Clinical Diagnostic Laboratory Tests and Use of Electronic Travel Logs (section III.M.)
- Medicare Provider and Supplier Enrollment Changes (section III.N.1.)
- Provider/Supplier Medical Review Requirements: Addition of Provider/Supplier Requirements related to Prepayment and Post-payment Reviews (section III.N.2.)
- Modifications Related to Medicare Coverage for Opioid Use Disorder (OUD) Treatment Services Furnished by Opioid Treatment Programs (OTPs) (section III.O.)
- Updates to the Physician Self-Referral Regulations (section III.P.)
- Requirement for Electronic Prescribing for Controlled Substances for a Covered Part D Drug under a Prescription Drug Plan or an MA-PD Plan (section 2003 of the SUPPORT Act) (section III.Q.)
- Open Payments (section III.R.)
- Updates to the Quality Payment Program (section IV.)
- Collection of Information Requirements (section V.)
- Response to Comments (section VI.)
- Regulatory Impact Analysis (section VII.) Start Printed Page 39106
3. Summary of Costs and Benefits
We have determined that this proposed rule is economically significant. For a detailed discussion of the economic impacts, see section VII., Regulatory Impact Analysis, of this proposed rule.
II. Provisions of the Proposed Rule for the PFS
A. Background
Since January 1, 1992, Medicare has paid for physicians' services under section 1848 of the Social Security Act (the Act), "Payment for Physicians' Services." The PFS relies on national relative values that are established for work, practice expense (PE), and malpractice (MP), which are adjusted for geographic cost variations. These values are multiplied by a conversion factor (CF) to convert the relative value units (RVUs) into payment rates. The concepts and methodology underlying the PFS were enacted as part of the Omnibus Budget Reconciliation Act of 1989 (OBRA '89) (Pub. L. 101-239, December 19, 1989), and the Omnibus Budget Reconciliation Act of 1990 (OBRA '90) (Pub. L. 101-508, November 5, 1990). The final rule published in the November 25, 1991 Federal Register (56 FR 59502) set forth the first fee schedule used for payment for physicians' services.
We note that throughout this proposed rule, unless otherwise noted, the term "practitioner" is used to describe both physicians and nonphysician practitioners (NPPs) who are permitted to bill Medicare under the PFS for the services they furnish to Medicare beneficiaries.
1. Development of the RVUs
a. Work RVUs
The work RVUs established for the initial fee schedule, which was implemented on January 1, 1992, were developed with extensive input from the physician community. A research team at the Harvard School of Public Health developed the original work RVUs for most codes under a cooperative agreement with the Department of Health and Human Services (HHS). In constructing the code-specific vignettes used in determining the original physician work RVUs, Harvard worked with panels of experts, both inside and outside the federal government, and obtained input from numerous physician specialty groups.
As specified in section 1848(c)(1)(A) of the Act, the work component of physicians' services means the portion of the resources used in furnishing the service that reflects physician time and intensity. We establish work RVUs for new, revised and potentially misvalued codes based on our review of information that generally includes, but is not limited to, recommendations received from the American Medical Association/Specialty Society Relative Value Scale Update Committee (RUC), the Health Care Professionals Advisory Committee (HCPAC), the Medicare Payment Advisory Commission (MedPAC), and other public commenters; medical literature and comparative databases; as well as a comparison of the work for other codes within the Medicare PFS, and consultation with other physicians and health care professionals within CMS and the federal government. We also assess the methodology and data used to develop the recommendations submitted to us by the RUC and other public commenters, and the rationale for their recommendations. In the CY 2011 PFS final rule with comment period (75 FR 73328 through 73329), we discussed a variety of methodologies and approaches used to develop work RVUs, including survey data, building blocks, crosswalk to key reference or similar codes, and magnitude estimation. More information on these issues is available in that rule.
b. Practice Expense RVUs
Initially, only the work RVUs were resource-based, and the PE and MP RVUs were based on average allowable charges. Section 121 of the Social Security Act Amendments of 1994 (Pub. L. 103-432, October 31, 1994), amended by section 1848(c)(2)(C)(ii) of the Act and required us to develop resource-based PE RVUs for each physicians' service beginning in 1998. We were required to consider general categories of expenses (such as office rent and wages of personnel, but excluding MP expenses) comprising PEs. The PE RVUs continue to represent the portion of these resources involved in furnishing PFS services.
Originally, the resource-based method was to be used beginning in 1998, but section 4505(a) of the Balanced Budget Act of 1997 (BBA `97) (Pub. L. 105-33, August 5, 1997) delayed implementation of the resource-based PE RVU system until January 1, 1999. In addition, section 4505(b) of the BBA `97 provided for a 4-year transition period from the charge-based PE RVUs to the resource-based PE RVUs.
We established the resource-based PE RVUs for each physicians' service in the November 2, 1998 final rule (63 FR 58814), effective for services furnished in CY 1999. Based on the requirement to transition to a resource-based system for PE over a 4-year period, payment rates were not fully based upon resource-based PE RVUs until CY 2002. This resource-based system was based on two significant sources of actual PE data: The Clinical Practice Expert Panel (CPEP) data; and the AMA's Socioeconomic Monitoring System (SMS) data. These data sources are described in greater detail in the CY 2012 PFS final rule with comment period (76 FR 73033).
Separate PE RVUs are established for services furnished in facility settings, such as a hospital outpatient department (HOPD) or an ambulatory surgical center (ASC), and in nonfacility settings, such as a physician's office. The nonfacility RVUs reflect all of the direct and indirect PEs involved in furnishing a service described by a particular HCPCS code. The difference, if any, in these PE RVUs generally results in a higher payment in the nonfacility setting because in the facility settings some resource costs are borne by the facility. Medicare's payment to the facility (such as the outpatient prospective payment system (OPPS) payment to the HOPD) would reflect costs typically incurred by the facility. Thus, payment associated with those specific facility resource costs is not made under the PFS.
Section 212 of the Balanced Budget Refinement Act of 1999 (BBRA) (Pub. L. 106-113, November 29, 1999) directed the Secretary of Health and Human Services (the Secretary) to establish a process under which we accept and use, to the maximum extent practicable and consistent with sound data practices, data collected or developed by entities and organizations to supplement the data we normally collect in determining the PE component. On May 3, 2000, we published the interim final rule (65 FR 25664) that set forth the criteria for the submission of these supplemental PE survey data. The criteria were modified in response to comments received, and published in the Federal Register (65 FR 65376) as part of a November 1, 2000 final rule. The PFS final rules published in 2001 and 2003, respectively, (66 FR 55246 and 68 FR 63196) extended the period during which we would accept these supplemental data through March 1, 2005.
In the CY 2007 PFS final rule with comment period (71 FR 69624), we revised the methodology for calculating direct PE RVUs from the top-down to the bottom-up methodology beginning in CY 2007. We adopted a 4-year transition to the new PE RVUs. This transition was completed for CY 2010. In the CY 2010 PFS final rule with Start Printed Page 39107 comment period, we updated the practice expense per hour (PE/HR) data that are used in the calculation of PE RVUs for most specialties (74 FR 61749). In CY 2010, we began a 4-year transition to the new PE RVUs using the updated PE/HR data, which was completed for CY 2013.
c. Malpractice RVUs
Section 4505(f) of the BBA `97 amended section 1848(c) of the Act to require that we implement resource-based MP RVUs for services furnished on or after CY 2000. The resource-based MP RVUs were implemented in the PFS final rule with comment period published November 2, 1999 (64 FR 59380). The MP RVUs are based on commercial and physician-owned insurers' MP insurance premium data from all the states, the District of Columbia, and Puerto Rico.
d. Refinements to the RVUs
Section 1848(c)(2)(B)(i) of the Act requires that we review RVUs no less often than every 5 years. Prior to CY 2013, we conducted periodic reviews of work RVUs and PE RVUs independently from one another. We completed 5-year reviews of work RVUs that were effective for calendar years 1997, 2002, 2007, and 2012.
Although refinements to the direct PE inputs initially relied heavily on input from the RUC Practice Expense Advisory Committee (PEAC), the shifts to the bottom-up PE methodology in CY 2007 and to the use of the updated PE/HR data in CY 2010 have resulted in significant refinements to the PE RVUs in recent years.
In the CY 2012 PFS final rule with comment period (76 FR 73057), we finalized a proposal to consolidate reviews of work and PE RVUs under section 1848(c)(2)(B) of the Act and reviews of potentially misvalued codes under section 1848(c)(2)(K) of the Act into one annual process.
In addition to the 5-year reviews, beginning for CY 2009, CMS and the RUC identified and reviewed a number of potentially misvalued codes on an annual basis based on various identification screens. This annual review of work and PE RVUs for potentially misvalued codes was supplemented by the amendments to section 1848 of the Act, as enacted by section 3134 of the Affordable Care Act, that require the agency to periodically identify, review and adjust values for potentially misvalued codes.
e. Application of Budget Neutrality to Adjustments of RVUs
As described in section VII. of this proposed rule, the Regulatory Impact Analysis, in accordance with section 1848(c)(2)(B)(ii)(II) of the Act, if revisions to the RVUs cause expenditures for the year to change by more than $20 million, we will make adjustments to ensure that expenditures do not increase or decrease by more than $20 million.
2. Calculation of Payments Based on RVUs
To calculate the payment for each service, the components of the fee schedule (work, PE, and MP RVUs) are adjusted by geographic practice cost indices (GPCIs) to reflect the variations in the costs of furnishing the services. The GPCIs reflect the relative costs of work, PE, and MP in an area compared to the national average costs for each component. Please refer to the CY 2020 PFS final rule for a discussion of the last GPCI update (84 FR 62615 through 62623).
RVUs are converted to dollar amounts through the application of a CF, which is calculated based on a statutory formula by CMS' Office of the Actuary (OACT). The formula for calculating the Medicare PFS payment amount for a given service and fee schedule area can be expressed as:
Payment = [(RVU work × GPCI work) + (RVU PE × GPCI PE) + (RVU MP × GPCI MP)] × CF
3. Separate Fee Schedule Methodology for Anesthesia Services
Section 1848(b)(2)(B) of the Act specifies that the fee schedule amounts for anesthesia services are to be based on a uniform relative value guide, with appropriate adjustment of an anesthesia CF, in a manner to ensure that fee schedule amounts for anesthesia services are consistent with those for other services of comparable value. Therefore, there is a separate fee schedule methodology for anesthesia services. Specifically, we establish a separate CF for anesthesia services and we utilize the uniform relative value guide, or base units, as well as time units, to calculate the fee schedule amounts for anesthesia services. Since anesthesia services are not valued using RVUs, a separate methodology for locality adjustments is also necessary. This involves an adjustment to the national anesthesia CF for each payment locality.
B. Determination of PE RVUs
1. Overview
Practice expense (PE) is the portion of the resources used in furnishing a service that reflects the general categories of physician and practitioner expenses, such as office rent and personnel wages, but excluding MP expenses, as specified in section 1848(c)(1)(B) of the Act. As required by section 1848(c)(2)(C)(ii) of the Act, we use a resource-based system for determining PE RVUs for each physicians' service. We develop PE RVUs by considering the direct and indirect practice resources involved in furnishing each service. Direct expense categories include clinical labor, medical supplies, and medical equipment. Indirect expenses include administrative labor, office expense, and all other expenses. The sections that follow provide more detailed information about the methodology for translating the resources involved in furnishing each service into service-specific PE RVUs. We refer readers to the CY 2010 PFS final rule with comment period (74 FR 61743 through 61748) for a more detailed explanation of the PE methodology.
2. Practice Expense Methodology
a. Direct Practice Expense
We determine the direct PE for a specific service by adding the costs of the direct resources (that is, the clinical staff, medical supplies, and medical equipment) typically involved with furnishing that service. The costs of the resources are calculated using the refined direct PE inputs assigned to each CPT code in our PE database, which are generally based on our review of recommendations received from the RUC and those provided in response to public comment periods. For a detailed explanation of the direct PE methodology, including examples, we refer readers to the 5-year review of work RVUs under the PFS and proposed changes to the PE methodology CY 2007 PFS proposed notice (71 FR 37242) and the CY 2007 PFS final rule with comment period (71 FR 69629).
b. Indirect Practice Expense per Hour Data
We use survey data on indirect PEs incurred per hour worked, in developing the indirect portion of the PE RVUs. Prior to CY 2010, we primarily used the PE/HR by specialty that was obtained from the AMA's SMS. The AMA administered a new survey in CY 2007 and CY 2008, the Physician Practice Expense Information Survey (PPIS). The PPIS is a multispecialty, Start Printed Page 39108 nationally representative, PE survey of both physicians and NPPs paid under the PFS using a survey instrument and methods highly consistent with those used for the SMS and the supplemental surveys. The PPIS gathered information from 3,656 respondents across 51 physician specialty and health care professional groups. We believe the PPIS is the most comprehensive source of PE survey information available. We used the PPIS data to update the PE/HR data for the CY 2010 PFS for almost all of the Medicare-recognized specialties that participated in the survey.
When we began using the PPIS data in CY 2010, we did not change the PE RVU methodology itself or the manner in which the PE/HR data are used in that methodology. We only updated the PE/HR data based on the new survey. Furthermore, as we explained in the CY 2010 PFS final rule with comment period (74 FR 61751), because of the magnitude of payment reductions for some specialties resulting from the use of the PPIS data, we transitioned its use over a 4-year period from the previous PE RVUs to the PE RVUs developed using the new PPIS data. As provided in the CY 2010 PFS final rule with comment period (74 FR 61751), the transition to the PPIS data was complete for CY 2013. Therefore, PE RVUs from CY 2013 forward are developed based entirely on the PPIS data, except as noted in this section.
Section 1848(c)(2)(H)(i) of the Act requires us to use the medical oncology supplemental survey data submitted in 2003 for oncology drug administration services. Therefore, the PE/HR for medical oncology, hematology, and hematology/oncology reflects the continued use of these supplemental survey data.
Supplemental survey data on independent labs from the College of American Pathologists were implemented for payments beginning in CY 2005. Supplemental survey data from the National Coalition of Quality Diagnostic Imaging Services (NCQDIS), representing independent diagnostic testing facilities (IDTFs), were blended with supplementary survey data from the American College of Radiology (ACR) and implemented for payments beginning in CY 2007. Neither IDTFs, nor independent labs, participated in the PPIS. Therefore, we continue to use the PE/HR that was developed from their supplemental survey data.
Consistent with our past practice, the previous indirect PE/HR values from the supplemental surveys for these specialties were updated to CY 2006 using the Medicare Economic Index (MEI) to put them on a comparable basis with the PPIS data.
We also do not use the PPIS data for reproductive endocrinology and spine surgery since these specialties currently are not separately recognized by Medicare, nor do we have a method to blend the PPIS data with Medicare-recognized specialty data.
Previously, we established PE/HR values for various specialties without SMS or supplemental survey data by crosswalking them to other similar specialties to estimate a proxy PE/HR. For specialties that were part of the PPIS for which we previously used a crosswalked PE/HR, we instead used the PPIS-based PE/HR. We use crosswalks for specialties that did not participate in the PPIS. These crosswalks have been generally established through notice and comment rulemaking and are available in the file titled "CY 2022 PFS proposed rule PE/HR" on the CMS website under downloads for the CY 2022 PFS proposed rule at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html.
For CY 2022, we have incorporated the available utilization data for two new specialties, each of which became a recognized Medicare specialty during 2020. These specialties are Micrographic Dermatologic Surgery (MDS) and Adult Congenital Heart Disease (ACHD). We are proposing to use proxy PE/HR values for these new specialties, as there are no PPIS data for these specialties, by crosswalking the PE/HR as follows from specialties that furnish similar services in the Medicare claims data:
- Micrographic Dermatologic Surgery (MDS) from Dermatology; and
- Adult Congenital Heart Disease (ACHD from Cardiology.
These updates are reflected in the "CY 2022 PFS proposed rule PE/HR" file available on the CMS website under the supporting data files for the CY 2022 PFS proposed rule at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html.
c. Allocation of PE to Services
To establish PE RVUs for specific services, it is necessary to establish the direct and indirect PE associated with each service.
(1) Direct Costs
The relative relationship between the direct cost portions of the PE RVUs for any two services is determined by the relative relationship between the sum of the direct cost resources (that is, the clinical staff, medical supplies, and medical equipment) typically involved with furnishing each of the services. The costs of these resources are calculated from the refined direct PE inputs in our PE database. For example, if one service has a direct cost sum of $400 from our PE database and another service has a direct cost sum of $200, the direct portion of the PE RVUs of the first service would be twice as much as the direct portion of the PE RVUs for the second service.
(2) Indirect Costs
We allocate the indirect costs at the code level based on the direct costs specifically associated with a code and the greater of either the clinical labor costs or the work RVUs. We also incorporate the survey data described earlier in the PE/HR discussion. The general approach to developing the indirect portion of the PE RVUs is as follows:
- For a given service, we use the direct portion of the PE RVUs calculated as previously described and the average percentage that direct costs represent of total costs (based on survey data) across the specialties that furnish the service to determine an initial indirect allocator. That is, the initial indirect allocator is calculated so that the direct costs equal the average percentage of direct costs of those specialties furnishing the service. For example, if the direct portion of the PE RVUs for a given service is 2.00 and direct costs, on average, represent 25 percent of total costs for the specialties that furnish the service, the initial indirect allocator would be calculated so that it equals 75 percent of the total PE RVUs. Thus, in this example, the initial indirect allocator would equal 6.00, resulting in a total PE RVU of 8.00 (2.00 is 25 percent of 8.00 and 6.00 is 75 percent of 8.00).
- Next, we add the greater of the work RVUs or clinical labor portion of the direct portion of the PE RVUs to this initial indirect allocator. In our example, if this service had a work RVU of 4.00 and the clinical labor portion of the direct PE RVU was 1.50, we would add 4.00 (since the 4.00 work RVUs are greater than the 1.50 clinical labor portion) to the initial indirect allocator of 6.00 to get an indirect allocator of 10.00. In the absence of any further use of the survey data, the relative relationship between the indirect cost portions of the PE RVUs for any two services would be determined by the relative relationship between these indirect cost allocators. For example, if one service had an indirect cost allocator of 10.00 and another service had an indirect cost allocator of 5.00, Start Printed Page 39109 the indirect portion of the PE RVUs of the first service would be twice as great as the indirect portion of the PE RVUs for the second service.
- Then, we incorporate the specialty-specific indirect PE/HR data into the calculation. In our example, if, based on the survey data, the average indirect cost of the specialties furnishing the first service with an allocator of 10.00 was half of the average indirect cost of the specialties furnishing the second service with an indirect allocator of 5.00, the indirect portion of the PE RVUs of the first service would be equal to that of the second service.
(3) Facility and Nonfacility Costs
For procedures that can be furnished in a physician's office, as well as in a facility setting, where Medicare makes a separate payment to the facility for its costs in furnishing a service, we establish two PE RVUs: Facility and nonfacility. The methodology for calculating PE RVUs is the same for both the facility and nonfacility RVUs, but is applied independently to yield two separate PE RVUs. In calculating the PE RVUs for services furnished in a facility, we do not include resources that would generally not be provided by physicians when furnishing the service. For this reason, the facility PE RVUs are generally lower than the nonfacility PE RVUs.
(4) Services With Technical Components and Professional Components
Diagnostic services are generally comprised of two components: A professional component (PC); and a technical component (TC). The PC and TC may be furnished independently or by different providers, or they may be furnished together as a global service. When services have separately billable PC and TC components, the payment for the global service equals the sum of the payment for the TC and PC. To achieve this, we use a weighted average of the ratio of indirect to direct costs across all the specialties that furnish the global service, TCs, and PCs; that is, we apply the same weighted average indirect percentage factor to allocate indirect expenses to the global service, PCs, and TCs for a service. (The direct PE RVUs for the TC and PC sum to the global.)
(5) PE RVU Methodology
For a more detailed description of the PE RVU methodology, we refer readers to the CY 2010 PFS final rule with comment period (74 FR 61745 through 61746). We also direct readers to the file titled "Calculation of PE RVUs under Methodology for Selected Codes" which is available on our website under downloads for the CY 2022 PFS proposed rule at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html. This file contains a table that illustrates the calculation of PE RVUs as described in this proposed rule for individual codes.
(a) Setup File
First, we create a setup file for the PE methodology. The setup file contains the direct cost inputs, the utilization for each procedure code at the specialty and facility/nonfacility place of service level, and the specialty-specific PE/HR data calculated from the surveys.
(b) Calculate the Direct Cost PE RVUs
Sum the costs of each direct input.
Step 1: Sum the direct costs of the inputs for each service.
Step 2: Calculate the aggregate pool of direct PE costs for the current year. We set the aggregate pool of PE costs equal to the product of the ratio of the current aggregate PE RVUs to current aggregate work RVUs and the projected aggregate work RVUs.
Step 3: Calculate the aggregate pool of direct PE costs for use in ratesetting. This is the product of the aggregate direct costs for all services from Step 1 and the utilization data for that service.
Step 4: Using the results of Step 2 and Step 3, use the CF to calculate a direct PE scaling adjustment to ensure that the aggregate pool of direct PE costs calculated in Step 3 does not vary from the aggregate pool of direct PE costs for the current year. Apply the scaling adjustment to the direct costs for each service (as calculated in Step 1).
Step 5: Convert the results of Step 4 to a RVU scale for each service. To do this, divide the results of Step 4 by the CF. Note that the actual value of the CF used in this calculation does not influence the final direct cost PE RVUs as long as the same CF is used in Step 4 and Step 5. Different CFs would result in different direct PE scaling adjustments, but this has no effect on the final direct cost PE RVUs since changes in the CFs and changes in the associated direct scaling adjustments offset one another.
(c) Create the Indirect Cost PE RVUs
Create indirect allocators.
Step 6: Based on the survey data, calculate direct and indirect PE percentages for each physician specialty.
Step 7: Calculate direct and indirect PE percentages at the service level by taking a weighted average of the results of Step 6 for the specialties that furnish the service. Note that for services with TCs and PCs, the direct and indirect percentages for a given service do not vary by the PC, TC, and global service.
We generally use an average of the 3 most recent years of available Medicare claims data to determine the specialty mix assigned to each code. Codes with low Medicare service volume require special attention since billing or enrollment irregularities for a given year can result in significant changes in specialty mix assignment. We finalized a policy in the CY 2018 PFS final rule (82 FR 52982 through 59283) to use the most recent year of claims data to determine which codes are low volume for the coming year (those that have fewer than 100 allowed services in the Medicare claims data). For codes that fall into this category, instead of assigning specialty mix based on the specialties of the practitioners reporting the services in the claims data, we use the expected specialty that we identify on a list developed based on medical review and input from expert stakeholders. We display this list of expected specialty assignments as part of the annual set of data files we make available as part of notice and comment rulemaking and consider recommendations from the RUC and other stakeholders on changes to this list on an annual basis. Services for which the specialty is automatically assigned based on previously finalized policies under our established methodology (for example, "always therapy" services) are unaffected by the list of expected specialty assignments. We also finalized in the CY 2018 PFS final rule (82 FR 52982 through 59283) a policy to apply these service-level overrides for both PE and MP, rather than one or the other category.
Step 8: Calculate the service level allocators for the indirect PEs based on the percentages calculated in Step 7. The indirect PEs are allocated based on the three components: the direct PE RVUs; the clinical labor PE RVUs; and the work RVUs.
For most services the indirect allocator is: Indirect PE percentage * (direct PE RVUs/direct percentage) + work RVUs.
There are two situations where this formula is modified:
- If the service is a global service (that is, a service with global, professional, and technical components), then the indirect PE allocator is: Indirect percentage (direct PE RVUs/direct percentage) + clinical labor PE RVUs + work RVUs.
- If the clinical labor PE RVUs exceed the work RVUs (and the service is not a global service), then the indirect Start Printed Page 39110 allocator is: Indirect PE percentage (direct PE RVUs/direct percentage) + clinical labor PE RVUs.
(Note: For global services, the indirect PE allocator is based on both the work RVUs and the clinical labor PE RVUs. We do this to recognize that, for the PC service, indirect PEs would be allocated using the work RVUs, and for the TC service, indirect PEs would be allocated using the direct PE RVUs and the clinical labor PE RVUs. This also allows the global component RVUs to equal the sum of the PC and TC RVUs.)
For presentation purposes, in the examples in the download file titled "Calculation of PE RVUs under Methodology for Selected Codes", the formulas were divided into two parts for each service.
- The first part does not vary by service and is the indirect percentage (direct PE RVUs/direct percentage).
- The second part is either the work RVU, clinical labor PE RVU, or both depending on whether the service is a global service and whether the clinical PE RVUs exceed the work RVUs (as described earlier in this step).
Apply a scaling adjustment to the indirect allocators.
Step 9: Calculate the current aggregate pool of indirect PE RVUs by multiplying the result of step 8 by the average indirect PE percentage from the survey data.
Step 10: Calculate an aggregate pool of indirect PE RVUs for all PFS services by adding the product of the indirect PE allocators for a service from Step 8 and the utilization data for that service.
Step 11: Using the results of Step 9 and Step 10, calculate an indirect PE adjustment so that the aggregate indirect allocation does not exceed the available aggregate indirect PE RVUs and apply it to indirect allocators calculated in Step 8.
Calculate the indirect practice cost index.
Step 12: Using the results of Step 11, calculate aggregate pools of specialty-specific adjusted indirect PE allocators for all PFS services for a specialty by adding the product of the adjusted indirect PE allocator for each service and the utilization data for that service.
Step 13: Using the specialty-specific indirect PE/HR data, calculate specialty-specific aggregate pools of indirect PE for all PFS services for that specialty by adding the product of the indirect PE/HR for the specialty, the work time for the service, and the specialty's utilization for the service across all services furnished by the specialty.
Step 14: Using the results of Step 12 and Step 13, calculate the specialty-specific indirect PE scaling factors.
Step 15: Using the results of Step 14, calculate an indirect practice cost index at the specialty level by dividing each specialty-specific indirect scaling factor by the average indirect scaling factor for the entire PFS.
Step 16: Calculate the indirect practice cost index at the service level to ensure the capture of all indirect costs. Calculate a weighted average of the practice cost index values for the specialties that furnish the service. (Note: For services with TCs and PCs, we calculate the indirect practice cost index across the global service, PCs, and TCs. Under this method, the indirect practice cost index for a given service (for example, echocardiogram) does not vary by the PC, TC, and global service.)
Step 17: Apply the service level indirect practice cost index calculated in Step 16 to the service level adjusted indirect allocators calculated in Step 11 to get the indirect PE RVUs.
(d) Calculate the Final PE RVUs
Step 18: Add the direct PE RVUs from Step 5 to the indirect PE RVUs from Step 17 and apply the final PE budget neutrality (BN) adjustment. The final PE BN adjustment is calculated by comparing the sum of steps 5 and 17 to the aggregate work RVUs scaled by the ratio of current aggregate PE and work RVUs. This adjustment ensures that all PE RVUs in the PFS account for the fact that certain specialties are excluded from the calculation of PE RVUs but included in maintaining overall PFS budget neutrality. (See "Specialties excluded from ratesetting calculation" later in this final rule.)
Step 19: Apply the phase-in of significant RVU reductions and its associated adjustment. Section 1848(c)(7) of the Act specifies that for services that are not new or revised codes, if the total RVUs for a service for a year would otherwise be decreased by an estimated 20 percent or more as compared to the total RVUs for the previous year, the applicable adjustments in work, PE, and MP RVUs shall be phased in over a 2-year period. In implementing the phase-in, we consider a 19 percent reduction as the maximum 1-year reduction for any service not described by a new or revised code. This approach limits the year one reduction for the service to the maximum allowed amount (that is, 19 percent), and then phases in the remainder of the reduction. To comply with section 1848(c)(7) of the Act, we adjust the PE RVUs to ensure that the total RVUs for all services that are not new or revised codes decrease by no more than 19 percent, and then apply a relativity adjustment to ensure that the total pool of aggregate PE RVUs remains relative to the pool of work and MP RVUs. For a more detailed description of the methodology for the phase-in of significant RVU changes, we refer readers to the CY 2016 PFS final rule with comment period (80 FR 70927 through 70931).
(e) Setup File Information
- Specialties excluded from ratesetting calculation: For the purposes of calculating the PE and MP RVUs, we exclude certain specialties, such as certain NPPs paid at a percentage of the PFS and low-volume specialties, from the calculation. These specialties are included for the purposes of calculating the BN adjustment. They are displayed in Table 1.
Start Printed Page 39111

- Crosswalk certain low volume physician specialties: Crosswalk the utilization of certain specialties with relatively low PFS utilization to the associated specialties.
- Physical therapy utilization: Crosswalk the utilization associated with all physical therapy services to the specialty of physical therapy.
- Identify professional and technical services not identified under the usual TC and 26 modifiers: Flag the services that are PC and TC services but do not use TC and 26 modifiers (for example, electrocardiograms). This flag associates the PC and TC with the associated global code for use in creating the indirect PE RVUs. For example, the professional service, CPT code 93010 (Electrocardiogram, routine ECG with at least 12 leads; interpretation and report only), is associated with the global service, CPT code 93000 (Electrocardiogram, routine ECG with at least 12 leads; with interpretation and report).
- Payment modifiers: Payment modifiers are accounted for in the creation of the file consistent with current payment policy as implemented in claims processing. For example, services billed with the assistant at surgery modifier are paid 16 percent of the PFS amount for that service; therefore, the utilization file is modified to only account for 16 percent of any service that contains the assistant at surgery modifier. Similarly, for those services to which volume adjustments are made to account for the payment modifiers, time adjustments are applied as well. For time adjustments to surgical services, the intraoperative portion in the work time file is used; where it is not present, the intraoperative percentage from the payment files used by contractors to process Medicare claims is used instead. Where neither is available, we use the payment adjustment ratio to adjust the time accordingly. Table 2 details the manner in which the modifiers are applied.
Start Printed Page 39112

We also make adjustments to volume and time that correspond to other payment rules, including special multiple procedure endoscopy rules and multiple procedure payment reductions (MPPRs). We note that section 1848(c)(2)(B)(v) of the Act exempts certain reduced payments for multiple imaging procedures and multiple therapy services from the BN calculation under section 1848(c)(2)(B)(ii)(II) of the Act. These MPPRs are not included in the development of the RVUs.
Beginning in CY 2022, section 1834(v)(1) of the Act requires that we apply a 15 percent payment reduction for outpatient occupational therapy services and outpatient physical therapy services that are provided, in whole or in part, by a physical therapist assistant (PTA) or occupational therapy assistant (OTA). Section 1834(v)(2)(A) of the Act required CMS to establish modifiers to identify these services, which we did in the CY 2019 PFS final rule (83 FR 59654 through 59661), creating the CQ and CO payment modifiers for services provided in whole or in part by PTAs and OTAs, respectively. These payment modifiers are required to be used on claims for services with dates of service beginning January 1, 2020, as specified in the CY 2020 PFS final rule (84 FR 62702 through 62708). We will apply the 15 percent payment reduction to therapy services provided by PTAs (using the CQ modifier) or OTAs (using the CO modifier), as required by statute. Under sections 1834(k) and 1848 of the Act, payment is made for outpatient therapy services at 80 percent of the lesser of the actual charge or applicable fee schedule amount (the allowed charge). The remaining 20 percent is the beneficiary copayment. For therapy services to which the new discount applies, payment will be made at 85 percent of the 80 percent of allowed charges. Therefore, the volume discount factor for therapy services to which the CQ and CO modifiers apply is: (0.20 + (0.80* 0.85), which equals 88 percent.
For anesthesia services, we do not apply adjustments to volume since we use the average allowed charge when simulating RVUs; therefore, the RVUs as calculated already reflect the payments as adjusted by modifiers, and no volume adjustments are necessary. However, a time adjustment of 33 percent is made only for medical direction of two to four cases since that is the only situation where a single practitioner is involved with multiple beneficiaries concurrently, so that counting each service without regard to the overlap with other services would overstate the amount of time spent by the practitioner furnishing these services.
- Work RVUs: The setup file contains the work RVUs from this final rule.
(6) Equipment Cost per Minute
The equipment cost per minute is calculated as:
(1/(minutes per year * usage)) * price * ((interest rate/(1−(1/((1 + interest rate)∧ life of equipment)))) + maintenance)
Where:
minutes per year = maximum minutes per year if usage were continuous (that is, usage = 1); generally 150,000 minutes.
usage = variable, see discussion below in this proposed rule.
price = price of the particular piece of equipment.
life of equipment = useful life of the particular piece of equipment.
maintenance = factor for maintenance; 0.05.
interest rate = variable, see discussion below in this proposed rule.
Usage: We currently use an equipment utilization rate assumption of 50 percent for most equipment, with the exception of expensive diagnostic imaging equipment, for which we use a 90 percent assumption as required by section 1848(b)(4)(C) of the Act.
Useful Life: In the CY 2005 PFS final rule we stated that we updated the useful life for equipment items primarily based on the AHA's "Estimated Useful Lives of Depreciable Hospital Assets" guidelines (69 FR 66246). The most recent edition of these guidelines was published in 2018. This reference material provides an estimated useful life for hundreds of different types of equipment, the vast majority of which fall in the range of 5 to 10 years, and none of which are lower than 2 years in duration. We believe that the updated editions of this reference material remain the most accurate source for estimating the useful life of depreciable medical equipment. Start Printed Page 39113
In the CY 2021 PFS final rule, we finalized a proposal to treat equipment life durations of less than 1 year as having a duration of 1 year for the purpose of our equipment price per minute formula. In the rare cases where items are replaced every few months, we noted that we believe it is more accurate to treat these items as disposable supplies with a fractional supply quantity as opposed to equipment items with very short equipment life durations. For a more detailed discussion of the methodology associated with very short equipment life durations, we refer readers to the CY 2021 PFS final rule (85 FR 84482 through 84483).
- Maintenance: We finalized the 5 percent factor for annual maintenance in the CY 1998 PFS final rule with comment period (62 FR 33164). As we previously stated in the CY 2016 PFS final rule with comment period (80 FR 70897), we do not believe the annual maintenance factor for all equipment is precisely 5 percent, and we concur that the current rate likely understates the true cost of maintaining some equipment. We also noted that we believe it likely overstates the maintenance costs for other equipment. When we solicited comments regarding sources of data containing equipment maintenance rates, commenters were unable to identify an auditable, robust data source that could be used by CMS on a wide scale. We noted that we did not believe voluntary submissions regarding the maintenance costs of individual equipment items would be an appropriate methodology for determining costs. As a result, in the absence of publicly available datasets regarding equipment maintenance costs or another systematic data collection methodology for determining a different maintenance factor, we did not propose a variable maintenance factor for equipment cost per minute pricing as we did not believe that we have sufficient information at present. We noted that we would continue to investigate potential avenues for determining equipment maintenance costs across a broad range of equipment items.
- Interest Rate: In the CY 2013 PFS final rule with comment period (77 FR 68902), we updated the interest rates used in developing an equipment cost per minute calculation (see 77 FR 68902 for a thorough discussion of this issue). The interest rate was based on the Small Business Administration (SBA) maximum interest rates for different categories of loan size (equipment cost) and maturity (useful life). The Interest rates are listed in Table 3.
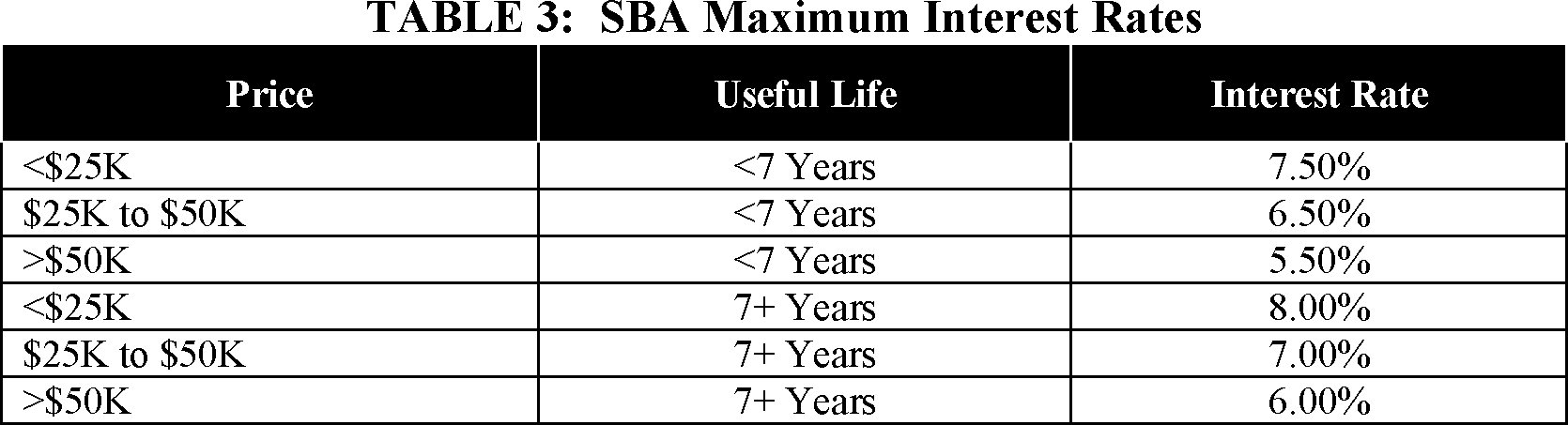
We are not proposing any changes to the equipment interest rates for CY 2022.
3. Changes to Direct PE Inputs for Specific Services
This section focuses on specific PE inputs. The direct PE inputs are included in the CY 2022 direct PE input public use files, which are available on the CMS website under downloads for the CY 2022 PFS proposed rule at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html.
a. Standardization of Clinical Labor Tasks
As we noted in the CY 2015 PFS final rule with comment period (79 FR 67640 through 67641), we continue to make improvements to the direct PE input database to provide the number of clinical labor minutes assigned for each task for every code in the database instead of only including the number of clinical labor minutes for the preservice, service, and post service periods for each code. In addition to increasing the transparency of the information used to set PE RVUs, this level of detail would allow us to compare clinical labor times for activities associated with services across the PFS, which we believe is important to maintaining the relativity of the direct PE inputs. This information would facilitate the identification of the usual numbers of minutes for clinical labor tasks and the identification of exceptions to the usual values. It would also allow for greater transparency and consistency in the assignment of equipment minutes based on clinical labor times. Finally, we believe that the detailed information can be useful in maintaining standard times for particular clinical labor tasks that can be applied consistently to many codes as they are valued over several years, similar in principle to the use of physician preservice time packages. We believe that setting and maintaining such standards would provide greater consistency among codes that share the same clinical labor tasks and could improve relativity of values among codes. For example, as medical practice and technologies change over time, changes in the standards could be updated simultaneously for all codes with the applicable clinical labor tasks, instead of waiting for individual codes to be reviewed.
In the CY 2016 PFS final rule with comment period (80 FR 70901), we solicited comments on the appropriate standard minutes for the clinical labor tasks associated with services that use digital technology. After consideration of comments received, we finalized standard times for clinical labor tasks associated with digital imaging at 2 minutes for "Availability of prior images confirmed", 2 minutes for "Patient clinical information and questionnaire reviewed by technologist, order from physician confirmed and exam protocoled by radiologist", 2 minutes for "Review examination with interpreting MD", and 1 minute for "Exam documents scanned into PACS" and "Exam completed in RIS system to generate billing process and to populate images into Radiologist work queue." In the CY 2017 PFS final rule (81 FR 80184 through 80186), we finalized a policy to establish a range of appropriate standard minutes for the clinical labor activity, "Technologist QCs images in PACS, checking for all images, reformats, and dose page." These standard minutes will be applied to new and revised Start Printed Page 39114 codes that make use of this clinical labor activity when they are reviewed by us for valuation. We finalized a policy to establish 2 minutes as the standard for the simple case, 3 minutes as the standard for the intermediate case, 4 minutes as the standard for the complex case, and 5 minutes as the standard for the highly complex case. These values were based upon a review of the existing minutes assigned for this clinical labor activity; we determined that 2 minutes is the duration for most services and a small number of codes with more complex forms of digital imaging have higher values. We also finalized standard times for a series of clinical labor tasks associated with pathology services in the CY 2016 PFS final rule with comment period (80 FR 70902). We do not believe these activities would be dependent on number of blocks or batch size, and we believe that the finalized standard values accurately reflect the typical time it takes to perform these clinical labor tasks.
In reviewing the RUC-recommended direct PE inputs for CY 2019, we noticed that the 3 minutes of clinical labor time traditionally assigned to the "Prepare room, equipment and supplies" (CA013) clinical labor activity were split into 2 minutes for the "Prepare room, equipment and supplies" activity and 1 minute for the "Confirm order, protocol exam" (CA014) activity. We proposed to maintain the 3 minutes of clinical labor time for the "Prepare room, equipment and supplies" activity and remove the clinical labor time for the "Confirm order, protocol exam" activity wherever we observed this pattern in the RUC-recommended direct PE inputs. Commenters explained in response that when the new version of the PE worksheet introduced the activity codes for clinical labor, there was a need to translate old clinical labor tasks into the new activity codes, and that a prior clinical labor task was split into two of the new clinical labor activity codes: CA007 (Review patient clinical extant information and questionnaire) in the preservice period, and CA014 (Confirm order, protocol exam) in the service period. Commenters stated that the same clinical labor from the old PE worksheet was now divided into the CA007 and CA014 activity codes, with a standard of 1 minute for each activity. We agreed with commenters that we would finalize the RUC-recommended 2 minutes of clinical labor time for the CA007 activity code and 1 minute for the CA014 activity code in situations where this was the case. However, when reviewing the clinical labor for the reviewed codes affected by this issue, we found that several of the codes did not include this old clinical labor task, and we also noted that several of the reviewed codes that contained the CA014 clinical labor activity code did not contain any clinical labor for the CA007 activity. In these situations, we continue to believe that in these cases, the 3 total minutes of clinical staff time would be more accurately described by the CA013 "Prepare room, equipment and supplies" activity code, and we finalized these clinical labor refinements. For additional details, we direct readers to the discussion in the CY 2019 PFS final rule (83 FR 59463 and 59464).
Following the publication of the CY 2020 PFS proposed rule, a commenter expressed concern with the published list of common refinements to equipment time. The commenter stated that these refinements were the formulaic result of the applying refinements to the clinical labor time and did not constitute separate refinements; the commenter requested that CMS no longer include these refinements in the table published each year. In the CY 2020 PFS final rule, we agreed with the commenter that these equipment time refinements did not reflect errors in the equipment recommendations or policy discrepancies with the RUC's equipment time recommendations. However, we believed that it was important to publish the specific equipment times that we were proposing (or finalizing in the case of the final rule) when they differed from the recommended values due to the effect that these changes can have on the direct costs associated with equipment time. Therefore, we finalized the separation of the equipment time refinements associated with changes in clinical labor into a separate table of refinements. For additional details, we direct readers to the discussion in the CY 2020 PFS final rule (84 FR 62584).
Historically, the RUC has submitted a "PE worksheet" that details the recommended direct PE inputs for our use in developing PE RVUs. The format of the PE worksheet has varied over time and among the medical specialties developing the recommendations. These variations have made it difficult for both the RUC's development and our review of code values for individual codes. Beginning with its recommendations for CY 2019, the RUC has mandated the use of a new PE worksheet for purposes of their recommendation development process that standardizes the clinical labor tasks and assigns them a clinical labor activity code. We believe the RUC's use of the new PE worksheet in developing and submitting recommendations will help us to simplify and standardize the hundreds of different clinical labor tasks currently listed in our direct PE database. As we did in previous calendar years, to facilitate rulemaking for CY 2022, we are continuing to display two versions of the Labor Task Detail public use file: one version with the old listing of clinical labor tasks, and one with the same tasks crosswalked to the new listing of clinical labor activity codes. These lists are available on the CMS website under downloads for the CY 2022 PFS proposed rule at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html.
b. Technical Corrections to Direct PE Input Database and Supporting Files
For CY 2022, we are proposing to address the following:
- Following the publication of the CY 2021 PFS proposed rule, several commenters questioned the proposed RVUs associated with several occupational therapy evaluation procedures (CPT codes 97165 through 97167). Commenters stated that the PE valuation for these codes appeared to be illogical as it was counterintuitive for the PE RVU to go down as the level of complexity increased. Commenters stated that the distribution of code usage has not changed in any manner to justify a reduction in the code values and that all three evaluation codes should reimburse at the same rate. In response to the commenters, we noted that although the three codes in question shared the same work RVU and the same direct PE inputs, they did not share the same specialty distribution in the claims data and therefore would not necessarily receive the same allocation of indirect PE. In the CY 2021 PFS final rule (85 FR 84490), we finalized the implementation of a technical change intended to ensure that these three services received the same allocation of indirect PE. We agreed with commenters that it was important to avoid a potential rank order anomaly in which the simple case for a service was valued higher than the complex case.
After the publication of the CY 2021 PFS final rule, stakeholders stated their appreciation for the technical change made in the final rule to ensure that the indirect PE allocation was the same for all three levels of occupational therapy evaluation codes. However, stakeholders expressed concern that the PE RVUs we finalized for CPT codes Start Printed Page 39115 97165-97167 decreased as compared to the PE RVUs we proposed for CY 2021. Stakeholders stated that nothing had occurred in the past year that would account for a reduction to the proposed PE for these codes, especially in a year where the proposed PE increased for the corresponding physical therapy evaluation procedures (CPT codes 97161-97163), and stakeholders questioned whether there had been an error in applying the indirect PE methodology.
We reviewed the indirect PE allocation for CPT codes 97165-97167 in response to the stakeholder inquiry and we do not agree that there was an error in applying the indirect PE methodology. We finalized a technical change in the CY 2021 PFS final rule intended to ensure that these three services received the same allocation of indirect PE, which achieved its desired goal of assigning equivalent indirect PE to these three services. However, by forcing CPT codes 97165-97167 to have the same indirect PE allocation, the indirect PE values for these codes no longer relied on the claims data, which ended up affecting the indirect practice cost index for the wider occupational therapy specialty. Because CPT codes 97165-97167 are high volume services, this resulted in a lower indirect practice cost index for the occupational therapy specialty and a smaller allocation of indirect PE for CY 2021 than initially proposed.
We are addressing this issue for CY 2022 by proposing to assign all claims data associated with CPT codes 97165-97167 to the occupational therapy specialty. This should ensure that CPT codes 97165-97167 would always receive the same indirect PE allocation as well as preventing any fluctuations to the indirect practice cost index for the wider occupational therapy specialty. This proposal is intended to avoid a potential rank order anomaly in which the simple case for a service is valued higher than the complex case. As the utilization for CPT codes 97165-97167 is overwhelmingly identified as performed by occupational therapists, we do not anticipate that assigning all of the claims data for these codes to the occupational therapy specialty will have a noticeable effect on their valuation. We are soliciting public comments regarding this proposal, and specifically on what commenters suggest as the most appropriate method of assigning indirect PE allocation for these services.
- In the CY 2020 PFS final rule (84 FR 63102 through 63104), we created two new HCPCS G codes, G2082 and G2083, effective January 1, 2020 on an interim final basis for the provision of self-administered esketamine. In the CY 2021 PFS final rule, we finalized a proposal to refine the values for HCPCS codes G2082 and G2083 using a building block methodology that summed the values associated with several codes (85 FR 84641 through 84642). Following the publication of the CY 2021 PFS final rule, stakeholders expressed their concern that the finalized PE RVU had decreased for HCPCS codes G2082 and G2083 as compared to the proposed valuation and as compared to the previous CY 2020 interim final valuation. Stakeholders questioned whether there had been an error in the PE allocation since CMS had finalized increases in the direct PE inputs for the services.
We reviewed the indirect PE allocation for HCPCS codes G2082 and G2083 in response to the stakeholder inquiry and discovered a technical change that was applied in error. Specifically, we inadvertently assigned a different physician specialty than we intended ("All Physicians") to HCPCS codes G2082 and G2083 for indirect PE allocation in our ratesetting process during valuation of these codes in the CY 2020 PFS final rule, and continued that assignment into the CY 2021 PFS proposed rule. This specialty assignment caused the PE value for these services to be higher than anticipated for CY 2020. We intended to revise the assigned physician specialty for these codes to "General Practice" in the CY 2021 PFS final rule; however, we neglected to discuss this change in the course of PFS rulemaking for CY 2021. Since we initially applied this technical change in the CY 2021 PFS final rule without providing an explanation, we issued a correction notice (86 FR 14690) to remove this change from the CY 2021 PFS final rule, and to instead maintain the All Physicians specialty assignment through CY 2021. We apologize for any confusion this may have caused.
For CY 2022, we are proposing to maintain the currently assigned physician specialty for indirect PE allocation for HCPCS codes G2082 and G2083. We are proposing to assign these two services to the All Physicians specialty for indirect PE allocation which will maintain payment consistency with the rates published in the CY 2020 PFS final rule and the CY 2021 PFS proposed rule. Although we had previously intended to assign the General Practice specialty to these codes, stakeholders have provided additional information about these services suggesting that maintaining the All Physicians specialty assignment for these codes will help maintain payment stability and preserve access to this care for beneficiaries. We are soliciting public comments to help us discern which specialty would be the most appropriate to use for indirect PE allocation for HCPCS codes G2082 and G2083. We note that the PE methodology, which relies on the allocation of indirect costs based on the magnitude of direct costs, should appropriately reflect the typical costs for the specialty the commenters suggest. For example, we do not believe it would be appropriate to assign the Psychiatry specialty for these services given that HCPCS codes G2082 and G2083 include the high direct costs associated with esketamine supplies. The Psychiatry specialty is an outlier compared to most other specialties, allocating indirect costs at a 15:1 ratio based on direct costs because psychiatry services typically have very low direct costs. Assignment of most other specialties would result in allocation of direct costs at roughly a 3:1 ratio. We request that commenters explain in their comments how the indirect PE allocation would affect the payment for these services. Specifically, to ensure appropriate payment for HCPCS codes G2082 and G2083, we would like to get a better understanding of the indirect costs associated with these services, relative to other services furnished by the suggested specialty.
- A stakeholder contacted us regarding a potential error involving the intraservice work time for CPT code 35860 (Exploration for postoperative hemorrhage, thrombosis or infection; extremity). The stakeholder stated that the RUC recommended an intraservice work time of 90 minutes for this code when it was last reviewed in the CY 2012 PFS final rule and we finalized the work time without refinement at 60 minutes (76 FR 73131). The stakeholder requested that the intraservice work time for CPT code 35860 should be updated to 90 minutes.
We reviewed the intraservice work time for CPT code 35860 and found that the RUC inadvertently recommended a time of 60 minutes for the code, which we proposed and finalized without comment in rulemaking for the CY 2012 PFS. As a result, we do not believe that this is a technical error on our part. However, since the stakeholder has clarified that the RUC intended to recommend 90 minutes of intraservice work time for CPT code 35860 based on the surveyed median time, we are proposing to update the intraservice work time to 90 minutes to match the survey results. Start Printed Page 39116
c. Updates to Prices for Existing Direct PE Inputs
In the CY 2011 PFS final rule with comment period (75 FR 73205), we finalized a process to act on public requests to update equipment and supply price and equipment useful life inputs through annual rulemaking, beginning with the CY 2012 PFS proposed rule. For CY 2022, we are proposing to update the price of six supplies and two equipment items in response to the public submission of invoices. Since this is the final year of the supply and equipment pricing update, the new pricing for each of these supply and equipment items will take effect for CY 2022 as there are no remaining years of the transition. The six supply and equipment items with proposed updated prices are listed in the valuation of specific codes section of the preamble under Table 16: CY 2022 Invoices Received for Existing Direct PE Inputs.
(1) Market-Based Supply and Equipment Pricing Update
Section 220(a) of the Protecting Access to Medicare Act of 2014 (PAMA) (Pub. L. 113-93, April 1, 2014) provides that the Secretary may collect or obtain information from any eligible professional or any other source on the resources directly or indirectly related to furnishing services for which payment is made under the PFS, and that such information may be used in the determination of relative values for services under the PFS. Such information may include the time involved in furnishing services; the amounts, types and prices of PE inputs; overhead and accounting information for practices of physicians and other suppliers, and any other elements that would improve the valuation of services under the PFS.
As part of our authority under section 1848(c)(2)(M) of the Act, we initiated a market research contract with StrategyGen to conduct an in-depth and robust market research study to update the PFS direct PE inputs (DPEI) for supply and equipment pricing for CY 2019. These supply and equipment prices were last systematically developed in 2004-2005. StrategyGen submitted a report with updated pricing recommendations for approximately 1300 supplies and 750 equipment items currently used as direct PE inputs. This report is available as a public use file displayed on the CMS website under downloads for the CY 2019 PFS final rule at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html.
The StrategyGen team of researchers, attorneys, physicians, and health policy experts conducted a market research study of the supply and equipment items currently used in the PFS direct PE input database. Resources and methodologies included field surveys, aggregate databases, vendor resources, market scans, market analysis, physician substantiation, and statistical analysis to estimate and validate current prices for medical equipment and medical supplies. StrategyGen conducted secondary market research on each of the 2,072 DPEI medical equipment and supply items that CMS identified from the current DPEI. The primary and secondary resources StrategyGen used to gather price data and other information were:
- Telephone surveys with vendors for top priority items (Vendor Survey).
- Physician panel validation of market research results, prioritized by total spending (Physician Panel).
- The General Services Administration system (GSA).
- An aggregate health system buyers database with discounted prices (Buyers).
- Publicly available vendor resources, that is, Amazon Business, Cardinal Health (Vendors).
- The Federal Register, current DPEI data, historical proposed and final rules prior to CY 2018, and other resources; that is, AMA RUC reports (References).
StrategyGen prioritized the equipment and supply research based on current share of PE RVUs attributable by item provided by CMS. StrategyGen developed the preliminary Recommended Price (RP) methodology based on the following rules in hierarchical order considering both data representativeness and reliability.
(1) If the market share, as well as the sample size, for the top three commercial products were available, the weighted average price (weighted by percent market share) was the reported RP. Commercial price, as a weighted average of market share, represents a more robust estimate for each piece of equipment and a more precise reference for the RP.
(2) If no data were available for commercial products, the current CMS prices were used as the RP.
GSA prices were not used to calculate the StrategyGen recommended prices, due to our concern that the GSA system curtails the number and type of suppliers whose products may be accessed on the GSA Advantage website, and that the GSA prices may often be lower than prices that are available to non-governmental purchasers. After reviewing the StrategyGen report, we proposed to adopt the updated direct PE input prices for supplies and equipment as recommended by StrategyGen.
StrategyGen found that despite technological advancements, the average commercial price for medical equipment and supplies has remained relatively consistent with the current CMS price. Specifically, preliminary data indicated that there was no statistically significant difference between the estimated commercial prices and the current CMS prices for both equipment and supplies. This cumulative stable pricing for medical equipment and supplies appears similar to the pricing impacts of non-medical technology advancements where some historically high-priced equipment (that is, desktop PCs) has been increasingly substituted with current technology (that is, laptops and tablets) at similar or lower price points. However, while there were no statistically significant differences in pricing at the aggregate level, medical specialties would experience increases or decreases in their Medicare payments if we were to adopt the pricing updates recommended by StrategyGen. At the service level, there may be large shifts in PE RVUs for individual codes that happened to contain supplies and/or equipment with major changes in pricing, although we note that codes with a sizable PE RVU decrease would be limited by the requirement to phase in significant reductions in RVUs, as required by section 1848(c)(7) of the Act. The phase-in requirement limits the maximum RVU reduction for codes that are not new or revised to 19 percent in any individual calendar year.
We believe that it is important to make use of the most current information available for supply and equipment pricing instead of continuing to rely on pricing information that is more than a decade old. Given the potentially significant changes in payment that would occur, both for specific services and more broadly at the specialty level, in the CY 2019 PFS proposed rule we proposed to phase in our use of the new direct PE input pricing over a 4-year period using a 25/75 percent (CY 2019), 50/50 percent (CY 2020), 75/25 percent (CY 2021), and 100/0 percent (CY 2022) split between new and old pricing. This approach is consistent with how we have previously incorporated significant new data into the calculation of PE RVUs, such as the 4-year transition period finalized in CY 2007 PFS final rule with comment period when changing to the "bottom- Start Printed Page 39117 up" PE methodology (71 FR 69641). This transition period will not only ease the shift to the updated supply and equipment pricing, but will also allow interested parties an opportunity to review and respond to the new pricing information associated with their services.
We proposed to implement this phase-in over 4 years so that supply and equipment values transition smoothly from the prices we currently include to the final updated prices in CY 2022. We proposed to implement this pricing transition such that one quarter of the difference between the current price and the fully phased-in price is implemented for CY 2019, one third of the difference between the CY 2019 price and the final price is implemented for CY 2020, and one half of the difference between the CY 2020 price and the final price is implemented for CY 2021, with the new direct PE prices fully implemented for CY 2022. An example of the transition from the current to the fully-implemented new pricing is provided in Table 4.
For new supply and equipment codes for which we establish prices during the transition years (CYs 2019, 2020 and 2021) based on the public submission of invoices, we proposed to fully implement those prices with no transition since there are no current prices for these supply and equipment items. These new supply and equipment codes would immediately be priced at their newly established values. We also proposed that, for existing supply and equipment codes, when we establish prices based on invoices that are submitted as part of a revaluation or comprehensive review of a code or code family, they will be fully implemented for the year they are adopted without being phased in over the 4-year pricing transition. The formal review process for a HCPCS code includes a review of pricing of the supplies and equipment included in the code. When we find that the price on the submitted invoice is typical for the item in question, we believe it would be appropriate to finalize the new pricing immediately along with any other revisions we adopt for the code valuation.
For existing supply and equipment codes that are not part of a comprehensive review and valuation of a code family and for which we establish prices based on invoices submitted by the public, we proposed to implement the established invoice price as the updated price and to phase in the new price over the remaining years of the proposed 4-year pricing transition. During the proposed transition period, where price changes for supplies and equipment are adopted without a formal review of the HCPCS codes that include them (as is the case for the many updated prices we proposed to phase in over the 4-year transition period), we believe it is important to include them in the remaining transition toward the updated price. We also proposed to phase in any updated pricing we establish during the 4-year transition period for very commonly used supplies and equipment that are included in 100 or more codes, such as sterile gloves (SB024) or exam tables (EF023), even if invoices are provided as part of the formal review of a code family. We would implement the new prices for any such supplies and equipment over the remaining years of the proposed 4-year transition period. Our proposal was intended to minimize any potential disruptive effects during the proposed transition period that could be caused by other sudden shifts in RVUs due to the high number of services that make use of these very common supply and equipment items (meaning that these items are included in 100 or more codes).
We believed that implementing the proposed updated prices with a 4-year phase-in would improve payment accuracy, while maintaining stability and allowing stakeholders the opportunity to address potential concerns about changes in payment for particular items. Updating the pricing of direct PE inputs for supplies and equipment over a longer timeframe will allow more opportunities for public comment and submission of additional, applicable data. We welcomed feedback from stakeholders on the proposed updated supply and equipment pricing, including the submission of additional invoices for consideration.
We received many comments regarding the market-based supply and equipment pricing proposal following the publication of the CY 2019 PFS proposed rule. For a full discussion of these comments, we direct readers to the CY 2019 PFS final rule (83 FR 59475 through 59480). In each instance in which a commenter raised questions about the accuracy of a supply or equipment code's recommended price, the StrategyGen contractor conducted further research on the item and its price with special attention to ensuring that the recommended price was based on the correct item in question and the clarified unit of measure. Based on the commenters' requests, the StrategyGen contractor conducted an extensive examination of the pricing of any supply or equipment items that any commenter identified as requiring additional review. Invoices submitted by multiple commenters were greatly appreciated and ensured that medical equipment and supplies were re-examined and clarified. Multiple researchers reviewed these specified supply and equipment codes for accuracy and proper pricing. In most cases, the contractor also reached out to a team of nurses and their physician panel to further validate the accuracy of the data and pricing information. In some cases, the pricing for individual items needed further clarification due to a lack of information or due to significant variation in packaged items. After consideration of the comments and this additional price research, we updated the recommended prices for approximately 70 supply and equipment codes identified by the commenters. Table 9 in the CY 2019 PFS final rule lists the supply and equipment codes with price changes based on feedback from the commenters and the resulting additional research into pricing (83 FR 59479 through 59480).
After consideration of the public comments, we finalized our proposals Start Printed Page 39118 associated with the market research study to update the PFS direct PE inputs for supply and equipment pricing. We continue to believe that implementing the updated prices with a 4-year phase-in will improve payment accuracy, while maintaining stability and allowing stakeholders the opportunity to address potential concerns about changes in payment for particular items. We continue to welcome feedback from stakeholders on the updated supply and equipment pricing, including the submission of additional invoices for consideration.
For CY 2022, we received invoice submissions from stakeholders for approximately half a dozen supply and equipment codes as part of the fourth year of the market-based supply and equipment pricing update. We used these submitted invoices in many cases to supplement the pricing originally proposed for the CY 2019 PFS rule cycle. We reviewed the invoices, as well as our own data for the relevant supply/equipment codes to make sure the item in the invoice was representative of the supply/equipment item in question and aligned with past research. Based on this review, we are proposing to update the prices of six supply items listed in the valuation of specific codes section of the preamble under Table 16: CY 2022 Invoices Received for Existing Direct PE Inputs. Since this is the final year of the supply and equipment pricing update, the new pricing for each of these supply and equipment items would take effect immediately for CY 2022.
The proposed prices for the supply and equipment items listed in Table 16 of CY 2022 were generally calculated following our standard methodology of averaging together the prices on the submitted invoices. In the case of the Liquid coverslip (Ventana 650-010) (SL479) supply, we are proposing a price of $0.051 based on the median invoice due to the presence of an outlier invoice that substantially increased the pricing when using an average. We believe that the proposed price of $0.051 would be more typical for the SL479 supply based on the pricing information contained on the other submitted invoices. We also received several invoices for the 3C patch system (SD343) supply; however, since we established a price of $625.00 for this supply in last year's CY 2021 PFS final rule and the submitted invoices had an average price of $612.50, we are not proposing to update the price. We believe that the submitted invoices confirm that the current pricing of $625.00 is typical for the SD343 supply.
(2) Invoice Submission
The full list of updated supply and equipment pricing as implemented over the 4-year transition period will be made available as a public use file displayed on the CMS website under downloads for the CY 2022 PFS proposed rule at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html.
We routinely accept public submission of invoices as part of our process for developing payment rates for new, revised, and potentially misvalued codes. Often these invoices are submitted in conjunction with the RUC-recommended values for the codes. To be included in a given year's proposed rule, we generally need to receive invoices by the same February 10th deadline we noted for consideration of RUC recommendations. However, we will consider invoices submitted as public comments during the comment period following the publication of the PFS proposed rule, and would consider any invoices received after February 10th or outside of the public comment process as part of our established annual process for requests to update supply and equipment prices. Stakeholders are encouraged to submit invoices with their public comments or, if outside the notice and comment rulemaking process, via email at PE_Price_Input_Update@cms.hhs.gov.
(3) Autologous Platelet-Rich Plasma (HCPCS Code G0460) Supply Inputs
We did not make any proposals associated with HCPCS code G0460 (Autologous platelet rich plasma for chronic wounds/ulcers, including phlebotomy, centrifugation, and all other preparatory procedures, administration and dressings, per treatment) in the CY 2021 PFS proposed rule. Following publication of the rule, stakeholders contacted CMS regarding the creation of a new 3C patch system supply, which is topically applied for the management of exuding cutaneous wounds, such as leg ulcers, pressure ulcers, and diabetic ulcers and mechanically or surgically-debrided wounds. Stakeholders first sought clarification on how CMS calculated the underlying nonfacility PE RVUs for HCPCS code G0460. Stakeholders also stated that autologous platelet rich plasma administration procedures furnished in clinical trials (including the new 3C patch system) are reported using HCPCS code G0460 and requested that CMS revalue the service to reflect the PEs associated with the new patch system supply. The stakeholders stated that the use of the new 3C patch system will represent the typical case for HCPCS code G0460, and suggested that, therefore, the cost inputs for this supply should be used to establish the RVUs for this code, as the current PFS payment rate is substantially less than the amount it costs to furnish the 3C patch.
We want to clarify that the direct PE inputs for HCPCS code G0460 increased for CY 2021 as a result of the ongoing market-based supply and equipment pricing update. However, there was also a minor decrease in the indirect PE allocation associated with this service for CY 2021, with the net result that the proposed PE RVU coincidentally ended up remaining the same as in the previous year. We also clarify that HCPCS code G0460 is not included in the Anticipated Specialty Assignment for Low Volume Services list, and therefore, was unaffected by low utilization in the claims data. In addition, as a contractor priced service, HCPCS code G0460 is unaffected by inclusion or exclusion from this list.
We share the concerns of the stakeholders that patient access to the 3C patch could be materially impacted if CMS maintains the current PE RVUs for HCPCS G0460. In the CY 2021 PFS final rule, we established contractor pricing for HCPCS code G0460 for CY 2021. We believe that the use of contractor pricing again for CY 2022 will allow us additional time to consider the most appropriate resource inputs and PE RVUs for HCPCS code G0460. We also added the 3C patch system to our supply database under supply code SD343 at a price of $625.00 based on an average of the submitted invoices. We are proposing to maintain contractor pricing for CY 2022 for HCPCS code G0460 as we do not currently have sufficient information to establish national pricing. It remains unclear to us what the typical supply inputs would be for HCPCS code G0460 and whether they would include the use of the new 3C patch system. We believe that it would be more appropriate to maintain contractor pricing for the service, which will allow for more flexibility in pricing. We are soliciting any additional information that commenters can supply that CMS should consider to establish national payment for HCPCS code G0460.
d. Clinical Labor Pricing Update
Section 220(a) of the PAMA provides that the Secretary may collect or obtain information from any eligible professional or any other source on the resources directly or indirectly related to furnishing services for which payment is made under the PFS, and Start Printed Page 39119 that such information may be used in the determination of relative values for services under the PFS. Such information may include the time involved in furnishing services; the amounts, types and prices of PE inputs; overhead and accounting information for practices of physicians and other suppliers, and any other elements that would improve the valuation of services under the PFS.
Since 2019, we have been updating the supply and equipment prices used for PE as part of a market-based pricing transition; CY 2022 will be the final year of this 4-year transition. We initiated a market research contract with StrategyGen to conduct an in-depth and robust market research study to update the supply and equipment pricing for CY 2019, and we finalized a policy in CY 2019 to phase in the new pricing over a period of 4 years. However, we did not propose to update the clinical labor pricing, and the pricing for clinical labor has remained unchanged during this pricing transition. Clinical labor rates were last updated for CY 2002 using Bureau of Labor Statistics (BLS) data and other supplementary sources where BLS data were not available; we refer readers to the full discussion in the CY 2002 PFS final rule for additional details (66 FR 55257 through 55262).
Stakeholders have raised concerns that the long delay since clinical labor pricing was last updated has created a significant disparity between CMS' clinical wage data and the market average for clinical labor. In recent years, a number of stakeholders have suggested that certain wage rates are inadequate because they do not reflect current labor rate information. Some stakeholders have also stated that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct PE. Since the pool of aggregated direct PE inputs is budget neutral, if these rates are not routinely updated, clinical labor may become undervalued over time relative to equipment and supplies, especially since the supply and equipment prices are in the process of being updated. There has been considerable stakeholder interest in updating the clinical labor rates, and when we solicited comment on this topic in past rules, such as in the CY 2019 PFS final rule (83 FR 59480), stakeholders supported the idea.
Therefore, we are proposing to update the clinical labor pricing for CY 2022, in conjunction with the final year of the supply and equipment pricing update. We believe it is important to update the clinical labor pricing to maintain relativity with the recent supply and equipment pricing updates. We are proposing to use the methodology outlined in the CY 2002 PFS final rule (66 FR 55257), which draws primarily from BLS wage data, to calculate updated clinical labor pricing. As we stated in the CY 2002 PFS final rule, the BLS' reputation for publishing valid estimates that are nationally representative led to the choice to use the BLS data as the main source. We believe that the BLS wage data continues to be the most accurate source to use as a basis for clinical labor pricing and this data will appropriately reflect changes in clinical labor resource inputs for purposes of setting PE RVUs under the PFS. We used the most current BLS survey data (2019) as the main source of wage data for this proposal.
We recognize that the BLS survey of wage data does not cover all the staff types contained in our direct PE database. Therefore, we crosswalked or extrapolated the wages for several staff types using supplementary data sources for verification whenever possible. In situations where the price wages of clinical labor types were not referenced in the BLS data, we have used the national salary data from the Salary Expert, an online project of the Economic Research Institute that surveys national and local salary ranges and averages for thousands of job titles using mainly government sources. (A detailed explanation of the methodology used by Salary Expert to estimate specific job salaries can be found at www.salaryexpert.com). We previously used Salary Expert information as the primary backup source of wage data during the last update of clinical labor pricing in CY 2002. If we did not have direct BLS wage data available for a clinical labor type, we used the wage data from Salary Expert as a reference for pricing, then crosswalked these clinical labor types to a proxy BLS labor category rate that most closely matched the reference wage data, similar to the crosswalks used in our PE/HR allocation. For example, there is no direct BLS wage data for the Mammography Technologist (L043) clinical labor type; we used the wage data from Salary Expert as a reference and identified the BLS wage data for Respiratory Therapists as the best proxy category. We calculated rates for the "blend" clinical labor categories by combining the rates for each labor type in the blend and then dividing by the total number of labor types in the blend.
As in the CY 2002 clinical labor pricing update, the proposed cost per minute for each clinical staff type was derived by dividing the average hourly wage rate by 60 to arrive at the per minute cost. In cases where an hourly wage rate was not available for a clinical staff type, the proposed cost per minute for the clinical staff type was derived by dividing the annual salary (converted to 2021 dollars using the Medicare Economic Index) by 2080 (the number of hours in a typical work year) to arrive at the hourly wage rate and then again by 60 to arrive at the per minute cost. To account for the employers' cost of providing fringe benefits, such as sick leave, we used the same benefits multiplier of 1.366 as employed in CY 2002. As an example of this process, for the Physical Therapy Aide (L023A) clinical labor type, the BLS data reflected an average hourly wage rate of $14.03, which we multiplied by the 1.366 benefits modifier and then divided by 60 minutes to arrive at the proposed per-minute rate of $0.32.
Table 5 lists our proposed updates to the clinical labor prices. The BLS occupational code used as a source of wage data is listed for each clinical labor type; for the "blend" clinical labor types, this may include multiple BLS occupational codes and other clinical labor types which were calculated separately and then averaged together. Clinical labor types without a direct BLS labor category where we are employing a proxy BLS wage rate are indicated with an asterisk in Table 5.
Start Printed Page 39120

We are proposing to use the 75th percentile of the average wage data for the Medical Physicist (L152A) clinical labor type because we believe this level Start Printed Page 39121 would most closely fit with the historic wage data for this clinical labor type. A Medical Physicist is a specific type of physicist, and the available BLS wage data describes the more general category of physicist which is paid at a lower rate. In this specific case, the 75th percentile more accurately describes the clinical labor type in question based on how it has historically been paid. We are also proposing to maintain the current clinical labor pricing for the Behavioral Health Care Manager (L057B) clinical labor type rather than update it. Although the BLS data reflected a decreased clinical labor rate for the Behavioral Health Care Manager labor type, we do not believe that the typical wages have decreased for this clinical labor type given that every other clinical labor type has increased over the past 5 years since the Behavioral Health Care Manager clinical labor type was created. The Behavioral Health Care Manager labor type was initially established in the CY 2017 PFS final rule (81 FR 80350). It seems more likely that we misidentified the proper BLS category for this clinical labor type than that wages have decreased since 2017. We believe that the clinical labor rate for the Behavioral Health Care Manager should be held constant for CY 2022 pending additional public feedback.
We are soliciting comments on the proposed updated clinical labor pricing. We are particularly interested in additional wage data for the clinical labor types for which we lacked direct BLS wage data and made use of proxy labor categories for pricing. We understand that the clinical labor undertaken by, for example, a Histotechnologist (L037B) is not the same as the clinical labor provided by the Health Information Technologist category of BLS wage data that we employed as a proxy for pricing. Although these occupations are not directly analogous to each other in terms of the work they do, we nonetheless believe that the proposed crosswalks are appropriate in terms of the resulting hourly wage data. We appreciate any additional information that commenters can supply both in terms of direct wage data, as well as identifying the most accurate types of BLS categories that could be used as proxies to update pricing for clinical labor types that lack direct BLS wage data. We isolated the anticipated effects of the clinical labor pricing update on specialty payment impacts by comparing the proposed CY 2022 PFS rates with and without the clinical labor pricing updates in place:
Start Printed Page 39122

Start Printed Page 39123
The potential effects of the clinical labor pricing update on specialty payment impacts are largely driven by the share that labor costs represent of the direct PE inputs for each specialty. Specialties with a substantially lower or higher than average share of direct costs attributable to labor would experience significant declines or increases, respectively, if this proposal is finalized. For example, the Family Practice specialty has a higher share of direct costs associated with clinical labor, and payments to services comprising the specialty would be expected to increase as a result of this clinical labor pricing update. In contrast, Diagnostic Testing Facilities have a lower share of direct costs that are associated with clinical labor, and payments to services comprising the specialty would be expected to decrease. Other specialty-level payment impacts for the proposed clinical labor pricing changes are driven by changes in wage rates for a clinical labor category that affects a given specialty more than average. One such example would be the proposed increase of 11 percent for Oncology nurses as opposed to the average increase for nurses of 63 percent. We emphasize that these are not the projected impacts by specialty of all the policies we are proposing in this proposed rule for CY 2022, only the anticipated effect of the isolated clinical labor pricing update, should this clinical labor pricing update be finalized as proposed.
When updates to our payment methodology based on new data produce significant shifts in payment, we often consider whether it would be appropriate to implement the updates through a phased transition across several calendar years. For example, we utilized a 4-year transition for the market-based supply and equipment pricing update concluding in CY 2022. We are considering the use of a similar 4-year transition to implement the clinical labor pricing update. A multi-year transition could smooth out the increases and decreases in payment caused by the pricing update for affected stakeholders, promoting payment stability. However, a phased transition would delay the full implementation of updated pricing and continue to rely in part on outdated data for clinical labor pricing. We discuss a potential 4-year transition for the clinical labor pricing update as an alternative considered in the Regulatory Impact Analysis (section VII.I) of this rule.
e. Proposal To Establish Values for Remote Retinal Imaging (CPT Code 92229), Comment Solicitation for Fractional Flow Reserve Derived From Computed Tomography (CPT Code 0503T), and Comment Solicitation for Codes Involving Innovative Technology
Rapid advances in innovative technology are having a profound effect on every facet of the economy, including in the delivery of health care. Emerging and evolving technologies are introducing advances in treatment options that have the potential to increase access to care for Medicare beneficiaries, improve outcomes, and reduce overall costs to the program. While new services have emerged over the last several years, it is possible that the COVID-19 public health emergency (PHE) could be accelerating the supply and demand for these innovations. Emerging and evolving technologies could be useful tools for improving disparities in care that have been exacerbated by the PHE. Some of these new applications have codes for which innovative technology is substituting for and/or augmenting physician work. For example, the CPT Editorial Panel created CPT code 92229 (Imaging of retina for detection or monitoring of disease; point-of-care automated analysis and report, unilateral or bilateral), a diagnostic test for diabetic retinopathy that uses a software algorithm, and the RUC provided valuation recommendations which included a retinal camera and an analysis fee for remote imaging. In the CY 2021 PFS final rule (85 FR 84629 through 84630), we considered CPT code 92229 to be a diagnostic service under the PFS, contractor-priced it, and stated that we would have ongoing conversations with stakeholders. The following section will discuss proposed policies to establish RVUs for CPT code 92229, solicit feedback to establish RVUs for CPT code 0503T (Noninvasive estimated coronary fractional flow reserve (FFR) derived from coronary computed tomography angiography data using computation fluid dynamics physiologic simulation software analysis of functional data to assess the severity of coronary artery disease; analysis of fluid dynamics and simulated maximal coronary hyperemia, and generation of estimated FFR model), and solicit feedback to help us better understand the resource costs for services involving the use of innovative technologies such as software algorithms and artificial intelligence (AI).
In our discussion of CPT code 92229 in the CY 2021 PFS final rule (85 FR 84629 through 84630), we wrote that as the data used in our PE methodology have aged, and more services have begun to include innovative technology such as software algorithms and AI, these innovative applications are not well accounted for in our PE methodology. As described earlier in this section, PE resources involved in furnishing services are characterized as either direct or indirect costs. Direct costs of the PE resources involved in furnish a service are estimated for each code and include clinical labor, medical supplies, and medical equipment. Indirect costs include administrative labor, office expenses, and all other expenses. Indirect PE is allocated to each service based on physician work, direct costs, and a specialty-specific indirect percentage. The source of the specialty specific indirect percentage was the Physician Practice Information Survey (PPIS), last administered in 2007 and 2008, when emerging technologies that rely primarily on software, licensing, and analysis fees, with minimal costs in equipment and hardware may not have been typical. Thus, these costs are not well accounted for in the PE methodology.
Consistent with our PE methodology and as we have stated in past PFS rulemaking (83 FR 59557), we have considered most computer software and associated analysis and licensing fees to be indirect costs tied to costs for associated hardware that is considered to be medical equipment. In the case of CPT code 92229, the hardware is a retinal camera used for remote imaging. Given that indirect costs are based on physician work, direct costs, and Start Printed Page 39124 specialty-specific indirect percentages that can include high-cost equipment, our concern is that if we were to consider an analysis fee to be a supply cost, as was recommended by the RUC, it is possible that we would inadvertently allocate too many indirect costs for a supply item that may not require additional indirect expenses. Unlike a piece of equipment, such as the retinal camera, an analysis fee for software does not require physical space in an office or administrative staff hours to maintain it.
However, increasingly, stakeholders have routinely expressed concerns with our policy to consider analysis fees as indirect costs, especially for evolving technologies that rely primarily on these fees with minimal costs in equipment or hardware. In comments in the CY 2021 PFS final rule (85 FR 84629 through 84630) responding to our proposal to price the analysis fee for remote imaging as an indirect cost, stakeholders stated that there would be no service if the software was not used. There are two aspects that distinguish CPT code 92229 from other services. First, most of the RUC's recommended resource costs for CPT code 92229 were for the analysis fee, rather than high-cost equipment or other supplies that require commensurate indirect costs to accommodate for space or administrative labor. Second, the innovative technology incorporated into the service is a software algorithm, which interprets data collected during the test, either augmenting the work of the physician or NPP performing the test, or in some cases replacing at least some work that a physician would typically furnish. In general, it is possible that physician work time and intensity of furnishing care to patients could be affected as more services that involve innovative technologies such as software algorithms or AI become available.
We finalized a policy to establish contractor pricing for CPT code 92229 (85 FR 84629 through 84630) because analysis fees for software algorithms and AI applications are not well accounted for our PE methodology, and to recognize that practitioners do incur resource costs for purchase and ongoing use of the software. We stated that we would continue to seek out new data sources and have ongoing conversations with stakeholders while also considering other approaches to reflect overall resource costs for these technologies in our PE methodology.
As we described in the CY 2021 PFS final rule (85 FR 84498 through 84499), the RAND Corporation is currently studying potential improvements to CMS' PE allocation methodology and the data that underlie it. RAND has found that the PPIS data last collected in 2007-2008 may no longer reflect the resource allocation, staffing arrangements, and cost structures that describe practitioners' resource requirements in furnishing services to Medicare beneficiaries, and consequently may not accurately capture the indirect PE resources required to furnish services to Medicare FFS beneficiaries. Our experience with the challenge of accurately accounting for resource costs for innovative and emerging technologies such as ongoing service-specific software costs that are included in CPT code 92229 is another reason we continue to be interested in potentially refining the PE methodology and updating the data used to establish RVUs and payment rates under the PFS. We commonly employ a crosswalk to recognize resource costs when we lack the inputs that we would need to calculate work, PE, and/or malpractice RVUs for a service otherwise. When we use a crosswalk to value a service, we substitute the established RVUs for other services with similar resource costs in the physician office setting to set RVUs and the national payment rates for that particular service.
For CY 2022, we are proposing to establish values for CPT code 92229 using our crosswalk approach, and thus this service would no longer be contractor-priced. We continue to believe that the software algorithm present in the analysis fee for CPT code 92229 is not well accounted for in our PE methodology; however, we recognize that practitioners are incurring resource costs for purchase of the software and its ongoing use. We are proposing to use a crosswalk that reflects the overall relative resource costs for this service while we continue to consider potentially refining the PE methodology and updating the data we use to establish PE RVUs under the PFS. Specifically, we are proposing a crosswalk to CPT code 92325 (Modification of contact lens (separate procedure), with medical supervision of adaptation), a PE-only code used for the eye, as we believe it reflects overall resource costs for CPT code 92229 in the physician office setting. We recognize that the services described by CPT code 92325 are not the same as the services in CPT code 92229; however, we believe that the total resource costs would be similar across these two codes. We believe that crosswalking the RVUs for CPT code 92229 to a code with similar resource costs allows CMS to recognize that practitioners are incurring resource costs for the purchase and ongoing use of the software employed in CPT code 92229, which would not typically be considered direct PE under our current methodology. We are also soliciting comments on our proposal to crosswalk CPT code 92229 to CPT code 92325, and whether other codes would provide a more appropriate crosswalk in terms of resource costs. In addition, as discussed in section II.E of this proposed rule, we are proposing to use our crosswalk approach for CPT code 77X01 (Trabecular bone score (TBS), structural condition of the bone microarchitecture; using dual X-ray absorptiometry (DXA) or other imaging data on gray-scale variogram, calculation, with interpretation and report on fracture risk) and CPT code 77X03 (Trabecular bone score (TBS), structural condition of the bone microarchitecture; using dual X-ray absorptiometry (DXA) or other imaging data on gray-scale variogram, calculation, with interpretation and report on fracture risk, technical calculation only).
We are aware of other services that use similar innovative technologies to those used for the diagnostic test for diabetic retinopathy and trabecular bone score, and that those technologies also are not well-accounted for in our PE methodology. For CY 2018, the AMA CPT Editorial Panel established four new Category III CPT codes for fractional flow reserve derived from computed tomography (FFRCT): CPT code 0501T (Noninvasive estimated coronary fractional flow reserve (FFR) derived from coronary computed tomography angiography data using computation fluid dynamics physiologic simulation software analysis of functional data to assess the severity of coronary artery disease; data preparation and transmission, analysis of fluid dynamics and simulated maximal coronary hyperemia, generation of estimated FFR model, with anatomical data review in comparison with estimated FFR model to reconcile discordant data, interpretation and report) CPT code 0502T (Noninvasive estimated coronary fractional flow reserve (FFR) derived from coronary computed tomography angiography data using computation fluid dynamics physiologic simulation software analysis of functional data to assess the severity of coronary artery disease; data preparation and transmission); CPT code 0503T (Noninvasive estimated coronary fractional flow reserve (FFR) derived from coronary computed tomography angiography data using computation fluid dynamics physiologic Start Printed Page 39125 simulation software analysis of functional data to assess the severity of coronary artery disease; analysis of fluid dynamics and simulated maximal coronary hyperemia, and generation of estimated FFR model); and CPT code 0504T (Noninvasive estimated coronary fractional flow reserve (FFR) derived from coronary computed tomography angiography data using computation fluid dynamics physiologic simulation software analysis of functional data to assess the severity of coronary artery disease; anatomical data review in comparison with estimated FFR model to reconcile discordant data, interpretation and report). FFRCT is a noninvasive diagnostic service that allows physicians to measure coronary artery disease in a patient through coronary CT scans. It uses a proprietary data analysis process performed at a central facility to develop a three-dimensional image of a patient's coronary arteries, which allows physicians to identify the fractional flow reserve to assess whether or not patients should undergo further invasive testing or treatment (typically, a coronary angiogram). We understand that FFRCT can show through non-invasive imaging whether a beneficiary has coronary artery disease thereby potentially avoiding an invasive coronary procedure. Medicare began payment for CPT code 0503T in the hospital outpatient department setting under the Outpatient Prospective Payment System (OPPS) in CY 2018 (82 FR 59284). For the PFS, we typically assign contractor pricing for Category III codes since they are temporary codes assigned to emerging technology and services. We followed this established process for Category III codes by assigning and listing them as contractor pricing in Appendix B in the CY 2018 PFS final rule (available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1676-F). We have since been trying to understand the costs of the PE resource inputs for CPT code 0503T in the physician office setting. In the CY 2021 PFS final rule, we stated that we found FFRCT to be similar to other technologies that use algorithms, artificial intelligence, or other innovative forms of analysis to determine a course of treatment, where the analysis portion of the service cannot adequately be reflected under the PE methodology; and that our recent reviews for the overall cost of CPT code 0503T have shown the costs in the physician office setting to be similar to costs reflected in payment under the OPPS (85 FR 84630). For the CY 2021 OPPS/ASC final rule, we found that the geometric mean cost reported by hospital outpatient departments for the service was $804.35 (85 FR 85943). We believe the costs reported under the OPPS are instructive as they reflect actual costs that hospitals incurred in furnishing the service described by CPT code 0503T to Medicare beneficiaries, and, as we stated in the CY 2021 PFS final rule, we believe that these costs would be similar in the physician office setting. Using the geometric mean costs under the OPPS as a proxy, we then searched for services paid under the PFS that could potentially serve as a crosswalk. Specifically, we looked for services paid under the PFS that include only a technical component because CPT code 0503T is a technical component-only service, and that have similar total costs to CPT code 0503T. We identified the following potential crosswalks, and seek public comment on which, if any of them, would be appropriate: CPT code 93455 (Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation; with catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) including intraprocedural injection(s) for bypass graft angiography) and CPT code 93458 (Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation; with left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed). We are also seeking comment on whether other codes would provide a more appropriate crosswalk in terms of resource costs.
We are also more broadly soliciting public comment to help us better understand the resource costs for services involving the use of innovative technologies, including but not limited to software algorithms and AI. Specifically, we are requesting commenters consider the following questions:
- To what extent are services involving innovative technologies such as software algorithms and/or AI substitutes and/or supplements for physician work? To what extent do these services involving innovative technology inform, augment, or replace physician work? For example, CPT code 92229 is a PE-only code in which the software algorithm may be substituting for some work of an ophthalmologist to diagnose/detect diabetic retinopathy. CPT code 77X01 is a service in which the trabecular bone score software may be supplementing physician work to predict and detect fracture risk. CPT code 0503T may be both substituting for, and supplementing physician work to detect coronary artery disease.
- How has innovative technology such as software algorithms and/or AI affected physician work time and intensity of furnishing services involving the use of such technology to Medicare beneficiaries? For example, if a new software algorithm or AI technology for a diagnostic test results in a reduction in the amount of time that a practitioner spends reviewing and interpreting the results of a diagnostic test that previously did not involve such software algorithm or AI technology, and if the software algorithm or AI could be considered in part a substitute for at least some physician work, it may follow that the intensity of the service decreases. It is also possible that a software algorithm for a diagnostic test that is supplementing other tests to establish a diagnosis or treatment pathway for a particular condition could result in an increase in the amount of time that a practitioner spends explaining the test to a patient and then reviewing the results.
- How is innovative technology such as software algorithms and/or AI changing cost structures in the physician office setting? As discussed previously, the PPIS data that underlie the PE methodology were last collected in 2007 and 2008, which was prior to the widespread adoption of electronic health records and services that involve care management, non-face-to-face and/or asynchronous remote care; the need to use electronic clinical quality measure data to support quality improvement, disparity identification and resolution, and value based payment; and the emergence of software algorithms and/or AI and other technologies that use data to inform, augment, or replace physician work in the delivery of health care. Do costs for innovative technology such as software algorithms and/or AI to furnish services to patients involve a one-time investment and/or recurring costs? How should CMS consider costs for software algorithms and/or AI that use patient data that were previously collected as part of another service? As technology adoption grows, do these costs decrease over time?
- How is innovative technology affecting beneficiary access to Medicare-covered services? How are services involving software algorithms and/or AI being furnished to Medicare beneficiaries and what is important for Start Printed Page 39126 CMS to understand as it considers how to accurately pay for services involving software algorithms and/or AI? For example, it is possible that services that involve software algorithms and/or AI may allow a practitioner to more efficiently furnish care to more Medicare beneficiaries, potentially increasing access to care. Additionally, to what extent have services that involve innovative technology such as software algorithms and/or AI affected access to Medicare-covered services in rural and/or underserved areas, or for beneficiaries that may face barriers (homelessness, lack of access to transportation, lower levels of health literacy, lower rates of internet access, mental illness, having a high number of chronic conditions/frailty, etc.) in obtaining health care?
- Compared to other services paid under the PFS, are services that are driven by or supported by innovative technology such as software algorithms and/or AI at greater risk of overutilization or more subject to fraud, waste, and abuse? As we are considering appropriate payment for services enabled by new technologies, there are considerations for program integrity. For example, section 218(b) of the PAMA required that we establish an Appropriate Use Criteria Program to promote appropriate use of advanced diagnostic imaging services provided to Medicare beneficiaries.[1] To what extent do services involving innovative technology require mechanisms such as appropriate use criteria to guard against overutilization, fraud, waste, or abuse?
- Compared to other services paid under the PFS, are services driven by or supported by innovative technology such as software algorithms and/or AI associated with improvements in the quality of care or improvements in health equity? For example, increased access to services to detect diabetic retinopathy such as the service described by CPT code 92229 could eventually lead to fewer beneficiaries losing their vision. Because CPT code 92229 can be furnished in a primary care practice's office and may not require the specialized services of an ophthalmologist, more beneficiaries could have access to a test, including those who live in areas with fewer ophthalmologists. Additionally, taking into consideration that a software algorithm and/or AI may introduce bias into clinical decision making that could influence outcomes for racial and ethnic minorities and people who are socioeconomically disadvantaged, are there guardrails, such as removing the source of bias in a software algorithm and/or AI, that Medicare should require as part of considering payment amounts for services enabled by software algorithm and/or AI?
- Our proposals to use crosswalks to set values for codes describing diabetic retinopathy and trabecular bone score would allow us to account for overall resource costs involved in furnishing the services. The possible crosswalks for FFRCT may also account for overall resource costs involved in furnishing the service. We also believe it is important to accurately account for resource costs for innovative and emerging technologies such as ongoing service-specific software costs and, as explained above, such costs are not well accounted for in the PE methodology. We continue to be interested in potentially refining the PE methodology and updating the underlying data, including the PPIS data that are the data source that underpins the indirect PE allocation. How might CMS consider updating such data to reflect ongoing advances in technology so that we could establish appropriate relative values without resorting to crosswalks? The RAND Corporation laid out a number of issues for CMS to consider in two reports. We refer readers to RAND's first phase of research, available at https://www.rand.org/pubs/research_reports/RR2166.html, and RAND's second phase of research, available at https://www.rand.org/pubs/research_reports/RR3248.html.
C. Potentially Misvalued Services Under the PFS
1. Background
Section 1848(c)(2)(B) of the Act directs the Secretary to conduct a periodic review, not less often than every 5 years, of the relative value units (RVUs) established under the PFS. Section 1848(c)(2)(K) of the Act requires the Secretary to periodically identify potentially misvalued services using certain criteria and to review and make appropriate adjustments to the relative values for those services. Section 1848(c)(2)(L) of the Act also requires the Secretary to develop a process to validate the RVUs of certain potentially misvalued codes under the PFS, using the same criteria used to identify potentially misvalued codes, and to make appropriate adjustments.
As discussed in section II.E. of this proposed rule, Valuation of Specific Codes, each year we develop appropriate adjustments to the RVUs taking into account recommendations provided by the American Medical Association (AMA) Resource-Based Relative Value Scale (RVS) Update Committee (RUC), the Medicare Payment Advisory Commission (MedPAC), and other stakeholders. For many years, the RUC has provided us with recommendations on the appropriate relative values for new, revised, and potentially misvalued PFS services. We review these recommendations on a code-by-code basis and consider these recommendations in conjunction with analyses of other data, such as claims data, to inform the decision-making process as authorized by statute. We may also consider analyses of work time, work RVUs, or direct PE inputs using other data sources, such as Department of Veteran Affairs (VA), National Surgical Quality Improvement Program (NSQIP), the Society for Thoracic Surgeons (STS), and the Merit-based Incentive Payment System (MIPS) data. In addition to considering the most recently available data, we assess the results of physician surveys and specialty recommendations submitted to us by the RUC for our review. We also consider information provided by other stakeholders. We conduct a review to assess the appropriate RVUs in the context of contemporary medical practice. We note that section 1848(c)(2)(A)(ii) of the Act authorizes the use of extrapolation and other techniques to determine the RVUs for physicians' services for which specific data are not available and requires us to take into account the results of consultations with organizations representing physicians who provide the services. In accordance with section 1848(c) of the Act, we determine and make appropriate adjustments to the RVUs.
In its March 2006 Report to the Congress (http://www.medpac.gov/docs/default-source/reports/Mar06_Ch03.pdf?sfvrsn=0), MedPAC discussed the importance of appropriately valuing physicians' services, noting that misvalued services can distort the market for physicians' services, as well as for other health care services that physicians order, such as hospital services. In that same report, MedPAC postulated that physicians' services under the PFS can become misvalued over time. MedPAC stated, "When a new service is added to the physician fee schedule, it may be assigned a relatively high value because of the time, technical skill, and psychological stress that are often required to furnish that service. Over time, the work Start Printed Page 39127 required for certain services would be expected to decline as physicians become more familiar with the service and more efficient in furnishing it." We believe services can also become overvalued when PE costs decline. This can happen when the costs of equipment and supplies fall, or when equipment is used more frequently than is estimated in the PE methodology, reducing its cost per use. Likewise, services can become undervalued when physician work increases or PE costs rises.
As MedPAC noted in its March 2009 Report to Congress (http://www.medpac.gov/docs/default-source/reports/march-2009-report-to-congress-medicare-payment-policy.pdf), in the intervening years since MedPAC made the initial recommendations, CMS and the RUC have taken several steps to improve the review process. Also, section 1848(c)(2)(K)(ii) of the Act augments our efforts by directing the Secretary to specifically examine, as determined appropriate, potentially misvalued services in the following categories:
- Codes that have experienced the fastest growth.
- Codes that have experienced substantial changes in PE.
- Codes that describe new technologies or services within an appropriate time-period (such as 3 years) after the relative values are initially established for such codes.
- Codes which are multiple codes that are frequently billed in conjunction with furnishing a single service.
- Codes with low relative values, particularly those that are often billed multiple times for a single treatment.
- Codes that have not been subject to review since implementation of the fee schedule.
- Codes that account for the majority of spending under the PFS.
- Codes for services that have experienced a substantial change in the hospital length of stay or procedure time.
- Codes for which there may be a change in the typical site of service since the code was last valued.
- Codes for which there is a significant difference in payment for the same service between different sites of service.
- Codes for which there may be anomalies in relative values within a family of codes.
- Codes for services where there may be efficiencies when a service is furnished at the same time as other services.
- Codes with high intraservice work per unit of time.
- Codes with high PE RVUs.
- Codes with high cost supplies.
- Codes as determined appropriate by the Secretary.
Section 1848(c)(2)(K)(iii) of the Act also specifies that the Secretary may use existing processes to receive recommendations on the review and appropriate adjustment of potentially misvalued services. In addition, the Secretary may conduct surveys, other data collection activities, studies, or other analyses, as the Secretary determines to be appropriate, to facilitate the review and appropriate adjustment of potentially misvalued services. This section also authorizes the use of analytic contractors to identify and analyze potentially misvalued codes, conduct surveys or collect data, and make recommendations on the review and appropriate adjustment of potentially misvalued services. Additionally, this section provides that the Secretary may coordinate the review and adjustment of any RVU with the periodic review described in section 1848(c)(2)(B) of the Act. Section 1848(c)(2)(K)(iii)(V) of the Act specifies that the Secretary may make appropriate coding revisions (including using existing processes for consideration of coding changes) that may include consolidation of individual services into bundled codes for payment under the PFS.
2. Progress in Identifying and Reviewing Potentially Misvalued Codes
To fulfill our statutory mandate, we have identified and reviewed numerous potentially misvalued codes as specified in section 1848(c)(2)(K)(ii) of the Act, and we intend to continue our work examining potentially misvalued codes in these areas over the upcoming years. As part of our current process, we identify potentially misvalued codes for review, and request recommendations from the RUC and other public commenters on revised work RVUs and direct PE inputs for those codes. The RUC, through its own processes, also identifies potentially misvalued codes for review. Through our public nomination process for potentially misvalued codes established in the CY 2012 PFS final rule with comment period, other individuals and stakeholder groups submit nominations for review of potentially misvalued codes as well. Individuals and stakeholder groups may submit codes for review under the potentially misvalued codes initiative to CMS in one of two ways. Nominations may be submitted to CMS via email or through postal mail. Email submissions should be sent to the CMS emailbox MedicarePhysicianFeeSchedule@cms.hhs.gov, with the phrase "Potentially Misvalued Codes" and the referencing CPT code number(s) and/or the CPT descriptor(s) in the subject line. Physical letters for nominations should be sent via the U.S. Postal Service to the Centers for Medicare & Medicaid Services, Mail Stop: C4-01-26, 7500 Security Blvd., Baltimore, Maryland 21244. Envelopes containing the nomination letters must be labeled "Attention: Division of Practitioner Services, Potentially Misvalued Codes". Nominations for consideration in our next annual rule cycle should be received by our February 10th deadline. Since CY 2009, as a part of the annual potentially misvalued code review and Five-Year Review process, we have reviewed over 1,700 potentially misvalued codes to refine work RVUs and direct PE inputs. We have assigned appropriate work RVUs and direct PE inputs for these services as a result of these reviews. A more detailed discussion of the extensive prior reviews of potentially misvalued codes is included in the Medicare Program; Payment Policies Under the Physician Fee Schedule, Five-Year Review of Work Relative Value Units, Clinical Laboratory Fee Schedule: Signature on Requisition, and Other Revisions to Part B for CY 2012; final rule (76 FR 73052 through 73055) (hereinafter referred to as the "CY 2012 PFS final rule with comment period"). In the CY 2012 PFS final rule with comment period (76 FR 73055 through 73958), we finalized our policy to consolidate the review of physician work and PE at the same time, and established a process for the annual public nomination of potentially misvalued services.
In the Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule, DME Face-to-Face Encounters, Elimination of the Requirement for Termination of Non-Random Prepayment Complex Medical Review and Other Revisions to Part B for CY 2013 (77 FR 68892) (hereinafter referred to as the "CY 2013 PFS final rule with comment period"), we built upon the work we began in CY 2009 to review potentially misvalued codes that have not been reviewed since the implementation of the PFS (so-called "Harvard-valued codes"). In the Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2009; and Revisions to the Amendment of the E-Prescribing Exemption for Computer Generated Facsimile Transmissions; Proposed Rule (73 FR 38589) (hereinafter referred to as Start Printed Page 39128 the "CY 2009 PFS proposed rule"), we requested recommendations from the RUC to aid in our review of Harvard-valued codes that had not yet been reviewed, focusing first on high-volume, low intensity codes. In the fourth Five-Year Review (76 FR 32410), we requested recommendations from the RUC to aid in our review of Harvard-valued codes with annual utilization of greater than 30,000 services. In the CY 2013 PFS final rule with comment period, we identified specific Harvard-valued services with annual allowed charges that total at least $10,000,000 as potentially misvalued. In addition to the Harvard-valued codes, in the CY 2013 PFS final rule with comment period we finalized for review a list of potentially misvalued codes that have stand-alone PE (codes with physician work and no listed work time and codes with no physician work that have listed work time). We continue each year to consider and finalize a list of potentially misvalued codes that have or will be reviewed and revised as appropriate in future rulemaking.
3. CY 2022 Identification and Review of Potentially Misvalued Services
In the CY 2012 PFS final rule with comment period (76 FR 73058), we finalized a process for the public to nominate potentially misvalued codes. In the CY 2015 PFS final rule with comment period (79 FR 67606 through 67608), we modified this process whereby the public and stakeholders may nominate potentially misvalued codes for review by submitting the code with supporting documentation by February 10th of each year. Supporting documentation for codes nominated for the annual review of potentially misvalued codes may include the following:
- Documentation in peer reviewed medical literature or other reliable data that demonstrate changes in physician work due to one or more of the following: Technique, knowledge and technology, patient population, site-of-service, length of hospital stay, and work time.
- An anomalous relationship between the code being proposed for review and other codes.
- Evidence that technology has changed physician work.
- Analysis of other data on time and effort measures, such as operating room logs or national and other representative databases.
- Evidence that incorrect assumptions were made in the previous valuation of the service, such as a misleading vignette, survey, or flawed crosswalk assumptions in a previous evaluation.
- Prices for certain high cost supplies or other direct PE inputs that are used to determine PE RVUs are inaccurate and do not reflect current information.
- Analyses of work time, work RVU, or direct PE inputs using other data sources (for example, VA, NSQIP, the STS National Database, and the MIPS data).
- National surveys of work time and intensity from professional and management societies and organizations, such as hospital associations.
We evaluate the supporting documentation submitted with the nominated codes and assess whether the nominated codes appear to be potentially misvalued codes appropriate for review under the annual process. In the following year's PFS proposed rule, we publish the list of nominated codes and indicate for each nominated code whether we agree with its inclusion as a potentially misvalued code. The public has the opportunity to comment on these and all other proposed potentially misvalued codes. In that year's final rule, we finalize our list of potentially misvalued codes.
a. Public Nominations
In this proposed rule, we are soliciting comments regarding the potentially misvalued codes nominated by the public to inform our decision on whether to establish the codes as potentially misvalued in the CY 2022 PFS final rule. We received public nominations for potentially misvalued codes by February 10th. We display these public nominations on our public website, including the submitter's name and their associated organization to provide full transparency. Among the public nominations that we received this year, one was a request for CMS to review a PE-related input for a code. We refer readers to section II.B. of this proposed rule, Determination of PE RVUs, for further discussion on the PE-related submission. The summary of this year's submissions under the potentially misvalued code initiative are discussed below.
A stakeholder nominated CPT code 22551 (Fusion of spine bones with removal of disc at upper spinal column, anterior approach, complex) "and common related services" as potentially misvalued. Citing the CY 2021 PFS final rule (84 FR 84501) where CMS agreed with the public nomination of CPT code 22867 (Insertion of interlaminar/interspinous process stabilization/distraction device, without fusion, including image guidance when performed, with open decompression, lumbar; single level) as potentially misvalued, and discussed the relationship between CPT code 22867 and CPT code 63047 (Laminectomy, facetectomy and foraminotomy (unilateral or bilateral with decompression of spinal cord, cauda equina and/or nerve root[s], [e.g., spinal or lateral recess stenosis]), single vertebral segment; lumbar), this stakeholder suggests that there are additional CPT code values related to spine procedures that are in need of contemporaneous review with CPT code 22867. The stakeholder believes that CMS has an interest in reviewing associated anterior cervical discectomy and fusion (ACDF) procedures as well, and suggests that CPT code 22551 "and common related services" can result in cumulative RVUs that do not sufficiently reflect physician work, time, or outcomes.
In their submission, the stakeholder expressed concern that there is a discrepancy between the typical total RVUs for codes billed for vertebral fusion procedures performed using three synthetic cage devices with plate and vertebral fusion procedures performed using three allografts with plate. Both methods of vertebral fusion are described by CPT code 22551 (includes a 90-day global period), which has a work RVU of 25.00. Both methods of vertebral fusion involve two units of CPT code 22552 (Arthrodesis, anterior interbody, including disc space preparation, discectomy, osteophytectomy and decompression of spinal cord and/or nerve roots; cervical below C2, each additional interspace (List separately in addition to code for primary procedure) (ZZZ global period)) with a total work RVU of 13.00 (6.50 × 2); and both methods of vertebral fusion involve 1 unit of CPT code 22846 (Anterior instrumentation; 4 to 7 vertebral segments (List separately in addition to code for primary procedure) (ZZZ global period)) with a work RVU of 12.40. The vertebral fusion method employing three synthetic cage devices with a plate would involve CPT code 22853 (Insertion of interbody biomechanical device (s) (e.g., synthetic cage, mesh) with integral anterior instrumentation for device anchoring (e.g., screws, flanges), when performed, to intervertebral disc space in conjunction with interbody arthrodesis, each interspace (List separately in addition to code for primary procedure) (ZZZ global period)) for the insertion of synthetic cage devices for a total work RVU of 12.75 (4.25 × 3), and CPT code 20930 (Allograft, morselized, or Start Printed Page 39129 placement of osteopromotive material, for spine surgery only (List separately in addition to code for primary procedure)) with a work RVU of 0.00 (because Medicare considers this code to be bundled into codes for other services). The stakeholder stated that the total work RVUs for the typical vertebral fusion employing three synthetic cage devices with plate would be 63.15 work RVUs.
In contrast, the stakeholder asserted that the vertebral fusion method employing three allografts with plate involves the same set of services and codes (CPT code 22551 (090 global period) and CPT code 22846 (ZZZ global period)), but instead of CPT codes 22853 or 20930, involve CPT code 20931 (Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) (ZZZ global period) with a work RVU of 1.81. Altogether, the total work RVUs for CPT codes involved in this vertebral fusion method is 52.21. The stakeholder suggested that this difference in total work RVUs, 63.15 versus 52.21, is evidence that these services are misvalued, and that the total work RVUs do not reflect the differences in the amount of work, resources, and intensity between the two vertebral fusion methods.
This stakeholder's description of the potential misvaluation of CPT code 22551 "and common related services" differs from the CMS approach to identifying potentially misvalued services by using certain criteria, as described in the beginning of this section. Our determination that one or more codes are potentially misvalued generally revolves around the specific RVUs assigned to an individual code, or several codes within a family of codes. CMS generally does not examine the summed differences in total RVUs based on billing patterns using different codes in different scenarios, representing different physician work, and then comparing the two methods of a procedure, in this case, the use or non-use, of the synthetic cage devices in the vertebral fusion with removal of the disc in the upper spinal column. We do not believe that the stakeholder has provided support for the premise that CPT code 22551 alone is misvalued, or that any of the codes identified as common related services are misvalued. Therefore, we are not inclined to propose this code as potentially misvalued. However, we welcome additional comment, including any analysis or studies demonstrating that one or more of these codes meet the criteria listed above under "Identification and Review of Potentially Misvalued Services," particularly in regard to any changes in the resources to providing a service, or are otherwise potentially misvalued.
A stakeholder nominated CPT code 49436 (Delayed creation of exit site from embedded subcutaneous segment of intraperitoneal cannula or catheter) as potentially misvalued, as it has not been valued for payment in the non-facility/office setting. This stakeholder did not include in their submission detailed recommendations for the items, quantities, and unit costs for the supplies, equipment types, and clinical labor (if any), that might be incurred in the non-facility/office setting, all of which are key factors when determining potential valuation or mis-valuation of a service. Medicare claims data for 2018, 2019, and 2020 show that CPT code 49436 is solely performed in the facility ambulatory surgical center (ASC) setting. We are not inclined to propose this code as potentially misvalued; however, we welcome additional comment, including any analysis or studies demonstrating that this code meets the criteria listed above under "Identification and Review of Potentially Misvalued Services," particularly in regard to any changes in the resources to providing a service, or is otherwise potentially misvalued.
A stakeholder nominated CPT code 55880 (Ablation of malignant prostate tissue, transrectal, with high intensity-focused ultrasound (HIFU), including ultrasound guidance) as potentially misvalued, as it has not been valued in the non-facility/office setting. This stakeholder also did not include in their submission detailed recommendations for items, quantities, and unit costs for the supplies, equipment types, and clinical labor (if any), that might be incurred in the non-facility/office setting, all of which are key factors when determining valuation or mis-valuation. This stakeholder stated that the advances in High Intensity Focused Ultrasound (HIFU) technology toward the destruction of cancerous tissues in the prostate gland have matured to the point where this procedure is now equally as effective and as safe as the cryoablation procedure described by CPT code 55873 (Cryosurgical ablation of the prostate (includes ultrasonic guidance and monitoring)), which is currently valued in the non-facility/office setting (186.69 total RVUs, approximately $6,514) and has been for approximately 10 years. We note that CPT code 55880 was reviewed and valued in the CY 2021 PFS final rule (85 FR 84614 through 84615) in the facility setting only. Accordingly, we do not have enough claims data for this code to make accurate comparisons to similar codes that may be furnished in non-facility settings. There is no case presented here that constitutes a misvaluation of CPT code 55880, and therefore, we are not inclined to put this code forward as potentially misvalued for CY 2022; however, we welcome additional comment, including any analysis or studies demonstrating that this code meets the criteria listed above under "Identification and Review of Potentially Misvalued Services," particularly in regard to any changes in the resources to providing a service, or is otherwise potentially misvalued.
A stakeholder nominated CPT code 59200 (Insertion cervical dilator (e.g., laminaria, prostaglandin) as potentially misvalued because the direct PE inputs do not include the supply item, Dilapan-S. This stakeholder had sought to establish a Level II HCPCS code for Dilapan-S, but CMS did not find sufficient evidence to support that request. The stakeholder now submits Dilapan-S to be considered as PE supply input to a Level I CPT code(s). This stakeholder seeks to add Dilapan-S to the nonfacility/office PE inputs for CPT code 59200. Specifically, the stakeholder recommends adding 4 rods of Dilapan-S at $80.00 per unit, for a total of $320.00, as a replacement for the current PE supply item, laminaria tent (a small rod of dehydrated seaweed that when inserted in the cervix, rehydrates, absorbing the water from the surrounding tissue in the woman's body), which is currently listed at $4.0683 per unit, with a total of 3 units, for a total of $12.20. We welcome additional comment, including any analysis or studies demonstrating that this code meets the criteria listed above under "Identification and Review of Potentially Misvalued Services," particularly in regard to any changes in the resources to providing a service, or is otherwise potentially misvalued.
A stakeholder nominated CPT codes 66982 through 66986 as potentially misvalued, as they have not been valued in the non-facility/office setting. This stakeholder did not submit other details or reasoning to support their nomination. We note that some of these cataract-related procedures were initially reviewed and valued in CY 2020 PFS final rule (84 FR 62751), and that presently, additional codes in this family are scheduled to be reviewed and valued in this CY 2022 PFS proposed rule (we refer readers to section II.E. of this proposed rule, Valuation of Specific Codes). The highest utilization of these cataract codes are CPT code 66982 (Extracapsular cataract removal with insertion of intraocular lens prosthesis Start Printed Page 39130 (1-stage procedure), manual or mechanical technique (e.g., irrigation and aspiration or phacoemulsification), complex, requiring devices or techniques not generally used in routine cataract surgery (e.g., iris expansion device, suture support for intraocular lens, or primary posterior capsulorrhexis) or performed on patients in the amblyogenic developmental stage; without endoscopic cyclophotocoagulation) and CPT code 66984 (Extracapsular cataract removal with insertion of intraocular lens prosthesis (1-stage procedure), manual or mechanical technique (e.g., irrigation and aspiration or phacoemulsification); without endoscopic cyclophotocoagulation). In 2018 and 2019, these services were almost all performed in the ASC facility setting, but based on 2020 claims, the most common setting appears to have shifted to the hospital inpatient or hospital outpatient facility setting. There is no case presented here that constitutes a misvaluation of CPT codes 66982 to 66986, and therefore, we are not inclined to put this code family forward as potentially misvalued for CY 2022; however, we welcome additional comment, including any analysis or studies demonstrating that one or more of these codes meet the criteria listed above under "Identification and Review of Potentially Misvalued Services," particularly in regard to any changes in the resources to providing a service, or are otherwise potentially misvalued.

D. Telehealth and Other Services Involving Communications Technology, and Interim Final Rule With Comment Period for Coding and Payment of Virtual Check-In Services—Payment for Medicare Telehealth Services Under Section 1834(m) of the Act
As discussed in prior rulemaking, several conditions must be met for Medicare to make payment for telehealth services under the PFS. See further details and full discussion of the scope of Medicare telehealth services in the CY 2018 PFS final rule (82 FR 53006) and CY 2021 PFS final rule (85 FR 84502) and in 42 CFR 410.78 and 414.65.
1. Payment for Medicare Telehealth Services Under Section 1834(m) of the Act
a. Proposed Changes to the Medicare Telehealth Services List
In the CY 2003 PFS final rule with comment period (67 FR 79988), we established a regulatory process for adding services to or deleting services from the Medicare telehealth services list in accordance with section 1834(m)(4)(F)(ii) of the Act (42 CFR 410.78(f)). This process provides the public with an ongoing opportunity to submit requests for adding services, which are then reviewed by us and assigned to categories established through notice and comment rulemaking. Specifically, we assign any submitted request to add to the Medicare telehealth services list to one of the following two categories:
- Category 1: Services that are similar to professional consultations, office visits, and office psychiatry services that are currently on the Medicare telehealth services list. In reviewing these requests, we look for similarities between the requested and existing telehealth services for the roles of, and interactions among, the beneficiary, the physician (or other practitioner) at the distant site and, if necessary, the telepresenter, a practitioner who is present with the beneficiary in the originating site. We also look for similarities in the telecommunications system used to deliver the service; for example, the use of interactive audio and video equipment.
- Category 2: Services that are not similar to those on the current Medicare telehealth services list. Our review of these requests includes an assessment of whether the service is accurately described by the corresponding code when furnished via telehealth and whether the use of a telecommunications system to furnish the service produces demonstrated clinical benefit to the patient. Submitted evidence should include both a description of relevant clinical studies that demonstrate the service furnished by telehealth to a Medicare beneficiary improves the diagnosis or treatment of an illness or injury or improves the functioning of a malformed body part, including dates and findings, and a list and copies of published peer reviewed articles relevant to the service when furnished via telehealth. Our evidentiary standard of clinical benefit does not include minor or incidental benefits. Some examples of other clinical benefits that we would consider include the following:
- Ability to diagnose a medical condition in a patient population without access to clinically appropriate in-person diagnostic services.
- Treatment option for a patient population without access to clinically appropriate in-person treatment options.
- Reduced rate of complications.
- Decreased rate of subsequent diagnostic or therapeutic interventions (for example, due to reduced rate of recurrence of the disease process).
- Decreased number of future hospitalizations or physician visits.
- More rapid beneficial resolution of the disease process treatment.
- Decreased pain, bleeding, or other quantifiable symptom.
- Reduced recovery time.
- Category 3: In the CY 2021 PFS final rule (85 FR 84507), we created a third category of criteria for adding services to the Medicare telehealth services list on a temporary basis following the end of the PHE for the COVID-19 pandemic. This new category describes services that were added to the Medicare telehealth services list during the PHE for which there is likely to be clinical benefit when furnished via telehealth, but there is not yet sufficient evidence available to consider the services for permanent addition under the Category 1 or Category 2 criteria. Services added on a temporary, Category 3 basis would ultimately need to meet the criteria under Category 1 or 2 in order to be permanently added to the Medicare telehealth services list. To add specific services on a Category 3 basis, we conducted a clinical assessment to identify those services for which we could foresee a reasonable potential likelihood of clinical benefit Start Printed Page 39131 when furnished via telehealth. We considered the following factors:
++ Whether, outside of the circumstances of the PHE for COVID-19, there are concerns for patient safety if the service is furnished as a telehealth service.
++ Whether, outside of the circumstances of the PHE for COVID-19, there are concerns about whether the provision of the service via telehealth is likely to jeopardize quality of care.
++ Whether all elements of the service could fully and effectively be performed by a remotely located clinician using two-way, audio/video telecommunications technology.
In the CY 2021 PFS final rule (85 FR 84507), we also temporarily added several services to the Medicare telehealth services list using the Category 3 criteria described above. In this proposed rule, we are considering additional requests to add services to the Medicare telehealth services list on a Category 3 basis using the previously described Category 3 criteria.
The Medicare telehealth services list, including the additions described later in this section, is available on the CMS website at https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/index.html.
Beginning in CY 2019, we stated that for CY 2019 and onward, we intend to accept requests through February 10, consistent with the deadline for our receipt of code valuation recommendations from the RUC (83 FR 59491). For CY 2022, requests to add services to the Medicare telehealth services list must have been submitted and received by February 10, 2021. Each request to add a service to the Medicare telehealth services list must have included any supporting documentation the requester wishes us to consider as we review the request. Because we use the annual PFS rulemaking process as the vehicle to make changes to the Medicare telehealth services list, requesters are advised that any information submitted as part of a request is subject to public disclosure for this purpose. For more information on submitting a request in the future to add services to the Medicare telehealth services list, including where to mail these requests, see our website at https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/index.html.
b. Requests To Add Services to the Medicare Telehealth Services List for CY 2022
Under our current policy, we add services to the Medicare telehealth services list on a Category 1 basis when we determine that they are similar to services on the existing Medicare telehealth services list for the roles of, and interactions among, the beneficiary, physician (or other practitioner) at the distant site and, if necessary, the telepresenter. As we stated in the CY 2012 PFS final rule with comment period (76 FR 73098), we believe that the Category 1 criteria not only streamline our review process for publicly requested services that fall into this category, but also expedite our ability to identify codes for the Medicare telehealth services list that resemble those services already on the Medicare telehealth services list.
We received several requests to permanently add various services to the Medicare telehealth services list effective for CY 2022. We found that none of the requests we received by the February 10th submission deadline met our Category 1 or Category 2 criteria for permanent addition to the Medicare telehealth services list. The requested services are listed in Table 8.
Start Printed Page 39132

Start Printed Page 39133
We remind stakeholders that the criterion for adding services to the Medicare telehealth list under Category 1 is that the requested services are similar to professional consultations, office visits, and office psychiatry services that are currently on the Medicare telehealth services list, and that the criterion for adding services under Category 2 is that there is evidence of clinical benefit if provided as telehealth. As explained below, we find that none of the requested services met the Category 1 criterion.
We received a request to permanently add CPT code 51741 (Complex uroflowmetry (e.g., calibrated electronic equipment)) to the Medicare telehealth services list. This CPT code describes the acquisition of uroflowmetric information and analysis of that information. The code includes a technical component and a professional component. The technical component describes the acquisition of the uroflowmetric information when billed as a standalone service. The professional component describes the analysis for the uroflowmetric information when it is billed as a standalone service. As we have explained in previous rulemaking (see 83 FR 59483), the remote interpretation of diagnostic tests is not considered to be a telehealth service under section 1834(m) of the Act or our regulation at § 410.78. We do not believe that the technical component, which would include acquisition of the uroflowmetric Start Printed Page 39134 information, would meet the criterion to be added on a Category 1 basis because it is not similar to other services on the Medicare telehealth list. Moreover, we do not believe the uroflowmetric information can be accurately and effectively collected using two-way, audio/video communication technology to the degree that would make the results clinically useful. We believe the patient would need to be in the same location as the equipment; thus, making it impracticable to achieve via telehealth. Due to these concerns, we do not believe that the submitted information demonstrates sufficient clinical benefit to support the addition of CPT code 51741 to the Medicare telehealth services list.
We received a request to permanently add several biofeedback, services, CPT codes 90901, 90912, and 90913, to the Medicare telehealth services list. We do not believe these services are similar to Category 1 services on the Medicare telehealth list in that these services describe the application of electrodes directly to the patient's skin and using them to monitor the patient's response. Therefore, we do not believe they meet the criterion for addition to the Medicare telehealth services list on a Category 1 basis. We also believe that proper application of electrodes and monitoring of the patient's response would require the furnishing practitioner to be in the same physical location as the beneficiary. As such, we do not believe these services would meet the criteria for addition to the Medicare telehealth list on a Category 2 basis. When we reviewed these biofeedback services on a Category 2 basis, we found that the information supplied with the requests was not detailed enough to determine if the objective functional outcomes (that is, Activities of Daily Living (ADLs) and Instrumental Activities of Daily Living (IADLs) of the telehealth patients) were similar to that of patients treated in person. Moreover, we believe that the ADLs/IADLs alone are not sufficient to determine if these services, when performed via telehealth, demonstrate a clinical benefit to a patient. We would request that stakeholders supply a more comprehensive set of objective data in order to fully illustrate any benefits, to better enable us to evaluate all outcomes.
We received requests to permanently add Neuropsychological/Psychological Testing services, CPT codes 96130-96133 and 96136-96139, to the Medicare telehealth services list. We separately reviewed each of the services in these two code families. In prior years' rulemaking, we have declined to add these services on a Category 1 basis because, in contrast to other services on the telehealth list these services require close observation by the furnishing practitioner to monitor how a patient responds and progresses through the testing (see 81 FR 80197). We continue to believe that this is the case. All of these codes describe services that involve a very thorough observation and testing process, and require the tester to observe the following: Speed of responses; the ability to adjust focus; written, sometimes manual tasks; following tasks that display the patients' visuospatial mapping abilities, pattern recognition, abstraction, calculation—all while appreciating that the patient may be distracted or aided by environmental cues. The tester must also maintain some subjective amount of flexibility to allow the patient to be in their environment. Additionally, the tester has to maintain professional scrutiny through dynamic tasks. Given all of the above, remote observation by the furnishing practitioner to accomplish the testing in question seems impractical and potentially creates the risk of inaccuracies in diagnosis and subsequent treatment. We note that the information supplied by stakeholders did not address these concerns, and as such, we have concerns over patient safety and the ability of these services to be accurately and thoroughly performed via telehealth to demonstrate a clinical benefit to Medicare beneficiaries. Therefore, we do not believe these services meet the Category 2 criteria for permanent addition to the Medicare telehealth list of services. Consequently, we are not proposing to add these services to the Medicare telehealth services list. We encourage stakeholders to submit information addressing the concerns we have stated in any future requests to have these services added to the Medicare telehealth list of services.
We received requests to add Therapy Procedures, CPT codes 97110, 97112, 97116, 97150, and 97530; Physical Therapy Evaluations, CPT codes 97161-97164; Therapy Personal Care services, CPT codes 97535, 97537, and 97542; and Therapy Tests and Measurements services, CPT codes 97750, 97755, and 97763, to the Medicare telehealth services list. In the CY 2017 PFS final rule (81 FR 80198), we noted that section 1834(m)(4)(E) of the Act specifies the types of practitioners who may furnish and bill for Medicare telehealth services as those practitioners under section 1842(b)(18)(C) of the Act. Physical therapists (PTs), occupational therapists (OTs), and speech-language pathologists (SLPs) are not among the practitioners identified in section 1842(b)(18)(C) of the Act. We also stated in the CY 2017 PFS final rule that, because these services are predominantly furnished by PTs, OTs, and SLPs, we did not believe it would be appropriate to add them to the Medicare telehealth services list at that time. In a subsequent request to consider adding these services for 2018, the original requester suggested that we might propose these services be added to the Medicare telehealth services list so that payment can be made for them when furnished via telehealth by physicians or practitioners who can serve as distant site practitioners. We stated that, since the majority of the codes are furnished over 90 percent of the time by therapy professionals who are not included on the statutory list of eligible distant site practitioners, we believed that adding therapy services to the Medicare telehealth services list could result in confusion about who is authorized to furnish and bill for these services when furnished via telehealth. We continue to believe this to be true; however, we reviewed each therapy service separately, and have categorized them together here for convenience as the same set of information accompanied the request for each of these services.
We determined that these services did not meet the Category 1 criteria for addition to the Medicare telehealth services because they are therapeutic in nature and in many instances involve direct physical contact between the practitioner and the patient. In assessing the evidence that was supplied by stakeholders in support of adding these services to the Medicare telehealth services list on a Category 2 basis, we concluded that it did not provide sufficient detail to determine whether all of the necessary elements of the service could be furnished remotely, and whether the objective functional outcomes of ADL and IADL for the telehealth patients were similar to those of patients receiving the services in person. As we stated above when discussing the request to add certain biofeedback services to the telehealth list, we do not believe ADLs and IADLS alone are sufficient to demonstrate clinical benefit to a Medicare beneficiary. We have enumerated above some examples of the types of clinical benefits we would consider when evaluating services using the Category 2 criterion.
Therefore, we do not believe the supplied information demonstrates that the services meet either the Category 1 or the Category 2 criteria. We are not Start Printed Page 39135 proposing to add these services to the Medicare telehealth services list. We continue to encourage commenters to supply sufficient data for us to be able to see all measurements/parameters performed, so that we may evaluate all outcomes.
We received requests to add the services in Table 9, and we note that these services are generally not separately payable under the Medicare PFS. Given that these services are not separately payable when furnished in-person, they would not be separately payable when furnished as telehealth. Section 1834(m)(2)(A) of the Act provides that payment for a service when furnished as a telehealth services is equal to the payment when the service is furnished in person. CPT code 90849 has a restricted payment status, indicating that claims must be adjudicated on a case-by-case basis when furnished in-person. Accordingly, any separate payment for that service would require special consideration and not be routine. Therefore, we do not believe this service should be added to the Medicare telehealth list. CPT codes 98960-98962 are bundled services, and therefore, payment for these services is always bundled into payment of other services. For that reason, we are not proposing to add them to the Medicare list of telehealth services.

We received requests to temporarily add Neurostimulators, CPT codes 95970-95972, and Neurostimulators, Analysis-Programming services, CPT codes 95983 and 95984, to the Medicare telehealth services list using the Category 3 criteria (see Table 10). In their submission, the requestor noted they would conduct a future study and would submit the study data to CMS at a later date. These services are on the expanded telehealth services list for the PHE, but were not added by CMS on a category 3 basis in the CY 2021 PFS final rule. We do not yet have sufficient information to adjudicate whether these services are likely to meet the category 1 or category 2 criteria given additional time on the Medicare telehealth services list, without having evaluated the full data, and we encourage commenters to submit all available information, when available, for future consideration. As a result, we are not proposing to add these services to the Medicare telehealth list of services on a Category 3 basis at this time.
Start Printed Page 39136

c. Revised Timeframe for Consideration of Services Added to the Telehealth List on a Temporary Basis
In the CY 2021 PFS final rule (85 FR 84506), in response to the PHE for COVID-19, we created a third category of criteria for adding services to the Medicare telehealth services list on a temporary basis. We included in this category the services that were added during the PHE for COVID-19 for which we believed there is likely to be clinical benefit when furnished via telehealth, but for which there is not yet sufficient evidence available to consider the services as permanent additions under Category 1 or Category 2 criteria. We recognized that the services we added on a temporary basis under Category 3 would ultimately need to meet the criteria under Categories 1 or 2 in order to be permanently added to the Medicare telehealth services list, and that there was a potential for evidence development that could continue through the Category 3 temporary addition period. We also stated that any service added on a temporary basis under Category 3 would remain on the Medicare telehealth services list through the end of the calendar year in which the PHE for COVID-19 ends.
We added 135 services to the Medicare telehealth list in CY 2020 on an interim basis in response to the PHE for COVID-19 through the interim final rule with comment period (IFC) (March 31st COVID-19 IFC (85 FR 19234-19243) and the subregulatory process established in the May 8th COVID-19 IFC (85 FR 27550-27649). Since the publication of the May 8th COVID-19 IFC, we have added several services to the Medicare telehealth list of services using this subregulatory process (please see https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes for the list of codes available for telehealth under the PFS). As discussed in the CY 2021 PFS final rule (FR 85 84507), at the conclusion of the PHE for COVID-19, associated waivers and interim policies will expire, payment for Medicare telehealth services will once again be limited by the requirements of section 1834(m) of the Act, and we will return to the policies established through the regular notice-and-comment rulemaking process, including the previously established Medicare telehealth services list, as modified by subsequent changes in policies and additions to the telehealth services list adopted through rulemaking. Services that were temporarily added on an interim basis during the PHE for COVID-19 would not be continued on the list after the end of the PHE for COVID-19.
Numerous stakeholders have continued to note that there is uncertainty about when the PHE for COVID-19 may end, and express concerns that the services added to the telehealth list on a temporary basis could be removed from the list before practitioners have had time to compile Start Printed Page 39137 and submit evidence to support the permanent addition of these services on a Category 1 or Category 2 basis. To respond to these continuing concerns, we are proposing to revise the timeframe for inclusion of the services we added to the Medicare telehealth services list on a temporary, Category 3 basis. Extending the temporary inclusion of these services on the telehealth list will allow additional time for stakeholders to collect, analyze and submit data on those services to support their consideration for permanent addition to the list on a Category 1 or Category 2 basis.
We propose to retain all services added to the Medicare telehealth services list on a Category 3 basis until the end of CY 2023. This will allow us time to collect more information regarding utilization of these services during the pandemic, and provide stakeholders the opportunity to continue to develop support for the permanent addition of appropriate services to the telehealth list through our regular consideration process, which includes notice-and-comment rulemaking. By keeping these services on the Medicare telehealth services list through CY 2023, we will facilitate the submission of requests to add services permanently to the Medicare telehealth services list for consideration in the CY 2023 PFS rulemaking process and for consideration in the CY 2024 PFS rule.
See Table 11 for a list of services that were added to the Medicare telehealth services list on an interim basis to respond to the PHE for COVID-19, but were not extended on a temporary Category 3 basis in the CY 2021 PFS final rule. Under our current policy, these services will be removed from the Medicare telehealth services list as of the date that the PHE for COVID-19 ends. We recognize that, during the time between the publication of the CY 2021 PFS final rule and this proposed rule, practitioners may have used that time to compile new evidence of clinical benefit to support addition to the Medicare telehealth services list on a category 3 basis, including information that suggests that a certain service would likely meet the category 1 or category 2 criteria if provided with more time. We are soliciting comment on whether any of the services that were added to the Medicare telehealth list for the duration of the PHE for COVID-19 should now be added to the Medicare telehealth list on a Category 3 basis to allow for additional data collection for submission for CMS to consider as part of the rulemaking process described in prior paragraphs.
Start Printed Page 39138

Start Printed Page 39139
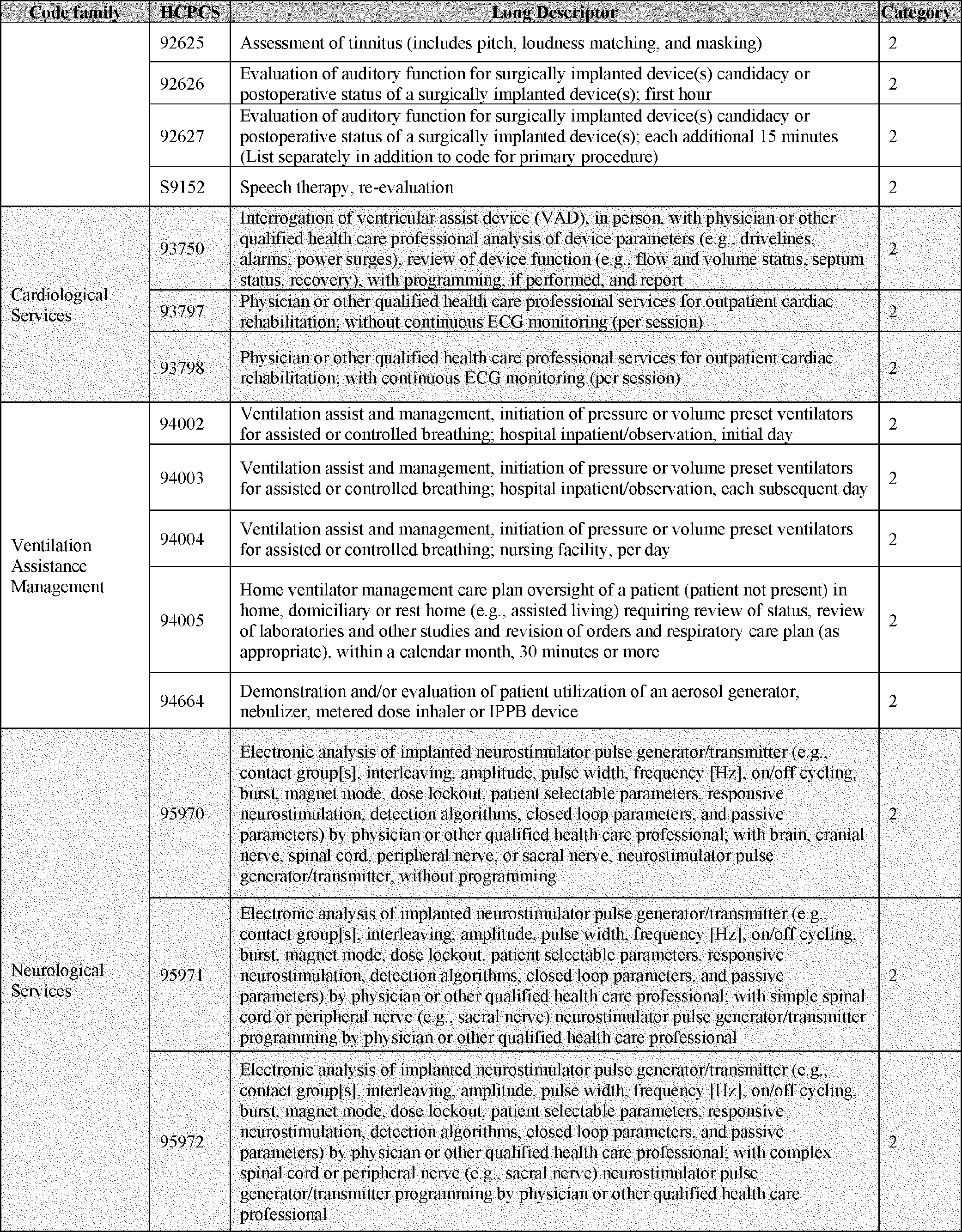
Start Printed Page 39140

Start Printed Page 39141

Start Printed Page 39142

Start Printed Page 39143

Start Printed Page 39144
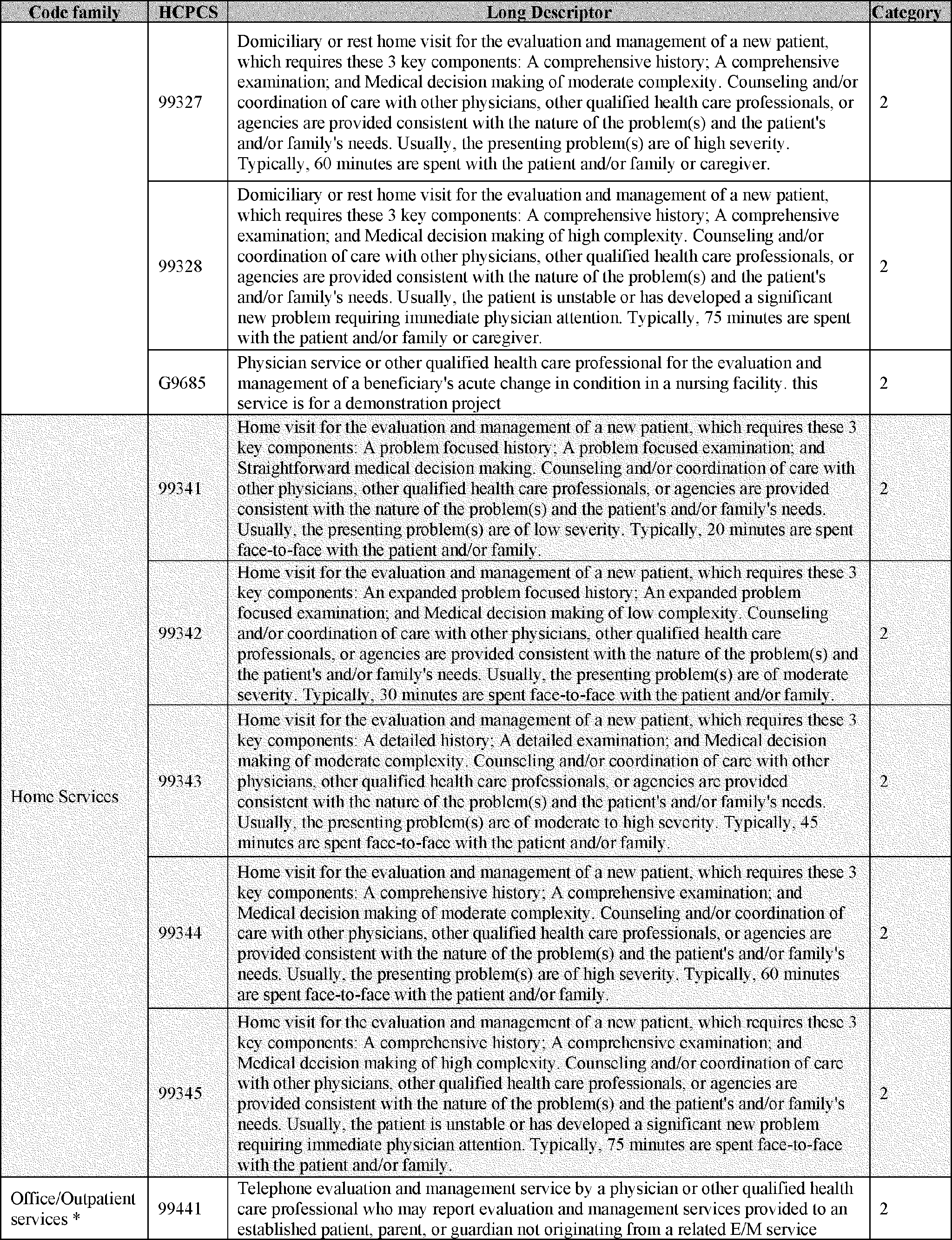
Start Printed Page 39145

d. Implementation of Provisions of the Consolidated Appropriations Act, 2021 (CAA)
The Consolidated Appropriations Act, 2021 (CAA) (Pub. L. 116-260, December 27, 2020) included a number of provisions pertaining to Medicare telehealth services. The Medicare telehealth statute at section 1834(m)(4)(C) of the Act generally limits the scope of telehealth services to those furnished in rural areas and in certain enumerated types of "originating sites" including physician offices, hospitals, and other medical care settings. Section 1834(m)(7) of the Act, (as added by section 2001(a) of the SUPPORT for Patients and Communities Act (Pub. L. 115-271, October 24, 2018), specifies that the geographic restrictions under section 1834(m)(4)(C)(i) of the Act do not apply, and includes the patient's home as a permissible originating site, for telehealth services furnished to a patient with a diagnosed substance use disorder (SUD) for treatment of that disorder or a co-occurring mental health disorder. Section 123(a) of Division CC of the CAA amended section 1834(m)(7)(A) of the Act to broaden the scope of services for which the geographic restrictions under section 1834(m)(4)(C)(i) of the Act do not apply and for which the patient's home is a permissible originating site to include telehealth services furnished for the purpose of diagnosis, evaluation, or treatment of a mental health disorder, effective for services furnished on or after the end of the PHE for COVID-19.[2]
Section 123(a) of the CAA also added subparagraph (B) to section 1834(m)(7) of the Act to prohibit payment for a telehealth service furnished in the patient's home under paragraph (7) unless the physician or practitioner furnishes an item or service in-person, without the use of telehealth, within 6 months prior to the first time the physician or practitioner furnishes a telehealth service to the beneficiary, and thereafter, at such times as the Secretary determines appropriate. However, section 123(a) of the CAA added a clarification at section 1834(m)(7)(B)(ii) of the Act that the periodic requirement for an in-person item or service does not apply if payment for the telehealth service furnished would have been allowed without the new amendments. As such, the requirement for a periodic in-person item or service applies only for telehealth services furnished for purposes of diagnosis, evaluation, or treatment of a mental health disorder other than for treatment of a diagnosed SUD or co-occurring mental health disorder, and only in locations that do not meet the geographic requirements in section 1834(m)(4)(C)(i) of the Act or when the originating site is the home of the patient, regardless of geography. We are seeking comment on whether we Start Printed Page 39146 should adopt a claims-based mechanism to distinguish between the mental health telehealth services that are within the scope of the CAA amendments and those that are not (in other words, the services for which payment was newly authorized by the CAA amendments, and those for which payment was authorized before the CAA amendments), and if so, what that mechanism should be. In the event that we need to distinguish between the mental health telehealth services that are within the scope of the CAA amendments and those that are not we are also seeking comment on whether a clarification should be added to the regulation at § 410.78 as follows (which would take into account the other amendments we are proposing to § 410.78):
The requirement that the physician or practitioner must furnish an item or service in person, without the use of telehealth, within a specified time frame shall not apply to telehealth services furnished for treatment of a diagnosed substance use disorder or co-occurring mental health disorder, or to services furnished in an originating site described in paragraphs (b)(3)(i) through (viii) or (xiii) that meets the geographic requirements specified in paragraph (b)(4) other than (b)(4)(iv)(D).
As we noted above, section 123(a) of the CAA amends section 1834(m)(7)(B)(i)(I) of the Act to prohibit payment for telehealth services under that paragraph unless the physician or practitioner furnished an item or service to the patient in person, without the use of telehealth, within 6 months before the first telehealth service. Thereafter, section 1834(m)(7)(B)(i)(II) of the Act leaves the Secretary discretion to specify the times or intervals at which an in-person, non-telehealth service is required as a condition of payment for these telehealth services. Therefore, in order to implement the new statutory requirement to specify when an in-person service is required, we propose that, as a condition of payment for a mental health telehealth service described in section 1834(m)(7)(A) of the Act other than services described in section 1834(m)(7)(B)(ii) of the Act (that is, services for which payment was authorized before the CAA amendments), the billing physician or practitioner must have furnished an in-person, non-telehealth service to the beneficiary within the 6-month period before the date of the telehealth service.
We are also seeking comment on whether the required in-person, non-telehealth service could also be furnished by another physician or practitioner of the same specialty and same subspecialty within the same group as the physician or practitioner who furnishes the telehealth service. We note that the language in the CAA states that the physician or practitioner furnishing the in-person, non-telehealth service must be the same person as the practitioner furnishing the telehealth service. There are several circumstances, however, under which we have historically treated the billing practitioner and other practitioners of the same specialty or subspecialty in the same group as if they were the same individual. For instance, for purposes of deciding whether a patient is a new or established patient, or whether to bill for initial or subsequent visit, practitioners of the same specialty/subspecialty in the same group are treated as the same person. For example, when Physician A and Physician B are of the same specialty and subspecialty and in the same group, if Physician A furnishes an initial critical care service to a patient, and Physician B subsequently furnishes additional critical care services to the same beneficiary for the same condition on the same day, Physician B would bill for a subsequent critical care service rather than an initial critical care visit. As we explain in in section II.F.2 of this proposed rule, because practitioners in the same specialty and same group often cover for one another to provide concurrent services, we believe the total time for critical care services furnished to a patient on the same day by the practitioners in the same group with the same specialty should be reflected as if it were a single set of critical care services furnished to the patient. See section II.F.2 of this proposed rule for further discussion of our current and proposed policies for billing critical care services. Similarly, if Physician A furnished a service to a patient, and then Physician B furnished a service to the patient a few months later, that patient would be considered an established patient with respect to both Physician A and Physician B. For example, Physician B could initiate care management services for the patient as an established patient. An example of guidance to this effect can be found in the Medicare Claims Processing Manual (IOM Pub. 100-04, Chapter 12, § 30.6.7), which defines "new patient" as a patient who has not received any professional services, that is, E/M service or other face-to-face service (for example, surgical procedure) from the physician or physician group (same physician specialty) within the previous 3 years, for E/M services.
We note that this manual provision is also consistent with CPT guidance on whether a patient is a new or established patient.[3]
We are interested in comments regarding the extent to which a patient routinely receiving mental health services from one practitioner in a group might have occasion to see a different practitioner of the same specialty in that group for treatment of the same condition. This might occur when practitioners in a group cover for each other when a particular practitioner is unavailable or when a practitioner has left the group, but the beneficiary continues to receive services furnished by the group. In addition, fee-for-time compensation arrangements (formerly referred to as locum tenens arrangements), as described in section 1842(b)(6)(D) of the Act, allow for payment to be made to a physician for physicians' services (and services furnished incident to such services) furnished by a second physician to patients of the first physician if the first physician is unavailable to provide the services, and the services are furnished pursuant to an arrangement that is either informal and reciprocal, or involves per diem or other fee-for-time compensation for such services.
Recognizing the importance of ensuring access to mental health telehealth services to beneficiaries who are unable to see the same practitioner who furnished the prerequisite in-person services due to the practitioner's unavailability, we are seeking comments on an alternative policy to also allow the prerequisite in-person, non-telehealth service for certain mental health telehealth services to be furnished by a practitioner in the same specialty/subspecialty in the same group when the physician or practitioner who furnishes the telehealth service is unavailable or the two professionals are practicing as a team.
As amended by the CAA, section 1834(m)(7)(B)(i)(II) of the Act specifies that for subsequent mental health telehealth service, an in-person, non-telehealth service is required at such times as the Secretary determines appropriate. We are proposing to require that an in-person, non-telehealth service must be furnished by the physician or practitioner at least once within 6 months before each telehealth service furnished for the diagnosis, evaluation, or treatment of mental health disorders by the same practitioner, other than for Start Printed Page 39147 treatment of a diagnosed SUD or co-occurring mental health disorder, and that the distinction between the telehealth and non-telehealth services must be documented in the patient's medical record. We distinguish between mental health services furnished for a diagnosed SUD or co-occurring mental health disorder and those furnished to beneficiaries without a SUD diagnosis on the basis of ICD-10 diagnosis codes included on claims when the services are billed. We chose this interval because we are concerned that an interval less than 6 months may impose potentially burdensome travel requirements on the beneficiary, but that an interval greater than 6 months could result in the beneficiary not receiving clinically necessary in-person care/observation. The proposed 6-month interval also matches the specified statutory interval for the initial telehealth service. We believe that a 6-month interval strikes an appropriate balance between these competing considerations, but are seeking comment on whether a different interval, whether shorter, such as 3-4 months or longer, such as 12 months, may be appropriate to balance program integrity and patient safety concerns with increased access to care. However, we note that regardless of the time interval we establish, the practitioner is not precluded from scheduling in-person visits at a more frequent interval should such visit be determined to be clinically appropriate or preferred by the patient.
As discussed below in this section of this proposed rule, "e. Payment for Medicare Telehealth Services Furnished Using Audio-Only Communication Technology," we are proposing to revise our regulatory definition of "interactive telecommunications system" to permit use of audio-only communications technology for mental health telehealth services under certain conditions when provided to beneficiaries located in their home. Therefore, we are also seeking comment on whether it would be appropriate to establish a different interval for these telehealth services, for the diagnosis, evaluation, or treatment of mental health disorders, other than for treatment of diagnosed SUD or co-occurring mental health disorder, when furnished as permitted through audio-only communications technology.
In any event, we propose that there would need to be an in-person visit within 6 months of any telehealth service furnished for the diagnosis, evaluation, or treatment of mental health disorders (other than for treatment of a diagnosed SUD or co-occurring mental health disorder), and the in-person visit would need to be documented in the patient's medical record. Payment would not be made for these telehealth services unless the required in-person service was furnished within 6 months of the telehealth service.
Given the addition of the home of the individual as a permissible originating site for telehealth services for purposes of diagnosis, evaluation, or treatment of a mental health disorder, we are proposing to revise our regulation at § 410.78(b)(3) to add a new paragraph (xiv) to identify the home of a beneficiary as an originating site for telehealth services for the diagnosis, evaluation, or treatment of a mental health disorder, effective for services furnished on or after the first day after the end of the PHE as defined § 400.200 of our regulations; and to provide that payment will not be made for a telehealth service furnished under this paragraph unless the physician or practitioner has furnished an item or service in person, without the use of telehealth, for which Medicare payment was made (or would have been made if the patient were entitled to, or enrolled for, Medicare benefits at the time the item or service is furnished) within 6 months of the telehealth service. We are also proposing to revise our regulation at § 410.78(b)(4)(iv)(D) to specify that the geographic restrictions in § 410.78(b)(4) do not apply to telehealth services furnished for the diagnosis, evaluation, or treatment of a mental health disorder, effective for services furnished on or after the first day after the end of the PHE as defined in our regulation at § 400.200.
In addition, section 125(c) of the CAA amended section 1834(m)(4)(C)(ii) of the Act to add to the list of permissible telehealth originating sites a rural emergency hospital, which is a new Medicare provider type added by section 125 of the CAA effective beginning in CY 2023.
We are also proposing to amend our regulation at § 410.78, Telehealth services, to conform with the statutory change to include rural emergency hospitals as telehealth originating sites beginning in CY 2023. In accordance with section 1834(m)(4)(C)(ii)(XI) of the Act, as added by section 125(c) of the CAA, we propose to revise § 410.78(b)(3) of our regulations to add a rural emergency hospital, as defined in section 1861(kkk)(2) of the Act, as a permissible originating site for telehealth services furnished on or after January 1, 2023.
e. Payment for Medicare Telehealth Services Furnished Using Audio-Only Communication Technology
Section 1834(m) of the Act outlines the requirements for Medicare payment for telehealth services that are furnished via a "telecommunications system," and specifies that, only for purposes of Medicare telehealth services through a federal telemedicine demonstration program conducted in Alaska or Hawaii, the term "telecommunications system" includes asynchronous, store-and-forward technologies. We further defined the term, "telecommunications system," in the regulation at § 410.78(a)(3) to mean an interactive telecommunications system, which is defined as multimedia communications equipment that includes, at a minimum, audio and video equipment permitting two-way, real-time interactive communication between the patient and distant site physician or practitioner.
During the PHE for COVID-19, we used waiver authority under section 1135(b)(8) of the Act to temporarily waive the requirement, for certain behavioral health and/or counseling services and for audio-only evaluation and management (E/M) visits, that telehealth services must be furnished using an interactive telecommunications system that includes video communications technology. Therefore, for certain services furnished during the PHE for COVID-19, we make payment for these telehealth services when they are furnished using audio-only communications technology. Emergency waiver authority is no longer available after the PHE for COVID-19 ends, and telehealth services will again be subject to all statutory and regulatory requirements.
In the CY 2021 PFS final rule (85 FR 84535), we noted that we continued to believe that our longstanding regulatory definition of "telecommunications system" reflected the intent of statute and that the term should continue to be defined as including two way, real-time, audio/video communication technology.
Historically, we have not proposed any permanent modifications to the definition of "interactive telecommunications system" to allow for use of audio-only communications technology due to our interpretation of the statutory requirements, as well as concerns over program integrity and quality of care. Specifically, we were concerned that the use of audio-only communications technology for Medicare telehealth services could lead to inappropriate overutilization, and believed that video visualization of the patient generally was necessary to fulfill Start Printed Page 39148 the full scope of service elements of the codes included on the Medicare telehealth list. We believe it is reasonable to reassess these concerns, given the now widespread utilization during the PHE for COVID-19 of Medicare telehealth services furnished using audio-only communication technology. Based upon an initial review of claims data collected during the PHE for COVID-19, which describe audio-only telephone E/M services, we observed that the audio-only E/M visits have been some of the most commonly performed telehealth services during the PHE, and that most of the beneficiaries receiving these services were receiving them for treatment of a mental health condition. Given the generalized shortage of mental health care professionals (https://bhw.hrsa.gov/sites/default/files/bureau-health-workforce/data-research/technical-documentation-health-workforce-simulation-model.pdf), and the existence of areas and populations where there is limited access to broadband due to geographic or socioeconomic challenges, we believe beneficiaries may have come to rely upon the use of audio-only communication technology in order to receive mental health services, and that a sudden discontinuation of this flexibility at the end of the PHE could have a negative impact on access to care.
As explained above, section 123 of the CAA removes the geographic restrictions for Medicare telehealth services for the diagnosis, evaluation, or treatment of a mental health disorder, and adds the patient's home as a permissible originating site for these telehealth services. We also believe that mental health services are different from most other services on the Medicare telehealth services list in that many of the services primarily involve verbal conversation where visualization between the patient and furnishing physician or practitioner may be less critical to provision of the service. While we continue to believe that two-way, audio/video communications technology is the appropriate, general standard for telehealth services, and that there may be particular instances where visual cues may help a practitioner's ability to assess and treat patients with mental health disorders, especially where opioids or other mental health medications are involved (for example, visual cues as to patient hygiene, or indicators of self-destructive behavior), we note that stakeholders have suggested to us that the availability of telehealth services for mental health care via audio-only communications technology would increase access to care. This is especially true in areas with poor broadband infrastructure and among patient populations that do not wish to use, do not have access to, and/or are unable to utilize devices that permit a two-way, audio/video interaction. Our preliminary analysis of Medicare claims data, as well as information provided to us by stakeholders on the popularity of these services, indicates that use of interactive communication technology for mental health care would likely continue to be high even beyond the circumstances of the COVID-19 pandemic. According to our analysis of Medicare Part B claims data for services furnished via Medicare telehealth during the PHE for COVID-19, utilization of telehealth for many professional services spiked around April 2020 and has diminished over the ensuing months. In contrast, preliminary analysis of Medicare claims data suggest that, for many mental health services that were permanently and temporarily added to the Medicare Telehealth list, there is a steady utilization trend from April 2020 and thereafter. Furthermore, as described above, according to preliminary analysis of claims data which examined utilization by diagnosis, the codes for audio-only E/M services have been highly utilized during the PHE, particularly for beneficiaries with mental health conditions.
Given these considerations, we now believe that it would be appropriate to revisit our regulatory definition of "interactive telecommunications system" beyond the circumstances of the PHE to allow for the inclusion of audio-only services under certain circumstances. Therefore, we are proposing to amend our regulation at § 410.78(a)(3) to define interactive telecommunications system to include audio-only communications technology when used for telehealth services for the diagnosis, evaluation, or treatment of mental health disorders furnished to established patients when the originating site is the patient's home. We believe this proposal is consistent with the expansion of at-home access to mental health telehealth services in section 1834(m)(7) of the Act, as amended by section 123 of the CAA, which required that the beneficiary must have received a Medicare-paid (or payable), in-person item or service from the physician or practitioner furnishing the mental health services through telehealth within 6 months of the first mental health telehealth service. We are proposing to adopt a similar ongoing requirement that an in-person item or service must be furnished within 6 months of such a mental health telehealth service. We reiterate that our proposed policy to permit audio-only telehealth services is limited to services where the home is the originating site. This is because the other enumerated telehealth originating sites are medical settings that are far more likely to have access to reliable broadband internet service. When a patient is located at one of these originating sites, access to care is far less likely to be limited by access to broadband that facilitates a video connection. In contrast, access to broadband, devices, and user expertise to enable a video connection is less likely to be available in the patient's home. As described in prior paragraphs, we also believe that mental health services are distinct from other kinds of services on the Medicare telehealth list in that many of the services do not necessarily require visualization of the patient to fulfill the full scope of service elements.
We are also proposing to limit payment for audio-only services to services furnished by physicians or practitioners who have the capacity to furnish two-way, audio/video telehealth services but are providing the mental health services via audio-only communication technology in an instance where the beneficiary is unable to use, does not wish to use, or does not have access to two-way, audio/video technology. We believe that this requirement will ensure that mental health services furnished via telehealth are only conducted using audio-only communication technology in instances where the use of audio-only technology is facilitating access to care that would be unlikely to occur otherwise, given the patient's technological limitations or preferences. In the interests of monitoring utilization and program integrity concerns for audio-only telehealth services furnished under the terms of this proposed exception, we are proposing to create a service-level modifier that would identify these mental health telehealth services furnished to a beneficiary in their home using audio-only communications technology. The use of this modifier would also serve to certify that the audio-only telehealth service meets the requirements for the exception specified in proposed on § 410.78(a)(3), including that the furnishing physician or practitioner has the capacity to furnish the service using interactive two-way, real-time audio/video communication technology, but instead used audio-only Start Printed Page 39149 technology under the conditions specified in the regulation.
We are proposing to amend our regulation at § 410.78(a)(3) to specify that an interactive telecommunications system can include interactive, real-time, two-way audio-only technology for telehealth services furnished for the diagnosis, evaluation, or treatment of a mental health disorder as described under paragraph (b)(4)(D), under the following conditions: The patient is located in their home at the time of service as described at § 410.78 (b)(3)(xiv); the distant site physician or practitioner has the technical capability at the time of the service to use an interactive telecommunications system that includes video; and the patient is not capable of, or does not consent to, the use video technology for the service.
We are seeking comment on these proposals, as well as what, if any, additional documentation should be required in the patient's medical record to support the clinical appropriateness of providing audio-only telehealth services for mental health in the event of an audit or claims denial. Additional required documentation could include information about the patient's level of risk and any other guardrails that are appropriate to demonstrate clinical appropriateness, and minimize program integrity and patient safety concerns.
We are also seeking comment on whether, for purposes of the proposed audio-only mental health telehealth services exception, we should exclude certain higher-level services, such as level 4 or 5 E/M visit codes, when furnished alongside add-on codes for psychotherapy, or codes that describe psychotherapy with crisis. We are seeking comment on whether the full scope of service elements for these codes could be performed via audio-only communication technology. However, we also note that maintaining the availability of these services through audio-only communication technology might give patients access to care needed to address their higher level or acute mental health needs in instances where they are unable to access two-way, audio/video communication technology.
2. Other Non-Face-to-Face Services Involving Communications Technology Under the PFS
a. Expiration of PHE Flexibilities for Direct Supervision Requirements
Under section 1861 of the Act and at § 410.32(b)(3) of the regulations, Medicare requires certain types of services to be furnished under specific levels of supervision of a physician or practitioner, including diagnostic tests, services incident to physician services, and other services. For professional services furnished incident to the services of a billing physician or practitioner (see § 410.26) and many diagnostic tests (see § 410.32), direct supervision is required. Additionally, for pulmonary rehabilitation services (see § 410.47) and for cardiac rehabilitation and intensive cardiac rehabilitation services (see § 410.49), requirements for immediate availability and accessibility of a physician are considered to be satisfied if the physician meets the requirements for direct supervision for physician office services at § 410.26 and for hospital outpatient services at § 410.27. Outside the circumstances of the PHE, direct supervision requires the immediate availability of the supervising physician or other practitioner, but the professional need not be present in the same room during the service, and we have interpreted this "immediate availability" requirement to mean in-person, physical, not virtual, availability.
Through the March 31st COVID-19 IFC, we changed the definition of "direct supervision" during the PHE for COVID-19 (85 FR 19245 through 19246) as it pertains to supervision of diagnostic tests, physicians' services, and some hospital outpatient services, to allow the supervising professional to be immediately available through virtual presence using real-time audio/video technology, instead of requiring their physical presence. In the CY 2021 PFS final rule (85 FR 84538 through 84540), we finalized continuation of this policy through the later of the end of the calendar year in which the PHE for COVID-19 ends or December 31, 2021. In that rule, we also solicited comment on issues related to the policy allowing virtual provision of direct supervision, specifically whether there should be any additional guardrails or limitations put in place to ensure patient safety/clinical appropriateness, beyond typical clinical standards, and whether we should consider potential restrictions to prevent fraud or inappropriate use. We also stated that we will consider this and other information as we contemplate future policy regarding use of communication technology to satisfy supervision requirements, as well as the best approach for safeguarding patient safety while promoting use of technology to enhance access.
We also note that the temporary exception to allow immediate availability for direct supervision through virtual presence facilitates the provision of telehealth services by clinical staff of physicians and other practitioners incident to their own professional services. This is discussed in the March 31st COVID-19 IFC (85 FR 19246). This is especially relevant for services such as physical therapy, occupational therapy, and speech language pathology services, since those practitioners can only bill Medicare directly for telehealth services under telehealth waivers that are effective only during the PHE for COVID-19. We note that sections 1834(m)(4)(D) and (E) of the Act specifies the types of clinicians who may furnish and bill for Medicare telehealth services, and include only physicians as defined in section 1861(r) of the Act and practitioners described in section 1842(b)(18)(C) of the Act.
We continue to seek information on whether this flexibility should be continued beyond the later of the end of the PHE for COVID-19 or CY 2021. Specifically, we are seeking comment on the extent to which the flexibility to meet the immediate availability requirement for direct supervision through the use of real-time, audio/video technology is being used during the PHE, and whether physicians and practitioners anticipate relying on this flexibility after the end of the PHE. We are seeking comment on whether this flexibility should potentially be made permanent, meaning that we would revise the definition of "direct supervision" at § 410.32(b)(3)(ii) to include immediate availability through the virtual presence of the supervising physician or practitioner using real-time, interactive audio/video communications technology without limitation after the PHE for COVID-19, or if we should continue the policy in place for a short additional time to facilitate a gradual sunset of the policy. We are soliciting comment on whether the current timeframe for continuing this flexibility at § 410.32(b)(3)(ii), which is currently the later of the end of the year in which the PHE for COVID-19 ends or December 31, 2021, remains appropriate, or if this timeframe should be extended through some later date to facilitate the gathering of additional information in recognition that, due to the on-going nature of the PHE for COVID-19, practitioners may not yet have had time to assess the implications of a permanent change in this policy. We also seek comment regarding the possibility of permanently allowing immediate availability for direct supervision through virtual presence using real-time audio/video technology for only a subset of services, as we recognize that it may be inappropriate to allow direct Start Printed Page 39150 supervision without physical presence for some services, due to potential concerns over patient safety if the practitioner is not immediately available in-person. We are also seeking comment on, were this policy to be made permanent, if a service level modifier should be required to identify when the requirements for direct supervision were met using two-way, audio/video communications technology.
b. Interim Final Provisions in the CY 2021 PFS Final Rule
In the CY 2021 PFS final rule (85 FR 84536), we finalized the establishment of HCPCS code G2252 (Brief communication technology-based service, e.g., virtual check-in service, by a physician or other qualified health care professional who can report evaluation and management services, provided to an established patient, not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment; 11-20 minutes of medical discussion) on an interim basis. We stated that, given the widespread concerns expressed by commenters about the continuing need for audio-only conversations with patients and our determination that we would not continue to pay for audio-only E/M visits after the conclusion of the PHE (see 85 FR 84533 through 84535 for further discussion of that policy), we believed it would be expedient to establish additional coding and payment for an extended virtual check-in, which could be furnished using any form of synchronous communication technology, including audio-only, on an interim basis for CY 2021. We stated that we believed establishing payment for this service on an interim basis will support access to care for beneficiaries who may be reluctant to return to in-person visits unless absolutely necessary, and allow us to consider whether this policy should be adopted on a permanent basis. In that rule, we finalized a direct crosswalk to CPT code 99442, the value of which we believe most accurately reflects the resources associated with a longer service delivered via synchronous communication technology, which can include audio-only communication. Commenters supported the creation and interim final adoption of this service. Commenters stated that, as beneficiaries and practitioners may be reluctant to return to primarily in-person services post-PHE, payment for a longer virtual check-in would be necessary to account for circumstances where more time is spent determining whether an in-person visit is needed beyond the 5-10 minutes accounted for by HCPCS code G2012 (Brief communication technology-based service, e.g., virtual check-in, by a physician or other qualified health care professional who can report evaluation and management services, provided to an established patient, not originating from a related e/m service provided within the previous 7 days nor leading to an e/m service or procedure within the next 24 hours or soonest available appointment; 5-10 minutes of medical discussion). Commenters also supported valuing HCPCS code G2252 through a direct crosswalk to CPT code 99442. We agree with commenters that additional time may be needed to assess the necessity of an in-person service given concerns over exposure to illnesses beyond the duration of the PHE for COVID-19 and that current coding may not accurately reflect that time. Based on support from commenters, we are proposing to permanently adopt coding and payment for CY 2022, HCPCS code G2252 as described in the CY 2021 PFS final rule.
E. Valuation of Specific Codes
1. Background: Process for Valuing New, Revised, and Potentially Misvalued Codes
Establishing valuations for newly created and revised CPT codes is a routine part of maintaining the PFS. Since the inception of the PFS, it has also been a priority to revalue services regularly to make sure that the payment rates reflect the changing trends in the practice of medicine and current prices for inputs used in the PE calculations. Initially, this was accomplished primarily through the 5-year review process, which resulted in revised work RVUs for CY 1997, CY 2002, CY 2007, and CY 2012, and revised PE RVUs in CY 2001, CY 2006, and CY 2011, and revised MP RVUs in CY 2010 and CY 2015. Under the 5-year review process, revisions in RVUs were proposed and finalized via rulemaking. In addition to the 5-year reviews, beginning with CY 2009, CMS and the RUC identified a number of potentially misvalued codes each year using various identification screens, as discussed in section II.C. of this proposed rule, Potentially Misvalued Services under the PFS. Historically, when we received RUC recommendations, our process had been to establish interim final RVUs for the potentially misvalued codes, new codes, and any other codes for which there were coding changes in the final rule with comment period for a year. Then, during the 60-day period following the publication of the final rule with comment period, we accepted public comment about those valuations. For services furnished during the calendar year following the publication of interim final rates, we paid for services based upon the interim final values established in the final rule. In the final rule with comment period for the subsequent year, we considered and responded to public comments received on the interim final values, and typically made any appropriate adjustments and finalized those values.
In the CY 2015 PFS final rule with comment period (79 FR 67547), we finalized a new process for establishing values for new, revised and potentially misvalued codes. Under the new process, we include proposed values for these services in the proposed rule, rather than establishing them as interim final in the final rule with comment period. Beginning with the CY 2017 PFS proposed rule (81 FR 46162), the new process was applicable to all codes, except for new codes that describe truly new services. For CY 2017, we proposed new values in the CY 2017 PFS proposed rule for the vast majority of new, revised, and potentially misvalued codes for which we received complete RUC recommendations by February 10, 2016. To complete the transition to this new process, for codes for which we established interim final values in the CY 2016 PFS final rule with comment period (81 FR 80170), we reviewed the comments received during the 60-day public comment period following release of the CY 2016 PFS final rule with comment period (80 FR 70886), and re-proposed values for those codes in the CY 2017 PFS proposed rule.
We considered public comments received during the 60-day public comment period for the proposed rule before establishing final values in the CY 2017 PFS final rule. As part of our established process, we will adopt interim final values only in the case of wholly new services for which there are no predecessor codes or values and for which we do not receive recommendations in time to propose values.
As part of our obligation to establish RVUs for the PFS, we thoroughly review and consider available information including recommendations and supporting information from the RUC, the Health Care Professionals Advisory Committee (HCPAC), public commenters, medical literature, Medicare claims data, comparative databases, comparison with other codes within the PFS, as well as consultation with other physicians and healthcare professionals within CMS and the Start Printed Page 39151 federal government as part of our process for establishing valuations. Where we concur that the RUC's recommendations, or recommendations from other commenters, are reasonable and appropriate and are consistent with the time and intensity paradigm of physician work, we proposed those values as recommended. Additionally, we continually engage with stakeholders, including the RUC, with regard to our approach for accurately valuing codes, and as we prioritize our obligation to value new, revised, and potentially misvalued codes. We continue to welcome feedback from all interested parties regarding valuation of services for consideration through our rulemaking process.
2. Methodology for Establishing Work RVUs
For each code identified in this section, we conduct a review that includes the current work RVU (if any), RUC-recommended work RVU, intensity, time to furnish the preservice, intraservice, and postservice activities, as well as other components of the service that contribute to the value. Our reviews of recommended work RVUs and time inputs generally include, but have not been limited to, a review of information provided by the RUC, the HCPAC, and other public commenters, medical literature, and comparative databases, as well as a comparison with other codes within the PFS, consultation with other physicians and health care professionals within CMS and the federal government, as well as Medicare claims data. We also assess the methodology and data used to develop the recommendations submitted to us by the RUC and other public commenters and the rationale for the recommendations. In the CY 2011 PFS final rule with comment period (75 FR 73328 through 73329), we discussed a variety of methodologies and approaches used to develop work RVUs, including survey data, building blocks, crosswalks to key reference or similar codes, and magnitude estimation (see the CY 2011 PFS final rule with comment period (75 FR 73328 through 73329) for more information). When referring to a survey, unless otherwise noted, we mean the surveys conducted by specialty societies as part of the formal RUC process.
Components that we use in the building block approach may include preservice, intraservice, or postservice time and post-procedure visits. When referring to a bundled CPT code, the building block components could include the CPT codes that make up the bundled code and the inputs associated with those codes. We use the building block methodology to construct, or deconstruct, the work RVU for a CPT code based on component pieces of the code. Magnitude estimation refers to a methodology for valuing work that determines the appropriate work RVU for a service by gauging the total amount of work for that service relative to the work for a similar service across the PFS without explicitly valuing the components of that work. In addition to these methodologies, we frequently utilize an incremental methodology in which we value a code based upon its incremental difference between another code and another family of codes. Section 1848(c)(1)(A) of the Act specifically defines the work component as the resources that reflect time and intensity in furnishing the service. Also, the published literature on valuing work has recognized the key role of time in overall work. For particular codes, we refine the work RVUs in direct proportion to the changes in the best information regarding the time resources involved in furnishing particular services, either considering the total time or the intraservice time.
Several years ago, to aid in the development of preservice time recommendations for new and revised CPT codes, the RUC created standardized preservice time packages. The packages include preservice evaluation time, preservice positioning time, and preservice scrub, dress and wait time. Currently, there are preservice time packages for services typically furnished in the facility setting (for example, preservice time packages reflecting the different combinations of straightforward or difficult procedure, and straightforward or difficult patient). Currently, there are three preservice time packages for services typically furnished in the nonfacility setting.
We developed several standard building block methodologies to value services appropriately when they have common billing patterns. In cases where a service is typically furnished to a beneficiary on the same day as an E/M service, we believe that there is overlap between the two services in some of the activities furnished during the preservice evaluation and postservice time. Our longstanding adjustments have reflected a broad assumption that at least one-third of the work time in both the preservice evaluation and postservice period is duplicative of work furnished during the E/M visit.
Accordingly, in cases where we believe that the RUC has not adequately accounted for the overlapping activities in the recommended work RVU and/or times, we adjust the work RVU and/or times to account for the overlap. The work RVU for a service is the product of the time involved in furnishing the service multiplied by the intensity of the work. Preservice evaluation time and postservice time both have a long-established intensity of work per unit of time (IWPUT) of 0.0224, which means that 1 minute of preservice evaluation or postservice time equates to 0.0224 of a work RVU.
Therefore, in many cases when we remove 2 minutes of preservice time and 2 minutes of postservice time from a procedure to account for the overlap with the same day E/M service, we also remove a work RVU of 0.09 (4 minutes × 0.0224 IWPUT) if we do not believe the overlap in time had already been accounted for in the work RVU. The RUC has recognized this valuation policy and, in many cases, now addresses the overlap in time and work when a service is typically furnished on the same day as an E/M service.
The following paragraphs contain a general discussion of our approach to reviewing RUC recommendations and developing proposed values for specific codes. When they exist we also include a summary of stakeholder reactions to our approach. We note that many commenters and stakeholders have expressed concerns over the years with our ongoing adjustment of work RVUs based on changes in the best information we had regarding the time resources involved in furnishing individual services. We have been particularly concerned with the RUC's and various specialty societies' objections to our approach given the significance of their recommendations to our process for valuing services and since much of the information we used to make the adjustments is derived from their survey process. We are obligated under the statute to consider both time and intensity in establishing work RVUs for PFS services. As explained in the CY 2016 PFS final rule with comment period (80 FR 70933), we recognize that adjusting work RVUs for changes in time is not always a straightforward process, so we have applied various methodologies to identify several potential work values for individual codes.
We have observed that for many codes reviewed by the RUC, recommended work RVUs have appeared to be incongruous with recommended assumptions regarding the resource costs in time. This has been the case for a significant portion of codes for which we recently established or proposed work RVUs that are based on refinements to the RUC-recommended values. When we have adjusted work Start Printed Page 39152 RVUs to account for significant changes in time, we have started by looking at the change in the time in the context of the RUC-recommended work RVU. When the recommended work RVUs do not appear to account for significant changes in time, we have employed the different approaches to identify potential values that reconcile the recommended work RVUs with the recommended time values. Many of these methodologies, such as survey data, building block, crosswalks to key reference or similar codes, and magnitude estimation have long been used in developing work RVUs under the PFS. In addition to these, we sometimes use the relationship between the old time values and the new time values for particular services to identify alternative work RVUs based on changes in time components.
In so doing, rather than ignoring the RUC-recommended value, we have used the recommended values as a starting reference and then applied one of these several methodologies to account for the reductions in time that we believe were not otherwise reflected in the RUC-recommended value. If we believe that such changes in time are already accounted for in the RUC's recommendation, then we do not make such adjustments. Likewise, we do not arbitrarily apply time ratios to current work RVUs to calculate proposed work RVUs. We use the ratios to identify potential work RVUs and consider these work RVUs as potential options relative to the values developed through other options.
We do not imply that the decrease in time as reflected in survey values should always equate to a one-to-one or linear decrease in newly valued work RVUs. Instead, we believe that, since the two components of work are time and intensity, absent an obvious or explicitly stated rationale for why the relative intensity of a given procedure has increased, significant decreases in time should be reflected in decreases to work RVUs. If the RUC's recommendation has appeared to disregard or dismiss the changes in time, without a persuasive explanation of why such a change should not be accounted for in the overall work of the service, then we have generally used one of the aforementioned methodologies to identify potential work RVUs, including the methodologies intended to account for the changes in the resources involved in furnishing the procedure.
Several stakeholders, including the RUC, have expressed general objections to our use of these methodologies and deemed our actions in adjusting the recommended work RVUs as inappropriate; other stakeholders have also expressed general concerns with CMS refinements to RUC-recommended values in general. In the CY 2017 PFS final rule (81 FR 80272 through 80277), we responded in detail to several comments that we received regarding this issue. In the CY 2017 PFS proposed rule (81 FR 46162), we requested comments regarding potential alternatives to making adjustments that would recognize overall estimates of work in the context of changes in the resource of time for particular services; however, we did not receive any specific potential alternatives. As described earlier in this section, crosswalks to key reference or similar codes are one of the many methodological approaches we have employed to identify potential values that reconcile the RUC-recommend work RVUs with the recommended time values when the RUC-recommended work RVUs did not appear to account for significant changes in time.
In response to comments, in the CY 2019 PFS final rule (83 FR 59515), we clarified that terms "reference services", "key reference services", and "crosswalks" as described by the commenters are part of the RUC's process for code valuation. These are not terms that we created, and we do not agree that we necessarily must employ them in the identical fashion for the purposes of discussing our valuation of individual services that come up for review. However, in the interest of minimizing confusion and providing clear language to facilitate stakeholder feedback, we will seek to limit the use of the term, "crosswalk," to those cases where we are making a comparison to a CPT code with the identical work RVU. We also occasionally make use of a "bracket" for code valuation. A "bracket" refers to when a work RVU falls between the values of two CPT codes, one at a higher work RVU and one at a lower work RVU.
We look forward to continuing to engage with stakeholders and commenters, including the RUC, as we prioritize our obligation to value new, revised, and potentially misvalued codes; and will continue to welcome feedback from all interested parties regarding valuation of services for consideration through our rulemaking process. We refer readers to the detailed discussion in this section of the valuation considered for specific codes. Table 13 contains a list of codes and descriptors for which we are proposing work RVUs; this includes all codes for which we received RUC recommendations by February 10, 2021. The proposed work RVUs, work time and other payment information for all CY 2022 payable codes are available on the CMS website under downloads for the CY 2022 PFS proposed rule at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html).
3. Methodology for the Direct PE Inputs To Develop PE RVUs
a. Background
On an annual basis, the RUC provides us with recommendations regarding PE inputs for new, revised, and potentially misvalued codes. We review the RUC-recommended direct PE inputs on a code by code basis. Like our review of recommended work RVUs, our review of recommended direct PE inputs generally includes, but is not limited to, a review of information provided by the RUC, HCPAC, and other public commenters, medical literature, and comparative databases, as well as a comparison with other codes within the PFS, and consultation with physicians and health care professionals within CMS and the federal government, as well as Medicare claims data. We also assess the methodology and data used to develop the recommendations submitted to us by the RUC and other public commenters and the rationale for the recommendations. When we determine that the RUC's recommendations appropriately estimate the direct PE inputs (clinical labor, disposable supplies, and medical equipment) required for the typical service, are consistent with the principles of relativity, and reflect our payment policies, we use those direct PE inputs to value a service. If not, we refine the recommended PE inputs to better reflect our estimate of the PE resources required for the service. We also confirm whether CPT codes should have facility and/or nonfacility direct PE inputs and refine the inputs accordingly.
Our review and refinement of the RUC-recommended direct PE inputs includes many refinements that are common across codes, as well as refinements that are specific to particular services. Table 14 details our refinements of the RUC's direct PE recommendations at the code-specific level. In section II.B. of this proposed rule, Determination of Practice Expense Relative Value Units (PE RVUs), we addressed certain refinements that would be common across codes. Refinements to particular codes are addressed in the portions of that section that are dedicated to particular codes. We noted that for each refinement, we Start Printed Page 39153 indicated the impact on direct costs for that service. We noted that, on average, in any case where the impact on the direct cost for a particular refinement is $0.35 or less, the refinement has no impact on the PE RVUs. This calculation considers both the impact on the direct portion of the PE RVU, as well as the impact on the indirect allocator for the average service. We also noted that many of the refinements listed in Table 14 result in changes under the $0.35 threshold and are unlikely to result in a change to the RVUs.
We also noted that the direct PE inputs for CY 2022 are displayed in the CY 2022 direct PE input files, available on the CMS website under the downloads for the CY 2022 PFS proposed rule at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html. The inputs displayed there have been used in developing the CY 2022 PE RVUs as displayed in Addendum B.
b. Common Refinements
(1) Changes in Work Time
Some direct PE inputs are directly affected by revisions in work time. Specifically, changes in the intraservice portions of the work time and changes in the number or level of postoperative visits associated with the global periods result in corresponding changes to direct PE inputs. The direct PE input recommendations generally correspond to the work time values associated with services. We believe that inadvertent discrepancies between work time values and direct PE inputs should be refined or adjusted in the establishment of proposed direct PE inputs to resolve the discrepancies.
(2) Equipment Time
Prior to CY 2010, the RUC did not generally provide CMS with recommendations regarding equipment time inputs. In CY 2010, in the interest of ensuring the greatest possible degree of accuracy in allocating equipment minutes, we requested that the RUC provide equipment times along with the other direct PE recommendations, and we provided the RUC with general guidelines regarding appropriate equipment time inputs. We appreciate the RUC's willingness to provide us with these additional inputs as part of its PE recommendations.
In general, the equipment time inputs correspond to the service period portion of the clinical labor times. We clarified this principle over several years of rulemaking, indicating that we consider equipment time as the time within the intraservice period when a clinician is using the piece of equipment plus any additional time that the piece of equipment is not available for use for another patient due to its use during the designated procedure. For those services for which we allocate cleaning time to portable equipment items, because the portable equipment does not need to be cleaned in the room where the service is furnished, we do not include that cleaning time for the remaining equipment items, as those items and the room are both available for use for other patients during that time. In addition, when a piece of equipment is typically used during follow-up postoperative visits included in the global period for a service, the equipment time would also reflect that use.
We believe that certain highly technical pieces of equipment and equipment rooms are less likely to be used during all of the preservice or postservice tasks performed by clinical labor staff on the day of the procedure (the clinical labor service period) and are typically available for other patients even when one member of the clinical staff may be occupied with a preservice or postservice task related to the procedure. We also note that we believe these same assumptions would apply to inexpensive equipment items that are used in conjunction with and located in a room with non-portable highly technical equipment items since any items in the room in question would be available if the room is not being occupied by a particular patient. For additional information, we refer readers to our discussion of these issues in the CY 2012 PFS final rule with comment period (76 FR 73182) and the CY 2015 PFS final rule with comment period (79 FR 67639).
(3) Standard Tasks and Minutes for Clinical Labor Tasks
In general, the preservice, intraservice, and postservice clinical labor minutes associated with clinical labor inputs in the direct PE input database reflect the sum of particular tasks described in the information that accompanies the RUC-recommended direct PE inputs, commonly called the "PE worksheets." For most of these described tasks, there is a standardized number of minutes, depending on the type of procedure, its typical setting, its global period, and the other procedures with which it is typically reported. The RUC sometimes recommends a number of minutes either greater than or less than the time typically allotted for certain tasks. In those cases, we review the deviations from the standards and any rationale provided for the deviations. When we do not accept the RUC-recommended exceptions, we refine the proposed direct PE inputs to conform to the standard times for those tasks. In addition, in cases when a service is typically billed with an E/M service, we remove the preservice clinical labor tasks to avoid duplicative inputs and to reflect the resource costs of furnishing the typical service.
We refer readers to section II.B. of this proposed rule, Determination of Practice Expense Relative Value Units (PE RVUs), for more information regarding the collaborative work of CMS and the RUC in improvements in standardizing clinical labor tasks.
(4) Recommended Items That Are Not Direct PE Inputs
In some cases, the PE worksheets included with the RUC's recommendations include items that are not clinical labor, disposable supplies, or medical equipment or that cannot be allocated to individual services or patients. We addressed these kinds of recommendations in previous rulemaking (78 FR 74242), and we do not use items included in these recommendations as direct PE inputs in the calculation of PE RVUs.
(5) New Supply and Equipment Items
The RUC generally recommends the use of supply and equipment items that already exist in the direct PE input database for new, revised, and potentially misvalued codes. However, some recommendations include supply or equipment items that are not currently in the direct PE input database. In these cases, the RUC has historically recommended that a new item be created and has facilitated our pricing of that item by working with the specialty societies to provide us copies of sales invoices. For CY 2022 we received invoices for several new supply and equipment items. Tables 16 and 17 detail the invoices received for new and existing items in the direct PE database. As discussed in section II.B. of this proposed rule, Determination of Practice Expense Relative Value Units, we encourage stakeholders to review the prices associated with these new and existing items to determine whether these prices appear to be accurate. Where prices appear inaccurate, we encourage stakeholders to submit invoices or other information to improve the accuracy of pricing for these items in the direct PE database by February 10th of the following year for consideration in future rulemaking, similar to our process for consideration of RUC recommendations. Start Printed Page 39154
We remind stakeholders that due to the relativity inherent in the development of RVUs, reductions in existing prices for any items in the direct PE database increase the pool of direct PE RVUs available to all other PFS services. Tables 16 and 17 also include the number of invoices received and the number of nonfacility allowed services for procedures that use these equipment items. We provide the nonfacility allowed services so that stakeholders will note the impact the particular price might have on PE relativity, as well as to identify items that are used frequently, since we believe that stakeholders are more likely to have better pricing information for items used more frequently. A single invoice may not be reflective of typical costs and we encourage stakeholders to provide additional invoices so that we might identify and use accurate prices in the development of PE RVUs.
In some cases, we do not use the price listed on the invoice that accompanies the recommendation because we identify publicly available alternative prices or information that suggests a different price is more accurate. In these cases, we include this in the discussion of these codes. In other cases, we cannot adequately price a newly recommended item due to inadequate information. Sometimes, no supporting information regarding the price of the item has been included in the recommendation. In other cases, the supporting information does not demonstrate that the item has been purchased at the listed price (for example, vendor price quotes instead of paid invoices). In cases where the information provided on the item allows us to identify clinically appropriate proxy items, we might use existing items as proxies for the newly recommended items. In other cases, we include the item in the direct PE input database without any associated price. Although including the item without an associated price means that the item does not contribute to the calculation of the final PE RVU for particular services, it facilitates our ability to incorporate a price once we obtain information and are able to do so.
(6) Service Period Clinical Labor Time in the Facility Setting
Generally speaking, our direct PE inputs do not include clinical labor minutes assigned to the service period because the cost of clinical labor during the service period for a procedure in the facility setting is not considered a resource cost to the practitioner since Medicare makes separate payment to the facility for these costs. We address code-specific refinements to clinical labor in the individual code sections.
(7) Procedures Subject to the Multiple Procedure Payment Reduction (MPPR) and the OPPS Cap
We note that the list of services for the upcoming calendar year that are subject to the MPPR on diagnostic cardiovascular services, diagnostic imaging services, diagnostic ophthalmology services, and therapy services; and the list of procedures that meet the definition of imaging under section 1848(b)(4)(B) of the Act, and therefore, are subject to the OPPS cap; are displayed in the public use files for the PFS proposed and final rules for each year. The public use files for CY 2022 are available on the CMS website under downloads for the CY 2022 PFS proposed rule at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html. For more information regarding the history of the MPPR policy, we refer readers to the CY 2014 PFS final rule with comment period (78 FR 74261 through 74263).
Effective January 1, 2007, section 5102(b)(1) of the Deficit Reduction Act of 2005 (Pub. L. 109-171) (DRA) amended section 1848(b)(4) of the Act to require that, for imaging services, if—(i) The technical component (including the technical component portion of a global fee) of the service established for a year under the fee schedule without application of the geographic adjustment factor, exceeds (ii) The Medicare OPD fee schedule amount established under the prospective payment system (PPS) for hospital outpatient department services under section 1833(t)(3)(D) of the Act for such service for such year, determined without regard to geographic adjustment under paragraph (t)(2)(D) of such section, the Secretary shall substitute the amount described in clause (ii), adjusted by the geographic adjustment factor [under the PFS], for the fee schedule amount for such technical component for such year. As required by the section 1848(b)(4)(A) of the statute, for imaging services furnished on or after January 1, 2007, we cap the TC of the PFS payment amount for the year (prior to geographic adjustment) by the Outpatient Prospective Payment System (OPPS) payment amount for the service (prior to geographic adjustment). We then apply the PFS geographic adjustment to the capped payment amount. Section 1848(b)(4)(B) of the Act defines imaging services as "imaging and computer-assisted imaging services, including X-ray, ultrasound (including echocardiography), nuclear medicine (including PET), magnetic resonance imaging (MRI), computed tomography (CT), and fluoroscopy, but excluding diagnostic and screening mammography." For more information regarding the history of the cap on the TC of the PFS payment amount under the DRA (the "OPPS cap"), we refer readers to the CY 2007 PFS final rule with comment period (71 FR 69659 through 69662).
For CY 2022, we identified new and revised codes to determine which services meet the definition of "imaging services" as defined above for purposes of this cap. Beginning for CY 2022, we are proposing to include the following services on the list of codes to which the OPPS cap applies: CPT codes 0633T (Computed tomography, breast, including 3D rendering, when performed, unilateral; without contrast material), 0634T (Computed tomography, breast, including 3D rendering, when performed, unilateral; with contrast material(s)), 0635T (Computed tomography, breast, including 3D rendering, when performed, unilateral; without contrast, followed by contrast material(s)), 0636T (Computed tomography, breast, including 3D rendering, when performed, bilateral; without contrast material(s)), 0637T (Computed tomography, breast, including 3D rendering, when performed, bilateral; with contrast material(s)), 0638T (Computed tomography, breast, including 3D rendering, when performed, bilateral; without contrast, followed by contrast material(s)), 0648T (Quantitative magnetic resonance for analysis of tissue composition (e.g., fat, iron, water content), including multiparametric data acquisition, data preparation and transmission, interpretation and report, obtained without diagnostic MRI examination of the same anatomy (e.g., organ, gland, tissue, target structure) during the same session), 0649T (Quantitative magnetic resonance for analysis of tissue composition (e.g., fat, iron, water content), including multiparametric data acquisition, data preparation and transmission, interpretation and report, obtained with diagnostic MRI examination of the same anatomy (e.g., organ, gland, tissue, target structure) (List separately in addition to code for primary procedure)), 77X01 (Trabecular bone score (TBS), structural condition of the bone microarchitecture; using dual X-ray absorptiometry (DXA) or other imaging data on gray-scale variogram, calculation, with interpretation and report on fracture risk), 77X02 Start Printed Page 39155 (Trabecular bone score (TBS), structural condition of the bone microarchitecture; using dual X-ray absorptiometry (DXA) or other imaging data on gray-scale variogram, calculation, with interpretation and report on fracture risk, technical preparation and transmission of data for analysis to be performed elsewhere), 77X03 (Trabecular bone score (TBS), structural condition of the bone microarchitecture; using dual X-ray absorptiometry (DXA) or other imaging data on gray-scale variogram, calculation, with interpretation and report on fracture risk, technical calculation only), 77X04 (Trabecular bone score (TBS), structural condition of the bone microarchitecture; using dual X-ray absorptiometry (DXA) or other imaging data on gray-scale variogram, calculation, with interpretation and report on fracture risk interpretation and report on fracture risk only, by other qualified health care professional), 9111X (Gastrointestinal tract imaging, intraluminal (e.g., capsule endoscopy), colon, with interpretation and report), and 933X0 (3D echocardiographic imaging and postprocessing during transesophageal echocardiography or transthoracic echocardiography for congenital cardiac anomalies for the assessment of cardiac structure(s) (e.g., cardiac chambers and valves, left atrial appendage, intraterial septum, interventricular septum) and function, when performed). We believe these codes meet the definition of imaging services under section 1848(b)(4)(B) of the Act, and thus, should be subject to the OPPS cap.
4. Proposed Valuation of Specific Codes for CY 2022
(1) Anesthesia for Cardiac Electrophysiologic Procedures (CPT Code 00537)
In October 2019, the RUC reviewed CPT code 00537 (Anesthesia for cardiac electrophysiologic procedures including radiofrequency ablation) and recommended that the code be surveyed for the October 2020 meeting. This service was identified by the RUC via the high volume growth screen for services with total Medicare utilization of 10,000 or more that have increased by at least 100 percent from 2009 through 2014. Additionally, at the October 2019 RUC meeting, the RUC approved an anesthesia reference service list (RSL) and a method to assess the relativity among services on the anesthesia fee schedule that uses a revised building block methodology and a regression line analysis. The RUC has stated that the revised building block methodology generates "proxy RVUs" that are then compared against the RSL regression line to assess relativity among anesthesia services. The RUC has indicated that their primary and approved method for anesthesia base unit valuation continues to be the anesthesia survey results, and that the building block and regression line analysis are used as a supplemental validation measure.
The RUC recommended a valuation of 12 base units for CPT code 00537.We disagree with the RUC-recommended valuation of 12 base units for CPT code 00537. After performing a RUC database search of codes with similar total times and post-induction period procedure anesthesia (PIPPA) times, 12 base units appears to be on the very high range. We are proposing a valuation of 10 base units supported by reference codes CPT code 00620 (anesthesia for procedures on the thoracic spine and cord, not otherwise specified) and CPT code 00600 (Anesthesia for procedures on cervical spine and cord; not otherwise specified), which both have a valuation of 10 base units. CPT code 00620 has a very similar total time of 235 minutes and CPT code 00600 has a higher total time of 257 minutes and the same base unit value of 10, which indicates that this is an appropriate valuation. Additionally, we note that the survey total time for CPT code 00537 increased from 150 to 238 minutes, resulting in a survey result 25th percentile valuation of 10 base units.
We are proposing the RUC-recommended direct PE inputs for CPT code 00537.
(2) Anesthesia Services for Image-Guided Spinal Procedures (CPT Codes 01XX2, 01XX3, 01XX4, 01XX5, 01XX6, and 01XX7)
In 2017, the RUC identified CPT code 01936 (Anesthesia for percutaneous image guided procedures on the spine and spinal cord; therapeutic) as possibly needing refinement due to inaccurate reporting via the high volume growth screen. The Relativity Assessment Workgroup reviewed data on what procedures were reported with this anesthesia code. In October 2019, the Workgroup reviewed this service and recommended that it be referred to the CPT Editorial Panel to create more granular codes. In October 2020, the CPT Editorial Panel replaced CPT codes 01935 and 01936 with six new codes to report percutaneous image-guided spine and spinal cord anesthesia procedures. These CPT codes are 01XX2 (Anesthesia for percutaneous image-guided injection, drainage or aspiration procedures on the spine or spinal cord; cervical or thoracic), 01XX3 (Anesthesia for percutaneous image guided injection, drainage or aspiration procedures on the spine or spinal cord; lumbar or sacral), 01XX4 (Anesthesia for percutaneous image guided destruction procedures by neurolytic agent on the spine or spinal cord; cervical or thoracic), 01XX5 (Anesthesia for percutaneous image guided destruction procedures by neurolytic agent on the spine or spinal cord; lumbar or sacral), 01XX6 (Anesthesia for percutaneous image guided neuromodulation or intravertebral procedures (e.g., Kyphoplasty, vertebroplasty) on the spine or spinal cord; cervical or thoracic) and 01XX7 (Anesthesia for percutaneous image guided neuromodulation or intravertebral procedures (e.g., Kyphoplasty, vertebroplasty) on the spine or spinal cord; lumbar or sacral).
We are proposing the RUC-recommended valuation of 4 base units for CPT codes 01XX2, 01XX3, 01XX4, and 01XX5.
We disagree with the RUC-recommend valuation of 6 base units for CPT codes 01XX6 and 01XX7. After performing a RUC database search of codes with similar total times and post-induction period procedure anesthesia (PIPPA) times, 6 base units for CPT codes 01XX6 and 01XX7 appears to be a high valuation. We are proposing a valuation of 5 base units for both codes supported by a reference code, CPT code 00813 (Anesthesia for combined upper and lower gastrointestinal endoscopic procedures, endoscope introduced both proximal to and distal to the duodenum). CPT code 00813 has a valuation of 5 base units with a higher PIPPA time of 40 minutes as well as a higher total time of 70 minutes. The RUC notes that CPT codes 01XX6 and 01XX7 should have a higher base unit valuation than the other similar codes within this family due to the complex nature of these procedures that have a more intensive anesthesia process. The RUC supports their recommendation with a crosswalk code, CPT code 00732 (Anesthesia for upper gastrointestinal endoscopic procedures, endoscope introduced proximal to duodenum; endoscopic retrograde cholangiopancreatography (ECRP)). CPT code 00732 has a valuation of 6 base units, a total time of 100 minutes, and a PIPPA time of 65 minutes. CPT codes 01XX6 and 01XX7 have a total time of 58 minutes and a PIPPA time of 20 minutes. We agree that a more complex procedure may require a higher base unit valuation within a code family; however, given the disparity in total and PIPPA time, we disagree with the use of Start Printed Page 39156 this crosswalk code to support a valuation of 6 base units and instead propose a valuation of 5 base units supported by reference CPT code 00813, which has higher times and the same base unit valuation.
We are proposing the RUC-recommended direct PE inputs for all six codes in the family.
(3) Closed Treatment of Nasal Bone Fracture (CPT Codes 21315 and 21320)
We agree with the RUC's recommendation to change CPT codes 21315 (Closed treatment of nasal bone fracture; without stabilization) and 21320 (Closed treatment of nasal bone fracture; with stabilization) to 000-day global period codes from 010-day global period codes to account for the degree of swelling within 10 days post-procedure, and because the patient can remove their own splint at home for CPT code 21320. For CPT codes 21315 and 21320, we disagree with the RUC-recommended work RVUs of 2.00 and 2.33, respectively, as we believe these values do not adequately reflect the surveyed reductions in physician time and the change to a 000-day global period from a 010-day global period for these CPT codes. We are proposing a work RVU of 0.96 for CPT code 21315 and 1.59 for CPT code 21320 based on the reverse building block methodology to remove the RVUs associated with the 010-day global period and the surveyed reductions in physician time. We believe that the proposed work RVU of 0.96 for CPT code 21315 adequately accounts for the 50 percent decrease in intraservice and postservice time, a 31-minute decrease in total time, and a change to a 000-day global period which will allow for separately billable E/M visits as medically necessary. We believe that the proposed work RVU of 1.59 for CPT code 21320 adequately accounts for the 5-minute decrease in intraservice time, 3-minute decrease in total time, and 48 percent decrease in postservice time. Absent an explicitly stated rationale for an intensity increase for CPT codes 21315 and 21320, we are proposing to adjust the work RVU to reflect significant decreases in surveyed physician time.
The global period changes from 010-day to 000-day allow for separately billable E/M visits relating to CPT codes 21315 and 21320, therefore we removed RVUs that we believed were attributable to the currently bundled E/M visits totaling 1.30 RVUs for CPT code 21315 and 0.35 RVUs for CPT code 21320. CPT code 21315 is currently bundled with one post-operative follow up office visit, CPT code 99213 (Office or other outpatient visit for the evaluation and management of an established patient, which requires a medically appropriate history and/or examination and low level of medical decision making. When using time for code selection, 20-29 minutes of total time is spent on the date of the encounter). CPT code 21320 is currently bundled with half of a post-operative follow up office visit, CPT code 99212 (Office or other outpatient visit for the evaluation and management of an established patient, which requires a medically appropriate history and/or examination and straightforward medical decision making. When using time for code selection, 10-19 minutes of total time is spent on the date of the encounter). We do not believe the RUC adequately accounted for the loss of these E/M visits in their recommended work RVUs for CPT codes 21315 and 21320. The RUC's recommendations also seem to dismiss the significant changes in surveyed physician time, without a persuasive explanation of a significant increase in IWPUT that results from the RUC's recommended work RVUs for CPT codes 21315 and 21320. We believe the surveyed decreases in physician time in conjunction with the loss of the post-operative visits for CPT codes 21315 and 21320 merit decreases in the work RVUs from the current work RVUs.
We considered using a modified total time ratio methodology given the age and potentially flawed methodology used to arrive at the current valuation. The modified total time ratio calculation does not include the loss of 8 minutes of post-operative time attributable to the change from a 010-day global period to a 000-day global period for CPT code 21320 and loss of 23 minutes of post-operative time for CPT code 23215. This modified time ratio methodology reflects how the physician time is changing in the pre-, intra-, and postservice periods when a code's global period is changing, given that E/M services can be billed as medically necessary and appropriate for a 000-day global code. The total time ratio between the current and proposed total times for CPT code 21315, excluding the 23 minutes of post-operative time in the current total time, equals 1.64. We arrived at 1.64 by modifying the original total time ratio equation to equal the proposed new total time divided by the current time, less any time attributable to the post-operative global period, then multiplied by the current work RVU. The current total time for CPT code 21315 without the 23 minutes of post-operative time that will be lost by going from a 010-day to a 000-day global period code is 76 minutes, therefore, the modified total time ratio = (68 minutes/(99 minutes−23 minutes)) * 1.83 = 1.64. When using the original total time ratio methodology for CPT code 21315, it shows a 31 percent decrease in total time [(68 minutes−99 minutes)/99 minutes = −0.31], whereas the modified methodology shows that there is only an 11 percent decrease in newly proposed pre-, intra-, and postservice time from the current times [(68 minutes−76 minutes)/76 minutes = −0.11]. The same modified total time ratio methodology could be applicable to CPT code 21320. The current total time for CPT code 21320 without the 8 minutes of post-operative time that will be lost by going from a 010-day to a 000-day global period code is 70 minutes, therefore, the modified total time ratio = (75 minutes/(78 minutes−8 minutes) * 1.88 = 2.01. The modified methodology shows that the pre-, intra-, and postservice time is increasing by 7 percent for CPT code 21320, whereas the original methodology, which accounts for the loss of the 8 post-operative minutes in the total time ratio, shows a 4 percent decrease in total time that would indicate the need for a work RVU decrease. We recognize that we have not previously used a modified total time approach to consider work RVU values when there is a change in the global period for a service in conjunction with significant surveyed changes to the pre-, intra-, and postservice times; therefore, we are seeking comment on application of the modified total time ratio approach to value services that have a global period change and significant surveyed physician time changes. We believe this methodology may account for the loss of post-operative visits and the surveyed changes in the pre-, intra-, and postservice times in this unique situation, given the potentially flawed methodology used to arrive at the current valuations for CPT codes 21315 and 21320 that are used in the total time ratios.
We are also proposing the RUC-recommended direct PE inputs without refinements and the surveyed physician times for CPT codes 21315 and 21320.
(4) Insertion of Interlaminar/Interspinous Device (CPT Code 22867)
We are proposing the RUC-recommended work RVU of 15.00 for CPT code 22867 (Insertion of interlaminar/interspinous process stabilization/distraction device, without fusion, including image guidance when performed, with open decompression, lumbar; single level). The RUC is not recommending changes to the current Start Printed Page 39157 PE inputs, and CMS is not proposing any changes to the current PE inputs.
(5) Treatment of Foot Infection (CPT Codes 28001, 28002, and 28003)
Through a screen of codes with 010-day global period service with more than one post-operative follow-up office visit, the RUC identified this family of major surgical codes that did not have consistent global periods. The RUC conducted a survey of these codes as 000-day globals for their April 2020 meeting, and the review was postponed until October 2020. CPT code 28001 (Incision and drainage, bursa, foot) (work RVU of 2.78 with 31 minutes of intraservice time) currently has a 010-day global period with one post-operative follow-up office visit, CPT code 99212 (Office or other outpatient visit for the evaluation and management of an established patient, which requires at least 2 of these 3 key components: A problem focused history; A problem focused examination; Straightforward medical decision making. Counseling and/or coordination of care with other physicians, other qualified health care professionals, or agencies are provided consistent with the nature of the problem(s) and the patient's and/or family's needs. Usually, the presenting problem(s) are self limited or minor. Typically, 10 minutes are spent face-to-face with the patient and/or family). Survey results from podiatrists and orthopedic surgeons yielded a median work RVU of 2.00 with 17 minutes of preservice evaluation time, 3 minutes of preservice positioning time, 5 minutes of preservice scrub/dress/wait time, 20 minutes intraservice time, and 15 minutes immediate postservice time for a total of 60 minutes total time. We are proposing the RUC-recommended work RVU of 2.00 and the surveyed physician times for this 000-day global code.
CPT code 28002 (Incision and drainage below fascia, with or without tendon sheath involvement, foot; single bursal space) (work RVU of 5.34 with 30 minutes of intraservice time) currently has a 010-day global period with two post-operative follow-up office visits, CPT code 99213 (Office or other outpatient visit for the evaluation and management of an established patient, which requires at least 2 of these 3 key components: An expanded problem focused history; An expanded problem focused examination; Medical decision making of low complexity. Counseling and coordination of care with other physicians, other qualified health care professionals, or agencies are provided consistent with the nature of the problem(s) and the patient's and/or family's needs. Usually, the presenting problem(s) are of low to moderate severity. Typically, 15 minutes are spent face-to-face with the patient and/or family); and a half day hospital discharge CPT code 99238 (Hospital discharge day management; 30 minutes or less). For CPT code 28002, the RUC recommended 30 minutes of preservice evaluation time, 5 minutes of preservice positioning time, 15 minutes of preservice scrub/dress/wait time, 30 minutes of intraservice time, and 20 minutes of immediate postservice time, for a total of 100 minutes total time. The RUC recommended a work RVU of 3.50 and the surveyed physician times for this 000-day global code.
We note that the result from the survey's 50th percentile work RVU was 3.73 and that the survey's 25th percentile work RVU was 2.80. As this CPT code is converting from a 010-day global to a 000-day global we find the reference CPT code 43193 (Esophagoscopy, rigid, transoral; with biopsy, single or multiple) as a more suitable value of 2.79 work RVUs with a similar 30 minutes of intraservice physician time and 106 minutes of total time. We are proposing a work RVU of 2.79 for CPT code 28002 and we are proposing the RUC surveyed physician times for this 000-day global code.
CPT code 28003 (Incision and drainage below fascia, with or without tendon sheath involvement, foot; multiple areas) currently has a 090-day global period with two post-operative follow-up office visits, CPT code 99212 (Office or other outpatient visit for the evaluation and management of an established patient, which requires at least 2 of these 3 key components: A problem focused history; A problem focused examination; Straightforward medical decision making. Counseling and/or coordination of care with other physicians, other qualified health care professionals, or agencies are provided consistent with the nature of the problem(s) and the patient's and/or family's needs. Usually, the presenting problem(s) are self limited or minor. Typically, 10 minutes are spent face-to-face with the patient and/or family); three post-operative follow-up office visits, CPT code 99213 (Office or other outpatient visit for the evaluation and management of an established patient, which requires at least 2 of these 3 key components: An expanded problem focused history; An expanded problem focused examination; Medical decision making of low complexity. Counseling and coordination of care with other physicians, other qualified health care professionals, or agencies are provided consistent with the nature of the problem(s) and the patient's and/or family's needs. Usually, the presenting problem(s) are of low to moderate severity. Typically, 15 minutes are spent face-to-face with the patient and/or family.); one post-operative CPT code 99231 (Subsequent hospital care, per day, for the evaluation and management of a patient, which requires at least 2 of these 3 key components: A problem focused interval history; A problem focused examination; Medical decision making that is straightforward or of low complexity. Counseling and/or coordination of care with other physicians, other qualified health care professionals, or agencies are provided consistent with the nature of the problem(s) and the patient's and/or family's needs. Usually, the patient is stable, recovering or improving. Typically, 15 minutes are spent at the bedside and on the patient's hospital floor or unit); one post-operative CPT code 99232 (Subsequent hospital care, per day, for the evaluation and management of a patient, which requires at least 2 of these 3 key components: An expanded problem focused interval history; An expanded problem focused examination; Medical decision making of moderate complexity. Counseling and/or coordination of care with other physicians, other qualified health care professionals, or agencies are provided consistent with the nature of the problem(s) and the patient's and/or family's needs. Usually, the patient is responding inadequately to therapy or has developed a minor complication. Typically, 25 minutes are spent at the bedside and on the patient's hospital floor or unit), and one hospital discharge CPT code 99238 (Hospital discharge day management; 30 minutes or less), for a total of eight post op follow-up visits, across five types of E/M and hospital care codes. For CPT code 28003, the RUC recommends 40 minutes of preservice evaluation time, 10 minutes of preservice positioning time, 15 minutes of preservice scrub/dress/wait time, 45 minutes of intraservice time, and 20 minutes of immediate postservice time, for a total time of 130 minutes. We are proposing the RUC-recommended work RVU of 5.28 and surveyed physician times for this 000-day global code.
In order to complete the adjustments for making these Treatment of Foot Infection codes consistent as 000-day global codes, the RUC adjusted the PE inputs for these codes to reflect their proposed global periods from 010 and 090-day globals to 000-day global, and to reflect the use of more typical supplies, equipment, and clinical labor Start Printed Page 39158 employed now, than what was necessary a decade ago. Some relatively small valued supply items were removed, while other items were added, and clinical labor times were largely adjusted to remove minutes from the post-operative follow-up office visit times in the 010 and 090-day global codes. We are proposing all of the PE refinements as recommended by the RUC for these codes, which can be found in section II.B. of this proposed rule, under the Determination of Practice Expense RVUs.
(6) Percutaneous Cerebral Embolic Protection (CPT Codes 33XXX)
CPT code 33XXX (Transcatheter placement and subsequent removal of cerebral embolic protection device(s), including arterial access, catheterization, imaging, and radiological supervision and interpretation, percutaneous (List separately in addition to code for primary procedure)) was created in October 2020, by the CPT Editorial Panel as a new add-on code to report transcatheter placement and subsequent removal of cerebral embolic protection device(s). The CPT Editorial Panel also added instructions to report the new code in the Aortic Valve guidelines. The RUC reviewed the survey results for the new add-on code and noted that the survey respondents likely overvalued the physician work involved in performing this service, with a 25th percentile work value of 3.43. The RUC recommends a work RVU of 2.50 for CPT code 33XXX.
We are proposing the RUC-recommended work RVU of 2.50 for CPT code 33XXX. This is a facility-based add-on code with no direct PE inputs.
(7) Exclusion of Left Atrial Appendage (CPT Codes 33XX3, 33XX4, and 33XX5)
In May 2020, the CPT Editorial Panel approved the creation of three new codes to describe open and thoracoscopic left atrial appendage management procedures when performed as stand-alone procedures or in conjunction with other procedures. The codes represent new technology and surgical techniques that may be used to treat atrial fibrillation at the time of another surgical procedure and include CPT code 33XX3 (Exclusion of left atrial appendage, open, any method (e.g., excision, isolation via stapling, oversewing, ligation, plication, clip), CPT code 33XX4 (Exclusion of left atrial appendage, open, performed at the time of other sternotomy or thoracotomy procedure(s), any method (e.g., excision, isolation via stapling, oversewing, ligation, plication, clip) (List separately in addition to code for primary procedure)), and CPT code 33XX5 (Exclusion of left atrial appendage, thoracoscopic, any method (e.g., excision, isolation via stapling, oversewing, ligation, plication, clip). CPT codes 33XX3 and 33XX5 are 090-day global codes while CPT code 33XX4 is a ZZZ global code.
In October 2020, the RUC reviewed and recommended work and PE values for the three new codes. Recommended work values include 18.50 RVUs for CPT code 33XX3, 2.50 work RVUs for CPT code 33XX4, and 14.31 work RVUs for CPT code 33XX5.
We are proposing the RUC-recommended work RVUs for the three new codes. We are also proposing the RUC-recommended direct PE inputs for CPT codes 33XX3 and 33XX5. We note that CPT code 33XX4 has no direct PE inputs.
(8) Endovascular Repair of Aortic Coarctation (CPT Codes 338X1, 338X2, and 338X0)
In October 2020, the CPT Editorial Panel created CPT codes 338X1 (Endovascular stent repair of coarctation of the ascending, transverse, or descending thoracic or abdominal aorta, involving stent placement; across major side branches) and 338X2 (Endovascular stent repair of coarctation of the ascending, transverse, or descending thoracic or abdominal aorta, involving stent placement; not crossing major side branches) to report endovascular stent repair of coarctation of the thoracic or abdominal aorta; and CPT code 338X0 (Percutaneous transluminal angioplasty of native or recurrent coarctation of the aorta) to report trans-liminal angioplasty for repair of native or recurrent percutaneous coarctation of the aorta. For CY 2022, the RUC recommended a work RVU of 21.70 for CPT code 338X1, a work RVU 17.97 for CPT code 338X2, and a work RVU 14.00 for CPT code 338X0.
We disagree with the RUC-recommended work RVUs for the CPT code family of 338X1, 338X2, and 338X0. We found that the recommended work RVUs for these CPT codes were high when compared to other codes with similar time values. Therefore, we are proposing the RUC survey 25th percentile of 18.27 as the work RVU for 338X1, we are proposing a work RVU of 14.54 for 338X2, and we are proposing a work RVU of 10.81 for 338X0.
When we reviewed CPT code 338X1, we found that the recommended work RVU was high compared to other codes with similar time values. The RUC survey 25th percentile of 18.27 falls within the range of RVUs with similar intra service time. This is supported by the reference CPT codes we compared to CPT code 338X1 with intra service time similar to the 134 minutes of intra service time for CPT code 338X1; reference CPT code 37231 (Revascularization, endovascular, open or percutaneous, tibial, peroneal artery, unilateral, initial vessel; with transluminal stent placement(s) and atherectomy, includes angioplasty within the same vessel, when performed) has a work RVU of 14.75 with 135 minutes of intra service time, and CPT code 93590 (Percutaneous transcatheter closure of paravalvular leak; initial occlusion device, mitral valve) has a work RVU of 21.70 with 135 minutes of intra service time. We note that the RUC-recommended RVU of 21.70 is a crosswalk from CPT code 93590 and is the highest value code within the range of reference codes we reviewed with similar intra service time. Again, we believe the RUC survey 25th percentile of 18.27 is a more appropriate value overall than 21.70 when compared to the range of codes with similar intra service time.
The RUC-recommended RVU of 17.97 for CPT code 338X2 was higher than other codes with the same 120 minutes of intra service time and similar total time. Although we disagree with the RUC-recommended work RVU for 338X2, we concur that the relative difference in work between CPT codes 338X1 and 338X2 is equivalent to the RUC-recommended interval of 3.73 RVUs. We believe the use of an incremental difference between these CPT codes is a valid methodology for setting values, especially in valuing services within a family of codes where it is important to maintain an appropriate intra-family relativity. Therefore, we are proposing a work RVU of 14.54 for CPT code 338X2, based on the RUC-recommended interval of 3.73 RVUs below our proposed work RVU of 18.27 for CPT code 338X1.
The RUC-recommended work RVU of 14.00 for CPT code 338X0 was higher than other codes with the same 90 minutes of intra service time and similar total time and we believe it would be more accurate to propose a work RVU that maintains the 3.73 incremental difference between the codes in this family. Therefore, for CPT code 338X0, we propose a work RVU of 10.81 which also continues the 3.73 incremental difference used between CPT codes 338X1 and 338X2, instead of the RUC incremental difference of 3.97 between CPT codes 338X2 and 338X0. Although Start Printed Page 39159 the work RVU of 10.81 we are proposing for CPT code 338X0 is lower than the RUC recommendation, the 3.73 incremental difference between CPT codes 338X2 and 338X0 we are proposing is more generous than the RUC incremental difference of 3.97 between CPT codes 338X2 and 338X0.
We are proposing no direct PE inputs for the CPT code family of 338X1, 338X2, and 338X0, as recommended by the RUC. These services are provided exclusively in the facility setting.
(9) Harvest of Upper Extremity Artery (CPT Codes 35XX0 and 35600)
In May 2020, the CPT Editorial Panel created CPT code 35XX0 (Harvest of upper extremity artery, 1 segment, for coronary artery bypass procedure, endoscopic) to describe endoscopic radial artery harvest via an endoscopic approach, and CPT code 35600 (Harvest of upper extremity artery, 1 segment, for coronary artery bypass procedure, open) was modified to only include an open approach for the upper extremity harvesting procedure. The RUC also stated that CPT codes 35XX0 and 35600 are almost always exclusively performed in conjunction with coronary artery bypass grafting (CABG) procedures. For CY 2022, the RUC-recommended a work RVU of 3.75 for CPT code 35XX0 and a work RVU of 4.00 for CPT code 35600.
We disagree with the RUC-recommended RVUs for the CPT code family of 35XX0 and 35600. We found that the recommended work RVUs for these CPT codes were high when compared to other codes with similar time values. Therefore, we are proposing 3.34 as the work RVU for 35XX0 and we are proposing a work RVU of 3.59 for 35600.
We disagree with the RUC-recommended work RVU for CPT code 35XX0 and are proposing an RVU of 3.34 which is a direct work RVU crosswalk from CPT code 35686 (Creation of distal arteriovenous fistula during lower extremity bypass surgery (non-hemodialysis) (List separately in addition to code for primary procedure)). The RUC-recommended value of 3.75 is higher than other codes with similar intra service time and total time. This is supported by the reference CPT codes we compared to CPT code 35XX0 with the same 35 minutes of intra service time and 35 minutes of total time as CPT code 35XX0; reference CPT code 74713 (Magnetic resonance (e.g., proton) imaging, fetal, including placental and maternal pelvic imaging when performed; each additional gestation (List separately in addition to code for primary procedure)) has a work RVU of 1.85, and CPT code 35686 has a work RVU of 3.34.
Although we disagree with the RUC-recommended work RVU for CPT code 35600, we concur that the relative difference in work between CPT codes 35XX0 and 35600 is equivalent to the RUC-recommended interval of 0.25 RVUs. We believe the use of an incremental difference between these CPT codes is a valid methodology for setting values, especially in valuing services within a family of codes where it is important to maintain an appropriate intra-family relativity. Therefore, we are proposing a work RVU of 3.59 for CPT code 35600, based on the RUC-recommended interval of 0.25 RVUs above our proposed work RVU of 3.34 for CPT code 35XX0.
We are proposing no direct PE inputs for the CPT code family of 35XX0 and 35600 as recommended by the RUC. These services are provided exclusively in the facility setting.
The RUC acknowledged that CPT codes 35XX0 and 35600 are almost always exclusively performed in conjunction with coronary artery bypass grafting (CABG) procedures. Such codes are designated as add-on procedures and are assigned a ZZZ-day global period (that is, code related to another service and is always included in the global period of the other service). The RUC also requested that the global period for both CPT codes 35XX0 and 35600 be an XXX-day global period (that is, global concept does not apply) and not a ZZZ-day global period as is customary for add-on codes. The RUC stated that an XXX-day global period would allow the individual that performs the harvest of upper extremity artery procedure (often separate from the surgeon performing the base CABG procedure) to report it under their own provider number. The RUC noted that it is often a nurse practitioner (NP) or physician's assistant (PA) who performs the harvest procedure. However, the RUC surveyed CPT codes 35XX0 and 35600 using reference codes with the ZZZ-day global period. Therefore, we believe it is appropriate to use that same ZZZ-day global period for CPT codes 35XX0 and 35600, and we are proposing to assign the ZZZ-day global period to CPT codes 35XX0 and 35600 for CY 2022. Through our scrutiny of comparing the code descriptions of codes with matching intra service times, we find much more clinically coherent similarities with codes with a ZZZ-day global period (procedures complementary, and sometimes necessary, to complete a larger procedure) than codes with an XXX-day global period.
However, we are compelled to understand more about the billing circumstances presented by the RUC and stakeholders that have presented this approach for CPT codes 35XX0 and 35600 to CMS for consideration. We are seeking comments and requesting information that may inform why CPT codes 35XX0 and 35600 should have an XXX-day global period instead of the ZZZ-day global period that is customary for add-on codes.
(10) Needle Biopsy of Lymph Nodes (CPT Code 38505)
CPT code 38505 (Biopsy or excision of lymph node(s); by needle, superficial (e.g., cervical, inguinal, axillary)) was identified in October 2019 as Harvard Valued with a utilization of over 30,000 claims. In January 2020, the RUC recommended that the code be surveyed for October 2020 RUC meeting. The RUC recommended increasing the work RVU to 1.59 which is the survey 25th percentile, acknowledging a change in the service, which now involves larger tissue samples as well as a change in technology, and a change in the dominant specialty now reporting the service.
We are proposing the RUC-recommended work RVU of 1.59 for CPT code 38505. We are also proposing the RUC-recommended direct PE inputs for this code.
(11) Drug Induced Sleep Endoscopy (CPT Codes 42XXX)
CPT code 42XXX (Drug induced sleep endoscopy; with dynamic evaluation of velum, pharynx, tongue base, and larynx for evaluation of sleep disordered breathing; flexible, diagnostic) is a new code created to report drug induced sleep endoscopy (DISE) flexible, diagnostic. The RUC recommended, and we agree, that the survey 25th percentile for the work RVU of 1.90 accurately reflects the typical physician work necessary to perform this service.
Since this is a drug induced sleep endoscopy, we are proposing CPT code 31575 (Diagnostic laryngoscopy) as the endoscopic base code for CPT code 42XXX because the description of the proposed CPT code is the same as what is described for CPT code 31575 with the additional component of the patient being sedated. The procedure is performed with a flexible endoscope or laryngoscope. CPT code 42XXX is not an add-on code, it has a 0-day global period. The endoscopic base code that it is using is a specific type of multiple procedure discount that applies to some endoscopy codes.
We are proposing the RUC-recommended work RVU of 1.90 for Start Printed Page 39160 CPT code 42XXX. We are also proposing the RUC-recommended direct PE inputs for this code.
(12) Per-Oral Endoscopic Myotomy (POEM) (CPT Codes 434XX)
In May 2020, the CPT Editorial Panel created a new CPT code 434XX (Lower esophageal myotomy, transoral (i.e., peroral endoscopic myotomy [POEM])) to describe a Per-Oral Endoscopic Myotomy (POEM), which involves the visualization and dissection of the esophageal muscle layers via an endoscope to treat esophageal motility disorders such as achalasia. This procedure accomplishes a comparable myotomy to what traditional open and laparoscopic myotomy (Heller) accomplishes. POEM utilizes an endoscope and specially designed dissecting, cutting, and cauterizing instruments to create a long submucosal tunnel beginning in the mid-esophagus and extending several centimeters into the cardia. For CY 2022, the RUC recommended a work RVU of 15.50 for CPT code 434XX.
We disagree with the RUC-recommended work RVU for CPT code 434XX and are proposing a work RVU of 13.29 based on a direct work RVU crosswalk from CPT code 36819 (Arteriovenous anastomosis, open; by upper arm basilic vein transposition). CPT code 36819 has the same 120 minutes of intra service time as CPT code 434XX, and has 283 minutes of total time, which is 2 minutes more than the 281 minutes of total time than for 434XX. The RUC used CPT codes 43279 (Laparoscopy, surgical, esophagomyotomy (Heller type), with fundoplasty, when performed) and 43180 (Esophagoscopy, rigid, transoral with diverticulectomy of hypopharynx or cervical esophagus (e.g., Zenker's diverticulum), with cricopharyngeal myotomy, includes use of telescope or operating microscope and repair, when performed) as reference codes for CPT code 434XX. However, the intra service time of 150 minutes and total time of 404 minutes for the RUC reference CPT code 43279, and intra service time of 60 minutes and total time of 201 minutes for the RUC reference CPT code 43180, are not adequate comparisons since they do not have similar time values to those of CPT code 434XX. Therefore, we believe the proposed work RVU of 13.29 for CPT code 434XX based on a direct work RVU crosswalk from CPT code 36819 is a better representation of the work being performed and is more appropriate based on the same intra service time and similar total time.
We are proposing the RUC-recommended direct PE inputs for CPT code 434XX without refinement.
(13) Placement-Removal of Seton (CPT Codes 46020 and 46030)
For CPT codes 46020 (Placement of seton) and 46030 (Removal of anal seton, other marker), we disagree with the RUC-recommended work RVUs of 3.50 and 2.00, respectively, as we believe these values do not adequately reflect the surveyed reductions in physician time for CPT code 46020 and the change to a 000-day global period from a 010-day global period for these CPT codes. Instead, we are proposing a work RVU of 1.86 for CPT code 46020 and 1.48 for CPT code 46030 based on a reverse building block methodology.
The survey showed that total time and intraservice time are decreasing for CPT code 46020 by 26 minutes and 5 minutes, respectively. We believe the surveyed decreases in physician time in conjunction with the loss of the post-operative visits for CPT code 46020 merit a decrease in work RVU from the current work RVU.
We note that the proposed work RVU of 1.48 for CPT code 46030 falls between CPT code 57410 (Pelvic examination under anesthesia (other than local)), which has a work RVU of 1.75, and CPT code 64487 (Transversus abdominis plane (TAP) block (abdominal plane block, rectus sheath block) unilateral; by continuous infusion(s) (includes imaging guidance, when performed)), which has a work RVU of 1.48. Both of these bracketing reference codes have identical intraservice times and similar total time values. While we understand that total time is going up for CPT code 46030, this increase is a result of significant increases to evaluation, positioning, and scrub, dress, wait preservice times, which is mostly low-intensity physician work.
We agree with the RUC's recommendation to change CPT codes 46020 and 46030 to 000-day global period codes from 010-day global period codes to account for the highly variable follow-up care for these services, but we note that the differences in RUC-recommended work RVUs and our proposed work RVUs largely reflect the change in global period and loss of physician time to provide the E/M services. The global period changes from 010-day to 000-day allow for separately billable E/M visits relating to CPT codes 46020 and 46030, therefore we removed RVUs that we believed were attributable to the currently bundled E/M visits totaling 2.04 RVUs for CPT code 46020 and 0.35 RVUs for CPT code 46030. CPT code 46020 is currently bundled with two post-operative follow up office visits, CPT code 99212 (Office or other outpatient visit for the evaluation and management of an established patient, which requires a medically appropriate history and/or examination and straightforward medical decision making. When using time for code selection, 10-19 minutes of total time is spent on the date of the encounter), and a half hospital discharge CPT code 99238 (Hospital discharge day management; 30 minutes or less). CPT code 46030 is currently bundled with half of a post-operative follow up office visit, CPT code 99212 (Office or other outpatient visit for the evaluation and management of an established patient, which requires a medically appropriate history and/or examination and straightforward medical decision making. When using time for code selection, 10-19 minutes of total time is spent on the date of the encounter). We do not believe the RUC adequately accounted for the loss of these E/M visits in their recommended work RVUs for CPT codes 46020 and 46030.
The RUC proposed the standard 090-day preservice times for the clinical labor activities CA001, CA002, CA003, CA004, and CA005 for CPT code 46020 in the facility. We note that the RUC recommended 090-day preservice clinical labor times despite surveying the service as a 000-day service. We disagree with the RUC-recommended 090-day preservice clinical labor times as we believe 000-day services should have times consistent with 000-day services, not 090-day services. However, we recognize there is time needed to coordinate this service. Therefore, we are proposing the following standard clinical labor times for extensive use of clinical staff for a 000-day global code:
- Complete preservice diagnostic and referral forms (CA001) 5 minutes.
- Coordinate pre-surgery services (including test results) (CA002) 10 minutes.
- Schedule space and equipment in facility (CA003) 5 minutes.
- Provide preservice education/obtain consent (CA004) 7 minutes.
- Complete pre-procedure phone calls and prescription (CA005) 3 minutes.
We are also proposing to refine the direct PE input for Coordinate post-procedure services (CA038) to 0 minutes from the RUC-recommended 3 minutes to align with 000-day standards instead of 090-day standards for CPT code 46020.
For CPT code 46030, the RUC recommended the standard 000-day Start Printed Page 39161 extensive use of clinical staff preservice times for clinical activities CA001, CA002, CA003, CA004, and CA005 in the facility and non-facility settings. Preservice times for 000-day codes are presumed to be zero unless there is sufficient justification that preservice time is warranted. We do not agree that sufficient justification was presented to warrant preservice time in the non-facility setting, therefore, we are proposing the following standard clinical labor times for use of clinical staff in the non-facility setting. We are also proposing the standards for minimal use of clinical staff in the facility setting, as we recognize there is time needed to coordinate this service for CPT code 46030:
- Complete preservice diagnostic and referral forms (CA001) 0 minutes for non-facility and 3 minutes for facility.
- Coordinate pre-surgery services (including test results) (CA002) 0 minutes for non-facility and 3 minutes for facility.
- Schedule space and equipment in facility (CA003) 0 minutes for non-facility and 3 minutes for facility.
- Provide preservice education/obtain consent (CA004) 0 minutes for non-facility and 3 minutes for facility.
- Complete pre-procedure phone calls and prescription (CA005) 0 minutes for non-facility and 3 minutes for facility.
We are also proposing to refine the direct PE input for Coordinate post-procedure services (CA038) to 0 minutes from the RUC-recommended 3 minutes to align with 000-day standards instead of 090-day standards for CPT code 46030.
(14) Periurethral Balloon Continence Device Procedures (CPT Codes 53XX1, 53XX2, 53XX3, and 53XX4)
In October 2020, the CPT Editorial Panel replaced four CPT Category III codes with four new CPT Category I codes to report periurethral adjustable balloon continence devices. Given the low utilization and the low survey response rate for the four new codes, the RUC recommended that CMS assign contractor pricing to these procedures. We agree with the RUC and we are proposing contractor pricing for all four codes in the family, CPT codes 53XX1 (Periurethral transperineal adjustable balloon continence device; bilateral insertion, including cystourethroscopy and imaging guidance), 53XX2 (Periurethral transperineal adjustable balloon continence device; unilateral insertion, including cystourethroscopy and imaging guidance), 53XX3 (Periurethral transperineal adjustable balloon continence device; removal, each balloon) and 53XX4 (Periurethral transperineal adjustable balloon continence device; percutaneous adjustment of balloon(s) fluid volume).
(15) Intracranial Laser Interstitial Thermal Therapy (LITT) (CPT Codes 617X1 and 617X2)
In October 2020, the CPT Editorial Panel approved the addition of two codes to report laser interstitial thermal therapy (LITT) of lesion, intracranial, including burr hole(s), with magnetic resonance (MR) imaging guidance for a single trajectory for 1 simple lesion and multiple trajectories for multiple or complex lesion(s). LITT is a novel procedure that involves multiple steps and movements of the patient through the hospital for different stages of the procedure. The typical facility does not have an interoperative MRI suite (a small minority of academic medical centers may), so patient transport is necessary.
The RUC recommended a work RVU of 20.00 for CPT code 617X1 (Laser interstitial thermal therapy (LITT) of lesion, intracranial, including burr hole(s), with magnetic resonance imaging guidance, when performed; single trajectory for 1 simple lesion) based on the survey median response. CPT code 617X1 was surveyed with having one subsequent hospital visit, CPT code 99232 (sbsq hospital care/day 25 minutes) and 40 minutes of immediate postservice time. The RUC noted that although the survey median immediate postservice time was 40 minutes, for 617X1, the CMS 23-Hour Stay Outpatient Surgical Services with Subsequent Hospital Visits Policy was applied which resulted in the 99232 visit being removed and its 20 minutes of intraservice time being applied to the 40 minutes of immediate postservice time resulting in 60 minutes of immediate postservice time. See the 2011 PFS final rule (75 FR 73226) for an in-depth explanation of the 23-hour policy. We believe the RUC partially applied the 23-hr policy when it applied the policy to the immediate post service time but not to the work RVU. We believe the 23-hour policy in its entirety should be applied to 671X1, which includes the work RVUs along with the immediate postservice time.
Following the valuation methodology we established for 23-hour stay services in the CY 2011 PFS final rule, 617X1 would have a work RVU of 19.06.
The steps are as follows:
- Step (1): CPT code 617X1 does not have a hospital discharge day management service; therefore, we would skip this step.
- Step (2): 20 − 1.39** = 18.61.
- Step (3): 18.61 + (20 minutes × 0.0224)*** = 19.06 RVUs.
* Value associated with 1/2 hospital discharge day management service.
** Value associated with an inpatient hospital visit, CPT code 99232.
*** Value associated with the reallocated intraservice time multiplied by the postservice intensity of the 23-hour stay code.
Therefore, for CY 2022 we are proposing a work RVU of 19.06 for CPT code 671X1.
In reviewing the RUC-recommended direct PE inputs for 671X1 we noticed the RUC proposed the standard 090-day preservice times for the following clinical labor activities:
- Complete preservice diagnostic and referral forms (CA001) 5 minutes.
- Coordinate pre-surgery services (including test results) (CA002) 20 minutes.
- Schedule space and equipment in facility (CA003) 8 minutes.
- Provide preservice education/obtain consent (CA004) 20 minutes.
Complete pre-procedure phone calls and prescription (CA005) 7 minutes.
We note that the RUC recommended 090-day preservice times despite surveying the service as a 000-day service. We disagree with the RUC-recommended 090-day times as we believe this is a 000-day service and should have times consistent with 000-day services. However, we recognize there is time needed to coordinate this service. Therefore, for CY 2022 we are proposing the following standard clinical labor times for a 000-day extensive:
- Complete preservice diagnostic and referral forms (CA001) 5 minutes.
- Coordinate pre-surgery services (including test results) (CA002) 10 minutes.
- Schedule space and equipment in facility (CA003) 5 minutes.
- Provide preservice education/obtain consent (CA004) 7 minutes.
- Complete pre-procedure phone calls and prescription (CA005) 3 minutes.
For CPT code 617X2 (Laser interstitial thermal therapy (LITT) of lesion, intracranial, including burr hole(s), with magnetic resonance imaging guidance, when performed; multiple trajectories for multiple or complex lesion(s)), the RUC recommended a work RVU of 24.00 which is the survey median. The RUC's recommendation also included 40 minutes of immediate postservice time and one hospital visit, CPT code 99233 (sbsq hospital care/day visit 35 minutes). We believe it would be appropriate to apply the 23-hr policy to CPT code 617X2 as well. Start Printed Page 39162
The steps are as follows:
- Step (1): CPT code 617X2 does not have a hospital discharge day management service. Therefore, we would skip this step.
- Step (2): 24 − 2** = 22
- Step (3): 22 + (30 minutes × 0.0224)*** = 22.67 RVUs
* Value associated with 1/2 hospital discharge day management service.
** Value associated with an inpatient hospital visit, CPT code 99233.
*** Value associated with the reallocated intraservice time multiplied by the postservice intensity of the 23-hour stay code.
This results in a work RVU of 22.67, and an immediate post service time of 70 minutes. Therefore, for CY 2022 we a proposing a work RVU of 22.67 and 70 minutes of immediate postservice time for CPT code 617X2.
For the direct PE, the RUC proposed identical preservice times for CPT codes 617X1 and 617X2. For the reasons stated above concerning the direct PE inputs for CPT code 671X1, we are proposing the standard clinical labor times associated with a 000-day extensive for CPT code 617X2 for CY 2022.
(16) Arthrodesis Decompression (CPT Codes 630XX and 630X1)
For CPT codes 630XX (Laminectomy, facetectomy, or foraminotomy (unilateral or bilateral with decompression of spinal cord, cauda equina and/or nerve root[s] [eg, spinal or lateral recess stenosis]), during posterior interbody arthrodesis, lumbar; single vertebral segment (List separately in addition to code for primary procedure)) and 630X1 (Laminectomy, facetectomy, or foraminotomy (unilateral or bilateral with decompression of spinal cord, cauda equina and/or nerve root[s] [eg, spinal or lateral recess stenosis]), during posterior interbody arthrodesis, lumbar; each additional segment (List separately in addition to code for primary procedure)), we disagree with the RUC-recommended work RVUs of 5.55 and 4.44, respectively, because these values are anomalously high in comparison to other similar add-on codes that have longer intraservice times, and we are proposing a work RVU of 3.08 for CPT code 630XX and a work RVU of 2.31 for CPT code 630X1.
CPT codes 630XX and 630X1 are new add-on codes to report decompression when performed in conjunction with posterior interbody arthrodesis at the same interspace. The proposed work RVU for CPT code 630XX is based on an intraservice time ratio between the proposed 40 minutes of intraservice time for CPT code 630XX and the 45 minutes of intraservice time for CPT code 63048 (Laminectomy, facetectomy and foraminotomy (unilateral or bilateral with decompression of spinal cord, cauda equina and/or nerve root[s], [eg, spinal or lateral recess stenosis]), single vertebral segment; each additional segment, cervical, thoracic, or lumbar (List separately in addition to code for primary procedure)). We believe that CPT code 63048 is a stronger reference code for CPT code 630XX than the RUC-recommended reference CPT codes 33924 (Ligation and takedown of a systemic-to-pulmonary artery shunt, performed in conjunction with a congenital heart procedure (List separately in addition to code for primary procedure)) and 22614 (Arthrodesis, posterior or posterolateral technique, single level; each additional vertebral segment (List separately in addition to code for primary procedure)) because of the similarities in the long descriptors, physician time, and intensity of intraservice work for CPT codes 630XX and 63048. The intraservice time ratio between CPT codes 63048 and 630XX equals a work RVU of 3.08 for CPT code 630XX ((40 minutes/45 minutes) * 3.47 = 3.08). Therefore, we are proposing a work RVU of 3.08 for CPT code 630XX. The intraservice time ratio between CPT codes 63048 and 630XX was selected to value CPT code 630XX because of the similarities in the descriptions of intraservice work provided in the RUC's summary of recommendations for CPT code 630XX and the RUC Database for CPT code 63048. We are proposing a work RVU of 2.31 for CPT code 630X1 based on an intraservice time ratio between the proposed 30 minutes of intraservice time for CPT code 630X1 and the proposed 40 minutes of intraservice time for CPT code 630XX ((30 minutes/40 minutes) * 3.08 = 2.31), given that the RUC contends that there are some efficiencies in providing an additional level of decompression, evidenced by the 10 minutes less of intraservice time for CPT code 630X1 compared to CPT code 630XX. These work RVU proposals are further supported by brackets of other 30 and 40 minute ZZZ codes.
We note that the proposed work RVU for CPT code 630XX falls between CPT code 19294 (Preparation of tumor cavity, with placement of a radiation therapy applicator for intraoperative radiation therapy (IORT) concurrent with partial mastectomy (List separately in addition to code for primary procedure)), which has a work RVU of 3.00, and CPT code 37185 (Primary percutaneous transluminal mechanical thrombectomy, noncoronary, non-intracranial, arterial or arterial bypass graft, including fluoroscopic guidance and intraprocedural pharmacological thrombolytic injection(s); second and all subsequent vessel(s) within the same vascular family (List separately in addition to code for primary mechanical thrombectomy procedure)), which has a work RVU of 3.28. Both of these bracketing reference codes have identical intraservice times as CPT code 630XX. The proposed work RVU for CPT code 630X1 falls between CPT code 43273 (Endoscopic cannulation of papilla with direct visualization of pancreatic/common bile duct(s) (List separately in addition to code(s) for primary procedure)), which has a work RVU of 2.24, and CPT code 22870 (Insertion of interlaminar/interspinous process stabilization/distraction device, without open decompression or fusion, including image guidance when performed, lumbar; second level (List separately in addition to code for primary procedure)), which has a work RVU of 2.34. Both of these bracketing reference codes have identical intraservice times as CPT code 630X1. When we compared the RUC-recommended work RVU of 5.55 for CPT code 630XX and 4.44 for CPT code 630X1 to other spinal add-on codes in the 63000 CPT code series in the RUC database, we found that CPT code 630XX would have the highest work RVU and the second shortest intraservice time (with CPT code 630X1 having the shortest intraservice time), and CPT code 630X1 would have the third highest work RVU and shortest intraservice time compared to the 10 other nationally-priced spinal add-on codes in the 63000 CPT code series. We do not agree that decompression when performed in conjunction with posterior interbody arthrodesis at the same interspace should have an anomalously high work value in comparison to other similar add-on codes that have longer intraservice times. We believe that our proposed work RVUs of 3.08 for CPT code 630XX and 2.31 for CPT code 630X1 better serve the interests of relativity. We note that the specialty societies did not survey the two new add-on codes with the base codes, which is a standard to provide assurance that the respondents followed instruction to only consider the work of the add-on codes. CPT codes 630XX and 630X1 were reviewed again with their base codes at the April 2021 RUC meeting. There were also revisions to the base codes' definitions, guidelines, and parenthetical instructions, which Start Printed Page 39163 were approved by the CPT Editorial Panel for CY 2022.
The RUC did not recommend any direct PE inputs for these codes and we are not proposing any direct PE inputs.
(17) Hypoglossal Nerve Stimulator Services (CPT Codes 645X1, 645X2, and 645X3)
In October 2020, the CPT Editorial Panel added three new CPT Category I codes to report open implantation, revision or replacement, and removal of hypoglossal nerve stimulator array. These new CPT codes replaced three CPT Category III codes which were reported with CPT codes 64568 (Incision for implantation of cranial nerve (eg, vagus nerve) neurostimulator electrode array and pulse generator), 64569 (Revision or replacement of cranial nerve (eg, vagus nerve) neurostimulator electrode array, including connection to existing pulse generator) and 64570 (Removal of cranial nerve (eg, vagus nerve) neurostimulator electrode array and pulse generator).
CPT code 645X1 (Open implantation of hypoglossal nerve neruostimulator array, pulse generator, and distal respiratory sensor electrode or electrode array) was previously reported using the now deleted Category III CPT code 0466T (Insertion of chest wall respiratory sensor electrode or electrode array, including connection to pulse generator (List separately in addition to code for primary procedure)) along with CPT code 64568. We are not proposing the RUC-recommendation to use the survey median work RVU of 16.00 for CPT code 645X1. We are proposing a work RVU of 14.00 based on the intraservice time ratio of CPT code 64568 compared to the RUC-recommended intraservice time for CPT code 645X1. CPT code 64568 has a work RVU of 9.00, intraservice time of 90 minutes and total time of 275 minutes. CPT code 645X1 has a RUC-recommended work RVU of 16.00, intraservice time of 140 minutes and total time of 294 minutes. Additionally, when we reviewed CPT code 645X1, we found that the RUC-recommended work RVU was higher than other global 90-day codes with similar time values. We do not agree that it would be typical to value this code so much higher than services with similar work time values. Additionally, we note that the proposed work RVU of 14.00 is also the survey 25th percentile. Therefore, as previously stated, we believe 14.00 is a more appropriate value overall than 16.00 when compared to the range of codes with similar work times.
We are not proposing the RUC-recommended work value of 16.50 for CPT code 645X2 (Revision or replacement of hypoglossal nerve neruostimulator array and distal respiratory sensor electrode or electrode array, including connection to an existing pulse generator), rather we are proposing a work RVU of 14.50. Although we disagree with the RUC-recommended work RVU, we concur that the relative difference in work between CPT codes 645X1 and 645X2 is equivalent to the recommended increment of 0.50 RVUs. Therefore, we are proposing a work RVU of 14.50 for CPT code 645X2 based on the recommended increment of 0.50 additional RVUs above our proposed work RVU of 14.00 for CPT code 645X1. We believe the use of an incremental difference between these CPT codes is a valid methodology for setting values, especially in valuing services within a family of codes where it is important to maintain an appropriate intra-family relativity. Additionally, we note that the proposed work RVU of 14.50 is also nearly identical to the 25th percentile survey value for CPT code 645X2 of 14.63. Therefore, as previously stated, we believe 14.50 is a more appropriate value than 16.50 to maintain an appropriate intra-family relativity.
We are not proposing the RUC-recommended work value of 14.00 for CPT code 645X3 (Removal of hypoglossal nerve neruostimulator array, pulse generator, and distal respiratory sensor electrode or electrode array), rather we are proposing a work RVU of 12.00. Although we disagree with the RUC-recommended work RVU, we concur that the relative difference in work between CPT codes 645X1 and 645X3 is equivalent to the recommended increment of −2.0 RVUs. We believe the use of an incremental difference between these CPT codes is a valid methodology for setting values, especially in valuing services within a family of codes where it is important to maintain an appropriate intra-family relativity. Therefore, we are proposing a work RVU of 12.00 for CPT code 645X3 based on the recommended increment of 2.0 RVUs below our proposed work RVU of 14.00 for CPT code 645X1. Additionally, we note that the proposed work RVU of 12.00 is also the RUC 25th percentile survey value for CPT code 645X3.
We are proposing the RUC-recommended direct PE inputs without refinements for CPT codes 645X1, 645X2 and 645X3.
(18) Destruction by Neurolytic Agent (CPT Codes 64633, 64634, 64635, and 64636)
In September 2014, the Relativity Assessment Workgroup identified a work neutrality issue for CPT codes 64633 (Destruction by neurolytic agent, paravertebral facet joint nerve(s), with imaging guidance (fluoroscopy or CT); cervical or thoracic, single facet joint), 64634 (Destruction by neurolytic agent, paravertebral facet joint nerve(s), with imaging guidance (fluoroscopy or CT); cervical or thoracic, each additional facet joint (List separately in addition to code for primary procedure)), 64635 (Destruction by neurolytic agent, paravertebral facet joint nerve(s), with imaging guidance (fluoroscopy or CT); lumbar or sacral, single facet joint), and 64636 (Destruction by neurolytic agent, paravertebral facet joint nerve(s), with imaging guidance (fluoroscopy or CT); lumbar or sacral, each additional facet joint (List separately in addition to code for primary procedure)) related to incorrect coding relative to how the services were originally valued. In May 2015, the CPT Editorial Panel revised the parenthetical instructions for the five codes describing paravertebral facet joint nerve destruction to clarify that these codes are reported per joint, not nerve. Due to the extensive growth and original incorrect assumptions about distribution of reporting, the RUC recommended that CPT codes 64633-64636 be surveyed. We are proposing the RUC-recommended work RVU of 1.32 for CPT code 64634 and the RUC-recommended work RVU of 1.16 for CPT code 64636.
For CPT codes 64633 and 64635, we are not proposing the RUC-recommended work RVU of 3.42 for both codes, as we believe this value understates the decrease in physician work time for these codes. An analysis of all 010-day global period codes indicates that these proposed values would place these codes among the highest valued for codes with similar time values. We are instead using a total-time ratio methodology to propose work RVUs of 3.31 for CPT code 64633 and 3.32 for CPT code 64635. We support these values by noting that they fall between CPT codes 54164 (Frenulotomy of penis), with a work RVU of 2.82, and CPT code 68371 (Harvesting conjunctival allograft, living donor), with a work RVU of 5.09; these reference codes have total time values that are similar to, and intraservice time values that are identical to those recommended for CPT codes 64633 and 64635. Start Printed Page 39164
We are proposing the RUC-recommended direct PE inputs without refinement.
(19) Destruction of Intraosseous Basivertebral Nerve (CPT Codes 646X0 and 646X1)
In October 2020, the CPT Editorial Panel added two Category I codes to report thermal destruction of intraosseous basivertebral nerve, inclusive of all imaging guidance for the first two vertebral bodies (lumbar or sacral) and for each additional vertebral body (lumbar or sacral).
We are not proposing the RUC-recommended work value of 8.25 for CPT code 646X0 (Thermal destruction of intraosseous basivertebral nerve, inclusive of all imaging guidance; first two vertebral bodies, lumbar or sacral). When we reviewed CPT code 646X0, we found that the RUC-recommended work RVU was higher than codes with the same 10-day global period, same intraservice time and similar total times. The RUC-recommended work RVU of 8.25 would value CPT code 646X0 at the 90th percentile of comparable 10-day globals and we do not agree that it would be typical to value this code so much higher than services with similar work time values. We believe it would be more accurate to propose a work RVU of 7.15 based on a crosswalk to CPT code 63650 (Percutaneous implantation of neurostimulator electrode array, epidural) with a work RVU of 7.15, identical intraservice time of 60, and similar total time of 170. We believe the crosswalk to CPT code 63650 serves as a more accurate valuation for CPT code 646X0.
We also are not proposing the RUC-recommended work value of 4.87 for CPT code 646X1 (Thermal destruction of intraosseous basivertebral nerve, inclusive of all imaging guidance; each additional vertebral body, lumbar or sacral (List separately in addition to code for primary procedure)). Although we disagree with the RUC-recommended work RVU, we concur that the relative difference in work between CPT codes 646X0 and 646X1 is equivalent to the recommended increment of −3.38 RVUs. However, since the recommended work RVU of code 646X0 was higher than other codes with the same 10-day global period, same intraservice time, and similar total times, we refined the work RVU for code 646X1 to preserve the incremental difference between the two codes. We believe that these refinements maintain the relationship between the two codes in the family while better preserving relativity with other similar 10-day global codes on the wider PFS. We believe the use of an incremental difference between these CPT codes is a valid methodology for setting values, especially in valuing services within a family of codes where it is important to maintain an appropriate intra-family relativity. Therefore, we are proposing a work RVU of 3.77 for CPT code 646X1 based on the recommended increment of 3.38 RVUs below our proposed work RVU of 7.15 for CPT code 646X0.
We are proposing the RUC-recommended direct PE inputs without refinements for CPT code 646X0. CPT code 646X1 is an add-on code and does not have any direct PE inputs.
(20) Dilation of Aqueous Outflow Canal (CPT Codes 66174 and 66175)
These services were identified through the New Technology/New Services List. In January 2020, the specialty societies submitted an action plan and the RUC recommended referral to the CPT Editorial Panel in 2020 to possibly revise the descriptor and add exclusionary parentheticals for CPT code 66174 (Transluminal dilation of aqueous outflow canal; without retention of device or stent). In October 2020, the CPT Editorial Panel revised this code to add a parenthetical to restrict reporting this code in conjunction with CPT code 65820 (Goniotomy).
We are not proposing the RUC-recommended work RVUs of 8.53 for CPT code 66174 and 10.25 for CPT code 66175 (Transluminal dilation of aqueous outflow canal; with retention of device or stent), as we believe these values do not adequately reflect the surveyed reductions in physician time. These RVUs would rank these codes among the highest valued 090-day global period codes of similar time values. We are proposing a work RVU of 9.34 for CPT code 66175 using a reverse building block methodology. We then subtract the incremental difference between the two RUC-recommended work RVUs, an increment of 1.72, from our proposed work RVU of 9.34 for CPT code 66175 to propose a work RVU of 7.62 for CPT code 66174. We believe this approach is consistent with the RUC's assumption that the intensity and complexity of CPT code 66174 is the same as that of CPT code 66175, the only difference between the two procedures being the additional intraservice time associated with placement of the stent. As further support for these values, we note that they fall between CPT code 66984 (Extracapsular cataract removal with insertion of intraocular lens prosthesis (1 stage procedure), manual or mechanical technique (eg, irrigation and aspiration or phacoemulsification); without endoscopic cyclophotocoagulation), with 7.35 work RVUs, and CPT code 15150 (Tissue cultured skin autograft, trunk, arms, legs; first 25 sq cm or less), with 9.39 work RVUs.
We are proposing the RUC-recommended PE inputs without refinement.
(21) Cataract Removal With Drainage Device Insertion (CPT Codes 669X1, 669X2, 66982, 66984, 66987, 66988, and 0X12T)
The RUC identified CPT code 0191T (Insertion of anterior segment aqueous drainage device, without extraocular reservoir, internal approach, into the trabecular meshwork; initial insertion) via the Category III codes with High Utilization screen (2018 estimated Medicare utilization over 1,000). In January 2020, the RUC recommended that the specialty societies develop a coding application for Category I status for CPT code 0191T and CPT code 0376T (each additional device insertion (List separately in addition to code for primary procedure). In October 2020, the CPT Editorial Panel replaced two Category III codes (CPT codes 0191T and 0376T) with two new codes, CPT codes 669X1 and 669X2, to report extracapsular cataract removal with insertion of intraocular lens prosthesis and one Category III code to report insertion of anterior segment aqueous drainage device without concomitant cataract removal.
The RUC recommended a work RVU of 12.13 for CPT code 669X1 (Extracapsular cataract removal with insertion of intraocular lens prosthesis (1-stage procedure), manual or mechanical technique (eg, irrigation and aspiration or phacoemulsification), complex, requiring devices or techniques not generally used in routine cataract surgery (eg, iris expansion device, suture support for intraocular lens, or primary posterior capsulorrhexis) or performed on patients in the amblyogenic developmental stage; with insertion of intraocular (eg, trabecular meshwork, supraciliary, suprachoroidal) anterior segment aqueous drainage device, without extraocular reservoir, internal approach, one or more) based on the survey 25th percentile.
In its recommendation, the RUC noted that the recommended intraservice time of 28 minutes for CPT code 669X1 is 2 minutes less than the intraservice time of 30 minutes associated with CPT code 66982 (Extracapsular cataract removal with insertion of intraocular lens prosthesis (1-stage procedure), manual Start Printed Page 39165 or mechanical technique (eg, irrigation and aspiration or phacoemulsification), complex, requiring devices or techniques not generally used in routine cataract surgery (eg, iris expansion device, suture support for intraocular lens, or primary posterior capsulorrhexis) or performed on patients in the amblyogenic developmental stage; without endoscopic cyclophotocoagulation). The RUC further noted this should not be the case, as the insertion of the intraocular lens prosthesis should take the same amount of time and be represented by the same relative work for both procedures and that it is counterintuitive that the intraservice time for CPT code 669X1 would be lower than the intraservice time for CPT code 66982, as CPT code 669X1 includes both complex cataract surgery and the insertion of the intraocular anterior segment aqueous drainage device. The specialty society that surveyed the codes explained that this is likely because the early adopters of this new technology service are highly skilled surgeons who would likely perform these procedures quickly. They stated that as this procedure diffuses into the wider population of ophthalmologic surgeons over the next few years, the intraservice time will likely rise above the intraservice time associated with CPT codes 66982 and 66984 and will come in line for both CPT codes 669X1 and 669X2.
CPT code 69982 has a work RVU of 10.25, 125 minutes of total time and 30 minutes of intraservice time. CPT code 669X1 has a RUC-recommended work RVU of 12.13, 176 minutes of total time and 28 minutes of intraservice time. We agree with the RUC assessment that both procedures, CPT code 66982 and CPT code 669X1, are almost identical in time and intensity. However, we disagree with the RUC-recommended work RVU of 12.13 for CPT code 669X1 noting that CPT code 66982 has a work RUV of 10.25. We are proposing a work RVU of 10.31 based on the current total time ratio of CPT code 66982 compared to the RUC-recommended total time for CPT code 669X1.
For CPT code 669X2, the RUC recommended a work RVU of 9.23. The RUC determined that it would be appropriate to use the increment between the 25th percentile work RVU value for CPT code 669X1 and the current RUC-reviewed work RVU value for CPT code 66982 to build a work RVU recommendation for CPT code 669X2. The RUC determined that the increment between the 25th percentile work RVU value for CPT code 669X1 (work RVU = 12.13) and the current RUC-reviewed work RVU value for CPT code 66982 (work RVU = 10.25) would yield an increment between those two codes of 1.88. The RUC added the 1.88 increment to 7.35, the current work RVU for 66984, which yields a RUC-recommended work RVU value of 9.23. This comparison results in a work RVU recommendation of 9.23 for CPT code 669X2. We are proposing a work RVU of 7.41, which is the increment between the current RUC-reviewed work RVU value for CPT code 66982 and CPT code 66984. The increment between CPT code 66982 (work RVU = 10.25) and CPT code 66984 (work RVU = 7.35) yields a work RUV of 2.90. We subtracted this 2.90 increment from 10.31, to determine our proposed work RVU of 7.41 for CPT code 669X1.
We are proposing the RUC-recommended indirect PE values for CPT codes 669X1 and 669X2.
We are not proposing any new valuations but reaffirming the work RVUs and direct PE inputs that we previously finalized for CPT codes 66982 (Extracapsular cataract removal with insertion of intraocular lens prosthesis (1-stage procedure), manual or mechanical technique (eg, irrigation and aspiration or phacoemulsification), complex, requiring devices or techniques not generally used in routine cataract surgery (eg, iris expansion device, suture support for intraocular lens, or primary posterior capsulorrhexis) or performed on patients in the amblyogenic developmental stage; without endoscopic cyclophotocoagulation) and 66984 (Extracapsular cataract removal with insertion of intraocular lens prosthesis (1 stage procedure), manual or mechanical technique (eg, irrigation and aspiration or phacoemulsification); without endoscopic cyclophotocoagulation). For CPT codes 66987 (Extracapsular cataract removal with insertion of intraocular lens prosthesis (1-stage procedure), manual or mechanical technique (eg, irrigation and aspiration or phacoemulsification), complex, requiring devices or techniques not generally used in routine cataract surgery (eg, iris expansion device, suture support for intraocular lens, or primary posterior capsulorrhexis) or performed on patients in the amblyogenic developmental stage; with endoscopic cyclophotocoagulation) and 66988 (Extracapsular cataract removal with insertion of intraocular lens prosthesis (1 stage procedure), manual or mechanical technique (eg, irrigation and aspiration or phacoemulsification); with endoscopic cyclophotocoagulation) we continue to believe these services should be contractor priced.
(22) Retinal Detachment Prophylaxis (CPT Codes 67141 and 67145)
CPT code 67145 (Prophylaxis of retinal detachment (eg, retinal break, lattice degeneration) without drainage, 1 or more sessions; photocoagulation (laser or xenon arc)) was identified in October 2019 as a Harvard Valued service with utilization over 30,000. In January 2020, the RUC agreed with the specialty societies that surveyed the service and recommended that CPT code 67145, as well as its parent CPT code 67141 (Prophylaxis of retinal detachment (eg, retinal break, lattice degeneration) without drainage, 1 or more sessions; cryotherapy, diathermy), be referred to the CPT Editorial Panel for a descriptor and global period change. The codes were edited to remove the reference to "1 or more sessions" so that the services may be valued as a 010-day procedure versus the current 090-day global. At the May 2020 CPT Editorial Panel meeting, the Panel approved revision of the two codes to remove "1 or more sessions" from the descriptors and deletion of the Eye and Ocular Adnexa Prophylaxis guidelines.
For CY 2022, we are proposing the RUC-recommended work RVU of 2.53 for CPT codes 67141 and 67145. We are also proposing the RUC-recommended direct PE inputs without refinements.
(23) Strabismus Surgery (CPT Codes 67311, 67312, 67314, 67316, 67318, 67320, 67331, 67332, 67334, 67335, and 67340)
In April 2020, The RUC recommend that add-on CPT codes 67320, 67331, 67332, 67334, 67335, and 67340 be surveyed along with the base codes in which these services are typically reported (CPT codes 67311, 67312, 67314, 67316 and 67318). When AMA staff compiled a list of 010-day and 090-day services for increases in physician work and time during the surgical global period, they noticed that several low volume codes that were converted to ZZZ global periods in 1999 still included office visits (specifically CPT codes 67320, 67331, 67332, 67334, 67340). It appeared that these office visits may not be appropriate for these services. This issue was deferred until October 2020.
We are proposing the RUC-recommended work RVUs for all base codes within this family. This includes a work RVU of 5.93 for CPT code 67311 (Strabismus surgery, recession or resection procedure; 1 horizontal muscle), 9.50 for CPT code 67312 Start Printed Page 39166 (Strabismus surgery, recession or resection procedure; 2 horizontal muscles), 5.93 for CPT code 67314 (Strabismus surgery, recession or resection procedure; 1 vertical muscle (excluding superior oblique), 10.31 for CPT code 67316 (Strabismus surgery, recession or resection procedure; 2 or more vertical muscles (excluding superior oblique)), and 9.80 for CPT code 67318 (Strabismus surgery, any procedure, superior oblique muscle).
We are also proposing the RUC-recommend work RVUs for all of the add-on codes within this family. This includes a work RVU of 3.00 for CPT code 67320 (Transposition procedure (eg, for paretic extraocular muscle), any extraocular muscle (specify) (List separately in addition to code)), 2.00 for CPT code 67331 (Strabismus surgery on patient with previous eye surgery or injury that did not involve the extraocular muscles (List separately in addition to code for primary procedure)), 3.50 for CPT code 67332 (Strabismus surgery on patient with scarring of extraocular muscles (eg, prior ocular injury, strabismus or retinal detachment surgery) or restrictive myopathy (eg, dysthyroid opthalmopathy) (List separately in addition to code for primary procedure)), 2.06 for CPT code 67334 (Strabismus surgery by posterior fixation suture technique, with or without muscle recession (List separately in addition to code for primary procedure)), 3.23 for CPT code 67335 (Strabismus surgery by posterior fixation suture technique, with or without muscle recession (List separately in addition to code for primary procedure)), and 5.00 for CPT code 67340 (Strabismus surgery by posterior fixation suture technique, with or without muscle recession (List separately in addition to code for primary procedure)).
We are proposing the RUC-recommended direct PE inputs for this code family without refinements.
(24) Lacrimal Canaliculus Drug Eluding Implant Insertion (CPT Codes 68XXX)
CPT code 68XXX (Insertion of drug-eluting implant, including punctal dilation, when performed, into lacrimal canaliculus, each) was recommended for RUC review in October 2020 since the CPT Editorial Panel replaced CPT Category III (temporary) code 0356T with a new CPT Category I code to report the insertion of a drug eluting implant into the lacrimal canaliculus. We are proposing the RUC-recommended work RVU of 0.49 for CPT code 68XXX.
For the direct PE inputs, we are proposing to refine the equipment time for the "lane, screening (oph)" (EL006) from the RUC-recommended 9 minutes of equipment time to the 5 minute equipment standard for CPT code 68XXX. Five minutes is the standard equipment time associated with EL006 for this procedure. The recommended materials for this code family from the RUC state that the screening lane is used for the duration of setup, procedure, cleaning, and counselling post procedure and that the standard formulas are applied. We believe that the RUC inadvertently failed to update the equipment time associated with this procedure when CPT code 68XXX was reviewed. The recommended materials for CPT code 68XXX state the standard equipment time formula would be typical for this service, which would be 5 minutes in this case (the CA013 and CA024 equipment times are included but not the CA035 equipment time). We are proposing to refine the equipment time for the equipment item lane, screening (oph) (EL006) from 9 minutes to 5 minutes to match this change in equipment time and are seeking additional comment from stakeholders regarding the RUC-recommended non-standard equipment time of 9 minutes. We do not agree that it would be typical for CPT code 68XXX to require an additional 4 minutes of equipment time totaling 9 minutes.
(25) Transcutaneous Passive Implant-Temporal Bone (CPT Codes 69714, 69717, 69X50, 69X51, 69X52, and 69X53)
In October 2020, the CPT Editorial Panel deleted two codes used for mastoidectomy and replaced them with four new codes for magnetic transcutaneous attachment to external speech processor. The CPT Editorial Panel made additional revisions to differentiate implantation, removal, and replacement of the implants.
We are proposing the RUC-recommended work RVU for all six of the codes in this family. We are proposing a work RVU of 8.69 for CPT code 69714 (Implantation, osseointegrated implant, skull; with percutaneous attachment to external speech processor), a work RVU of 9.77 for CPT code 69X50 (Implantation, osseointegrated implant, skull; with magnetic transcutaneous attachment to external speech processor), a work RVU of 8.80 for CPT code 69717 (Revision/replacement (including removal of existing device), osseointegrated implant, skull; with percutaneous attachment to external speech processor), a work RVU of 9.77 for CPT code 69X51 (Revision/replacement (including removal of existing device), osseointegrated implant, skull; with magnetic transcutaneous attachment to external speech processor), a work RVU of 5.93 for CPT code 69X52 (Removal, osseointegrated implant, skull; with percutaneous attachment to external speech processor), and a work RVU of 7.13 for CPT code 69X53 (Removal, osseointegrated implant, skull; with magnetic transcutaneous attachment to external speech processor).
For the direct PE inputs, we are proposing to refine the clinical labor time for the "Post-operative visits (total time)" (CA039) activity from the RUC-recommended 108 minutes to 99 minutes for CPT codes 69714 and 69717. 99 minutes is the clinical labor time associated with one Level 2 postoperative office visit and two Level 3 postoperative office visits; we believe that the RUC inadvertently failed to update the clinical labor time associated with these postoperative office visits when CPT codes 69714 and 69717 were reviewed. We are also proposing to refine the equipment time for all equipment items other than the basic instrument pack (EQ137) from 108 minutes to 99 minutes to match this change in clinical labor time.
(26) X-Rays at Surgery Add-On (CPT Code 74301)
The RUC recommended that CPT code 74301 (Cholangiography and/or pancreatography; additional set intraoperative, radiological supervision and interpretation (List separately in addition to code for primary procedure)) be deleted for October 2020. The specialty societies that typically bill for this service submitted a code change application to delete CPT code 74301 at the February 2020 CPT meeting. However, the specialty societies withdrew the deletion request after receiving feedback from the dominant provider of CPT code 74301 (general surgery), indicating the code is still necessary and should not be deleted. The RUC recommended to maintain the work RVU of 0.21 for CPT code 74301. The specialty societies did not resurvey CPT code 74301 due to its low utilization (2019 Medicare utilization = 63) and the difficulty of obtaining 30 survey responses from providers with experience in the past 12 months. Since there was no survey done, there is no new information and the RUC recommended to maintain the current value. The work RVU suggested by the RUC is a reaffirmation of the current value.
We are proposing the RUC-recommended work RVU of 0.21 for Start Printed Page 39167 CPT code 74301. This is an add-on code with no direct PE inputs.
(27) Trabecular Bone Score (TBS) (CPT Codes 77X01, 77X02, 77X03, and 77X04)
We are proposing the RUC-recommended work RVUs of 0.20 for CPT codes 77X01 (Trabecular bone score (TBS), structural condition of the bone microarchitecture; using dual X-ray absorptiometry (DXA) or other imaging data on gray-scale variogram, calculation, with interpretation and report on fracture risk) and 77X04 (Trabecular bone score (TBS), structural condition of the bone microarchitecture; using dual X-ray absorptiometry (DXA) or other imaging data on gray-scale variogram, calculation, with interpretation and report on fracture risk interpretation and report on fracture risk only, by other qualified health care professional). CPT codes 77X02 (Trabecular bone score (TBS), structural condition of the bone microarchitecture; technical preparation and transmission of data for analysis to be performed elsewhere) and 77X03 (Trabecular bone score (TBS), structural condition of the bone microarchitecture; technical calculation only) are PE only codes; the RUC did not recommend and we are not proposing a work RVU for these codes.
The RUC PE recommendations for CPT codes 77X01 and 77X03 include a new "TBS iNsight Software" supply input. The submitted invoice for this supply indicates that it is a licensing fee associated with the use of the software, which is not typically considered to be a form of direct PE under our methodology. Historically, we have considered most computer software and associated licensing fees to be indirect costs tied to associated costs for hardware considered to be medical equipment. However, as we noted in section II.B. of this proposed rule (the PE section), stakeholders have routinely expressed concerns with this policy, especially for evolving technologies that rely primarily on software and licensing fees with minimal costs in equipment or hardware. Most of the recommended resource costs for CPT codes 77X01 and 77X03 are for this analysis fee and these costs are not well accommodated by the PE methodology since these sorts of technological applications did not exist when the data that underlie the PE allocation was last collected in 2007 through 2008.
We are therefore proposing to value the PE for CPT codes 77X01 and 77X03 through the use of a crosswalk to a comparable service, CPT code 71101 (Radiologic examination, ribs, unilateral; including posteroanterior chest, minimum of 3 views), which, for CY 2021, had a PE RVU of 0.94. We are proposing that the PE RVU for CPT code 77X03 equals the PE RVU from code 77X01 minus the PE RVU from codes 77X02 and 77X04 so that the three codes sum to the valuation of code 77X01. (CPT code 77X01 is the global code in this family and CPT codes 77X02, 77X03, and 77X04 must sum together to equal the value of 77X01.) CPT code 71101 is another type of bone imaging procedure that we believe reflects codes 77X01 and 77X03 similar direct PE resource costs as CPT codes 77X01 and 77X03. We recognize that the services being performed in this crosswalk code are not the same as the services in CPT codes 77X01 and 77X03, however we believe that the direct resource costs would typically be analogous across these codes. We believe that this is the most accurate way to incorporate the costs of the software employed in CPT codes 77X01 and 77X03 which would not typically be considered direct PE under our current methodology. We are soliciting comments, both on the specific proposal for the Trabecular Bone Score codes as well as our broader discussion of this topic in section II.B. of this proposed rule.
(28) Pathology Clinical Consult (CPT Codes 80XX0, 80XX1, 80XX2, and 80XX3)
The Relativity Assessment Workgroup identified CPT code 80500 (Clinical pathology consultation; limited, without review of patient's history and medical records) via the CMS/Other source codes with the Medicare utilization over 20,000 screen. In October 2019, the RUC referred this issue to the CPT Editorial Panel to define this service more specifically as the current descriptor is vague. In October 2020, the CPT Editorial Panel replaced CPT codes 80500 and 80502 (Clinical pathology consultation; comprehensive, for a complex diagnostic problem, with review of patient's history and medical records) with four new codes, CPT codes 80XX0 (Pathology clinical consultation; for a clinical problem with limited review of patient's history and medical records and straightforward medical decision making. When using time for code selection, 5-20 minutes of total time is spent on the date of the consultation. (For consultations involving the examination and evaluation of the patient, see 99241, 99242, 99243, 99244, 99245, 99251, 99252, 99253, 99254, 99255)), 80XX1 (for a moderately complex clinical problem, with review of patient's history and medical records and moderate level of medical decision making. When using time for code selection, 21-40 minutes of total time is spent on the date of the consultation), 80XX2 (for a highly complex clinical problem, with comprehensive review of patient's history and medical records and high level of medical decision making. When using time for code selection, 41-60 minutes of total time is spent on the date of the consultation), and 80XX3 (prolonged service, each additional 30 minutes (List separately in addition to code for primary procedure) (Use 80XX3 in conjunction with 80XX2) (Do not report 80XX0, 80XX1, 80XX2, 80XX3 in conjunction with 88321, 88323, 88325) (Prolonged pathology clinical consultation service of less than 15 additional minutes is not reported separately) (For consultations involving the examination and evaluation of the patient, see 99241-99255)) to report pathology clinical consultation and creation of guidelines to select and document the appropriate level of service.
The RUC recommended a work RVU of 0.50 for CPT code 80XX0 based on the 25th percentile of the survey. The RUC-recommended 15 minutes of intraservice and total times for CPT code 80XX0 are 2 minutes above the current instraservice and total times for CPT code 80500. This represents a 15 percent increase in the respective times. However, the RUC-recommended work RVU of 0.50 is 35 percent higher than the current work RVU of 0.37 for CPT code 80500. We believe that the increase or decrease in times should be commensurate with the increase or decrease in the work RVU. Therefore, we are proposing a work RVU of 0.43. This represents the ratio of total time between the current total time of CPT code 80500 and the proposed total time of CPT code 80XX0 (0.15) applied to the current value of CPT code 80500 (0.37 × 0.15 = 0.43).
We are proposing the RUC-recommended work RVU of 0.91 without refinements for CPT code 80XX1.
The RUC recommended a work RVU of 1.80 for CPT code 80XX2 based on the 25th percentile of the survey. The current intraservice and total times for CPT code 80502 are 42 minutes. The RUC-recommended times for CPT code 80XX2 are 54 minutes. Similar to the scenario described above for CPT code 80XX0, the intraservice and total times for CPT code 80XX2 increased 28.6 percent while the work RVU increased 35 percent. As stated above, we believe the increase or decrease in time should be commensurate with the increase or Start Printed Page 39168 decrease in the work RVU. Therefore, for CPT code 80XX2 we are proposing a work RVU of 1.71, which is the current total time ratio of CPT code 80502 compared to the RUC-recommended total time for CPT code 80XX2.
We are proposing the RUC-recommended work RVU of 0.80 for CPT code 80XX3 without refinement.
For the direct PE inputs of CPT codes 80XX0, 80XX1, and 80XX2, we are proposing to refine the time associated with the clinical labor activity PA001 (Accession and enter information) from the RUC-recommended time of 4 minutes to 0 minutes as we believe the time is duplicative with clinical labor activity PA008 (File specimen, supplies, and other materials).
The RUC recommended 15, 30, 54, and 30 minutes of equipment time for EP024 (microscope, compound) for CPT codes 80XX0, 80XX1, 80XX2, and 80XX3, respectively. We note that there is no indication from the code descriptors that the pathologist is reviewing physical slides. The code descriptor and description of work indicate that the pathologist is reviewing paper records and/or EHR and therefore we are proposing to remove the equipment time associated with EP024 (microscope, compound) from CPT codes 80XX0, 80XX1, 80XX2, and 80XX3.
Additionally, the proposed Levels of Decision Making for Table for Pathology Clinical Consult codes includes "Assessment requiring an independent historian(s)" as an element of "Amount and/or Complexity of Data to be Reviewed and Analyzed *—Each unique test, order, or document contributes to the combination of 2 or combination of 3 in Category 1 below." Neither the code descriptors nor the descriptions of work indicate that this type of assessment is typical in a pathology clinical consult as was discussed for the office visit Levels of Decision Making table. For these reasons, CMS proposes that this element not be included as an element that CMS would recognize as an element of medical decision making. We note that CMS will monitor the use of these replacement codes per our usual practice to ensure appropriate billing and inform future rulemaking as needed. We are also seeking comment on how these replacement codes would most typically be billed relative to use of existing pathology coding. Such information would also inform future rulemaking as needed.
(29) Revaluing End Stage Renal Disease (ESRD) Monthly Capitation Payment Services (MCP) (CPT Code 90954)
In the CY 2021 PFS final rule (85 FR 84551 through 84554), we revalued most, but not all, of the ESRD MCP services. We finalized an increase in valuations for those ESRD MCP codes with values tied to the values of Outpatient/Office Evaluation and Management (O/O E/M) codes. We did not revalue CPT code 90954 (End-stage renal disease (ESRD) related services monthly, for patients 2-11 years of age to include monitoring for the adequacy of nutrition, assessment of growth and development, and counseling of parents; with 4 or more face-to-face visits by a physician or other qualified health care professional per month) because it was originally valued by a crosswalk.
Stakeholders stated that CPT code 90954 was different from the other ESRD MCP codes. Rather than using an O/O E/M code building block methodology as had been used originally to value the other ESRD MCP codes, CPT code 90954 was valued based upon a crosswalk to CPT code 99293 (Inpatient pediatric critical care provided for children age 29 days through 24 months old, per day). When CPT code 99293 was deleted, the value of CPT code 90954 was crosswalked to a replacement code, CPT code 99471 (Initial inpatient pediatric critical care, per day, for the evaluation and management of a critically ill infant or young child, 29 days through 24 months of age). By crosswalking CPT code 90954 to CPT code 99471, the rank order across the ESRD MCP code family at that time was preserved.
Since we finalized the revalued ESRD MCP values for CY 2021, stakeholders have requested that we revalue CPT code 90954 because by not updating it, we created a rank order anomaly for work RVUs and time within the ESRD MCP code family. A stakeholder suggested that we address the rank order anomaly by revaluing CPT code 90954 based upon a new crosswalk to CPT code 33977 (Removal of a ventricular assist device; extracorporeal, single ventricle). The stakeholder stated that CPT code 33977 more appropriately represented the time and effort of the service provided over one month than the existing crosswalk to CPT code 99471 relative to the revalued services within the MCP code family.
In response to stakeholder requests to update the value of CPT code 90954, we are proposing to increase the value of CPT code 90954, a global code with a current work RVU of 15.98, by crosswalking it to CPT code 33977, a 090-day procedural code with a work RVU of 20.86 to preserve relativity within the ESRD MCP family. We are also seeking comment on our proposal to increase the value of CPT code 90954.
(30) Colon Capsule Endoscopy (CPT Codes 91110, 91111, and 9111X)
In October 2020, the CPT Editorial Panel replaced Category III code 0355T (Gastrointestinal tract imaging, intraluminal (eg, capsule endoscopy), colon, with interpretation and report) with a new Category I code 9111X (Gastrointestinal tract imaging, intraluminal (eg, capsule endoscopy), colon, with interpretation and report) to report gastrointestinal tract imaging. CPT codes 91110 (Gastrointestinal tract imaging, intraluminal (eg, capsule endoscopy), esophagus through ileum, with interpretation and report) and 91111 (Gastrointestinal tract imaging, intraluminal (eg, capsule endoscopy), esophagus with interpretation and report) were added as part of the family and surveyed for the January 2021 RUC meeting.
We are proposing the RUC-recommended work RVU for two of the codes in this family. We are proposing a work RVU of 2.24 for CPT code 91110 and a work RVU of 2.41 for CPT code 9111X as recommended by the RUC in both cases. For CPT code 91111, we disagree with the RUC-recommended work RVU of 1.00 and we are proposing a work RVU of 0.90 based on a crosswalk to CPT code 95923 (Testing of autonomic nervous system function; sudomotor, including 1 or more of the following: Quantitative sudomotor axon reflex test (QSART), silastic sweat imprint, thermoregulatory sweat test, and changes in sympathetic skin potential). CPT code 95923 is an autonomic nervous system testing procedure that shares the identical intraservice work time of 15 minutes with CPT code 91111 and has 5 additional minutes of immediate postservice work time. When we reviewed CPT code 91111, we noted that the surveyed intraservice work time had decreased by 3 minutes, from 18 minutes to 15 minutes, while the RUC recommended maintaining the current work RVU of 1.00. Although we do not imply that the decrease in time as reflected in survey values must equate to a one-to-one or linear decrease in the valuation of work RVUs, we believe that since the two components of work are time and intensity, decreases in time should typically be reflected in decreases to work RVUs. In the case of CPT code 91111, we believe that it would be more accurate to propose a work RVU of 0.90 based on a crosswalk Start Printed Page 39169 to CPT code 95923 to account for these decreases in the surveyed work time.
For the direct PE inputs, we are proposing to refine the clinical labor time for the "Prepare, set-up and start IV, initial positioning and monitoring of patient" (CA016) activity from the RUC-recommended 9 minutes to 6 minutes for CPT code 91111. The recommended materials for this code family state that the 6 minutes for the CA016 activity are used to connect the equipment, fit belt to patient, put data recorder on patient, and sync capsule to each sensor on belt. This description of this clinical labor activity is identical for CPT codes 91110 and 9111X and each code has the same recommended time of 6 minutes. However, the recommended materials for CPT code 91111 state that 6 minutes are used to connect the equipment, fit belt, put data recorder on patient, sync capsule to each sensor and then an additional 3 minutes are used to position the patient (assist patient onto table lying down on right side and then into a sitting position after the capsule is swallowed). We do not agree that it would be typical for CPT code 91111 to require an additional 3 minutes for positioning as compared with the other codes in the family, particularly in light of the clinical similarities between these services. We are refining the clinical labor time to 6 minutes for CPT code 91111 to maintain relativity within the family.
We are also proposing to refine the equipment time for the capsule endoscopy recorder kit (EQ146) from 64 minutes to 61 minutes and the exam table (EF023) from 44 minutes to 41 minutes to match this change in clinical labor time for CPT code 91111.
(31) External Cardiovascular Device Monitoring (CPT Codes 93228 and 93229)
For CPT code 93228 (External mobile cardiovascular telemetry with electrocardiographic recording, concurrent computerized real time data analysis and greater than 24 hours of accessible ECG data storage (retrievable with query) with ECG triggered and patient selected events transmitted to a remote attended surveillance center for up to 30 days; review and interpretation with report by a physician or other qualified health care professional), we disagree with the RUC-recommended work RVU of 0.52, and we are proposing a work RVU of 0.43. The proposed work RVU is based on an intraservice time ratio between the current and RUC-recommended intraservice times for CPT code 93228 ((10 minutes/12 minutes)*0.52), yielding a work RVU of 0.43. This proposed work RVU reflects the decrease in total time and is a direct work RVU crosswalk to CPT code 93290 (Interrogation device evaluation (in person) with analysis, review and report by a physician or other qualified health care professional, includes connection, recording and disconnection per patient encounter; implantable cardiovascular physiologic monitor system, including analysis of 1 or more recorded physiologic cardiovascular data elements from all internal and external sensors). CPT code 93290 has the same pre-, intra-, and postservice times as the survey times for CPT code 93228 and was reviewed in October 2016. While we recognize that the number of ECG tracings and daily reports have increased because of the increase in average wear time from 14 days to 20 days, the specialty societies and the RUC contend that this is offset by technology advancements, integrations with EHRs, and online portals that make it easier to manage and review the data in a chronological and efficient manner. Therefore, we are recommending a work RVU that accounts for decrease in total time to provide this service, given that the increased tracings and daily reports are offset by the efficiencies gained by technological advancements.
The RUC recommended 10 minutes for "Provide education/obtain consent" (CA011) for CPT code 93228, based on a direct crosswalk and duplication of CPT code 93229 (External mobile cardiovascular telemetry with electrocardiographic recording, concurrent computerized real time data analysis and greater than 24 hours of accessible ECG data storage (retrievable with query) with ECG triggered and patient selected events transmitted to a remote attended surveillance center for up to 30 days; review and interpretation with report by a physician or other qualified health care professional). We disagree with the RUC-recommended duplication of clinical labor to provide education that the patient will hear for a second time from the IDTF technician. While we understand that the duplication is by design, we do not agree with a direct crosswalk from CPT code 93229, because the provider of CPT code 93229 will likely have more in-depth education, specific to the patient, including materials and instructions for the patient to review. Therefore, we are proposing the standard 2 minutes for CA011 in the non-facility for CPT code 93228.
The RUC recommended the addition of 24 minutes for quality assurance "overread" done by a second, senior technician, Clinical Activity Code CA021, Line 67 on the RUC-recommended PE Spreadsheet, for CPT code 93229. This is a new clinical activity for CPT code 93228, and we are seeking public comment about the typicality of a second senior technician. We are requesting additional information about the IDTF's current quality assurance measures and parameters within the ECG recording program that should act as some degree of quality assurance. We are also seeking additional information from IDTFs about the current error rate for improperly transmitted tracings to the physician that would indicate that it is typical for a second, senior technician to perform "overread." We are proposing 0 minutes for Clinical Activity Code CA021, Line 67 on the RUC-recommended PE Spreadsheet, unless commenters can provide compelling information that a second, senior technician typically performs quality assurance measures. Otherwise, we agree with the RUC-recommended direct PE inputs and are proposing the refinements as recommended.
In addition to the proposed work RVU and direct PE input refinements, we are requesting additional information about the acquisition costs for equipment item EQ340 Patient Worn Telemetry System. Due to the proprietary nature of this equipment, invoices were unattainable to update this equipment item. Substantial technological improvements have been made to these devices since the last update in 2008, but they are proprietary devices, owned and manufactured for each IDTF. We are seeking public comment on the manufacturing costs and other information to help update the equipment item for CY 2022. Second, we are requesting additional information about the useful lifetime of EQ340. CMS currently assigns 3 years of useful life to EQ340, but the RUC notes that this is the only equipment item and CPT code 93228 is the only CPT code with an equipment item that has more than 500 minutes of equipment time and a useful life of 3 years or less. We are seeking public comment to help update the useful life of EQ340, as it has not been updated since 2008, and the device has experienced significant technological changes.
(32) Electrophysiologic Evaluation (CPT Code 93621)
In October 2019, the RUC identified CPT code 93621 (Comprehensive electrophysiologic evaluation including insertion and repositioning of multiple electrode catheters with induction or attempted induction of arrhythmia; with left atrial pacing and recording from coronary sinus or left atrium (List separately in addition to code for Start Printed Page 39170 primary procedure) as a high-growth service. It is an add-on code that can be used with several different procedures—base codes or other add-on codes, diagnostic as well as therapeutic. CPT code 93621 is furnished in the facility only and thus has no direct PE inputs.
We disagree with the RUC-recommended work RVU of 1.75 based on a crosswalk to CPT code 36483 (Endovenous ablation therapy of incompetent vein, extremity, by transcatheter delivery of a chemical adhesive (eg, cyanoacrylate) remote from the access site, inclusive of all imaging guidance and monitoring, percutaneous; subsequent vein(s) treated in a single extremity, each through separate access sites (List separately in addition to code for primary procedure). We are proposing a work RVU of 1.50 based on a crosswalk to CPT code 16036 (Escharotomy; each additional incision). CPT code 16036 is also an add-on code for a surgical incision that shares both an identical intraservice work time and a total time of 20 minutes with CPT code 93621. While the RUC's recommended crosswalk code also has 20 minutes of intraservice and total time, CPT code 36483 is more intense than CPT code 93621, whereas CPT code 16036 has a similar level of intensity as CPT code 93621.
The RUC did not recommend and we are not proposing any direct PE inputs for CPT code 93621.
(33) Cardiac Ablation Services Bundling (CPT Codes 93653, 93654, 93655, 93656, and 93657)
The technologies and clinical practices associated with Cardiac Ablation Services have changed enough over the past decade (since 2011 when they were first developed) that the specialty societies recommended referring theses codes to CPT Editorial Panel to have the code descriptors for Cardiac Ablation Services updated to create new and more complete descriptors reflecting the fact that many of these services are commonly performed together and should be incorporated and bundled. In October 2020, the CPT Editorial Panel revised the three existing cardiac ablation codes to be bundled with 3D mapping and to include "induction or attempted induction of an arrhythmia with right atrial pacing and recording, and catheter ablation of arrhythmogenic focus," and "left atrial pacing and recording from coronary sinus or left atrium" and "intracardiac echocardiography including imaging supervision and interpretation" into their descriptors.
A survey of the Cardiac Ablation Services was sent out using the newly revised CPT code descriptors asking cardiac electrophysiologists about the revised language in the existing CPT codes. From the survey results, the RUC advisory committee believes that many of the survey respondents may not have realized that the code descriptors had been substantially revised and that they may not have read the updated code descriptors thoroughly enough to understand that services that are separately billed, were now combined into the existing codes (since CPT did not issue new codes for the revised descriptors). The RUC recommended that these services be valued as interim to allow for re-survey and subsequent review at the April 2021 RUC meeting.
CPT code 93653 (Comprehensive electrophysiologic evaluation with insertion and repositioning of multiple electrode catheters, induction or attempted induction of an arrhythmia with right atrial pacing and recording, and catheter ablation of arrhythmogenic focus, including intracardiac electrophysiologic 3-dimensional mapping, right ventricular pacing and recording, left atrial pacing and recording from coronary sinus or left atrium, and His bundle recording, when performed; treatment of supraventricular tachycardia by ablation of fast or slow atrioventricular pathway, accessory atrioventricular connection, cavo-tricuspid isthmus or other single atrial focus or source of atrial re-entry) (previous work RVU of 14.75 with 000-day global) is now bundled with the add-on CPT codes 93613 (Intracardiac electrophysiologic 3-dimensional mapping (List separately in addition to code for primary procedure))(work RVU of 5.23 with 90 minutes of intraservice time) and the add-on CPT code 93621 (Comprehensive electrophysiologic evaluation including insertion and repositioning of multiple electrode catheters with induction or attempted induction of arrhythmia; with left atrial pacing and recording from coronary sinus or left atrium (List separately in addition to code for primary procedure))(work RVU of 2.10 with 30 minutes of intraservice time). The RUC-recommended work RVU for CPT code 93653 is 18.49, with 40 minutes of preservice evaluation, 3 minutes of preservice positioning, 15 minutes of preservice scrub/dress/wait time, 125 minutes of intraservice time and 30 minutes of immediate postservice time.
Since the two add-on codes are combined with the primary CPT code 93653, one would expect the intraservice time to have increased or remained similar to the current 180 minutes. Instead, the RUC-recommended intraservice time has decreased to 125 minutes. Accounting for changes in technologies and clinical practices from over 10 years since this code family's last review, we would expect better efficiencies and reductions in work times, but with the addition of two add-on codes whose work is mostly, if not all, added to the intraservice time, one would not expect a net decrease in minutes. This is not what the collected responses from this survey show and it is a concern. Some of CPT code 93653 add-on service times may have shifted over to the increases in preservice times, but there does appear to be a collective misunderstanding in the survey's work RVUs and physician work time responses.
In light of the RUC's intention to resurvey and re-review CPT code 93653 (and this family of codes) at the April 2021 RUC meeting, and to resolve any flaws from the initial survey, such as survey respondents probably not realizing that a new descriptor describing the inclusion of services is now bundled to the existing CPT code (and not a newly issued CPT code), we are proposing to maintain the current physician times and current work RVU of 14.75, until the AMA RUC returns with a more definitive and accurate valuation.
For CPT code 93654 (Comprehensive electrophysiologic evaluation with insertion and repositioning of multiple electrode catheters, induction or attempted induction of an arrhythmia with right atrial pacing and recording, and catheter ablation of arrhythmogenic focus, including intracardiac electrophysiologic 3-dimensional mapping, right ventricular pacing and recording, left atrial pacing and recording from coronary sinus or left atrium, and His bundle recording, when performed; with treatment of ventricular tachycardia or focus of ventricular ectopy including left ventricular pacing and recording, when performed) (work RVU of 19.75), the RUC recommends 40 minutes of preservice evaluation, 3 minutes of preservice positioning, 20 minutes of preservice scrub/dress/wait time, 240 minutes of intraservice time and 33 minutes of immediate postservice time for a total of 336 minutes, an increase to the code's current 309 total minutes. Unlike CPT codes 93653 and 93656, CPT code 93654 already accounts for the work RVUs and physician times for 3-dimensional mapping of add-on CPT code 93613. The RUC recommended maintaining the current work RVU Start Printed Page 39171 value of 19.75. We are proposing the RUC-recommended updates to the physician times (net increase in total minutes) and to maintain the same work RVUs for CPT code 93654 for CY 2022.
CPT code 93655 (Intracardiac catheter ablation of a discrete mechanism of arrhythmia which is distinct from the primary ablated mechanism, including repeat diagnostic maneuvers, to treat a spontaneous or induced arrhythmia (List separately in addition to code for primary procedure)) has a current work RVU of 7.50 with a physician intraservice time of 90 minutes. The RUC recommended a revised intraservice time of 60 minutes and 6.50 work RVUs. The primary change to CPT code 93655 is the reduction of the intraservice time of about 67 percent, which we use as a guide to determine a work RVU. We compare add-on CPT code 22854 (Insertion of intervertebral biomechanical device(s) (e.g., synthetic cage, mesh) with integral anterior instrumentation for device anchoring (e.g., screws, flanges), when performed, to vertebral corpectomy(ies) (vertebral body resection, partial or complete) defect, in conjunction with interbody arthrodesis, each contiguous defect (List separately in addition to code for primary procedure)) also with 60 minutes of intraservice and total time and a work RVU of 5.50 to CPT code 93655 and we believe that this is a more accurate valuation than the RUC's work RVU crosswalk to CPT code 34709 (Placement of extension prosthesis(es) distal to the common iliac artery(ies) or proximal to the renal artery(ies) for endovascular repair of infrarenal abdominal aortic or iliac aneurysm, false aneurysm, dissection, penetrating ulcer, including pre-procedure sizing and device selection, all nonselective catheterization(s), all associated radiological supervision and interpretation, and treatment zone angioplasty/stenting, when performed, per vessel treated (List separately in addition to code for primary procedure)) with a work RVU of 6.50 and an intraservice and total time of 60 minutes because the proportional reduction in physician time should also reflect a similar proportional reduction in work RVUs. We are proposing the RUC-recommended 60 minutes of intraservice and total time, but instead propose a work RVU of 5.50 for CPT code 93655.
CPT code 93656 (Comprehensive electrophysiologic evaluation including transseptal catheterizations, insertion and repositioning of multiple electrode catheters with intracardiac catheter ablation of atrial fibrillation by pulmonary vein isolation, including intracardiac electrophysiologic 3-dimensional mapping, intracardiac echocardiography including imaging supervision and interpretation, induction or attempted induction of an arrhythmia including left or right atrial pacing/recording, right ventricular pacing/recording, and His bundle recording, when performed) is now bundled with the add-on CPT codes 93613 (Intracardiac electrophysiologic 3-dimensional mapping (List separately in addition to code for primary procedure)) (work RVU of 5.23 with 90 minutes of intraservice time) and the add-on CPT code 93662 (Intracardiac echocardiography during therapeutic/diagnostic intervention, including imaging supervision and interpretation (List separately in addition to code for primary procedure) (work RVU currently carrier-priced with 25 minutes of intraservice time) which previously were separately reported add-on services, similar to above CPT code 93653 and its add-on codes.
The RUC-recommended work RVU for CPT code 93656 is 20.00, with 40 minutes of preservice evaluation, 3 minutes of preservice positioning, 20 minutes of preservice scrub/dress/wait time, 210 minutes of intraservice time and 33 minutes of immediate postservice time, for a total of 306 minutes. The current physician times for CPT code 93656 are 23 minutes of preservice evaluation, 1 minutes of preservice positioning, 5 minutes of preservice scrub/dress/wait time, 240 minutes of intraservice time, and 40 minutes of immediate postservice time, for a total of 309 minutes, which is a net difference of 3 minutes less in the total proposed minutes, and the RUC is recommending a work RVU of 20.00, which is 0.23 more work RVUs than the current work RVU of 19.77.
In light of the RUC's intention to resurvey and review CPT code 93653 (and this family of codes) with its new bundling at their April 2021 RUC meeting to resolve any flaws from the initial survey, where many of the survey respondents may not have realized that the code descriptors had been substantially revised and that they may not have read the updated code descriptors thoroughly enough to respond correctly, we believe CPT code 93656 is in the same situation with its new bundling thus, we are proposing the RUC-recommended updates to the physician times (a net decrease of 3 minutes in total time) and to maintain the current work RVU of 19.77.
From the survey of CPT code 93657 (Additional linear or focal intracardiac catheter ablation of the left or right atrium for treatment of atrial fibrillation remaining after completion of pulmonary vein isolation (List separately in addition to code for primary procedure)), a value of 8.00 work RVUs was obtained at the 25th percentile for this add-on code. The RUC recommended a work RVU of 6.50, for the 60 minutes of intraservice and total physician time. The current work RVU is 7.50, for 90 minutes of intraservice and total physician time.
We compare add-on CPT code 22854 (Insertion of intervertebral biomechanical device(s) (e.g., synthetic cage, mesh) with integral anterior instrumentation for device anchoring (e.g., screws, flanges), when performed, to vertebral corpectomy(ies) (vertebral body resection, partial or complete) defect, in conjunction with interbody arthrodesis, each contiguous defect (List separately in addition to code for primary procedure)) with 60 minutes of intraservice and total time and 5.50 work RVUs to CPT code 93657 and we believe that this is a more accurate valuation, since the primary change to CPT code 93657 is the reduction of the intraservice time of about 67 percent, which we use as a guide to determining a work RVU. The RUC-recommended work RVU is crosswalked from CPT code 34709 (Placement of extension prosthesis(es) distal to the common iliac artery(ies) or proximal to the renal artery(ies) for endovascular repair of infrarenal abdominal aortic or iliac aneurysm, false aneurysm, dissection, penetrating ulcer, including pre-procedure sizing and device selection, all nonselective catheterization(s), all associated radiological supervision and interpretation, and treatment zone angioplasty/stenting, when performed, per vessel treated (List separately in addition to code for primary procedure)) with a work RVU of 6.50 and an intraservice and total time of 60 minutes, does not reflect the proportional reductions to the intraservice time and work. For CPT code 93657, we are proposing the RUC-recommended 60 minutes of intraservice and total time, and a work RVU of 5.50, crosswalked from CPT code 22854. There are no direct PE inputs for these facility-only CPT codes.
(34) 3D Imaging of Cardiac Structures (CPT Codes 933X0)
In May 2020, the CPT Editorial Panel created one new add-on code to describe the 3D echocardiographic imaging and postprocessing during transesophageal or transthoracic echocardiography for congenital cardiac anomalies for the assessment of cardiac Start Printed Page 39172 structure(s). The 3D imaging could be performed as a follow-up to a 2D transthoracic echocardiogram.
We are proposing the RUC-recommended work RVU of 0.50 for CPT code 93XX0 (3D echocardiographic imaging and postprocessing during transesophageal echocardiography, or during transthoracic echocardiography for congenital cardiac anomalies, for the assessment of cardiac structure(s) (eg, cardiac chambers and valves, left atrial appendage, interatrial septum, interventricular septum) and function, when performed (List separately in addition to code for echocardiographic imaging).
While we are proposing no refinements to the direct PE inputs, we are requesting additional information about the 3D echocardiography probe equipment item. The RUC recommended that a 3D probe was required in addition to the base echocardiography machine. We received an invoice for $31,754.30 for this equipment item. It was unclear if the invoice reflected both the 3D probe and the base echocardiography machine or only the probe itself. We are seeking additional information to know if this equipment item reflected both the 3D probe and the base echocardiography machine or only the probe.
(35) Cardiac Catheterization for Congenital Defects (CPT Codes 93X1X, 93X2X, 93X3X, 93X4X, 93X5X, and 93X6X)
In May 2020, the CPT Editorial Panel replaced a family of four cardiac catheterization codes with five new codes (CPT codes 93X1X-93X5X) to describe cardiac catheterization for congenital cardiac defect(s). The CPT Editorial Panel also replaced two cardiac output measurement codes with one new add-on code (CPT code 93X6X) to report cardiac output measurement(s), performed during cardiac catheterization for congenital cardiac defects.
We are proposing the RUC-recommended work RVU for two of the codes in this family. We are proposing a work RVU of 3.99 for CPT code 93X1X (Right heart catheterization for congenital heart defect(s) including imaging guidance by the proceduralist to advance the catheter to the target zone; normal native connections) and a work RVU of 6.10 for CPT code 93X2X (Right heart catheterization for congenital heart defect(s) including imaging guidance by the proceduralist to advance the catheter to the target zone; abnormal native connections) as recommended by the RUC in both cases.
For CPT code 93X3X (Left heart catheterization for congenital heart defect(s) including imaging guidance by the proceduralist to advance the catheter to the target zone, normal or abnormal native connections), we disagree with the RUC-recommended work RVU of 6.00 and we are instead proposing a work RVU of 5.50 based on a crosswalk to CPT code 32607 (Thoracoscopy; with diagnostic biopsy(ies) of lung infiltrate(s) (eg, wedge, incisional), unilateral). CPT code 32607 is a thorascopy procedure with three fewer minutes of intraservice work time (45 minutes) than CPT code 93X3X but a higher total work time of 178 minutes. CPT code 93X3X has similar surveyed work time to CPT code 93X1X but the RUC recommended a work RVU of 3.99 for the first code in the family as compared to 6.00 for CPT code 93X3X. While we agree that CPT code 93X3X is a more intensive procedure, we do not agree that it should be valued more than two full RVUs higher as compared to the first code in the family. We believe that it would be more accurate to propose a work RVU of 5.50 based on the aforementioned crosswalk to CPT code 32607. We note that the intensity of CPT code 93X3X remains higher than the first two codes in the family at the proposed work RVU of 5.50.
For CPT code 93X4X (Right and left heart catheterization for congenital heart defect(s) including imaging guidance by the proceduralist to advance the catheter to the target zone(s); normal native connections), we disagree with the RUC-recommended work RVU of 7.91 and we are instead proposing a work RVU of 6.84 based on a crosswalk to CPT code 32608 (Thoracoscopy; with diagnostic biopsy(ies) of lung nodule(s) or mass(es) (eg, wedge, incisional), unilateral). CPT code 32608 is another thorascopy procedure from the same family as CPT code 32607, with the same 60 minutes of intraservice work time as CPT code 93X4X and a higher total work time of 195 minutes. In the same fashion as the previous code, CPT code 93X4X has similar surveyed work time to CPT code 93X2X but the RUC recommended a work RVU of 6.10 for the second code in the family as compared to 7.91 for CPT code 93X4X. While we agree that CPT code 93X4X is a more intensive procedure, we do not agree that it should be valued almost two full RVUs higher as compared to the second code in the family. We believe that it would be more accurate to propose a work RVU of 6.84 based on the aforementioned crosswalk to CPT code 32608. We note that the intensity of CPT code 93X4X remains the highest among the first four codes in the family at the proposed work RVU of 6.84. We believe that our proposed RVUs for CPT codes 93X3X and 93X4X better preserve relativity both within the family and also with other services on the PFS.
For CPT code 93X5X (Right and left heart catheterization for congenital heart defect(s) including imaging guidance by the proceduralist to advance the catheter to the target zone(s); abnormal native connections), we disagree with the RUC-recommended work RVU of 9.99 and we are instead proposing a work RVU of 8.88 based on the median work RVU from the survey. The RUC's recommendation of a work RVU of 9.99, based on maintaining the prior work RVU of deleted CPT code 93532 (Combined right heart catheterization and transseptal left heart catheterization through intact septum with or without retrograde left heart catheterization, for congenital cardiac anomalies), was nearly equal to the 75th percentile work RVU from the survey at 10.00. Since the RUC recommended the survey median work RVU for the other four non-measurement codes in the family, we do not understand the recommendation of a value for CPT code 93X5X that sits within 0.01 RVUs of the survey 75th percentile. The survey for CPT code 93X5X also revealed that it typically requires far less work time to perform as compared with predecessor code 93532 (83 minutes of intraservice work time as compared to 175 minutes for the predecessor code). Although we agree that CPT code 93X5X is a more intensive procedure than its predecessor code, we do not believe that the work RVU should remain unchanged given the greatly reduced work time in the new procedure. Since the two components of work are time and intensity, we believe that decreases in time should typically be reflected in decreases to work RVUs. We are therefore proposing a work RVU of 8.88 for CPT code 93X5X based on the survey median outcome. We believe that our proposed RVU more accurately accounts for these changes in surveyed work time and better preserves relativity with the rest of the family.
For CPT code 93X6X (Cardiac output measurement(s), thermodilution or other indicator dilution method, performed during cardiac catheterization for the evaluation of congenital heart defects), we disagree with the RUC-recommended work RVU of 1.75 and we are instead proposing a work RVU of 1.44 based on a crosswalk Start Printed Page 39173 to CPT code 37253 (Intravascular ultrasound (noncoronary vessel) during diagnostic evaluation and/or therapeutic intervention, including radiological supervision and interpretation; each additional noncoronary vessel). CPT code 37253 is an intravascular ultrasound procedure that shares the same intraservice work time of 20 minutes as CPT code 93X6X and has 1 additional minute of immediate postservice time. We note that the intensity of CPT code 93X6X as recommended by the RUC at a work RVU of 1.75 would be the second-highest in the family, higher than CPT code 93X5X for example. We do not agree that this cardiac output measurement code would typically be more intensive to perform than the two types of heart catheterization taking place in CPT code 93X5X.
We also note that the recommended work RVU for CPT code 93X6X was higher than the sum of its two predecessor codes. Former CPT codes 93561 (Indicator dilution studies such as dye or thermodilution, including arterial and/or venous catheterization; with cardiac output measurement) and 93562 (Indicator dilution studies such as dye or thermodilution, including arterial and/or venous catheterization; subsequent measurement of cardiac output) had CY 2021 work RVUs of 0.95 and 0.77 respectively. These two codes sum together to a work RVU of 1.72 which would be lower than the RUC's recommendation of 1.75 for CPT code 93X6X. The RUC's recommendation suggests that there would be no efficiencies gained or savings created in the process of creating CPT code 93X6X; we believe that the survey for the new code indicates otherwise, as the predecessor codes had work times of 15 minutes and 12 minutes respectively (27 minutes total) as compared to 20 minutes of surveyed work time for the new code. This lower work time suggests that the creation of CPT code 93X6X has led to greater efficiencies in the service which, under the resource-based nature of the RVU system, lends further support for a reduction in the work RVU as compared to a sum of the predecessor codes. We therefore believe that it would be more accurate to propose a work RVU of 1.44 based on the aforementioned crosswalk to CPT code 37253.
The RUC did not recommend any direct PE inputs for these six codes and we are not proposing any direct PE inputs.
(36) Outpatient Pulmonary Rehabilitation Services (CPT Codes 946X1 and 946X2)
CPT code 946X1 (Physician or other qualified health care professional services for outpatient pulmonary rehabilitation; without continuous oximetry monitoring (per session)) and CPT code 946X2 (Physician or other qualified health care professional services for outpatient pulmonary rehabilitation; with continuous oximetry monitoring (per session) (Do not report 946X1, 946X2 in conjunction with 94760, 94761)) are two new codes created by the CPT Editorial Panel to take the place of the HCPCS G-code G0424 (Pulmonary rehabilitation, including exercise (includes monitoring), one hour, per session, up to two sessions per day) which was created in 2010. The RUC recommended work RVUs for CPT codes 946X1 and 946X2 of 0.55 and 0.69 respectively. We disagree with the RUC-recommended work RVUs for both CPT code 946X1 and 946X2. Although the pulmonary rehab service as described by these new codes have not changed, the RUC recommendation included an increase in intraservice and total time for the services. As the survey time increased for the pulmonary rehabilitation codes, an increase in work value may be appropriate.
Based on a comparison of intraservice time for the current code relative the recommended values, we are proposing a work RVU of 0.36 for CPT code 946X1 and a work RVU of 0.56 for CPT code 946X2, which is an increase to the work RVU from the HCPCS G-code G0424 that these two codes are replacing and reflects a commensurate increase in work relative to the increase in intraservice time.
For the direct PE inputs, we are proposing to refine the clinical labor time for the "Provide education/obtain consent" (CA011) activity from the RUC-recommended 15 minutes to 2 minutes for both CPT codes 946X1 and 946X2. The recommended materials for this code family state that the 15 minutes for the CA011 activity are used for education which is an integral component of pulmonary rehabilitation programs. There is education provided at each separate session following a curriculum outlined in the guideline and covers both educational topics concerning self-management and educational topics concerning advance care planning which is different at every session.
We do not agree that it would be typical for CPT codes 946X1 and 946X2 to require an additional 13 minutes for education and consent given the patient is seen two to three times a week for pulmonary rehabilitation and the education can be covered during those sessions. We are refining the clinical labor time to 2 minutes for both CPT codes 946X1 and 946X2 to maintain relativity, particularly in light of the clinical similarities between these services. The education would be done during the "Perform procedure/service—NOT directly related to physician work time" (CA021), as stated above, as the patient is seen two to three times a week for pulmonary rehabilitation.
We are also proposing to refine the equipment time and lower the pulse oximeter w-printer (EQ211) and exercise equipment (treadmill, bike, stepper, UBE, pulleys, balance board) (EQ118) equipment times from 93 minutes to 80 minutes to match this change in clinical labor time for CPT codes 946X1 and 946X2.
Additionally, we are proposing to revise the utilization that we would use to set rates for CPT code 946X2 to reflect our understanding that pulmonary rehabilitation is always done with pulse oximetry. Thus, we are proposing to update our analytic crosswalk to reflect our belief that 100 percent of the utilization for the pulmonary rehabilitation services currently billed using HCPCS code G0424 will now be billed using CPT code 946X2. We believe that it is unlikely that these services would typically be billed using CPT code 946X1 since it is our understanding that pulmonary rehabilitation is typically provided with pulse oximetry, and therefore, we expect little to no utilization for CPT code 946X1. We are seeking comment from stakeholders on our understanding and proposal to revise the utilization as stated.
(37) Remote Therapeutic Monitoring (CPT Codes 989X1, 989X2, 989X3, 989X4, and 989X5)
Remote Therapeutic Monitoring (RTM) is a family of five codes created by the CPT Editorial Panel in October 2020 and valued by the RUC at its January 2021 meeting. The RTM family includes three PE-only codes and two codes that include professional work.
In recent years, we have finalized seven codes in the Remote Physiological Monitoring (RPM) family that include services similar to the new RTM codes. (See the CY 2021 PFS final rule at 85 FR 84542 through 84546 for more information.) Based upon our analysis, the services and code structure of RTM resemble those of RPM. For example, the RTM codes reflect similar staff and Start Printed Page 39174 physician work, although the specific equipment used is different.
While there are notable similarities between the two sets of code descriptors, there are two primary differences. One difference is that according to RUC documents, primary billers of RTM codes are projected to be nurses and physical therapists. Stakeholders have suggested that the new RTM coding was created to allow practitioners who cannot bill RPM codes to furnish and bill for services that look similar to those of RPM. RPM services are considered to be E/M services and physical therapists, for example, are practitioners who cannot bill E/M services. The RTM codes, instead, are general medicine codes.
In our review of the new codes, we identified an issue that disallows physical therapists and other practitioners, who are not physicians or NPPs, to bill the RTM codes. By modeling the new RTM codes on the RPM codes, "incident to" services became part of the three direct practice expense-only (PE-only) codes (that is, CPT codes 989X1, 989X2, and 989X3) as well as the two professional work codes (that is, CPT codes 989X4 and 989X5). As a result, the RTM codes as constructed currently cannot be billed by, for example, physical therapists. We describe "incident to" services in the CMS Medicare Benefit Policy Manual, Chapter 15, beginning at section 60 and note that only physicians and certain other practitioners are authorized to furnish and bill "incident to" services. Incident to services are:
- An integral, although incidental, part of the physician's professional service (see § 60.1);
- Commonly rendered without charge or included in the physician's bill (see § 60.1A);
- Of a type that are commonly furnished in physician's offices or clinics (see § 60.1A); and
- Furnished by the physician or by auxiliary personnel under the physician's direct supervision (see § 60.1B).
Additionally, we designated the treatment management RPM codes (that is, CPT codes 99457 and 99458) as care management services (84 FR 62697 through 62698), which allow general supervision rather than direct supervision for incident to services. The treatment management RTM codes (CPT codes 989X4 and 989X5), because they are not E/M codes, cannot be designated as care management services. As a result, we are seeking comment on how we might remedy the issues related to the RTM code construction in order to permit practitioners who are not physicians or NPPs to bill the RTM codes.
The second primary difference between the RTM and RPM codes is the nature of the data to be collected and how it is collected. According to the code descriptors, RTM codes monitor health conditions, including musculoskeletal system status, respiratory system status, therapy (medication) adherence, and therapy (medication) response, and as such, allow non-physiologic data to be collected. Reportedly, data also can be self-reported as well as digitally uploaded. RPM requires that data be physiologic and be digitally uploaded. We note that, for both sets of codes, the device used must meet the FDA definition of a medical device as described in section 201(h) of the Federal Food, Drug and Cosmetic Act (FFDCA). We are seeking comment on the typical type of device(s) and associated costs of the device(s) that might be used to collect the various kinds of data included in the code descriptors (for example, respiratory system status, musculoskeletal status, medication adherence, pain) for the RTM services.
For CY 2022, we are proposing the RUC-recommended work RVU of 0.62 for CPT code 989X4 (Remote therapeutic monitoring treatment management services, physician/other qualified health care professional time in a calendar month requiring at least one interactive communication with the patient/caregiver during the calendar month; first 20 minutes) and the RUC-recommended work RVU of 0.61 for its add-on code, CPT code 989X5 (Remote therapeutic monitoring treatment management services, physician/other qualified health care professional time in a calendar month requiring at least one interactive communication with the patient/caregiver during the calendar month; each additional 20 minutes (List separately in addition to code for primary procedure)) as a means of maintaining parity with the two RPM treatment management codes (CPT codes 99457 and 99458) upon which the two RTM codes are based. We also are proposing the RUC-recommended direct PE inputs for the two treatment management codes, CPT codes 989X4 and 989X5, without refinement.
We are proposing to refine the direct PE inputs for the three PE-only codes: CPT code 989X1 (Remote therapeutic monitoring (e.g., respiratory system status, musculoskeletal system status, therapy adherence, therapy response); initial set-up and patient education on use of equipment), CPT code 989X2 (Remote therapeutic monitoring (e.g., respiratory system status, musculoskeletal system status, therapy adherence, therapy response); device(s) supply with scheduled (e.g., daily) recording(s) and/or programmed alert(s) transmission to monitor respiratory system, each 30 days), and CPT code 989X3 (Remote therapeutic monitoring (e.g., respiratory system status, musculoskeletal system status, therapy adherence, therapy response); device(s) supply with scheduled (e.g., daily) recording(s) and/or programmed alert(s) transmission to monitor musculoskeletal system, each 30 days). We are proposing to value the PE for CPT code 989X1 by crosswalking to the PE RVU for RPM code 99453 upon which the new RTM code was based. We also are proposing to value the PE for CPT codes 989X2 and 989X3 by crosswalking to the PE RVU for comparable RPM code 99454, a code that includes payment for the medical device used to collect and transmit data. We note that the only input to CPT code 989X2 is a monthly fee of $25, which would not be paid as a direct cost under the PFS. Historically, we have considered most computer software and associated licensing fees to be indirect costs. However, as we noted in section II.B. of this proposed rule (the PE section), stakeholders have routinely expressed concerns with this policy, especially for evolving technologies that rely primarily on software and licensing fees with minimal costs in equipment or hardware.
(38) Principal Care Management and Chronic Care Management (CPT Codes 99490, 99439, 99491, 99X21, 99487, 99489, 99X22, 99X23, 99X24, and 99X25)
In recent years, we have engaged in efforts to update and improve the relative value of care management and coordination services within the PFS by identifying gaps in payment and coding. One of those PFS services is Chronic Care Management (CCM). CCM services, which include management and support services provided by clinical staff under the supervision of a physician or NPP or services provided personally by a physician or NPP, have received ongoing refinements related to payment and coding since CY 2013.
Beginning in the CY 2014 PFS final rule (78 FR 74414 through 74427), we noted that physicians and NPPs who furnish care to patients with multiple chronic conditions require greater resources than are required to support patient care in a typical E/M service. In response, we finalized a separately payable HCPCS code, GXXX1 (Chronic Care Management (CCM) services Start Printed Page 39175 furnished to patients with multiple (2 or more) chronic condition expected to last at least 12 months, or until the death of the patient; 20 minutes or more per in 30 days of chronic care management services provided by clinical staff and directed by a physician or other qualified health care practitioner). For CY 2015 (79 FR 67715 through 67730), we refined aspects of the existing CCM policies and adopted separate payment for CCM services under CPT code 99490 (Chronic care management services (CCM), at least 20 minutes of clinical staff time directed by a physician or other qualified health professional, per calendar month, with the following required elements: Multiple (two or more) chronic conditions expected to last at least 12 months, or until the death of the patient; Chronic conditions place the patient at significant risk of death, acute exacerbation/decompensation, or functional decline; Comprehensive care plan established, implemented, revised, or monitored). For CY 2017 (81 FR 80244), we adopted CPT codes 99487 (Complex chronic care management (CCCM) services with the following required elements: Multiple (two or more) chronic conditions expected to last at least 12 months, or until the death of the patient, chronic conditions place the patient at significant risk of death, acute exacerbation/decompensation, or functional decline, comprehensive care plan established, implemented, revised, or monitored, moderate or high complexity medical decision making; first 60 minutes of clinical staff time directed by a physician or other qualified health care professional, per calendar month) and 99489 (Complex chronic care management (CCCM) services with the following required elements: Multiple (two or more) chronic conditions expected to last at least 12 months, or until the death of the patient, chronic conditions place the patient at significant risk of death, acute exacerbation/decompensation, or functional decline, comprehensive care plan established, implemented, revised, or monitored, moderate or high complexity medical decision making; each additional 30 minutes of clinical staff time directed by a physician or other qualified health care professional, per calendar month (List separately in addition to code for primary procedure)). Then, in the CY 2019 PFS final rule (83 FR 59577), we adopted a new CPT code, 99491 (Chronic care management services, provided personally by a physician or other qualified health care professional, at least 30 minutes of physician or other qualified health care professional time, per calendar month, with the following required elements: Multiple (two or more) chronic conditions expected to last at least 12 months, or until the death of the patient; chronic conditions place the patient at significant risk of death, acute exacerbation/decompensation, or functional decline; comprehensive care plan established, implemented, revised, or monitored), to describe at least 30 minutes of CCM services performed personally by a physician or NPP. In the CY 2020 PFS final rule (84 FR 62690), we established payment for an add-on code to CPT code 99490 by creating HCPCS code G2058 (Chronic care management services, each additional 20 minutes of clinical staff time directed by a physician or other qualified healthcare professional, per calendar month). We also created two new HCPCS G codes, G2064 and G2065 (84 FR 62692 through 62694), representing comprehensive services for a single high-risk disease (that is, principal care management). In the CY 2021 PFS final rule (85 FR 84639), we finalized a RUC-recommended replacement code for HCPCS code G2058, CPT code 99439, which was given the same valuation and the identical descriptor as G2058.
For CY 2022, the RUC resurveyed the CCM code family, including Complex Chronic Care Management (CCCM) and Principal Care Management (PCM), and added five new CPT codes: 99X21 (Chronic care management services each additional 30 minutes by a physician or other qualified health care professional, per calendar month (List separately in addition to code for primary procedure)), 99X22 (Principal care management services for a single high-risk disease first 30 minutes provided personally by a physician or other qualified health care professional, per calendar month), 99X23 (Principal care management services for a single high-risk disease each additional 30 minutes provided personally by a physician or other qualified health care professional, per calendar month (List separately in addition to code for primary procedure), 99X24 (Principal care management services, for a single high-risk disease first 30 minutes of clinical staff time directed by physician or other qualified health care professional, per calendar month), and 99X25 (Principal care management services, for a single high-risk disease each additional 30 minutes of clinical staff time directed by a physician or other qualified health care professional, per calendar month (List separately in addition to code for primary procedure)). The CCM/CCCM/PCM code family now includes five sets of codes, each set with a base code and an add-on code. The sets vary by the degree of complexity of care (that is, CCM, CCCM, or PCM), who furnishes the care (that is, clinical staff or the physician or NPP), and the time allocated for the services. The RUC-recommended values for work RVUs and direct PE inputs for CY 2022 derive from the recent RUC specialty society survey (see Table 12).
We reviewed the RUC-recommended values for the 10 codes in the CCM family and are proposing to accept the recommended work values for the codes. We are proposing the RUC-recommended direct PE inputs without refinements. We believe that proposing to accept these updated values is consistent with our goals of ensuring continued and consistent access to these crucial care management services and acknowledges our longstanding concern about undervaluation of care management under the PFS. We are seeking comment, however, on whether keeping professional PCM and CCM at the same value creates an incentive to bill CCM instead of billing PCM when appropriate.
In addition to the proposals on the values for CCM codes, we are interested in understanding more about the standard practice used by practitioners to obtain beneficiary consent for these services. We have received questions from stakeholders regarding the consent requirements for CCM services. We believe that these questions have arisen because of the many flexibilities allowed in response to the PHE for COVID-19. In particular, during the PHE for COVID-19, we allowed stakeholders to obtain beneficiary consent for certain services under general supervision (85 FR 19230, April 6, 2020). Before the PHE for COVID-19, we required that beneficiary consent be obtained either by or under the direct supervision of the primary care practitioner. This requirement is consistent with the conditions of payment for this service under the PFS. As we consider what policies implemented during the PHE for COVID-19 should remain in effect beyond the PHE, we are interested in understanding how billing practitioners furnishing CCM at different service sites (for example, physician office settings, RHCs, FQHCs) have been obtaining beneficiary consent over the past year and how different levels of supervision impact this activity. We welcome public comment on the issue, specifically on what levels of supervision are necessary to obtain beneficiary consent when furnishing CCM services and will Start Printed Page 39176 consider such comments in future rulemaking.
We also are proposing to adopt CPT codes 99X22 (PCM First 30 minutes provided personally by a physician or other qualified health care professional, per calendar month) and 99X24 (PCM First 30 minutes of clinical staff time directed by physician or other qualified health care professional, per calendar month) to replace HCPCS codes G2064 and G2065 in the calculation of the rate for HCPCS code G0511 for General Care Management services billed by RHCs and FQHCs. The payment rate for HCPCS code G0511 is calculated based on the average of the national non-facility PFS payment rate for care management and general behavioral health integration codes (CPT codes 99484, 99487, 99490, and 99491) as well as HCPCS codes G2064 and G2065 which describe PCM services billed under the PFS. The payment rate for HCPCS code G0511 is updated annually based on the PFS amounts for these codes.

(39) Moderate Sedation (HCPCS Code G0500)
Following the publication of the CY 2021 PFS final rule, a stakeholder contacted us regarding what they believed to be an error in the intraservice work time for HCPCS code G0500 (Moderate sedation services provided by the same physician or other qualified health care professional performing a gastrointestinal endoscopic service that sedation supports, requiring the presence of an independent trained observer to assist in the monitoring of the patient's level of consciousness and physiological status; initial 15 minutes of intra-service time; patient age 5 years or older (additional time may be reported with 99153, as appropriate)). We established HCPCS code G0500 in CY 2017 to more accurately capture the work of administering moderate sedation for gastrointestinal endoscopic procedures for patients 5 years of age or older. We based the physician work and time for HCPCS code G0500 on data from the 100 gastroenterologists who completed the survey of CPT code 99152 (Moderate sedation services provided by the same physician or other qualified health care professional performing the diagnostic or therapeutic service that the sedation supports, requiring the presence of an independent trained observer to assist in the monitoring of the patient's level of consciousness and physiological status; initial 15 minutes of intraservice time, patient age 5 years or older) presented at the October 2015 RUC meeting. The survey data for CPT code 99152 showed a significant bimodal distribution with data from gastroenterologists performing endoscopic procedures demonstrating a markedly different and lesser amount of physician work for moderate sedation compared to other specialties. The stakeholder stated that the finalization of 12 minutes of intraservice work time for HCPCS G0500 appeared to be an error and asked CMS to correct it to reflect the 5 minutes of intraservice work time indicated by survey data when gastroenterologists performed endoscopic procedures.
While we appreciate the feedback from the stakeholder, we disagree that the finalization of 12 minutes of intraservice work time for HCPCS code G0500 (matching CPT code 99152) was an error. The work time for HCPCS code G0500 was proposed and finalized at 12 minutes in CY 2017, with the intention that it would match the work time for CPT code 99152. This was the rationale behind the descriptor for HCPCS code G0500 listing that the code was intended for the initial 15 minutes of intraservice time. Furthermore, several commenters questioned the work time for HCPCS code G0500 in the CY 2017 PFS final rule (81 FR 80341) and we stated in response that we expected that practitioners would report the appropriate CPT or HCPCS code that most accurately described the services performed during a patient encounter, including those services performed concurrently and in support of a procedural service consistent with CPT guidance. We noted that the commenters referred to the time for moderate sedation in the survey data, while the time thresholds for the moderate sedation codes were intended to match the intraservice time of the procedure itself. For a full discussion of this topic, we refer readers to the CY Start Printed Page 39177 2017 PFS final rule (81 FR 80339 through 80349).
Although we are not proposing a change in the work time for HCPCS code G0500, we are soliciting comments on this issue in the interest of gaining additional information about the typical use of this procedure.
(40) Payment for Synthetic Skin Substitutes (HCPCS Codes GXXAB, GXXAC, GXXAD, GXXAE, GXXAF, GXXAG, GXXAH, and GXXAI)
On July 1, 2020, Medicare implemented HCPCS code C1849 (Skin substitute, synthetic, resorbable, per square centimeter) and made it payable under the OPPS. In the CY 2021 OPPS final rule (85 FR 86064 through 86067) Medicare finalized payment for C1849—and the associated synthetic skin substitute products—allowing it to be billed with graft skin substitute procedure CPT codes 15271 through 15278. We note that under the OPPS, payment for C1849 is packaged into the payment for the graft skin substitute procedure, and its costs are reflected in the development of the payment rates for those services. The creation of the C-code and the CY 2021 OPPS rulemaking addressed the need for a mechanism to pay for graft skin substitute application services performed with synthetic graft substitute products in the outpatient hospital setting, which is comparable to how Medicare pays for graft skin substitute application services performed with graft skin substitutes that are regulated by the Food and Drug Administration (FDA) under its regulatory framework at section 361 of the Public Health Service (PHS) Act for human cells, tissues, and cellular and tissue-based products (HCT/Ps). We want to clarify that the availability of a HCPCS code for a particular HCT/P does not mean that the product is appropriately regulated solely under section 361 of the PHS Act and the FDA regulations in 21 CFR part 1271. Manufacturers of HCT/Ps should consult with the FDA Tissue Reference Group (TRG) or obtain a determination through a Request for Designation (RFD) on whether their HCT/Ps are appropriately regulated solely under section 361 of the PHS Act and the regulations in 21 CFR part 1271 (85 FR 86058). We note that in a response to the CY 2021 OPPS proposal, a commenter noted that the use of a C-code meant that synthetic graft skin substitute products would only be payable under the OPPS, and would not be able to be reported for graft skin substitute services using a synthetic product in the physician office setting (85 FR 86066).
Currently, graft skin substitute application services are paid separately from the (HCT/Ps) skin substitutes under the PFS. Specifically, when a physician or NPP furnishes a surgical service to apply a (HCT/Ps) skin substitute in a non-facility setting, they may bill Medicare for the surgical service (as described by CPT codes 15271 through 15278), and separately bill for the (HCT/Ps) skin substitute. For CY 2022, in order to reconcile the gap in payment for synthetic products in the physician office setting, we are proposing to create eight HCPCS codes (parallel to the aforementioned existing surgical codes) that would include the synthetic graft skin substitute product as a supply cost in determining the PFS rate. We believe that it would be appropriate to consider these products as incident to supplies in the office setting, and as such they should be built in as a supply cost in calculating the PFS rate. Therefore, we are proposing to consider these products as incident to supplies in the office setting.
The codes and long descriptors for the proposed synthetic graft skin substitute services are:
- HCPCS Code GXXAB: Application of synthetic skin substitute graft to trunk, arms, legs, total wound surface area up to 100 sq cm, including provision of synthetic skin substitute; first 25 sq cm or less wound surface area.
- HCPCS Code GXXAC: Application of synthetic skin substitute graft to trunk, arms, legs, total wound surface area up to 100 sq cm, including provision of synthetic skin substitute; each additional 25 sq cm wound surface area, or part thereof (List separately in addition to code for primary procedure).
- HCPCS Code GXXAD: Application of synthetic skin substitute graft to trunk, arms, legs, total wound surface area greater than or equal to 100 sq cm, including provision of synthetic skin substitute; first 100 sq cm wound surface area, or 1% of body area of infants and children.
- HCPCS Code GXXAE: Application of synthetic skin substitute graft to trunk, arms, legs, total wound surface area greater than or equal to 100 sq cm, including provision of synthetic skin substitute; each additional 100 sq cm wound surface area, or part thereof, or each additional 1% of body area of infants and children, or part thereof (List separately in addition to code for primary procedure).
- HCPCS Code GXXAF: Application of synthetic skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area up to 100 sq cm, including provision of synthetic skin substitute; first 25 sq cm or less wound surface area.
- HCPCS Code GXXAG: Application of synthetic skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area up to 100 sq cm, including provision of synthetic skin substitute; each additional 25 sq cm wound surface area, or part thereof (List separately in addition to code for primary procedure).
- HCPCS Code GXXAH: Application of synthetic skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area greater than or equal to 100 sq cm, including provision of synthetic skin substitute; first 100 sq cm wound surface area, or 1% of body area of infants and children.
- HCPCS Code GXXAI: Application of synthetic skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area greater than or equal to 100 sq cm, including provision of synthetic skin substitute; each additional 100 sq cm wound surface area, or part thereof, or each additional 1% of body area of infants and children, or part thereof (List separately in addition to code for primary procedure).
We are proposing contractor pricing for these codes for CY 2022; we note that there is limited data available on the cost of synthetic skin substitute products in physician offices, so we are also seeking comment and documentation regarding the appropriate values for these services for consideration of national pricing in future rulemaking.
Though we are proposing contractor pricing in the interim, we also considered an alternative approach that would use crosswalks to value these services in the physician office setting in a way that is commensurate with the rates paid under the OPPS. Though limited data exists on the cost of graft synthetic skin substitute products in physician offices, hospitals began reporting costs associated with synthetic skin substitute products in CY 2020 after C1849 became effective and payable under the OPPS starting in July, 2020. We analyzed CY 2020 OPPS claims data and estimate hospital outpatient department costs for graft synthetic skin substitute products averaged $1,500. We note that under the OPPS, outpatient departments are paid separately for the primary surgical application codes (CPT codes 15271, 15273, 15275, 15277), and the costs associated with the synthetic products Start Printed Page 39178 as well as the add-on services (described by CPT codes 15272, 15274, 15276, 15278) are packaged into the payment for the primary procedure.
Under this alternative, we considered following an approach similar to that under the OPPS where the cost of the supply would be included in the primary codes (described by HCPCS GXXAB, GXXAD, GXXAF, and GXXAH) and not the add-on codes (described by HCPCS GXXAC, GXXAE, GXXAG, and GXXAI), though the add-on would continue to be reported and paid separately. Specifically, we would use direct crosswalks for the work RVUs, MP RVUs, and facility PE RVUs from the current surgical application codes (that is, CPT codes 15271 through 15278) as we believe that these payment components for the synthetic graft skin substitute services, described by the aforementioned HCPCS codes, would be similar.
However, with regards to the non-facility PE RVUs, we recognize that there are significant supply costs associated with synthetic skin substitute products. As described previously, we estimate that hospitals face average costs associated with synthetic skin substitute products of $1,500. We note that the PE methodology, which relies on the allocation of indirect costs based on the magnitude of direct costs, may not be appropriate for these types of services because the specialists that typically furnish these types of services do not typically have significant supply costs within the methodology. As such, we used the hospital reported costs and we looked to other codes where specialists frequently have similarly high supply costs in order to crosswalk the non-facility PE RVUs. We considered services that have a significant proportion of supply costs and are furnished by specialists who typically have higher supply costs as potential crosswalks for the non-facility PE RVUs. For example, we considered a crosswalk to CPT code 21461 (Open treatment of mandibular fracture; without interdental fixation) for HCPCS codes GXXAB and GXXAF, and a crosswalk to CPT code 21462 (Open treatment of mandibular fracture; with interdental fixation) for HCPCS codes GXXAD and GXXAH. As an estimate of non-facility PE, we believe these would be appropriate codes for crosswalking non-facility PE RVUs. As previously discussed, for the purposes of the work RVUs, MP RVUs, and facility PE RVUs, we believe direct crosswalks to the current surgical application codes would be appropriate as those values would generally not be impacted by the addition of a synthetic skin substitute product. We realize this alternative considered would follow a similar coding and payment approach established under the OPPS, and that potential adoption of this alternative would mean that the cost of the products is included in the primary codes and not included in the add-on codes. We welcome feedback on our proposal to treat synthetic skin substitute products as incident to supplies in the physician office, the proposal to have contractor pricing for these codes, and other ways we could obtain detailed and reliable cost information on synthetic skin substitutes that are furnished in the non-facility setting. We are also seeking comment on the alternative approach that we considered (using crosswalks to value these services in the physician office setting). Additionally, we are seeking comment on potential ways to reconcile these coding and payment differences across settings to yield a more consistent and rational payment approach for synthetic and HCT/P graft skin substitutes.
(41) External Extended ECG Monitoring (CPT Codes 93241, 93242, 93243, 93244, 93245, 93246, 93247, and 93248)
In the CY 2021 PFS proposed rule (85 FR 50164), we proposed to adopt the RUC recommendations for CPT codes 93241 (External electrocardiographic recording for more than 48 hours up to 7 days by continuous rhythm recording and storage; includes recording, scanning analysis with report, review and interpretation), 93242 (External electrocardiographic recording for more than 48 hours up to 7 days by continuous rhythm recording and storage; recording (includes connection and initial recording)), 93243 (External electrocardiographic recording for more than 48 hours up to 7 days by continuous rhythm recording and storage; scanning analysis with report), 93244 (External electrocardiographic recording for more than 48 hours up to 7 days by continuous rhythm recording and storage; review and interpretation), 93245 (External electrocardiographic recording for more than 7 days up to 15 days by continuous rhythm recording and storage; includes recording, scanning analysis with report, review and interpretation), 93246 (External electrocardiographic recording for more than 7 days up to 15 days by continuous rhythm recording and storage; recording (includes connection and initial recording)), 93247 (External electrocardiographic recording for more than 7 days up to 15 days by continuous rhythm recording and storage; scanning analysis with report), and 93248 (External electrocardiographic recording for more than 7 days up to 15 days by continuous rhythm recording and storage; review and interpretation).
We noted that the recommendations for this family of codes contain one new supply item, the "extended external ECG patch, medical magnetic tape recorder" (SD339). We did not receive a traditional invoice to establish a price for this supply item. Instead we received pricing information from two sources: A weighted median of claims data with the cost of the other direct PE inputs removed, and a top-down approach calculating the cost of the supply per service based on summing the total costs of the health care provider and dividing by the total number of tests furnished. The former methodology yielded a supply price of approximately $440 while the latter methodology produced an estimated supply price of $416.85. Stakeholders also submitted a series of invoices from the clinical study marketplace with a price of $595, which we rejected as we typically require an invoice representative of commercial market pricing to establish a national price for a new supply or equipment item.
After consideration of the information, we proposed to employ a crosswalk to an existing supply for use as a proxy price until we received pricing information to use for the "extended external ECG patch, medical magnetic tape recorder" item. We proposed to use the "kit, percutaneous neuro test stimulation" (SA022) supply as our proxy item at a price of $413.24. We believed the kit to be the closest match from a pricing perspective to employ as a proxy until we would be able to arrive at an invoice that is representative of commercial market pricing. We welcomed the submission of invoices or other additional information for use in pricing the "extended external ECG patch, medical magnetic tape recorder" supply. In response to our proposal, we received conflicting information from commenters and in the CY 2021 PFS final rule (85 FR 84631), we ultimately finalized contractor pricing for CY 2021 for the four codes that include this supply input (CPT codes 93241, 93243, 93245, and 93247) to allow additional time to receive more pricing information.
We note that stakeholders have continued to engage with CMS and the MACs on payment for this service. We remain concerned that we continue to hear that the supply costs as initially considered in our CY 2021 PFS proposal are much higher than they should be. At the same time we also have heard that Start Printed Page 39179 the resource costs, as reflected in the contractor based payments do not adequately cover the incurred cost for the SD339 supply that is used to furnish these services. In consideration of continued access to these services for Medicare beneficiaries, we are once again seeking public comment and information to support CMS' future rulemaking to establish a uniform national payment that appropriately reflects the PE that are used to furnish these services. As previously stated, invoices or other additional information, including for example, which proxy supply items could be used to establish cost for the SD339 supply, information on use/application and potential alternatives (as appropriate) to the supply items, would be ideal for us to use in establishing fair and stable pricing for these services. We note that in the absence of such additional and actionable information (that is, information that provides further context to information that has already been considered) we are proposing to maintain contractor pricing for these services.
(42) Comment Solicitation for Impact of Infectious Disease on Codes and Ratesetting
During the PHE for COVID-19, several stakeholders have contacted CMS with concerns about the additional costs borne by physician and NPPs due to the pandemic that may impact the professional services furnished to Medicare beneficiaries. For example, we have heard from stakeholders about higher costs due to additional supplies, such as personal protective equipment, and increased time that physicians, NPPs and their clinical staff may spend with patients to mitigate further spread of infection when, for example, stakeholders are working to rule out a COVID-19 infection, or furnishing other services to a patient with a confirmed COVID-19 infection. While costs such as these may diffuse into Medicare payment rates over a period of time, our payment systems, including the PFS, are not generally designed to accommodate more acute increases in resource costs, even if they are widespread. We acknowledge the circumstances stakeholders have identified that may lead to additional costs borne by physicians and NPPs during the PHE, and we have developed and implemented policies, as appropriate and where possible, to maintain beneficiary access to necessary services during the PHE. CMS is continuing to think broadly about the concerns raised, and specifically about the types of resource costs that may not be fully reflected in payment rates for existing services, or costs that could be accounted for by establishing new payment rates for new services. We are interested in feedback from stakeholders about additional strategies to account for PHE-related costs, including feedback on the specific types of services and costs that may benefit from further review, such as infectious disease control measures, research-related activities and services, or PHE-related preventive or therapeutic counseling services. We are interested in detailed feedback from stakeholders to help inform whether we should consider making changes to payments for services or develop separate payments for such services in future rulemaking.
(43) Comment Solicitation on Separate PFS Coding and Payment for Chronic Pain Management
Adequate treatment of pain is a significant public health challenge. Centers for Disease Control and Prevention (CDC) data indicate 50 million adults in the United States have chronic daily pain, with nearly 20 million experiencing high impact pain that interferes with daily life or work. Pain is the most common reason individuals seek medical care, and more than 20 percent of office visits are associated with pain.[4] In the United States, 42.6 percent of adults report having pain on some days in the past 6 months,[5] and chronic pain and high-impact chronic pain are experienced by 20.4 percent and 8 percent of adults, respectively.[6] The high prevalence of pain exacts a substantial economic toll: Medical expenditures and lost productivity related to pain result in a cost to the United States estimated at up to $635 billion.[7]
In 2010, HHS, through the National Institutes of Health (NIH), contracted with the Institute of Medicine to make recommendations "to increase the recognition of pain as a significant public health problem in the United States." In its 2011 report entitled Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research, the Institute of Medicine, through a study mandated by Congress, recommended significant improvements in pain prevention, care, education, and research and development of a population health-level strategy to address pain care.[8] The report described that the unique experience of pain requires a combination of person-centered therapies and coping techniques influenced by genes, cultural attitudes, stress, depression, ability to understand health information, and other behavioral, cultural, and emotional factors. It noted that individualized care can require adequate extra time to counsel patients and caregivers, promote self-management, and consult with other providers, but current reimbursement systems are not designed to efficiently pay for this approach. HHS subsequently convened an expert committee to oversee creation of the National Pain Strategy (NPS), issued in 2016.[9] The NPS addressed six key areas of care: Population research, prevention and care, disparities, service delivery and payment, professional education and training, and public education/communication. In this report, NPS' vision is to "decrease the prevalence of pain across its continuum from acute to high-impact chronic pain and its associated morbidity and disability across the lifespan," and aim "to reduce the burden of pain for individuals, their families, and society as a whole."
This work was followed by HHS's 2019 release of its Pain Management Best Practices Inter-Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations (PMTF Report).[10] The PMTF Report focuses on the development of patient-centered pain treatment plans to establish diagnosis and set measurable outcomes such as improvements in quality of life, function, and activities of daily living. It emphasized multi-modal, multi-disciplinary approaches that include various modalities for acute and chronic pain. The PMTF Report also identified five broad treatment categories: Medications including opioids and non-opioids, restorative therapies, interventional approaches, behavioral approaches, and complementary and integrative health. It stressed the importance of special populations including older adults and persons with Start Printed Page 39180 relapsing conditions, Veterans, and people who receive palliative care. The PMTF Report recognized the importance of proper opioid stewardship for individuals who need opioids to effectively manage their pain. As the Task Force noted, there are ongoing concerns regarding suicide and suicidal ideation due to pain, and a lack of access to pain treatment, including appropriate access to opioid medications. The PMTF Report noted that management of pain conditions often requires multidisciplinary coordination among health care professionals, and that the experience of pain can intensify other health issues such as delayed recovery from surgery, or exacerbate behavioral health conditions. Many health care professionals, including primary care providers, have opted out entirely in treating pain, worsening an existing shortage of pain specialists and making chronic pain care hard to access, including for people who frequently experience disparities in pain care such as rural dwellers, racial/ethnic minorities, and people with disabilities. The COVID-19 Public Health Emergency has also had an impact on the ability of many older adults and people with disabilities' access to care, although telehealth modalities have shown promise in broadening access to services and supports.
At the same time individuals are experiencing difficulties finding pain care, the country is also coping with a worsening opioid and SUD crisis. The current environment involves shifting "waves" of overdose deaths associated with heroin, synthetic opioids, and prescription drugs, and intensifying stimulant and polysubstance use. Preliminary Centers for Disease Control and Prevention data released in April 2021 show a 29 percent rise in overdose deaths from October 2019 through September 2020—the most recent data available—compared with the previous 12-month period.[11] Illicitly manufactured fentanyl and other synthetic opioids were the primary drivers, although many fatal overdoses have also involved stimulant drugs, particularly methamphetamine. In December 2020, the Substance Abuse and Mental Health Services Administration (SAMHSA) released a preliminary report from its Drug Abuse Warning Network, which captures data on emergency department (ED) visits related to recent substance use and misuse such as alcohol use, illicit drug use, suicide attempts, and nonmedical use of pharmaceuticals. Most commonly associated with ED visits in the participating hospitals are illicit substances and central nervous system agents. Among illicit drugs, stimulants (including methamphetamine and illicit amphetamine) are the most common, followed by cannabinoids (including marijuana and synthetic cannabinoids).[12]
The PMTF Report urged clinicians to use a comprehensive, individualized, person-centered approach to the diagnosis and treatment of pain featuring multiple therapeutic modalities. The uptake of this approach is an urgent concern as growing numbers of older adults are enrolling in Medicare. Some estimates indicate about half of older adults have pain that interferes with function. Primary care clinicians and specialists are already facing challenges in treating pain and associated chronic disease in the Medicare population, where conditions such as arthritis, bone/joint disorders, back and neck pain, cancer and other conditions that inform and at times inhibit employing the full spectrum of pain management therapies are common. We believe untreated and inappropriately treated pain may translate to increased costs to the Medicare program as more beneficiaries experience functional decline, incapacitation, and frailty. Additional risks in untreated pain include individuals using illicit drugs such as cannabis; inadequate treatment of mental disorders such as depression and anxiety, misuse of prescription drugs, alcohol and other drug use disorder, and increased suicide risk and suicide.
In 2019 HHS issued the Guide for Clinicians on the Appropriate Dosage Reduction or Discontinuation of Long-Term Opioid Analgesics (the Guide) to support the thoughtful, deliberative, and measured discontinuation of long-term opioid analgesics, and mitigate harm and risk to patients who are working with their clinicians to undergo appropriate tapering or discontinuation.[13] The Guide notes that decisions to continue or reduce opioid medications for pain should be collaborative and based on the individual patient's goals and circumstances and clinicians should consider, for example, whether opioid medications continue to support patients meeting treatment goals; if opioids are exposing the person to an increased risk for serious adverse events or an opioid use disorder; and whether benefits continue to outweigh risks of opioids. Whether or not opioids are used in treatment, safe and effective non-opioid treatments can be integrated into patients' pain management plans based on an individualized assessment of benefits and risks, and considering the patient's diagnosis, goals and circumstances.[14] Unique needs and coordination across the health care team is critical and clinicians and care teams have a responsibility to provide, or arrange for, coordinated management of patients' pain including any medication-related issues. The system of care should not ultimately result in patient abandonment. The FDA issued a safety announcement in 2019, advising that health care professionals should not abruptly discontinue opioids in patients who are physically dependent and that patient-specific plans should be created to gradually taper off opioids, in part due to the risk of adverse events including abrupt withdrawal symptoms, increased pain, mood changes, mental health impact, psychosocial impact, and importantly, suicide risk.[15]
In 2020 the National Academy of Medicine, as part of its "Action Collaborative to Countering the U.S. Opioid Epidemic," began an effort to understand more about the state of chronic pain management, and to bring greater awareness to any intended and unintended consequences of opioid prescribing metrics as they pertain to the delivery, access, and coordination of chronic pain management and care. CMS is one of the sponsors of this work. The aim of this project is to visually illustrate the chronic pain management journey and accelerate the uptake of a range of pain treatments by outlining approaches to effective communication that leads to strong clinical relationships and optimal quality of life for people with pain.[16]
The SUPPORT Act (Pub. L. 115-271, October 24, 2018) outlines national strategies to help address America's opioid and substance use disorders (SUD) crisis, and advances policies to improve the treatment of pain and SUD. The SUPPORT Act recognizes the importance of opioid-related medication management, as well as the overall need to identify SUD in the Medicare Start Printed Page 39181 beneficiary population. Sections 2002 and 6086 of the SUPPORT Act are of particular importance regarding pain management. For beneficiaries with chronic pain, section 2002 of the SUPPORT Act amended sections 1861(ww) and (hhh)(2) of the Act to include a review of any current opioid prescriptions in conjunction with the initial preventive physical examination (the "Welcome to Medicare" visit) and annual wellness visit (AWV). The opioid prescription review is to include a review of the potential risk factors to the individual for opioid use disorder, an evaluation of the individual's pain severity and current treatment plan, the provision of information on non-opioid treatment options, and referral to a specialist, if appropriate. Section 2002 also amended sections 1861(ww) and (hhh)(2) of the Act to add a screening for potential SUDs to the Welcome to Medicare visit and the AWV, and to add referral to a specialist, as appropriate, to the AWV.
Section 6086 of the SUPPORT Act, the Dr. Todd Graham Pain Management Study, will provide HHS and CMS with key information about services delivered to Medicare beneficiaries with acute or chronic pain, help in understanding the current landscape of pain relief options for Medicare beneficiaries, and inform decisions around payment and coverage for pain management interventions, including those that minimize the risk of SUD. CMS has worked with the Agency for Healthcare Research and Quality, which has undertaken three topic briefs and two systematic reviews to inform Medicare coverage for the treatment of acute and chronic pain. CMS has also worked with HHS's Office of the Secretary for Planning and Evaluation to write a Report on the Study, which will be submitted to Congress. CMS will post a completed copy of the Report on our website. The Report will address questions regarding coverage and payment for evidence-based interventions for acute and chronic pain in Medicare, barriers to access, costs and benefits of expanding or revising benefits not currently covered, and legislative and administrative options to improve pain interventions.
We believe it is important to highlight the role of a person-centered approach to pain care. The National Quality Forum, which as its core work defines measures and health care practices as the best, evidence-based approaches to improving care, has defined person-centered planning as "a facilitated, individual-directed, positive approach to the planning and coordination of a person's services and supports based on individual aspirations, needs, preferences, and values," and stated that the "goal of person-centered planning is to create a plan that would optimize the person's self-defined quality of life, choice, and control, and self-determination through meaningful exploration and discovery of unique preferences and needs and wants in areas including, but not limited to, health and well-being, relationships, safety, communication, residence, technology, community, resources, and assistance."[17] These general principles should also apply in the treatment of individuals with pain, where clinicians confirm and affirm the individual's recovery and/or maintenance goals, and focus on those, where treatment is a means to an end.[18] For example, one goal might be to not rely on aiming to reduce a simple pain score, such as a numeric or visual score, but to evaluate function for example, through a tool such as the Defense and Veterans Pain Rating scale,[19] which integrates functional status, and then aim to optimize physical function and mental function in the beneficiary with chronic pain.
We recognize that there are no existing codes that specifically describe the work of the clinician involved in performing the tasks necessary to perform pain management care. We believe there are complexities in treating pain management patients that could include lifestyle discussion, ongoing medication management (such as opioid tapering or discontinuation, when appropriate), behavioral health care, preparation and updating of a care plan, consideration of federal and other opioid prescribing limits and guidelines, Prescription Drug Monitoring Program checks, electronic prescribing requirements, special licensing requirements (controlled substance licenses; buprenorphine "X-waivers"), interdisciplinary interactions, prescription drug coverage, CMS high-prescriber oversight, consideration of out-of-pocket costs, and other issues. As one example, decreasing or discontinuing opioid treatment requires careful, person-centered consideration of all of these aspects of providing care. These unique challenges often adversely impact the delivery of care, and subsequent access to care, for beneficiaries with chronic pain. Current Medicare payment methodologies such as Chronic Care Management (CCM) support chronic disease management, though may not provide adequate payment to health care providers or systems to holistically care for beneficiaries with chronic pain; we believe the complexity and resources required for safe and effective pain management may not be adequately captured and paid through these codes.
We believe that creating separate or add-on payment for care and management for people with pain might provide opportunities to better leverage services furnished using telecommunications technology and non face-to-face care while expanding access to treatment for pain. Such an additional payment could potentially be effective in preventing or reducing the need for acute services such as fall avoidance, and reduce the need for treatment for mental disorders such as depression, anxiety, and sleep disorders which may occur in some individuals with pain. There is also reason to believe that addressing chronic pain (for example, pain that lasts more than 3 months) early in its course may result in averting the development of "high-impact" chronic pain in some individuals, where they experience at least one major activity restriction (for example, unable to work, go to school, perform household chores). These individuals report more severe pain, more difficulty with self-care, and higher health care use than others with chronic pain. From a social determinants of health perspective, Blacks, Native Americans, persons of Asian/Indian descent, older adults, and people with less education, and single individuals report more high impact chronic pain.[20]
In 2019, 12.2 million individuals were enrolled in both Medicaid and Medicare, including people age 65 and older and younger beneficiaries with disabilities. Many have multiple chronic conditions, physical disabilities, behavioral health conditions, and cognitive impairments and on average, use more services and supports than those enrolled in only Medicaid or Medicare, with higher per capita costs. Dually eligible beneficiaries often have multiple social risk factors such as housing insecurity and homelessness, food insecurity, inadequate access to transportation, and low health literacy. A 2019 study[21] on dually eligible beneficiaries using "high dose" opioids to treat pain between 2006 through 2015 Start Printed Page 39182 indicated that the common conditions in beneficiaries studied were chronic pain, migraine, rheumatoid arthritis, osteoporosis, HIV/AIDS, viral hepatitis, and SUD.[22]
We are soliciting comment on whether we should consider creating separate coding and payment for medically necessary activities involved with chronic pain management and achieving safe and effective dose reduction of opioid medications when appropriate, or whether the resources involved in furnishing these services are appropriately recognized in current coding and payment. These activities could include, but are not limited to the following:
- Diagnosis;
- Assessment and monitoring;
- Administration of a validated rating scale(s);
- Development and maintenance of a person-centered care plan;
- Overall treatment management;
- Facilitation and coordination of any needed behavioral health treatment;
- Medication management;
- Patient education and self-management;
- Crisis care;
- Specialty care coordination such as complementary and integrative pain care, and SUD care; and
- Other aspects of pain and/or behavioral health services, including care rendered through telehealth modalities.
We are interested in feedback regarding whether the resource costs involved in furnishing these activities would be best captured through an add-on code to be billed with an E/M visit or a standalone code. To price such a code, we could consider using a crosswalk to the valuation and inputs for reference codes such as CPT code 99483 (Assessment of and care planning for a patient with cognitive impairment), HCPCS code G2064 (Comprehensive care management services for a single high-risk disease, e.g., principal care management, at least 30 minutes of physician or other qualified health care professional time per calendar month), HCPCS code G0108 (Diabetes outpatient self-management training services, individual, per 30 minutes), or other services paid under the PFS with similar resource costs.
We also seek information on which healthcare settings and stages in treatment these transitions from opioid dependence are occurring, as well as what types of practitioners furnish these services. We are soliciting comments on whether the specific activities we identify above are appropriate, and whether there are other activities that should be included. We are interested in stakeholder feedback regarding how we could define and value separate coding or an E/M add-on code. We also seek comment on whether any components of the service could be provided "incident to" the services of the billing physician who is managing the beneficiary's overall care similar to the structure of the Behavioral Health Integration (BHI) codes, which can include BHI services that are not delivered personally by the billing practitioner and delivered by other members of the care team (except the beneficiary), under the direction of the billing practitioner on an incident to basis (as an integral part of services delivered by the billing practitioner), subject to applicable state law, licensure, and scope of practice. The other care team members are either employees or working under contract to the practitioner who bills for BHI services.
We welcome feedback from stakeholders and the public on potential separate coding or an E/M add-on code for chronic pain management for our consideration for CY 2022 or for future rulemaking.
Start Printed Page 39183
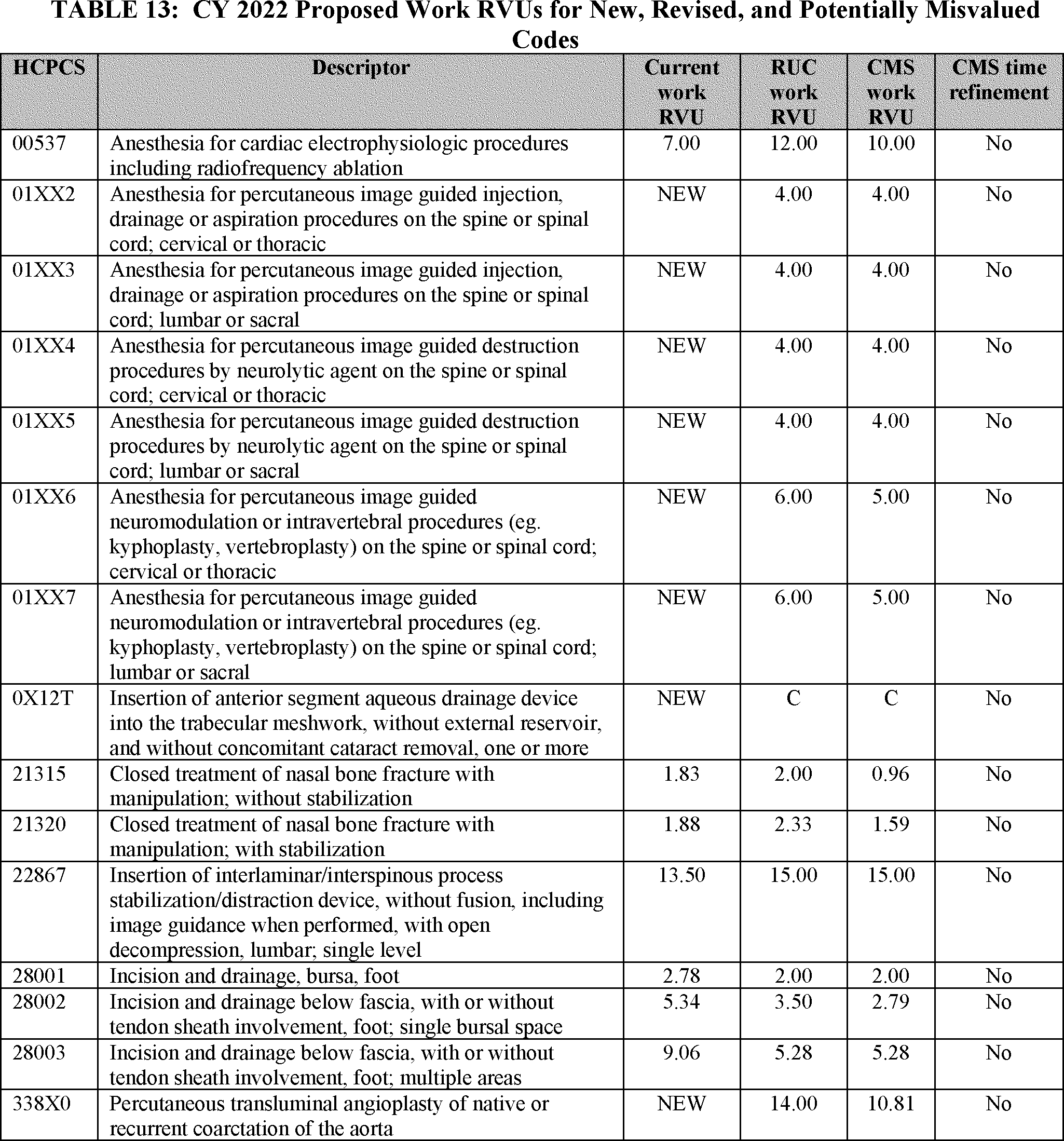
Start Printed Page 39184

Start Printed Page 39185

Start Printed Page 39186

Start Printed Page 39187

Start Printed Page 39188

Start Printed Page 39189
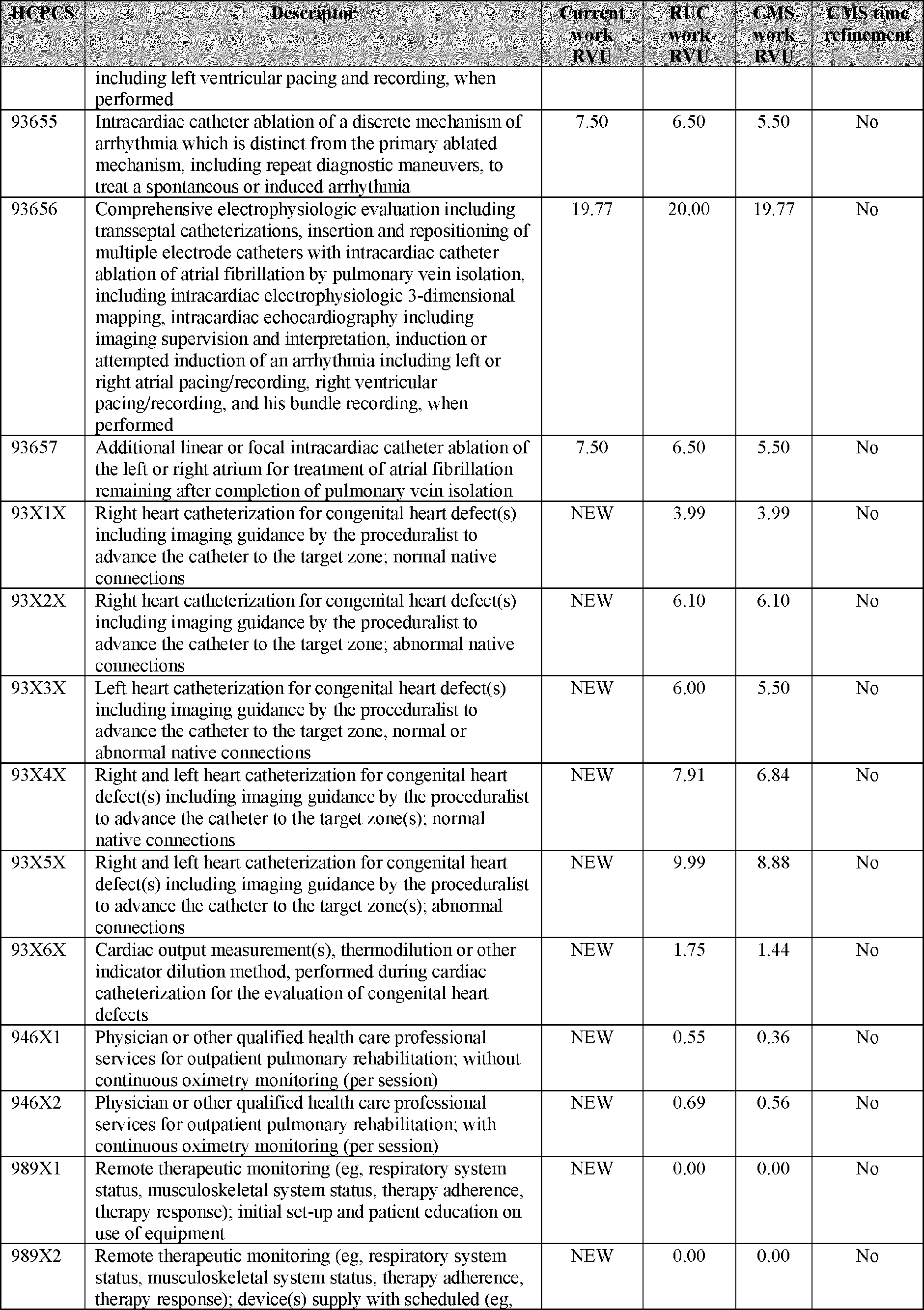
Start Printed Page 39190

Start Printed Page 39191

Start Printed Page 39192

Start Printed Page 39193
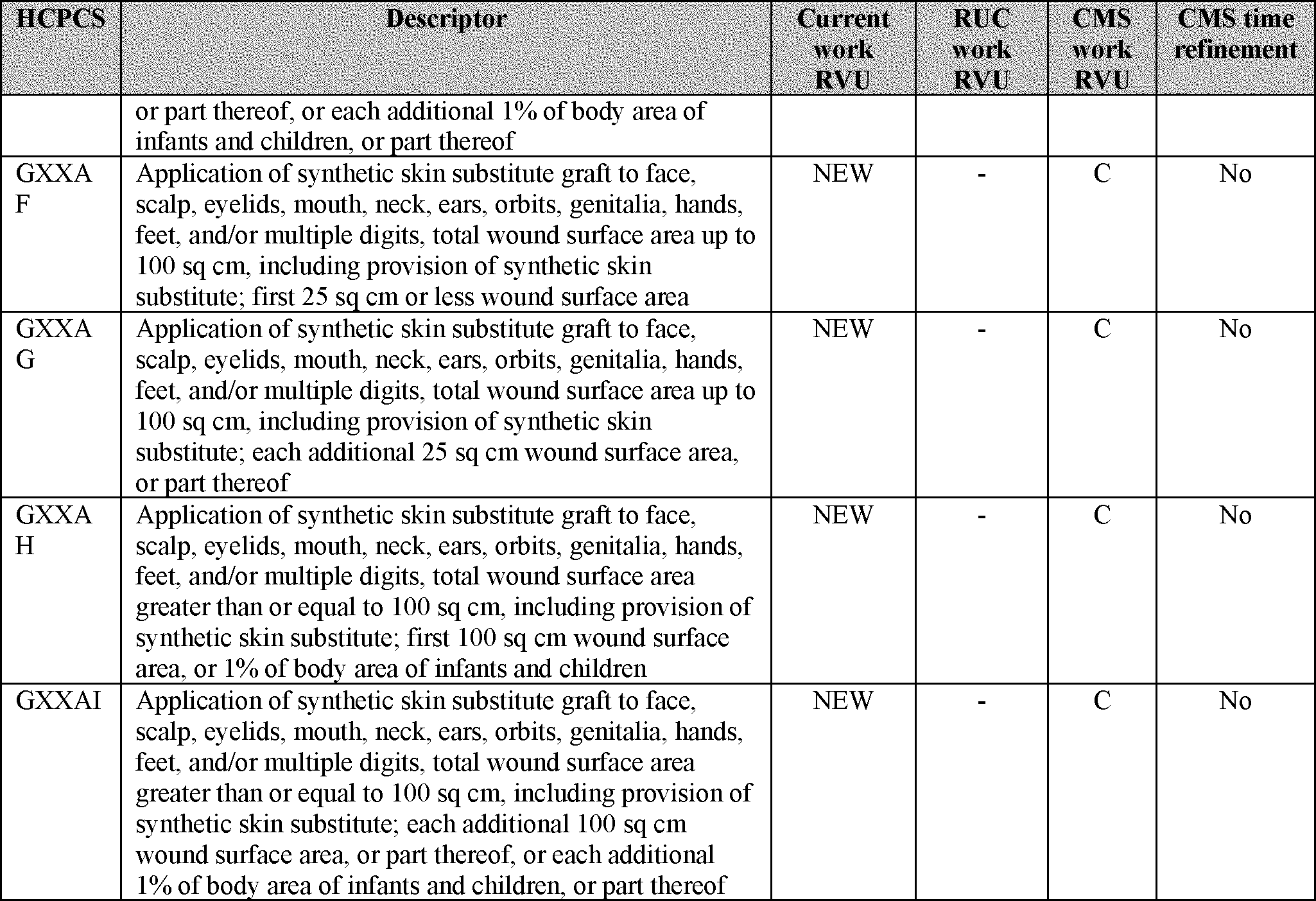
Start Printed Page 39194

Start Printed Page 39195

Start Printed Page 39196

Start Printed Page 39197
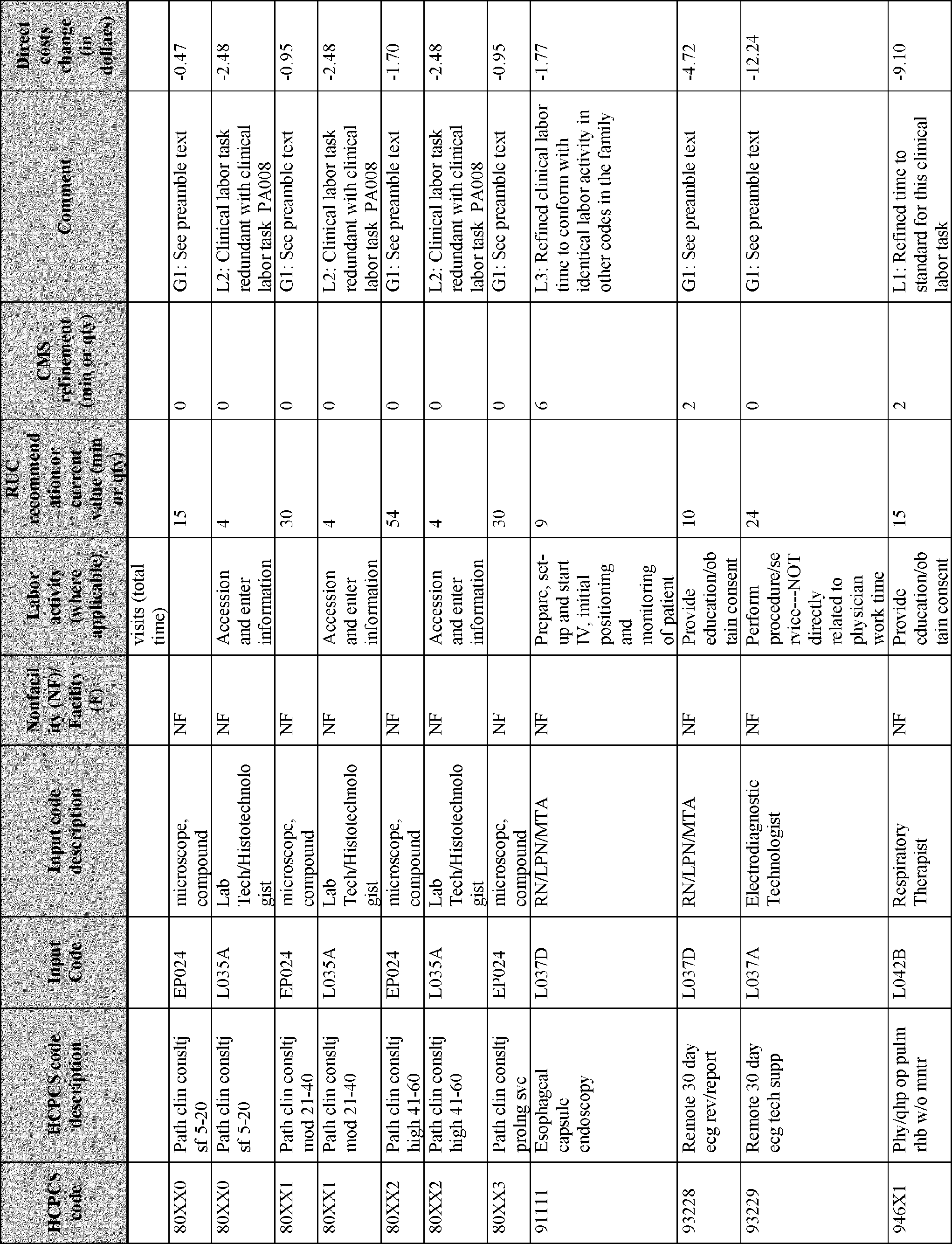
Start Printed Page 39198
Start Printed Page 39199
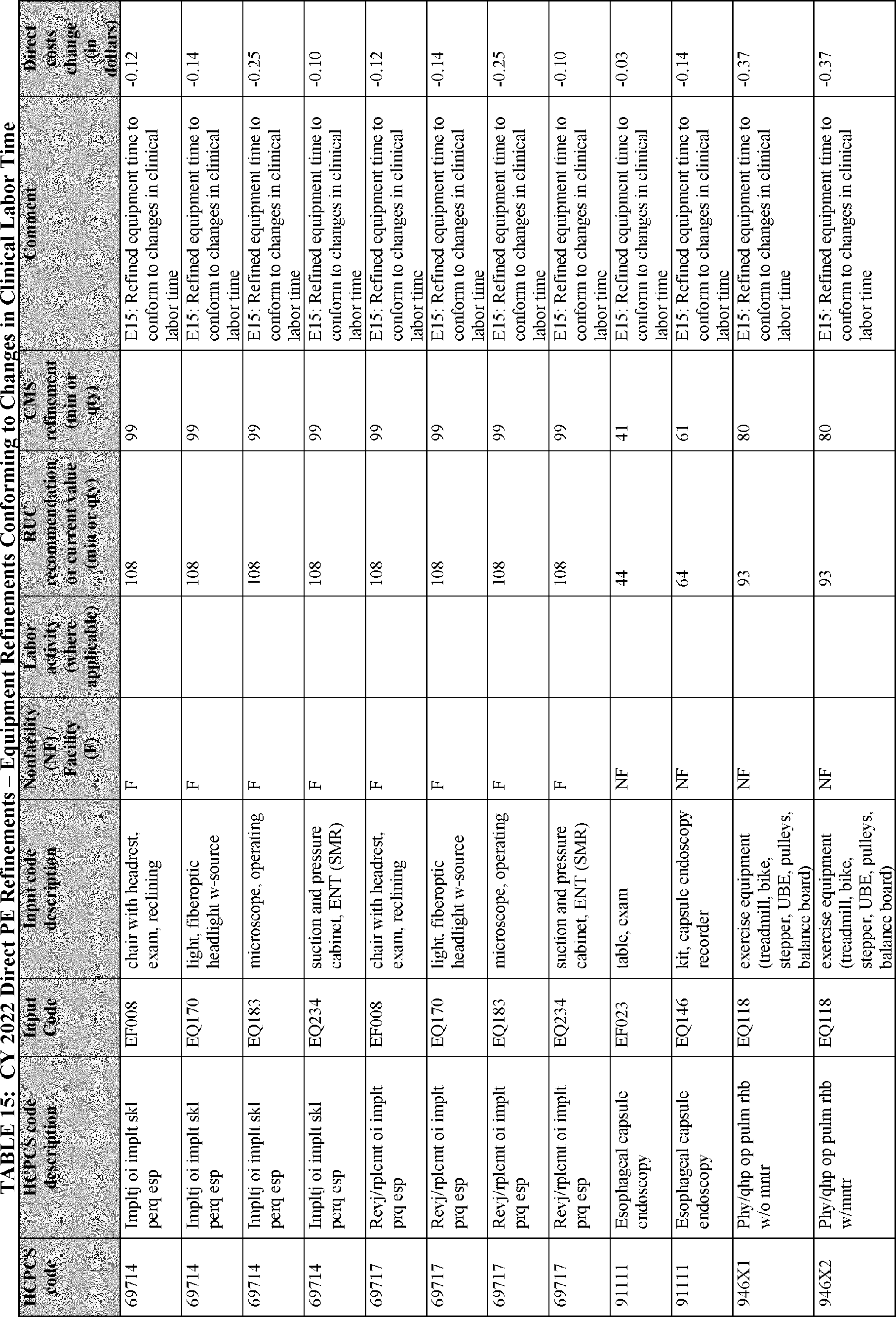
Start Printed Page 39200

Start Printed Page 39201
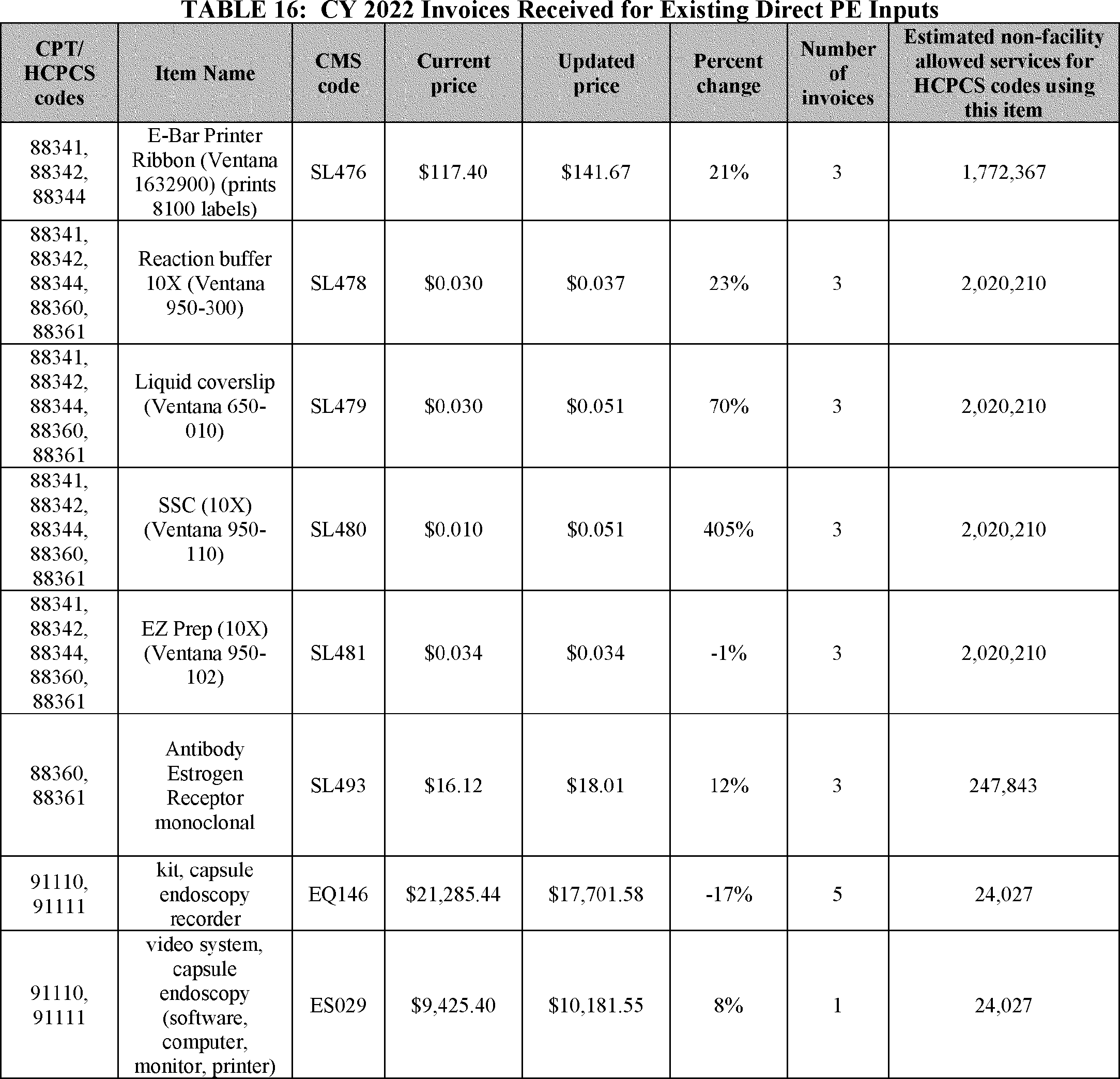
Start Printed Page 39202

Start Printed Page 39203

F. Evaluation and Management (E/M) Visits
Over the past several years, CMS has engaged with the AMA and other stakeholders in a process to update coding and payment for office/outpatient evaluation and management (E/M) visits, with recent changes taking effect January 1, 2021 (see 85 FR 84548 through 84574). In light of these changes, we are engaged in an ongoing review of other E/M visit code sets and are proposing a number of refinements to our current policies. The following section will discuss proposed policies regarding split (or shared) visits, critical care services, and teaching physician visits.
1. Split (or Shared) Visits
a. Background
A split (or shared) visit refers to an E/M visit that is performed ("split" or "shared") by both a physician and a NPP who are in the same group. Because the Medicare statute provides a higher PFS payment rate for services furnished by physicians than services furnished by NPPs, we need to address whether and when the physician can bill for split (or shared) visits. For visits in the non-facility (for example, office) setting for which the physician and NPP each perform portions of the visit, the physician can bill for the visit rather than the NPP as long as the visit meets the conditions of payment in our regulations at § 410.26(b)(1) for services furnished "incident to" a physician's professional services. However, for visits furnished under similar circumstances in facility settings (for example, in a hospital), our current regulations provide for payment only to the physician or NPP who personally performs all elements of the service, and no payment is made for services furnished "incident to" the billing professional's services.
As stated in our regulation at § 410.26(b)(1), Medicare Part B pays for services and supplies furnished "incident to" a physician's (or other practitioner's) professional services if those services and supplies are furnished in a noninstitutional setting to noninstitutional patients. In certain institutional (or "facility") settings, our longstanding split (or shared) billing Start Printed Page 39204 policy allows a physician to bill for an E/M visit when both the billing physician and an NPP in their group each perform portions of the visit, but only if the physician performs a substantive portion of the visit. When the physician bills for such a split (or shared) visit, in accordance with section 1833(a)(1)(N) of the Act, the Medicare Part B payment is equal to 80 percent of the payment basis under the PFS which, under section 1848(a)(1) of the Act, is the lesser of the actual charge or the fee schedule amount for the service. In contrast, if the physician does not perform a substantive portion of such a split (or shared) visit and the NPP bills for it, in accordance with section 1833(a)(1)(O) of the Act, the Medicare Part B payment is equal to 80 percent of the lesser of the actual charge or 85 percent of the fee schedule rate.
Previously, our policy for billing these split (or shared) visits was reflected in several provisions of our Medicare Claims Policy Manual (sections 30.6.1(B), 30.6.12, and 30.6.13(H)) which were withdrawn effective May 9, 2021, in response to a petition under the Department's Good Guidance regulations at 45 CFR 1.5 (see Transmittal 10742 available on the CMS website at https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Transmittals/r10742cp). In the absence of these manual provisions, the Medicare statute and various broadly applicable regulations continue to apply. In addition to withdrawing the manual provisions, we issued our response to the petition and an accompanying enforcement instruction on May 26, 2021, available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Evaluation-and-Management-Visits). In those documents, we indicated that we intend to address split (or shared) visits and critical care services (addressed below) through rulemaking; and that until we do, we will limit review to the applicable statutory and regulatory requirements for purposes of assessing payment compliance.
The list of applicable statutory and regulatory requirements includes the CY 2021 PFS final rule (85 FR 84549), where CMS generally adopted new CPT prefatory language and code descriptors for office/outpatient E/M visits. The new CPT guidelines for E/M services introduced a CPT definition of a split (or shared) visit for the first time, effective January 1, 2021. This new CPT definition was part of CPT's new guidelines indicating how to select the visit level based on time, which can be done for all office/outpatient E/M visits starting in 2021. The CPT guidelines that we are referring to are published in the CPT Codebook, in a section titled "Evaluation and Management Services (E/M) Guidelines."[23] In this section of our proposed rule, we use the term "CPT E/M Guidelines" to refer to this material.
In the CY 2021 PFS final rule (85 FR 84549), we stated that we are generally adopting the CPT E/M Guidelines for the new office/outpatient E/M visit codes. However, the CPT E/M Guidelines do not address many issues that arise in the context of PFS payment for split (or shared) visits, such as which practitioner should report the visit when elements of the visit are performed by different practitioners; whether a substantive portion of the visit must be performed by the billing practitioner; whether practitioners must be in the same group to bill for a split (or shared) visit; or the settings of care where split (or shared) visits may be furnished and billed. The CPT E/M Guidelines simply state, "A split or shared visit is defined as a visit in which a physician and other qualified health care professional(s) jointly provide the face-to-face and non-face-to-face work related to the visit. When time is being used to select the appropriate level of services for which time-based reporting of shared or split visits is allowed, the time personally spent by the physicians and other qualified health care professional(s) assessing and managing the patient on the date of the encounter is summed to define total time. Only distinct time should be summed for split or shared visits (that is, when two or more individuals jointly meet with or discuss the patient, only the time of one individual should be counted)."[24]
In contrast, to ensure appropriate PFS payment, our policy for split (or shared) visits, as expressed in the recently withdrawn manual provisions, is that the physician may bill for a split (or shared) visit only if they perform a substantive portion of the visit, and the practitioners must be in the same group and furnishing the visit in specified settings in order to bill for a split (or shared) visit. Our manual also limited billing for split (or shared) visits to services furnished to established patients. In this proposed rule, we are making a number of proposals to address the recently withdrawn manual sections and improve transparency and clarity regarding our policies on billing for split (or shared) visits, to update them to account for recent revisions to E/M visit coding and payment, and to revise our regulations to reflect these policies.
b. Definition of Split (or Shared) Visits
We are proposing to define a split (or shared) visit as an E/M visit in the facility setting that is performed in part by both a physician and an NPP who are in the same group, in accordance with applicable laws and regulations. We propose to add this definition to a new section of our regulations at § 415.140.
Additionally, we propose to define split (or shared) visits as those that:
- Are furnished in a facility setting by a physician and an NPP in the same group, where the facility setting is defined as an institutional setting in which payment for services and supplies furnished incident to a physician or practitioner's professional services is prohibited under our regulation at § 410.26(b)(1).
- Are furnished in accordance with applicable law and regulations, including conditions of coverage and payment, such that the E/M visit could be billed by either the physician or the NPP if it were furnished independently by only one of them in the facility setting (rather than as a split (or shared) visit).
We are proposing to revise our regulations at § 415.140 to codify this definition.
We believe that limiting the definition of split (or shared) visits to include only E/M visits in institutional settings, for which "incident to" payment is not available, will allow for improved clarity, and clearly distinguish, the policies applicable to split (or shared) visits, from the policies applicable to services furnished incident to the professional services of a physician. We do not see a need for split (or shared) visit billing in the office setting, because the "incident to" regulations govern situations where an NPP works with a physician who bills for the visit, rather than billing under the NPP's own provider number.
We are also proposing to modify our policy to allow physicians and NPPs to bill for split (or shared) visits for both new and established patients, and for critical care and certain Skilled Nursing Facility/Nursing Facility (SNF/NF) E/M visits. We are proposing these modifications to the current policy and conditions of payment for split (or shared) visits, discussed below, to account for changes that have occurred in medical practice patterns, including Start Printed Page 39205 the evolving role of NPPs as part of the medical team.
c. Definition of Substantive Portion
(1) More Than Half of the Total Time
As stated earlier, only the physician or NPP who performs the substantive portion of the split (or shared) visit would bill for the visit. We are proposing to define "substantive portion" as more than half of the total time spent by the physician and non-physician practitioner performing the visit. We note that our withdrawn manual instructions contained a few definitions of "substantive portion." For example, one section defined substantive portion as any face-to-face portion of the visit, while another section defined it as one of the three key components of an E/M visit—either the history of present illness (HPI), physical exam, and/or medical decision-making (MDM). Given recent changes in the CPT E/M Guidelines, HPI and physical exam are no longer necessarily included in all E/M visits, because as noted above, for office/outpatient E/M visits, the visit level can now be selected based on either MDM or time, and history and exam are performed only as medically appropriate. Accordingly, defining "substantive portion" as one of these three key components is no longer a viable approach. Similarly, MDM is not easily attributed to a single physician or NPP when the work is shared, because MDM is not necessarily quantifiable and can depend on patient characteristics (for example, risk). We believe that time is a more precise factor than MDM to use as a basis for deciding which practitioner performs the substantive portion of the visit.
We also do not believe it would be appropriate to consider the performance of any portion of the visit—with or without direct patient contact—as a substantive portion. For instance, we do not believe it would be appropriate to consider a brief or minor interaction, with or without direct patient contact, such as where the physician merely "pokes their head" into the room, to be a substantive portion of the visit. Therefore, we are proposing to define "substantive portion" as more than half of the total time spent by the physician and NPP performing the split (or shared) visit. We are proposing to revise our regulation at § 415.140 to codify this definition.
We recognize that the billing practitioner, who would be the practitioner providing the substantive portion of the visit, could select the level for the split (or shared) visit based on MDM, but we nonetheless propose to base the definition of substantive portion on the amount of time spent by the physician and NPP providing the visit. We recognize that this policy would necessitate the practitioners' tracking and documenting the time they spent for these visits. However, we believe that practitioners are likely to increasingly time their visits for purposes of visit level selection independent of our split (or shared) visit policies, given recent changes to the CPT E/M Guidelines, and the fact that critical care visits are already timed. Accordingly, we do not believe this would comprise a substantial new burden.
(2) Distinct Time
We propose that the distinct time of service spent by each physician or NPP furnishing a split (or shared) visit would be summed to determine total time and who provided the substantive portion (and therefore bills for the visit). This would be consistent with the CPT E/M Guidelines stating that, for split (or shared) visits, when two or more individuals jointly meet with or discuss the patient, only the time of one individual should be counted).[25] For example, if the NPP first spent 10 minutes with the patient and the physician then spent another 15 minutes, their individual time spent would be summed to equal a total of 25 minutes. The physician would bill for this visit since they spent more than half of the total time (15 of 25 total minutes). If, in the same situation, the physician and NPP met together for five additional minutes (beyond the 25 minutes) to discuss the patient's treatment plan, that overlapping time could only be counted once for purposes of establishing total time and who provided the substantive portion of the visit. The total time would be 30 minutes, and the physician would bill for the visit since they spent more than half of the total time (20 of 30 total minutes).
(3) Qualifying Time
Drawing on the CPT E/M Guidelines, we are proposing a listing of activities that could count toward total time for purposes of determining the substantive portion. For visits that are not critical care services, we are proposing the same listing of activities that can count when time is used to select E/M visit level, specifically the following activities, when performed and regardless of whether or not they involve direct patient contact:
- Preparing to see the patient (for example, review of tests).
- Obtaining and/or reviewing separately obtained history.
- Performing a medically appropriate examination and/or evaluation.
- Counseling and educating the patient/family/caregiver.
- Ordering medications, tests, or procedures.
- Referring and communicating with other health care professionals (when not separately reported).
- Documenting clinical information in the electronic or other health record.
- Independently interpreting results (not separately reported) and communicating results to the patient/family/caregiver.
- Care coordination (not separately reported).
Practitioners would not count time spent on the following:
- The performance of other services that are reported separately.
- Travel.
- Teaching that is general and not limited to discussion that is required for the management of a specific patient.[26]
Since critical care services can include additional activities that are bundled into the critical care visit code(s), we are proposing a different listing of qualifying activities, discussed in our section below on split (or shared) critical care services. Additionally, we are seeking public comment on whether there should be a different listing of qualifying activities for purposes of determining the total time and substantive portion of split (or shared) emergency department visits, since those visits also have a unique construct.
(4) Application to Prolonged Services
For office/outpatient E/M visits, as discussed in our CY 2021 PFS final rule (85 FR 84572), HCPCS code G2212 can be used to report prolonged services in 15-minute increments of time beyond the maximum time for a level 5 office/outpatient E/M visit. For all other E/M visits (except critical care and emergency department visits), CPT codes 99354-9 can be used to report prolonged time with or without direct patient contact, when required time increments above the typical time is spent (see CY 2017 PFS final rule, 81 FR 80228-80230 and the Medicare Claims Processing Manual (Pub. 100-02), chapter 12, section 30.6.15 available on our website at https://www.cms.gov/ Start Printed Page 39206 Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c12.pdf).
Our withdrawn manual provisions instructed that practitioners cannot bill prolonged services as a split (or shared) visit. Having reviewed this policy, we believe that codes that are billed as add-on codes for prolonged service time for an E/M visit, which could be furnished and billed as a split (or shared) visit under our proposed policy, should be considered to be part of that E/M visit. Therefore, we are proposing to change our policy to allow a practitioner to bill for a prolonged E/M visit as a split (or shared) visit. Specifically, the physician or practitioner who spent more than half the total time (that is, performed the substantive portion described above) would bill for the primary E/M visit and the prolonged service code(s) when the service is furnished as a split (or shared) visit, if all other requirements to bill for the services were met. The physician and NPP would sum their time together, and whomever furnished more than half of the total time, including prolonged time, (that is, the substantive portion) would report both the primary service code and the prolonged services add-on code(s), assuming the time threshold for reporting prolonged services is met. We note that for critical care visits, the practitioner would not bill prolonged E/M services because the practitioners would instead aggregate their time, as proposed below, to report additional units of critical care services.
d. New and Established Patients, and Initial and Subsequent Visits
Our withdrawn manual provisions stated that when an E/M service is furnished as a split or shared encounter, between a physician and an NPP (that is, an NP, PA, CNS or CNM), the service is considered to have been performed "incident to" if the requirements for "incident to" are met and the patient is an established patient. This provision was generally interpreted to mean that split (or shared) visits cannot be billed for new patients. The withdrawn manual provisions also did not specify whether the practitioner who bills for the split or shared visit could bill for initial, versus subsequent, split (or shared) visits in the facility setting. After conducting an internal review, including consulting our medical officers, we believe that the practice of medicine has evolved toward a more team-based approach to care, and greater integration in the practice of physicians and NPPs, particularly when care is furnished by practitioners in the same group in the facility setting. Given this evolution in medical practice, the concerns that may have been present when we issued the manual instructions may no longer be as relevant. We understand that there have been changes in the practice of medicine over the past several years, some facilitated by the advent of electronic health records (EHRs) and other systems, toward a more team-based approach to care. There has also been an increase in alternative payment models that employ a more team-based approach to care. After considering and reevaluating our policy, we see no reason to preclude the physician or NPP from billing for split (or shared) visits for a new patient, in addition to an established patient, or for initial and subsequent split (or shared) visits. Therefore, we are proposing to permit the physician or NPP to bill for split (or shared) visits for both new and established patients, as well as for initial and subsequent visits. We believe this approach is also consistent with the CPT E/M Guidelines for split (or shared) visits, which does not exclude these types of visits from being billed when furnished as split (or shared) services.
e. Settings of Care
The concept of split (or shared) visits was developed as an analog in the facility setting to payment policies for services and supplies furnished incident to a physician's or an NPP's professional services in the non-institutional setting. Section 410.26(a)(6) of our regulations defines the non-institutional setting as all settings other than a hospital or SNF. We are proposing to allow billing of split (or shared) visits, including critical care visits, when they are performed in any institutional setting and are proposing to codify the definition of facility setting in the regulation at § 415.140. We discuss our proposals regarding billing for critical care split (or shared) E/M services below (see section II.F. of this proposed rule).
Our withdrawn manual provisions did not allow practitioners to bill for split (or shared) visits that are critical care services or SNF/NF visits. The manual stated that the split (or shared) E/M policy did not apply to critical care services or procedures, and that a split (or shared) E/M service performed by a physician and a qualified NPP of the same group (or employed by the same employer) cannot be reported as a critical care service. It also stated that a split (or shared) E/M visit cannot be reported in the SNF/NF setting. We propose to define split (or shared) visits to be limited to services furnished in institutional settings, as discussed above. As discussed below, we do not see any reason to preclude billing for split (or shared) visits for critical care services, although we are seeking public comment on this issue in particular. We understand that there have been changes in the practice of medicine over the past several years, some facilitated by the advent of EHRs and other systems, toward a more team-based approach to care. There has also been an increase in alternative payment models that employ a more team-based approach to care. Where a physician and NPP in the same group take a team approach to furnishing care, as would be the case for split (or shared) visits, even for new patients, initial visits, critical care visits, or SNF/NF visits, we are less concerned about potential disruptions in continuity of care than we might once have been. Rather, we believe that when a visit is shared between a physician and an NPP in the same group, there would be close coordination and an element of collaboration in providing care to the beneficiary.
We do not see any reason to preclude billing for split (or shared) visits for the subset of SNF/NF visits that are not required by our regulations to be performed in their entirety by a physician. Under our current policy, no E/M services can be furnished and billed as split (or shared) visits in the SNF setting. We refer readers to our Conditions of Participation in 42 CFR 483.30 for information regarding the SNF/NF visits that are required to be performed in their entirety by a physician. That regulation requires that certain SNF/NF visits must be furnished directly and solely by a physician. If finalized, our proposal would not apply to the SNF/NF visits that are required to be performed in their entirety by a physician; any SNF/NF visit that is required to be performed in its entirety by a physician cannot and would not be able to be billed as a split (or shared) visit. However, for other visits to which the regulation at § 483.30 does not apply, there is no requirement for a physician to directly and solely perform the visit. We propose that those visits could be furnished and billed as split (or shared) visits.
f. Same Group
In accordance with the current policy outlined in the withdrawn manual provisions, we are proposing that a physician and NPP must be in the same group in order for the physician and NPP to bill for a split (or shared) visit. We believe that in circumstances when a split or (shared) visit is appropriately billed, a physician and NPP are working jointly to furnish all of the work related to the visit with the patient. However, if a physician and NPP are in different groups, we would expect the physician Start Printed Page 39207 and NPP to bill independently, and only for the services they specifically and fully furnish. Further, consistent with our withdrawn manual guidance, we note that Medicare does not pay for partial physician's visits, so CPT modifier −52 (reduced services) could not be used to report split (or shared) visits. Thus, if a physician and an NPP who are in different groups each furnish part of an E/M service, but not all of it, then we would not consider either service to be a billable service. Similarly, if two physicians, each in their own private practice, both saw the same patient in the hospital, but neither one fully furnished a billable service—there would be no basis on which to combine their efforts or minutes of service into one billable E/M visit.
We are seeking public comment on whether we should further define "group" for purposes of split (or shared) visit billing. While we are not proposing a definition in this proposed rule, we have considered several options, such as requiring that the physician and NPP must be in the same clinical specialty, in which case we would use the approach outlined in the CPT E/M Guidelines; that is the NPP is considered to be in the same specialty and subspecialty as the physician with whom they are working.[27] We are also considering an approach under which we would align the definition of "group" with the definition of "physician organization" at § 411.351. The term "physician organization" is defined at § 411.351 for purposes of section 1877 of the Act and our regulations in 42 CFR part 411, subpart J (collectively, the physician self-referral law), and explained further in frequently asked questions available on the CMS website at https://www.cms.gov/Medicare/Fraud-and-Abuse/PhysicianSelfReferral/Downloads/FAQs-Physician-Self-Referral-Law.pdf. Another approach would be to consider practitioners with the same billing tax identification number as being in the same group. We are concerned that this particular approach may be too broad in multi-specialty groups or health care systems that include many practitioners who do not typically work together to furnish care to patients in the facility setting. We note that some of these approaches may not align with the definition of "group" used for purposes of Medicare enrollment.
g. Medical Record Documentation
To ensure program integrity and quality of care, we are proposing that documentation in the medical record must identify the two individual practitioners who performed the visit. The individual who performed the substantive portion (and therefore bills the visit) would be required to sign and date the medical record. We are proposing to revise our regulation at § 415.140 to reflect the conditions of payment for split (or shared) visits as discussed in this section.
h. Claim Identification
We are proposing to create a modifier to describe split (or shared) visits, and we are proposing to require that the modifier must be appended to claims for split (or shared) visits, whether the physician or NPP bills for the visit. Currently, we cannot identify through claims that a visit was performed as a split (or shared) visit, which means that we could know that a visit was performed as a split (or shared) visit only through medical record review. We believe it is important for program integrity and quality considerations to have a way to identify who is providing which E/M services, and how often we are paying at the physician rate for services provided in part by NPPs. (Please see the documentation section below for additional information). The proposed modifier, if finalized, would give CMS insight, directly through our claims data instead of only through medical record review, into the specific circumstances under which these split (or shared) visits are furnished. Such information would be helpful to CMS for program integrity purposes, and could be instructive in considering whether we may need to offer additional clarification to the public, or further revise the policy for these E/M visits in future rulemaking.
We are proposing to revise our regulation at § 415.140 to reflect the conditions of payment for split (or shared) visits as discussed in this section.
Consistent with our current policy, Medicare does not pay for partial E/M visits for which all elements of the service are not furnished. Therefore, we are proposing that the modifier identified by CPT for purposes of reporting partial services (modifier −52 (reduced services)) could not be used to report partial E/M visits, including any partial services furnished as split (or shared) visits. We are also considering whether it is necessary to amend our regulations to explicitly state that Medicare does not pay for partial E/M visits and are interested in public comments on this issue.
2. Critical Care Services (CPT Codes 99291-99292)
As stated previously, in light of updates that we previously finalized for coding and payment for office/outpatient E/M visits, we are proposing a number of refinements to other E/M code sets. Historically, our policy for billing critical care services was reflected in several provisions of the Medicare Claims Processing Manual (sections 30.6.1(B), 30.6.12, and 30.6.13(H)) which were withdrawn effective May 9, 2021, in response to a petition under the Department's Good Guidance regulation at 45 CFR 1.5 (see Transmittal 10742 available on the CMS website at https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Transmittals/r10742cp). In the absence of these manual provisions, the Medicare statute and various broadly applicable regulations continue to apply. In addition to withdrawing the manual provisions, we issued our response to the petition and accompanying enforcement instruction issued on May 26, 2021, available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Evaluation-and-Management-Visits. In those documents, we indicated that we intend to address split (or shared) visits (addressed above) and critical care services (addressed below) through rulemaking; and that until we do, we will limit review to the applicable statutory and regulatory requirements for purposes of assessing payment compliance. The list of applicable statutory and regulatory requirements includes the CY 2021 PFS final rule (85 FR 84549), where CMS generally adopted new CPT prefatory language and code descriptors for office/outpatient E/M visits. Therefore, in this section of the proposed rule, we are proposing to update our critical care E/M visit policies to improve transparency and clarity, and to account for recent revisions to E/M visit coding and payment.
The CPT 2021® Professional Codebook (hereafter, CPT Codebook) provides guidelines for critical care services in the CPT E/M Guidelines on pp. 5-9 and in prefatory language, code descriptors, and parentheticals on pp. 31-33. We are proposing to adopt the CPT prefatory language for critical care services as currently described in the CPT Codebook, except as otherwise specified in this section of the proposed rule. Should CPT make changes to the guidance for critical care services in a Start Printed Page 39208 subsequent edition of the CPT Codebook, we could revisit these policies in future rulemaking.
We are also proposing to clarify our definition of critical care visits, and the requirements governing how critical care visits are reported when more than one practitioner or specialty is involved in furnishing critical care services to a patient. Further, we are proposing to prohibit a practitioner that reports critical care services furnished to a patient from also reporting any other E/M visit for that same patient on the same calendar day that the critical care services are furnished to that patient, and vice versa. Additionally, we are proposing to prohibit practitioners from reporting critical care visits during the same time-period as a procedure with a global surgical period.
a. Definition of Critical Care
Critical care visits are described by CPT codes 99291 (Critical care, evaluation and management of the critically ill or critically injured patient; first 30-74 minutes) and 99292 (each additional 30 minutes (List separately in addition to code for primary service). As stated above, the CPT Codebook defines critical care services in prefatory language on pp. 31-33.
Critical care services were defined in the withdrawn provisions of the Medicare Claims Processing Manual, and that definition tracked closely with the CPT prefatory language regarding critical care services. To improve transparency and clarity, we are proposing to adopt the CPT prefatory language as the definition of critical care services. The CPT prefatory language states that critical care is the direct delivery by a physician(s) or other qualified healthcare professional (QHP) of medical care for a critically ill/injured patient in which there is acute impairment of one or more vital organ systems, such that there is a probability of imminent or life-threatening deterioration of the patient's condition.[28] It involves high complexity decision-making to treat single or multiple vital organ system failure and/or to prevent further life-threatening deterioration of the patient's condition. We continue to believe that the CPT Codebook appropriately delineates coding and definitions for critical care services in order to distinguish them as more intense services that are valued relatively higher than other E/M services. Thus, we are proposing to adopt the CPT prefatory language as the definition of critical care services, and refer readers to the CPT Codebook for additional details.
Under current Medicare policy, a QHP is an individual who is qualified by education, training, licensure/regulation (when applicable), facility privileging (when applicable), and the applicable Medicare benefit category to perform a professional service within their scope of practice and independently report that service (see, for example, 80 FR 70957; 85 FR 84543, 84593). Because the CPT Codebook provides that critical care services can be delivered by a physician or QHP, we are proposing that critical care services may be reported by a physician or NPP who is a QHP as explained above.
The CPT prefatory language specifies that critical care may be furnished on multiple days, and is typically furnished in a critical care area, which can include an intensive care unit or emergency care facility. CPT prefatory language also states that critical care requires the full attention of the physician or NPP, and therefore, for any given time-period spent providing critical care services, the practitioner cannot provide services to any other patient during the same period of time. We are proposing to adopt this CPT prefatory language to improve transparency and clarity of our policy for this service for Medicare billing purposes.
CPT prefatory language and billing and coding guidance bundles several services into critical care visits furnished by a given practitioner when performed during the critical period by the practitioners providing critical care. We are proposing to adopt CPT's listing of bundled services that are part of critical care visits to improve transparency and clarity of our policy for this service. Therefore, we are proposing that the following services would be bundled into critical care visits: Interpretation of cardiac output measurements (93561, 93562), chest X rays (71045, 71046), pulse oximetry (94760, 94761, 94762), blood gases, and collection and interpretation of physiologic data (for example, ECGs, blood pressures, hematologic data); gastric intubation (43752, 43753); temporary transcutaneous pacing (92953); ventilator management (94002-94004, 94660, 94662); and vascular access procedures. As a result, these codes would not be separately billable by a practitioner during the time-period when the practitioner is providing critical care for a given patient. We are also proposing to adopt the CPT prefatory language stating that time spent performing separately reportable procedures or services should be reported separately and should not be included in the time reported as critical care time.
b. Critical Care by a Single Physician or NPP
Our withdrawn manual provisions and the prefatory language in the CPT Codebook cited above both describe the time duration for the correct reporting of critical care services by a single physician or NPP. To improve transparency and clarity of our policy for this service, we are proposing to adopt the CPT prefatory language. Under our proposal, the physician or NPP would report CPT code 99291 for the first 30-74 minutes of critical care services provided to a patient on a given date. Thereafter, they would report CPT code 99292 for additional 30-minute time increments provided to the same patient. We refer readers to the CPT Codebook for examples of the total duration of critical care visits.[29] The prefatory language states that CPT codes 99291 and 99292 are used to report the total duration of time spent by the physician or QHP providing critical care services to a critically ill or critically injured patient, even if the time spent by the practitioner on that date is not continuous; and that non-continuous time for medically necessary critical care services may be aggregated. The CPT Codebook indicates that CPT code 99291 is used to report the first 30-74 minutes of critical care on a given date, and that the code should be used only once per date even if the time spent by the practitioner is not continuous on that date. We are proposing to adopt this rule for critical care services furnished by a single physician or NPP. We note that the prefatory language does not indicate how practitioners should report critical care when a service lasts beyond midnight. We are seeking comment about how practitioners should report CPT codes 99291 and 99292 when a service extends beyond midnight to the following calendar day.
c. Critical Care Services Furnished Concurrently by Different Specialties
The CPT Codebook does not provide any special instructions regarding how to report critical care furnished by more than one physician or practitioner, whether in a split (or shared) visit context or other contexts that might be relevant given the unique nature of critical care and the long timeframes over which patients may receive these services. The CPT E/M Guidelines state broadly that concurrent care is the provision of similar services (for Start Printed Page 39209 example, hospital visits) to the same patient by more than one physician or other QHP on the same day. The CPT E/M Guidelines state that when concurrent care is provided, no special reporting is required.[30] The CPT E/M Guidelines also state broadly that when time is being used to select the appropriate level of services for which time-based reporting of split (or shared) visits is allowed, the time personally spent by the physician and other QHP(s) assessing and managing the patient on the date of the encounter is summed to define total time; and that only distinct time should be summed for split (or shared) visits (that is, when two or more individuals jointly meet with or discuss the patient, only the time of one individual should be counted).[31]
In the context of critical care services, our withdrawn manual provisions provided guidance on concurrent care, and stated that there are situations where physicians or NPPs within a group provide coverage or follow-on care for one another on a single day. The manual also stated that critically ill or injured patients may require the care of more than one practitioner from more than one specialty (regardless of group affiliation), and this work could transpire simultaneously or overlap. Consistent with our current policy, and to improve transparency and clarity of our policy for critical care services, we are proposing that concurrent care occurs where more than one physician or qualified NPP furnishes services to the same patient on the same day. In general, concurrent care is covered when the services of each practitioner are medically necessary, and not duplicative. For example, concurrent care may be medically necessary because of the existence of more than one medical condition requiring diverse specialized medical services, that is, more than one specialty (which can include a qualified NPP as a specialty). In the context of critical care services, a critically ill patient may have more than one medical condition requiring diverse specialized medical services and thus requiring more than one practitioner having different specialties to play an active role in the patient's treatment. Thus, we are proposing that critical care services may be furnished as concurrent care (or concurrently) to the same patient on the same day by more than one practitioner in more than one specialty (for example, an internist and a surgeon, allergist and a cardiologist, neurosurgeon and NPP), regardless of group affiliation, if the service meets the definition of critical care and is not duplicative of other services. However, as for most Medicare-covered services, these critical care services would need to be medically reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member. We are seeking comment on this proposal to better understand current clinical practice for critical care, and when it would be appropriate for more than one physician or NPP of the same or different specialties, and within the same or a different group, to provide critical care services.
d. Critical Care Furnished Concurrently by Practitioners in the Same Specialty and Same Group (Follow-Up Care)
Physician(s) or NPP(s) in the same specialty and in the same group may provide concurrent follow-up care, such as a critical care visit subsequent to another practitioner's critical care visit. This may be as part of continuous staff coverage or follow-up care to critical care services furnished earlier in the day on the same calendar date. According to CPT coding and billing conventions that we generally acknowledge, a practitioner who furnishes a timed service such as critical care would typically need to report the primary service or procedure code before reporting an add-on code. However, we are proposing that when critical care is furnished concurrently by two or more practitioners in the same specialty and in the same group to the same patient on the same day, the individual physician(s) or NPP(s) providing the follow-up or subsequent care would report their time using the code for subsequent time intervals (CPT code 99292), and would not report the primary service code (CPT code 99291). CPT code 99291 would not be reported more than once for the same patient on the same day by these practitioners. This proposal recognizes that multiple practitioners in the same specialty and the same group can maintain continuity of care by providing follow-up care for the same patient on the same day, and is consistent with our current policy as described in the withdrawn manual provisions. Because practitioners in the same specialty and same group cover for one another to provide concurrent critical care services, we believe the total time for critical care services furnished to a patient on the same day by the practitioners in the same group with the same specialty should be reflected as if it were a single set of critical care services furnished to the patient. The practitioner furnishing the initial critical care service would report CPT code 99291, and the practitioner(s) reporting subsequent critical care service time would report CPT code 99292.
Under our current policy, the initial critical care service must be performed by a single physician or qualified NPP. In considering and reevaluating this policy, we believe it would better reflect current medical practice to allow critical care service time spent by more than one practitioner in the same group with the same specialty to be added together for the purposes of meeting the time requirement to bill for the initial critical care service using CPT code 99291. We are proposing this policy for two main reasons. First, we believe this proposal would appropriately recognize that multiple practitioners in the same specialty and group can concurrently furnish critical care services to a patient on a single day. Second, this proposal would conform our policy for the initial critical care service with our proposal described above for multiple practitioners in the same specialty and same group to report CPT code 99292 for their cumulative critical care service time. Thus, we are proposing that where one practitioner begins furnishing the initial critical care service but does not meet the time required to report CPT code 99291, and another practitioner in the same specialty and group continues to deliver critical care to the same patient on the same day, the time spent by those practitioners could be aggregated to meet the time requirement to bill CPT code 99291. Under our proposal, once the cumulative required critical care service time is met to report CPT code 99291, CPT code 99292 would not be reported by the practitioner or another practitioner in the same specialty and group unless and until an additional 30 minutes of critical care services are furnished to the same patient on the same day (114 total minutes). Finally, consistent with our current policy, we are proposing that the aggregated time spent on critical care visits must be medically necessary and each visit must meet the definition of critical care in order to add the times for purposes of meeting the time requirement to bill CPT code 99291. We are seeking comment on this proposal to better understand current clinical practice for critical care, and when it would be appropriate for more than one physician or NPP of the same or different specialties, and within the same or a different group, to provide Start Printed Page 39210 critical care services to a patient on a single day.
e. Split (or Shared) Critical Care Services
Under current CMS policy, critical care services cannot be billed as split (or shared) E/M services. As previously discussed in section II.F.1. of this proposed rule for split (or shared) visits, we believe the practice of medicine has evolved toward a more team-based approach to care, and greater integration in the practice of physicians and NPPs, particularly when care is furnished by clinicians in the same group in the facility setting. Given this evolution in medical practice, the concerns that may have been present when we issued current policy may no longer be as relevant. We understand that there have been changes in the practice of medicine over the past several years, some facilitated by the advent of EHRs and other systems, toward a more team-based approach to care. There has also been an increase in alternative payment models that employ a more team-based approach to care. In considering and reevaluating this policy, we believe it would be appropriate to revise our policy to allow critical care services to be reported when furnished as split (or shared) services. Therefore, we are proposing that critical care visits may be furnished as split (or shared) visits. The proposals described in section II.F.1. of this proposed rule for split (or shared) visits would apply (with one exception discussed below), and service time would be counted for CPT code 99292 in the same way as for prolonged E/M services. In other words, we are proposing that the total critical care service time provided by a physician and NPP in the same group on a given calendar date to a patient would be summed, and the practitioner who furnishes the substantive portion of the cumulative critical care time would report the critical care service(s).
In section II.F.1. of this proposed rule, drawing on the CPT E/M Guidelines, we proposed a list of activities that could count toward total time for purposes of determining the substantive portion. We stated that since critical care services can include additional activities that are bundled into the critical care visits code(s), we are proposing a different listing of qualifying activities for split (or shared) critical care. These qualifying activities are described in prefatory language on pp. 31-32 of the CPT Codebook. Thus, when critical care services are furnished as a split (or shared) visit, we are proposing to define the substantive portion as more than half the cumulative total time in qualifying activities that are included in CPT codes 99291 and 99292. Additionally, the billing practitioner would first report CPT code 99291 and, if 75 or more cumulative total minutes were spent providing critical care, one or more units of CPT code 99292. We would require practitioners to include the proposed split (or shared) visit modifier on the claim, and we are proposing that the documentation and other rules proposed in section II.F.1. of this proposed rule for split (or shared) visits would apply to split critical care services. We note that, in contrast to our proposals regarding concurrent critical care services above, we are proposing that when a critical care service is furnished as a split (or shared) visit, when two or more practitioners spend time jointly meeting with or discussing the patient, the time may be counted only once for purposes of reporting the split (or shared) critical care visit. This proposed policy accords with our proposed policy for all split (or shared) visits. It also accords with the CPT E/M Guidelines stating that, for split (or shared) visits, when two or more individuals jointly meet with or discuss the patient, only the time of one individual should be counted).[32]
We are seeking comment on these proposals to ensure they reflect a clinically appropriate approach, and intend to assess whether we should instead require that an individual physician or NPP directly perform the entirety of each critical care visit. We are seeking comment on this proposal to better understand current clinical practice for critical care, and when it would be appropriate for more than one physician or NPP of the same or different specialties, and within the same or a different group, to provide critical care to a patient.
f. Critical Care Visits and Same-Day Emergency Department, Inpatient or Office/Outpatient Visits
The CPT Codebook states that critical care and other E/M services may be provided to the same patient on the same date by the same individual. However, our general policy as described in the Medicare Claims Processing Manual states that physicians in the same group who are in the same specialty must bill and be paid for services under the PFS as though they were a single physician. If more than one E/M visit is provided on the same day to the same patient by the same physician, or by more than one physician in the same specialty in the same group, only one E/M service may be reported unless the E/M services are for unrelated problems. Instead of billing separately, the physicians should select a level of service representative of the combined visits and submit the appropriate code for that level.[33] This policy is intended to ensure that multiple E/M visits for a patient on a single day are medically necessary and not duplicative. With respect to office/outpatient E/M visits specifically, our current manual instructs, "As for all other E/M services except where specifically noted, the Medicare Administrative Contractors (MACs) may not pay two E/M office visits billed by a physician (or physician of the same specialty from the same group) for the same beneficiary on the same day unless the physician documents that the visits were for unrelated problems in the office, off campus-outpatient hospital, or on campus-outpatient hospital setting which could not be provided during the same encounter."[34] With respect to hospital visits, hospital ED visits, and critical care services furnished on the same day, the Medicare Claims Processing Manual states, "When a hospital inpatient or office/outpatient E/M service are furnished on a calendar date at which time the patient does not require critical care and the patient subsequently requires critical care both the critical care services (CPT codes 99291 and 99292) and the previous E/M service may be paid on the same date of service. Hospital ED services are not paid for the same date as critical care services when provided by the same physician to the same patient."[35]
We are concerned that adopting the CPT rule that critical care and other E/M visits may be furnished to the same patient on the same date by the same practitioner could have unintended consequences for the Medicare program. We have previously expressed concerns that multiple E/M visits by the same practitioner, or by practitioners in the same specialty within a group, on the same day as another E/M service ordinarily would not be medically necessary (83 FR 59639). It is possible that adopting the CPT rule allowing billing for critical care and other E/M Start Printed Page 39211 visits on the same day, by practitioners in the same group and of the same specialty, could lead to duplicative payment, particularly given the frequently long duration of critical care services, the CPT prefatory language indicating that time spent furnishing critical care may be non-continuous, and the relatively higher valuation of critical care services compared to other E/M services. Thus, we are proposing that no other E/M visit can be billed for the same patient on the same date as a critical care service when the services are furnished by the same practitioner, or by practitioners in the same specialty in the same group.
There are possible alternative approaches to address our concerns about medical necessity and duplicative payment for E/M services furnished to a patient on the same day by the same practitioner or a practitioner in the same group. We have previously considered a Multiple Procedure Payment Reduction (MPPR) for standalone office/outpatient E/M visits that occur on the same day as a procedure to address efficiencies (for example, in preservice and postservice clinician work and PE) that are not accounted for in the current payment rates (83 FR 59639). These visits could be identified on the claim with modifier −25 (significant, separately identifiable E/M service by the same physician on the same day of the procedure or other service) and CMS could assign a reduced payment rate to one of the visits. CMS could also use documentation requirements to support the medical necessity and non-duplicative nature of a claim for critical care services on the same calendar date as another E/M visit provided to a patient by the same practitioner or practitioner of the same specialty in a group. We also recognize that our proposal not to allow an E/M visit to be billed for the same patient on the same date as a critical care service when the services are furnished by the same practitioner, or by practitioners in the same specialty within a group, may be appropriate only in certain clinical situations. For example, it may be possible that a patient would not require critical care services at the time of an ED visit, but then be admitted to the hospital on the same calendar date as the ED visit and require care that meets the definition of critical care services. It may also be possible that the practitioner who furnished the ED visit later provided critical care services to the same patient on the same calendar date. Thus, we are seeking comment on this proposal to better understand clinical practice for critical care, whether and how CMS could pay for E/M services furnished on the same date as critical care services when provided by the same practitioner, or practitioners in the same specialty within a group, while also reducing the potential for duplicative payment.
g. Critical Care Visits and Global Surgery
Critical care services may be needed on the on the same calendar date as a procedure code with a global surgical period. In many cases, preoperative and postoperative critical care visits are included in procedure codes that have a global surgical period. In the CY 2015 PFS final rule, we discussed the challenges of accurately accounting for the number of visits included in the valuation of 10- and 90-day global packages (79 FR 67548, 67582). The 10- and 90-day global packages can include critical care visits. We finalized a policy to change all global periods to 0-day global periods, and to allow separate payment for post-operative E/M visits. Our concerns were based on a number of key points including: The lack of sufficient data on the number of visits typically furnished during the global periods, questions about whether we will be able to adjust values on a regular basis to reflect changes in the practice of medicine and health care delivery, and concerns about how our global payment policies could affect the services that are actually furnished. Section 1848(c)(8)(B) of the Act, which was added by section 523(a) of the Medicare Access and CHIP Reauthorization Act (MACRA), required us to collect data to value surgical services. Because critical care visits are included in some 10- and 90-day global packages, we are proposing to bundle critical care visits with procedure codes that have a global surgical period. We note that this proposal contrasts with the current policy as described in the Medicare Claims Processing Manual which states that critical care visits are unbundled from procedures with a global surgical period as long as the critical care service was unrelated to the procedure.[36] As we have made clear in previous rulemaking, we are continuing to assess values for global surgery procedures (84 FR 2452), including in particular the number and level of preoperative and postoperative visits, which can include critical care services. Because this work is still ongoing, we are proposing to bundle critical care visits with procedure codes that have a global surgical period.
h. Documentation Requirements
Because critical care is a time-based service, we are proposing to require practitioners to document in the medical record the total time that critical care services were provided by each reporting practitioner (not necessarily start and stop times). The documentation would also need to indicate that the services furnished to the patient, including any concurrent care by the practitioners, were medically reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member. To support coverage and payment determinations regarding concurrent care, services would need to be sufficiently documented to allow a medical reviewer to determine the role each practitioner played in the patient's care (that is, the condition or conditions for which the practitioner treated the patient). To support coverage and payment determinations regarding split (or shared) critical care services, the documentation requirements proposed above for all split (or shared) E/M visits would also apply to critical care visits (see section II.F. of this proposed rule).
3. Payment for the Services of Teaching Physicians
As part of the CPT office/outpatient E/M visit coding framework that we finalized beginning for CY 2021 (85 FR 84548 through 84574), practitioners can select the office/outpatient E/M visit level to bill, based either on the total time personally spent by the reporting practitioner or MDM. Stakeholders have asked us how teaching physicians who involve residents in furnishing care should consider time spent by the resident in selecting the office/outpatient E/M visit level.
For teaching physicians, section 1842(b) of the Act specifies that in the case of physicians' services furnished to a patient in a hospital with a teaching program, the Secretary shall not provide payment for such services unless the physician renders sufficient personal and identifiable physicians' services to the patient to exercise full, personal control over the management of the portion of the case for which payment is sought.
Regulations regarding PFS payment for teaching physician services are codified in 42 CFR part 415. In general, under § 415.170, payment is made under the PFS for services furnished in a teaching hospital setting if the services are personally furnished by a physician Start Printed Page 39212 who is not a resident, or the services are furnished by a resident in the presence of a teaching physician, with exceptions as specified in subsequent regulatory provisions in part 415. Medicare separately pays for the time spent by the resident through direct graduate medical education (GME) under Medicare Part A.
a. General Policy for Evaluation and Management Visits
Under our regulation at § 415.172 and absent a public health emergency (PHE), if a resident participates in a service furnished in a teaching setting, a teaching physician can bill for the service only if they are present for the key or critical portion of the service. For residency training sites that are located outside a metropolitan statistical area, PFS payment may also be made if a teaching physician is present through audio/video real-time communications technology (that is, "virtual presence"). In the case of E/M services, the teaching physician must be present during the portion of the service that determines the level of service billed.
We are proposing that when total time is used to determine the office/outpatient E/M visit level, only the time that the teaching physician was present can be included. We believe it is appropriate to include only the time of the teaching physician because the Medicare program makes separate payment for the program's share of the resident's graduate medical training program, which includes time spent by a resident furnishing services with a teaching physician, under Medicare Part A. During the PHE, the time of the teaching physician when they are present through audio/video real-time communications technology may also be included in the total time considered for visit level selection. We note that, outside the circumstances of the COVID-19 PHE, the teaching physician presence requirement can be met virtually, through audio/video real-time communications technology, only in residency training sites that are located outside of a metropolitan statistical area.
This proposal is consistent with our previously finalized policy that practitioners can use total time personally spent by the reporting practitioner to select office/outpatient E/M visit level. It is also consistent with our regulation at § 415.172 that states that PFS payment is made when a teaching physician involves a resident in providing care only if the teaching physician is present for the key or critical portions of the service, including the portion that is used to select the visit level.
b. Primary Care Exception Policy
The regulation at § 415.174 sets forth an exception to the conditions for PFS payment for services furnished in teaching settings in the case of certain E/M services furnished in certain primary care centers. Under the so-called "primary care exception," Medicare makes PFS payment in certain teaching hospital primary care centers for certain services of lower and mid-level complexity furnished by a resident without the physical presence of a teaching physician. We expanded the list of services that residents could furnish without the physical presence of the teaching physician for the duration of the PHE to include all levels of an office/outpatient E/M visit, among other services. Upon the conclusion of the PHE, levels 4-5 office/outpatient E/M visits will no longer be included in the primary care exception (85 FR 84585 through 84590).
Section 415.174(a)(3) requires that the teaching physician must not direct the care of more than four residents at a time, and must direct the care from such proximity as to constitute immediate availability (that is, provide direct supervision), and must review with each resident during or immediately after each visit, the beneficiary's medical history, physical examination, diagnosis, and record of tests and therapies. Section 415.174(a)(3) also requires that the teaching physician must have no other responsibilities at the time, assume management responsibility for the beneficiaries seen by the residents, and ensure that the services furnished are appropriate.
We are proposing that under the primary care exception, only MDM can be used to select office/outpatient E/M visit level. The intent of the primary care exception as described in § 415.174 is that E/M visits of lower and mid-level complexity furnished by residents are simple enough to permit a teaching physician to be able to direct and manage the care of up to four residents at any given time and direct the care from such proximity as to constitute immediate availability. In the context of teaching hospital primary care centers that are staffed by residents and teaching physicians, we believe that MDM would be a more accurate indicator of the complexity of the visit as opposed to time. Because residents are in training, they may need more time than is reflected in the code descriptor to furnish a visit that has a low-level of medical decision making. For example, CPT code 99213 (Office or other outpatient visit for the evaluation and management of an established patient, which requires a medically appropriate history and/or examination and low level of medical decision making. When using time for code selection, 20-29 minutes of total time is spent on the date of the encounter) involves a low level of MDM and between 20-29 minutes of total time. If time was used for level selection instead of MDM, it is possible that residents may need more than 20-29 minutes of time, including any conferring with the teaching physician, to furnish CPT code 99213. Thus, residents may be less efficient relative to a teaching physician in furnishing care.
Office/outpatient E/M visits requiring 30 or more minutes of total time are described by visit levels 4-5. After the expiration of the COVID-19 PHE, office/outpatient levels 4-5 will no longer be included in the primary care exception. In the CY 2021 PFS final rule, we expressed concern that the teaching physician may not be able to maintain sufficient personal involvement in all of the care to warrant PFS payment for the services being furnished by up to four residents when some or all of the residents might be furnishing services that are more than lower and mid-level complexity. We noted that when the teaching physician is directing the care of a patient that requires moderate or higher medical decision-making, the ability to be immediately available to other residents could be compromised, potentially putting patients at risk (85 FR 84586). Thus, to guard against the possibility of residents furnishing visits that are of more than lower and mid-level complexity, we are proposing that only MDM may be used for office/outpatient E/M visit level selection for services furnished by residents under the primary care exception.
We acknowledge that under the new CPT office/outpatient E/M visit coding framework, it is possible that time is an accurate indicator of the complexity of the visit. Thus, we are seeking comment on this proposal, including our assumption that MDM is a more accurate indicator of the appropriate level of the visit relative to time in the context of the primary care exception for services furnished by residents and billed by teaching physicians in primary care centers. We are also seeking comment on whether time is an accurate indicator of the complexity of the visit and how teaching physicians might select office/outpatient E/M visit level using time when directing the care of a patient that is being furnished by a resident in the context of the primary care exception. Start Printed Page 39213
G. Billing for Physician Assistant (PA) Services
Under the respective Medicare statutory benefit categories for services of Physician assistants (PAs), nurse practitioners (NPs), and clinical nurse specialists (CNSs), these practitioners are authorized to furnish services that would be physicians' services if they were furnished by a physician, and which they are legally authorized to perform by the state in which the services are furnished; and such services that are furnished incident to the practitioners' professional services (but only if no facility or other provider charges or is paid any amount for the services). Additionally, the payment amount for the services of PAs, NPs, and CNSs, as specified under section 1833(a)(1)(O) of the Act, is equal to 80 percent of the lesser of the practitioner's actual charge or 85 percent of the amount that would be paid to a physician under the PFS. However, while NPs and CNSs are authorized to bill the Medicare program and be paid directly for their professional services, section 1842(b)(6)(C)(i) of the Act has required since the inception of the PA benefit (with a narrow exception not relevant here) that payment for PA services must be made to the PA's employer. Accordingly, our regulation at § 410.74(a)(2)(v) specifies that PA services are covered under Medicare Part B only when billed by the PA's employer. Our regulation that addresses to whom Medicare Part B payment is made, at § 410.150(b)(15), further provides that payment is made to the qualified employer of a PA, and specifies that the PA could furnish services under a W-2 employment relationship, an employer-employee relationship, or as an independent contractor through a 1099 employment relationship. The regulation also specifies that a group of PAs that incorporate to bill for their services is not a qualified employer. Given the statutory requirement that we make payment to the PA's employer, PAs are precluded from directly billing the Medicare program and receiving payment for their services, and do not have the ability to reassign Medicare payment rights for their services to any employer, facility, or billing agent.
Section 403 of the Consolidated Appropriations Act, 2021 (CAA) (Pub. L. 116-260, December 27, 2020), amends section 1842(b)(6)(C)(i) of the Act to remove the requirement to make payment for PA services only to the employer of a PA effective January 1, 2022. With the removal of this requirement, PAs will be authorized to bill the Medicare program and be paid directly for their services in the same way that NPs and CNSs do. Effective with this amendment, PAs also may reassign their rights to payment for their services, and may choose to incorporate as a group comprised solely of practitioners in their specialty and bill the Medicare program, in the same way that NPs and CNSs may do. We note that the amendment made by section 403 of the CAA changed only the statutory billing construct for PA services. It neither changed the statutory benefit category for PA services, including the requirement that PA services are performed under physician supervision, at section 1861(s)(2)(K)(i) of the Act, nor did it change the statutory payment percentage applicable to PA services specified in section 1833(a)(1)(O) of the Act.
We are proposing to amend pertinent sections of our regulations to reflect the amendment made by section 403 of the CAA. Specifically, we are proposing to amend § 410.74(a)(2)(v) to specify that the current requirement that PA services must be billed by the PA's employer in order to be covered under Medicare Part B is effective only until January 1, 2022. We are also proposing to amend § 410.150(b) to redesignate the current requirements in paragraph (b)(15) as § 410.150(b)(15)(i), and to amend that paragraph to provide that Medicare payment is made for PA services to the qualified employer of the PA for services furnished prior to January 1, 2022. In § 410.150, we further propose to add a new paragraph (b)(15)(ii) to state that, effective for services furnished on or after January 1, 2022, payment is made to a PA for their professional services, including services and supplies furnished incident to their services. We would conform this new paragraph with the regulation at § 410.150(b)(16) regarding to whom payment is made for NP or CNS services. As such, the proposed new paragraph at § 410.150(b)(15)(ii) would provide that payment will be made to a PA for professional services furnished by a PA in all settings in both rural and non-rural areas; and that payment is made only if no facility or other provider charges or is paid any amount for services furnished by a PA. We also intend to update our program manual instructions to reflect the statutory change made by section 403 of the CAA and the changes to our regulations.
H. Therapy Services
We are implementing the third and final part of the amendments made by section 53107 of the Bipartisan Budget Act (BBA of 2018) (Pub. L. 115-123, February 9, 2018). The BBA of 2018 added a new section 1834(v) of the Act. Section 1834(v)(1) of the Act requires CMS to make a reduced payment for physical therapy and occupational therapy services furnished in whole or in part by physical therapist assistants (PTAs) and occupational therapy assistants (OTAs) at 85 percent of the otherwise applicable Part B payment for the service, effective January 1, 2022.
Section 1834(v)(2) of the Act requires that: (1) By January 1, 2019, CMS must establish a modifier to indicate that a therapy service was furnished in whole or in part by a PTA or OTA; and, (2) beginning January 1, 2020, each claim for a therapy service furnished in whole or in part by a PTA or an OTA must include the modifier. Section 1834(v)(3) of the Act requires CMS to implement these amendments through notice and comment rulemaking.
In the CY 2019 PFS final rule (83 FR 59654 through 59660), we established the CQ and CO modifiers that were required to be used by the billing practitioner or therapy provider to identify therapy services provided in whole or in part by PTAs and OTAs, respectively, beginning January 1, 2020. We require these payment modifiers to be appended on claims for therapy services, alongside the GP and GO therapy modifiers which are used to indicate the services are furnished under a physical therapy or occupational therapy plan of care, respectively. The payment modifiers are defined as follows:
- CQ modifier: Physical therapy services furnished in whole or in part by PTAs.
- CO modifier: Occupational therapy services furnished in whole or in part by OTAs. In the CY 2019 PFS final rule (83 FR 59654 through 59660), we did not finalize our proposed definition of "furnished in whole or in part by a PTA or OTA" as a service for which any minute of a therapeutic service is furnished by a PTA or OTA. Instead, in response to public comments, we finalized a de minimis standard under which a service is considered to be furnished in whole or in part by a PTA or OTA when more than 10 percent of the service is furnished by the PTA or OTA.
In the CY 2019 PFS proposed and final rules (83 FR 35850 through 35852, and 83 FR 59654 through 59660, respectively), we explained that the CQ and CO modifiers would not apply to claims for outpatient therapy services that are furnished by, or incident to, the services of, physicians or NPPs including NPs, PAs, and CNSs. This is because our outpatient physical and Start Printed Page 39214 occupational therapy services regulations require that the individual who performs outpatient therapy services incident to the services of a physician or NPP must meet the qualifications and standards for a therapist (other than state licensure). As such, only therapists, and not therapy assistants, can perform outpatient therapy services incident to the services of a physician or NPP (83 FR 59655 through 59656); and the modifiers to describe services furnished in whole or in part by a PTA or OTA are not applicable to the claim for a therapy service billed by a physician or NPP incident to their professional services. We indicated that we would add this distinction in the provision of the Medicare Benefit Policy Manual (MBPM) Chapter 15 that discusses therapy services furnished incident to the physician's or NPP's services at section 230.5, as well as the sections that discuss PTA and OTA services at sections 230.1 and 230.2, respectively.
In the CY 2020 PFS proposed and final rules (84 FR 40558 through 40564 and 62702 through 62708, respectively), we explained that the CQ/CO modifiers and the de minimis policy would apply to both untimed and timed codes. The untimed codes are evaluation and reevaluation codes, group therapy and supervised modalities, and when these are billed, only one unit is reflected in the "units" portion of the claim. When the PTA/OTA provides more than 10 percent of the service, the code is billed with a CQ/CO modifier. For timed codes, that is, those codes defined in 15-minute increments, the services are typically performed in multiple units of the same and/or different codes for a patient on one treatment day. We explained that under our policy, the therapist or therapy assistant needs to find the total time of all these 15-minute timed codes in order to determine the number of units that can be billed for that day. For example, if the PT/OT and/or the PTA/OTA, as appropriate, furnished between 8 minutes through 22 minutes, one unit can be billed; if 23 minutes through 37 minutes are provided, 2 units can be billed; if 38 minutes through 52 minutes are furnished, 3 units can be billed. Once the total number of units to bill is determined, the qualified professional (therapist or assistant) then needs to decide whether the CQ/CO modifier is applicable.
In the CY 2020 PFS proposed rule (84 FR 40558 through 40564), we proposed that the time the PTA/OTA spent together with the PT/OT in performing a service, as well as the time the PTA/OTA spent independent of the PT/OT treating the patient, is considered time for which the service is furnished in whole or in part by the PTA/OTA. As explained in the CY 2020 PFS final rule (84 FR 62702 through 62708), many commenters objected to our proposal to include as time that the therapy service is furnished "in whole or in part" by the PTA/OTA both the minutes spent by the PTA/OTA concurrently with and separately from the therapist. These commenters also expressed concerns that this policy would unfairly discount services that are fully furnished by therapists, and in which the therapy assistant supports them while they provide a service. We were persuaded by commenters to finalize a policy to not include as minutes furnished in whole or in part by a PTA/OTA the minutes in which the PTA/OTA worked concurrently with the PT/OT. We agreed with the commenters that when a therapy assistant and therapist furnish care to a patient at the same time, the patient requires both professionals, and this reflects a clinical scenario where the assistant is helping the therapist to provide a highly skilled procedure or one in which both professionals are needed for safety reasons. We modified our proposed regulation text at §§ 410.59 (outpatient occupational therapy), 410.60 (physical therapy), and 410.105 (for PT and OT CORF services) accordingly.
For purposes of deciding whether the 10 percent de minimis standard is exceeded, we offered two different ways to compute this.
- The simple method: Divide the total of the PTA/OTA + PT/OT minutes by 10, round to the nearest integer then add 1 minute to get the number of minutes needed to exceed the de minimis standard at and above which the CQ/CO modifier applies.
- The percentage method: Divide the PTA/OTA minutes by the sum of the PTA/OTA and therapist minutes and then multiply this number by 100 to calculate the percentage of the service that involves the PTA/OTA, if this number is greater than 10 percent the CQ/CO modifier applies.
Hypothetical examples of each of these methods are included later in this section. In response to our proposal that all the units of one service needed to be considered when determining if the de minimis is applied, commenters requested that we consider each 15-minute unit instead—noting that they would be able to apply the CQ/CO modifier on one claim line for a service that was provided by the PTA/OTA and report another claim line without the CQ/CO for the service provided by the PT/OT. We were persuaded by stakeholders, and finalized a policy under which the de minimis standard is applied for each 15-minute unit of a service. This allows the separate reporting, on two different claim lines, of the number of 15-minute units of a code to which the therapy assistant modifiers do not apply, and the number of 15-minute units of a code to which the therapy assistant modifiers do apply. However, we neglected to modify the text of our regulations to reflect this final policy for applying the de minimis standard; therefore, we are proposing to revise our regulation text to specify that the de minimis rule is applied to each 15-minute unit of a service, rather than to all the units of a service at §§ 410.59(a)(4)(iii)(B), 410.60(a)(4)(iii)(B), and 410.105(d)(3)(ii). The specific proposed revisions are discussed below.
To recap, we finalized a de minimis standard to identify when the CQ/CO modifiers apply and when they do not apply as follows:
- Portions of a service furnished by the PTA/OTA independent of the physical therapist/occupational therapist, as applicable, that do not exceed 10 percent of the total service (or 15-minute unit of a service) are not considered to be furnished in whole or in part by a PTA/OTA, so are not subject to the payment reduction;
- Portions of a service that exceed 10 percent of the total service (or 15-minute unit of a service) when furnished by the PTA/OTA independent of the therapist must be reported with the CQ/CO modifier, alongside of the corresponding GP/GO therapy modifier; are considered to be furnished in whole or in part by a PTA/OTA, and are subject to the payment reduction; and
- Portions of a service provided by the PTA/OTA together with the physical therapist/occupational therapist are considered for this purpose to be services provided by the therapist.
In the CY 2020 PFS proposed rule (84 FR 40558 through 40564), we proposed to adopt a documentation requirement that a short phrase or statement must be added to the daily treatment note to explain whether the therapy assistant modifier was or was not appended for each therapy service furnished. We also sought comment on whether it would be appropriate to also require documentation of the minutes spent by the therapist or therapy assistant along with the CQ/CO modifier explanation as a means to avoid possible additional burden associated with a contractor's medical review process conducted for these services. Many commenters stated that: (1) The statute does not require documentation to explain why a Start Printed Page 39215 modifier was or was not applied for each code; (2) the proposed documentation requirements are exceedingly burdensome and conflict with the agency's "Patients over Paperwork Initiative"; (3) the proposed documentation requirement that calls for a narrative phrase in the treatment note and requires documentation of the minutes is duplicative of current requirements that requires adding the total timed code minutes and total treatment time (includes timed and untimed codes) to the daily treatment note; and, (4) the Medicare Benefit Policy Manual (MBPM) already includes extensive documentation requirements. In response to the feedback, we did not finalize the proposed documentation requirement; nor did we finalize a requirement that the therapist and therapy assistant minutes be included in the documentation. Instead, we reminded therapists and therapy providers that correct billing requires sufficient documentation in the medical record to support the codes and units reported on the claim, including those reported with and without an assistant modifier. Further, in agreement with many commenters, we clarified that we would expect the documentation in the medical record to be sufficient to know whether a specific service was furnished independently by a therapist or a therapist assistant, or was furnished "in part" by a therapist assistant, in sufficient detail to permit the determination of whether the 10 percent standard was exceeded.
In the CY 2020 PFS proposed rule, we also provided multiple typical clinical billing scenarios to illustrate when the CQ/CO modifier would and would not be applicable. Because these clinical scenarios did not convey our finalized policies as modified in response to public comments, we indicated in the CY 2020 PFS final rule that we would provide further detail regarding the clinical scenario examples to illustrate how to use the therapy assistant modifiers through information we would post on the cms.gov website. We clarified that our revised finalized policy applied generally in the same way as illustrated in those examples, except for the difference in the minutes of time that are counted toward the 10 percent standard (not counting the minutes furnished together by a therapist and therapy assistant), the application of the 10 percent standard to each billed unit of a timed code rather than to all billed units of a timed code, and the billing on two separate claim lines of the units of a timed code to which the therapy assistant modifiers do and do not apply.
In early March 2021, we posted on our Therapy Services website at https://www.cms.gov/Medicare/Billing/TherapyServices general guidance on how to assign the CQ/CO modifiers for multiple billing scenarios. In the guidance, we provided general examples for 8 different billing scenarios in which multiple units of 15-minute codes are provided by PTs/OTs and PTAs/OTAs and one billing example that used the untimed code for group therapy performed for equal minutes by a PT and a PTA.
We noted that prior to applying our rules to determine appropriate application of the CQ/CO modifiers, the PTA/OTA or PT/OT first needs to determine how many 15-minute units can be billed in a single treatment day for a patient. For information on this topic, we referred readers to the chart in section 20.2.C of Chapter 5 of the Medicare Claims Processing Manual (MCPM) that describes how to count minutes for timed codes defined by 15-minute units, since the therapist or assistant should use the same counting rule, commonly known as the "8-minute rule," that they have used previously.
Once the therapist or therapy assistant has identified the number of 15-minute units that can be billed for a patient on a single treatment day, we provided the following information to clarify how to apply our policy for application of the CQ and CO modifiers, as follows:
Step 1. Identify the Timed HCPCS Codes Furnished for 15 Minutes or More: List the code numbers of each of the services furnished along with the number of minutes in total done by the PT, PTA, OT, or OTA. When a PT, PTA, OT, or OTA provides at least 15 minutes and less than 30 minutes of a service on a single treatment day, assign 1 unit; when multiples of 15 minutes are furnished, for example, 30 minutes (assign 2 units) and 45 minutes (assign 3 units), etc. This needs to be the first step whenever it is applicable to the billing scenario. When any of these services, that is, full 15-minute increments, are provided by a PTA/OTA, the CQ/CO modifiers apply.
Step 2. Identify Services for Which the PT/OT and PTA/OTA Provide Minutes of the Same HCPCS Code: After applying Step 1, where applicable, identify any minutes (including remaining minutes from Step 1) performed by a PT/OT and PTA/OTA for the same service/code. Add the minutes furnished by the PT/OT and the PTA/OTA together, then divide the total by 10 and round to the nearest integer—this is the 10 percent de minimis time standard. Then add 1 minute to get the fewest number of minutes performed by the PTA/OTA that would exceed the 10 percent time standard for that service—if the PTA/OTA minutes meet or exceed this number, the CQ/CO modifier would be appended. This is the "simple" method for calculating the de minimis number of minutes.
Step 3. Identify Services Where the PT/OT and PTA/OTA Furnish Services of Two Different Timed HCPCS Codes: After applying Step 1 for each service, compare the remaining minutes furnished by the PT/OT for one service with the remaining minutes furnished by the PTA/OTA for a different service. Assign the CQ/CO modifier to the service provided by the PTA/OTA when the time they spent is greater than the time spent by the PT/OT performing the different service. The CQ/CO modifier does not apply when the minutes spent delivering a service by the PT/OT are greater than the minutes spent by the PTA/OTA delivering a different service.
Step 4. Identify the Different HCPCS Codes Where the PT/OT and the PTA/OTA Each Independently Furnish the Same Number of Minutes: Once Step 1 is completed for each service (when applicable), and when the remaining minutes for each service—one provided by the PT/OT and the other provided by the PTA/OTA—are the same, either service may be billed. If the service provided by the PT/OT is billed, the CQ/CO modifier does not apply. However, if the service provided by the PTA/OTA is billed, the CQ/CO modifier does apply.
The below two examples are taken from our guidance on the CMS website. These are examples of when the PT and PTA provide minutes of the same service:
Example #1
PTA—23 minutes 97110
PT—13 minutes 97110
PT—30 minutes 97140
Total = 66 minutes—qualifies for billing 4 units (53 minutes through 67 minutes).
Billing Explanation:
- First Step: Assign units to services based on those that have at least 15 minutes or codes that were provided in multiples of 15 minutes. For 97110, assign one unit of 97110 with the CQ modifier because the PTA furnished at least 15 minutes of 97110 (therapeutic exercise). Then, assign two units of 97140 without the modifier, because the PT furnished the full 30 minutes of manual therapy.
- Second Step: Determine if the PTA furnished more than 10 percent of the remaining minutes of the 97110 service. To do this via the simple method: Add Start Printed Page 39216 the PTA's 8 remaining minutes to the PT's 13 minutes for a total time of 21 minutes. Divide the total by 10 to get 2.1 minutes and round to the nearest integer, which is 2 minutes (the 10 percent time standard for this service). Add 1 minute to find the threshold number of minutes that would exceed the de minimis standard, which in this example is 3 minutes. Using the percentage method, divide the PTA's remaining 8 minutes by the total 21 minutes of the service (8 PTA + 13 PT = 21 minutes) to get 0.38, then multiply the result × 100 = 38 percent.
Final Step: Because 8 minutes meets or exceeds the 3-minute threshold, and 38 percent is greater than 10 percent, a second unit of 97110 is billed with the CQ modifier.
Example #2
PTA—19 minutes of 97110
PT—10 minutes of 97110
Total = 29 minutes—two units of 97110 can be billed (23 minutes through 37 minutes).
Billing Explanation:
- First Step: Bill one unit of 97110 with the CQ modifier because a full 15 minutes was provided by the PTA, with 4 minutes remaining.
- Second Step: Determine if the PTA's 4 remaining minutes exceed the 10 percent de minimis standard. Simple method: Add together the PTA's 4 remaining minutes and the 10 PT minutes to get the total time of 14 minutes and divide by ten to get 1.4 minutes and round to the nearest integer = 1 minute to get the 10 percent de minimis standard. Then add 1 minute to get a threshold minimum of 2 minutes for PTA time. If the PTA minutes are at or above the threshold, the CQ modifier applies. Percentage method: Divide the PTA's 4 remaining minutes by the total time of 14 to get 0.29 then multiply by 100 = 29 percent. If the resulting percentage is greater than 10 percent, the PTA modifier applies.
- Final Step: Bill another unit of 97110 with the CQ modifier since 4 minutes is greater than the 2-minute threshold minimum and 29 percent is greater than 10 percent.
After reviewing the information posted on the CMS Therapy Services web page, therapy stakeholders reached out to CMS to express concern that certain aspects of the billing scenarios described in the guidance contradict their interpretation of our de minimis policy, especially as it applies to a final unit of a multiple-unit timed service. The therapy stakeholders suggested that the guidance we offered would lead to confusion for the same-service billing scenarios (including examples #1 and #2 above). We consider the unit of measure for a timed therapy service code to be 15 minutes. In billing scenarios with multiple units, we would consider the combined time for same or different services in 15-minute unit increments.
The stakeholders agree that the de minimis standard is applied to the last unit of a timed therapy service code in two separate cases. The first case happens when the PTA/OTA and the PT/OT each furnish less than 8 minutes for that final unit of a service. For example, if the PTA/OTA provided 7 minutes and the PT/OT furnished 5 minutes—using the simple method: 12 minutes divided by 10 equals 1.2, rounded to the nearest integer is 1, plus 1 equals 2—if the PTA/OTA provides 2 or more minutes, the CQ/CO modifier is applied. The second case occurs when the PTA/OTA provides 8 or more minutes and the PT/OT furnishes less than 8 minutes—in which event, the de minimis standard is exceeded and the CQ/CO modifier is applied.
We note that the therapy stakeholders' interpretation of when the de minimis policy applies for a final 15-minute unit of a multiple unit timed service is based on what is commonly termed the "8-minute rule" which recognizes a unit of a 15-minute timed therapy service code as 8 minutes (more than the midpoint of the service or 7.5 minutes), but only when it applies to the final unit billed. Applied to the above two examples, the stakeholders informed us that they believe the second unit of CPT code 97110 in both examples should not be billed with an assistant modifier because the therapist provided enough minutes of the service on their own, that is, 8 minutes or more, to bill for the last unit without the assistant's additional minutes. The stakeholders indicated that the therapist would have a financial incentive to not have the PTA/OTA provide the additional minutes at all if the CQ or CO modifier would apply. We note that, in addition to the two cases discussed above, there is another billing scenario to address in the context of our de minimis policy—specifically, where the PT/OT and PTA/OTA each furnish between 9 and 14 minutes of a 15-minute timed service when the total time of therapy services furnished in combination by the PTA/OTA and PT/OT is at least 23 but no more than 28 minutes, and there are two remaining units left to be billed. These "two remaining unit" cases with time ranges between 9 and 14 minutes include the following PTA/OTA:PT/OT (or vice versa) time splits: 9:14, 10:13, 11:12, 12:12, 12:13, 12:14, 13:13; 13:14; and 14:14.
We believe that the stakeholder's interpretation of the de minimis standard is not consistent with the de minimis policy we finalized in the CY 2020 PFS final rule (84 FR 62702 through 62708). However, in working through the billing scenarios with the stakeholders, we identified where we could make refinements to our policy to address some of the confusion and concerns expressed by stakeholders and to address the "two remaining unit" cases noted above. These refinements may also avoid implementing a payment policy that could be perceived to penalize the provision of additional care by a therapy assistant when those minutes of service would lead to a reduced payment for a unit of a service. The stakeholders criticized the finalized de minimis policy because they believed it provides an inherent financial incentive for the therapist to ensure that PTAs/OTAs provide services in exactly 15-minute intervals—to avoid any leftover PTA/OTA minutes that could necessitate application of the CQ/CO modifier, and reduced payment, for the service that the therapist is also providing—without regard to the clinical needs of the individual patient. The stakeholders suggested that if we were to recognize their "8-minute rule" and recommended policy, we would remove the incentive for the therapist to avoid providing appropriate minutes of therapy services performed by the PTA/OTA.
To address the concerns expressed by the stakeholders and the "two remaining unit" cases we identified in our review, we propose to modify our existing policy, specifically for billing scenarios when only one unit of a timed therapy service remains to be billed (the majority of all billing scenarios) and the "two remaining unit" cases described above. As shown in Table 19, this proposal would require application of the CQ/CO modifier when the PTA/OTA provides at least 8 minutes or more and the PT/OT provides less than 8 minutes of the service; or, when both the PT/OT and the PTA/OTA provide less than 8 minutes of the same service.
Start Printed Page 39217

Under this proposed modification, the CQ/CO modifier would not apply when the PT/OT furnishes 8 minutes or more, or both the PT/OT and the PTA/OTA furnish 8 minutes or more, of a timed service. This proposed "midpoint rule" policy was suggested to us by the therapy stakeholders. We agree that since, in this circumstance, the PT/OT provided enough minutes of the service on their own to bill the last unit of the service, the additional minutes of service performed by the PTA/OTA are not material, and thus, should be disregarded, as shown in the examples in Table 20.

With these proposed policy adjustments, the CQ/CO modifiers apply when the PTA/OTA provides all the minutes of a timed service, and to some services (as illustrated in Table 19) when the PTA/OTA and PT/OT each, independent of the other, furnish portions of the same timed service. The CQ/CO modifiers also apply if the portion of an untimed code furnished by the PTA/OTA exceeds the de minimis standard. The CQ/CO modifiers do not apply when the PTA/OTA and the PT/OT furnish different services. Time spent by the PT/OT and PTA/OTA providing services together is considered time spent by the PT/OT for purposes of applying the de minimis standard. Finally, we propose to modify our policy so that the CQ/CO modifiers would not apply when the PT/OT provides enough minutes of the service on their own to bill for the last unit of a timed service, (more minutes than the midpoint or 8 minutes of a 15-minute timed code) regardless of any additional minutes for the service provided by the PTA/OTA.
Examples of Billing Scenarios using the CQ/CO modifiers when the de minimis standard applies, and the proposed policy for the last billed unit of a service:
Example #A:
PTA—10 minutes of 97110
PT—5 minutes of 97110
Total = 15 minutes—qualifies to bill one 15-minute unit (8 minute to 22 minutes).
Analysis: Bill one unit of 97110 with the CQ modifier because the PTA provided 8 minutes or more and the PT provided less than 8 minutes. The de minimis standard applies in these cases.
Example #B:
PTA—5 minutes of 97110
PT—6 minutes of 97110
Total = 11 minutes—qualifies to bill one 15-minute unit (8 minute through 22 minutes).
Analysis: Bill one unit of 97110 with the CQ modifier because the PTA and the PT both provided less than 8 minutes. In this case, the PT provided 6 minutes and the PTA furnished 5 minutes independent of each other. The de minimis standard applies in these cases.
Example #C:
PTA-22 minutes of 97110
PT—23 minutes of 97110
Total = 45 minutes—qualifies to bill three 15-minute units (38 minutes through 52 minutes).
Analysis:
- Apply Step One of the general policy rules and bill one unit of 97110 with the CQ modifier because the PTA provided 15 full minutes with 7 minutes remaining.
- Apply Step One to the PT's 23 minutes and bill one unit without the assistant modifier with 8 minutes remaining.
- The third unit of 97110 is billed without the assistant modifier because the therapist provided enough minutes (8 or more minutes) without the PTAs minutes to bill the final unit. Start Printed Page 39218
Example #D—also see the below regulatory proposal using this `two remaining unit' example.
PT—12 minutes of 97110
PTA—14 minutes of 97110
PT—20 minutes of 97140
Total = 46 minutes—qualifies to bill three units (38 minutes through 52 minutes).
Analysis:
- Apply Step One of the general policy rules and bill one unit of 97140 without the CQ modifier because the PT provided 15 full minutes of one unit with 5 minutes remaining.
- Two units remain to be billed and the PT and the PTA each provided between 9 and 14 minutes independent of one another with a total time between 23 and 28 minutes—in these "two remaining unit" scenarios, one unit is billed with the CQ modifier for the PTA and the other unit is billed without it for the PT.
- The PT's 5 remaining minutes of 97140 are counted towards the total timed minutes but are not billable in this scenario.
Example #E
OTA—11 minutes of 97535
OT—11 minutes of 97530
Total = 22 minutes—qualifies to bill one (1) unit (8 minutes through 22 minutes).
Billing Analysis:
Since two different services were furnished for an equal number of minutes—the "tie-breaker" scenario applies. Either code 97530 by the OT or code 97535 by the OTA can be billed in accordance with a billing example in the MCPM, Chapter 5, section 20.2.C. Either one unit of 97530 is billed without the CO modifier or one unit of 97535 is billed with the CO modifier.
Example #F: Untimed code—1 unit is billed for all untimed codes including evaluations, reevaluations, supervised modalities, and group therapy.
OTA—20 minutes 97150 independent of the OT
OT—20 minutes 97150 independent of the OTA
Total = 40 minutes of Group Therapy = 1 unit of 97150 is billed for each group member.
Billing Analysis: One unit of group therapy 97150 is billed with the CO modifier because the OTA provided more than the 10 percent time standard in this example. Either method can be used to determine if the OTA's time exceeded the 10 percent time standard for this clinical scenario, see below:
- The simple method: First add the OTA's 20 minutes to the OT's 20 minutes to get 40, then divide by 10 to get 4.0 and add 1 to equal 5 minutes. The OTA's 20 minutes is equal to or greater than 5 minutes so the CO modifier is required on the claim.
- The percentage method: Divide the number of minutes that an OTA independently furnished a service by the total number of minutes the service was furnished as a whole—20 divided by 40 equals 0.50. Then multiple by 100 to get 50 percent, which is greater than 10 percent. The CO modifier is applied to 97150.
- Tie breaker: The tie breaker does not apply in this scenario because the example does not contain two different timed codes described in 15-minute intervals. For "tie breaker" see Example #F above.
As noted above and illustrated in Example #D, there are a finite number of cases where there are two 15-minute units left to bill. In these "two remaining unit" cases, the PTA/OTA and the PT/OT each provide between 9 and 14 minutes with a total time of at least 23 minutes through 28 minutes. Under our proposed policy, one unit of the service would be billed with the CQ/CO modifier for the minutes furnished by the PTA/OTA (who furnished between 9 and 14 minutes of the service), and one unit would be billed without the CQ/CO modifier for the service provided by the PT/OT (who also furnished between 9 and 14 minutes of the same service). This is because the PTA/OTA and the PT/OT each independently furnished part of each unit of the same service, and these cases are not addressed by the proposed midpoint rule that would apply when there is only one single unit left to bill. We are proposing to amend our regulation to address the scenario where there are two remaining 15-minute units of the same service for which the PTA/OTA and the PT/OT each provided between 9 and 14 minutes with a total time of at least 23 minutes and no more than 28 minutes. In this scenario, we propose that one unit of the service would be billed with the CQ/CO modifier and the other unit of the service would be billed without the assistant modifier. We are proposing to add this policy to our regulations at §§ 410.59(a)(4)(v) and 410.60(a)(4)(v) for outpatient occupational therapy and physical therapy services, respectively and at § 410.105(d)(3)(iv) for Comprehensive Outpatient Rehabilitation Facility (CORF) services.
As noted above, when we finalized the policy to consider each 15-minute unit of a service for purposes of determining whether the de minimis standard applies, we neglected to revise our regulations at §§ 410.59, 410.60 and 410.105 to reflect this change. As such, we are proposing to amend the regulations at §§ 410.59(a)(4)(iii)(B) and 410.60(a)(4)(iii)(B) for outpatient occupational therapy and physical therapy services, respectively, and at § 410.105(d)(3)(ii) for CORF services to specify that we consider a service to be furnished in part by a PTA or an OTA when the PTA/OTA furnishes a portion of a service, or in the case of a 15-minute timed code, a portion of a unit of a service, separately from the portion of the service or unit of service furnished by the therapist such that the minutes for that portion of a service or a unit of a service furnished by the PTA/OTA exceed 10 percent of the total minutes for that service or unit of a service.
To accommodate the proposed refinement of the de minimis policy, we are proposing to amend the same regulations at §§ 410.59(a)(4)(iv) and 410.60(a)(4)(iv) for outpatient occupational therapy and physical therapy services, respectively, and at § 410.105(d)(3)(iii) for CORF services to provide that, for the final 15-minute unit billed for a patient for a date of service, when the PT/OT provides more than the midpoint (at least 8 minutes) of a service such that they could bill for the service without any additional minutes being furnished by the PTA/OTA, the service may be billed without a CQ or CO modifier, and any remaining minutes of service furnished by the PTA/OTA are considered immaterial.
Beginning January 1, 2022, therapy services furnished in whole or in part by a PTA or OTA will be identified based on the inclusion by the billing therapy services provider (whether a therapist in private practice or therapy provider) of the CQ or CO modifier, respectively, on claim lines for therapy services, and the payment for those services will be adjusted as required by section 1834(v)(1) of the Act. Per our usual system update process, we plan to issue instructions in a change request to prepare our shared systems and Medicare Administrative Contractors (MACs) to pay the reduced amount for therapy services furnished in whole or in part by a PTA or OTA. We will issue an MLN article once the CR is released, after the CY 2022 PFS final rule is issued.
We are seeking comment on all of our proposals.
I. Changes to Beneficiary Coinsurance for Additional Procedures Furnished During the Same Clinical Encounter as Certain Colorectal Cancer Screening Tests
Section 122 of the Consolidated Appropriations Act (CAA) of 2021, Waiving Medicare Coinsurance for Start Printed Page 39219 Certain Colorectal Cancer Screening Tests, amends section 1833(a) of the Act to offer a special coinsurance rule for screening flexible sigmoidoscopies and screening colonoscopies, regardless of the code that is billed for the establishment of a diagnosis as a result of the test, or for the removal of tissue or other matter or other procedure, that is furnished in connection with, as a result of, and in the same clinical encounter as the colorectal cancer screening test. The reduced coinsurance will be phased-in beginning January 1, 2022. Currently, the addition of any procedure beyond a planned colorectal cancer screening test (for which there is no coinsurance), results in the beneficiary having to pay coinsurance.
Section 1861(pp) of the Act defines "colorectal cancer screening tests" and, under sections 1861(pp)(1)(B) and (C) of the Act, identifies "screening flexible sigmoidoscopy" and "screening colonoscopy" as two of the recognized procedures. During the course of either one of these two procedures, removal of tissue or other matter may become necessary for diagnostic purposes. Among other things, section 1861(pp)(1)(D) of the Act authorizes the Secretary to include in the definition other tests or procedures and modifications to the tests and procedures described under this subsection, with such frequency and payment limits as the Secretary determines appropriate, in consultation with appropriate organizations. Section 1861(s)(2)(R) of the Act includes colorectal cancer screening tests in the definition of the medical and other health services that fall within the scope of Medicare Part B benefits described in section 1832(a)(1) of the Act. Section 1861(ddd)(3) of the Act includes colorectal cancer screening tests within the definition of "preventive services." In addition, section 1833(a)(1)(Y) of the Act provides for payment for a preventive service under the PFS at 100 percent of the lesser of the actual charge or the fee schedule amount for these colorectal cancer screening tests, and under the OPPS at 100 percent of the OPPS payment amount, when the preventive service is recommended by the United States Preventive Services Task Force (USPSTF) with a grade of A or B. As such, there is no beneficiary coinsurance for recommended colorectal cancer screening tests as defined in section 1861(pp)(1) of the Act.
Under these statutory provisions, we have issued regulations governing payment for colorectal cancer screening tests at § 410.152(l)(5). We pay 100 percent of the Medicare payment amount established under the applicable payment methodology for the setting for providers and suppliers, and beneficiaries are not required to pay Part B coinsurance for colorectal cancer screening tests (except for barium enemas, which are not recommended by the USPSTF with a grade of A or B).[37]
In addition to colorectal cancer screening tests, which typically are furnished to patients in the absence of signs or symptoms of illness or injury, Medicare also covers various diagnostic tests (see § 410.32). In general, diagnostic tests must be ordered by the physician or practitioner who is treating the beneficiary and who uses the results of the diagnostic test in the management of the patient's specific medical condition. Under Part B, Medicare may cover flexible sigmoidoscopies and colonoscopies as diagnostic tests when those tests are reasonable and necessary as specified in section 1862(a)(1)(A) of the Act. When these services are furnished as diagnostic tests rather than as screening tests, patients are responsible for the Part B coinsurance (20 or 25 percent depending upon the setting) associated with these services.
We define colorectal cancer screening tests in our regulation at § 410.37(a)(1) to include "flexible screening sigmoidoscopies" and "screening colonoscopies, including anesthesia furnished in conjunction with the service." Under our current regulations, we exclude from the definition of colorectal screening services, colonoscopies and sigmoidoscopies that begin as screening services, but where a polyp or other growth is found and removed as part of the procedure. The exclusion of these services from the definition of colorectal cancer screening tests is based upon longstanding provisions under sections 1834(d)(2)(D) and (d)(3)(D) of the Act dealing with the detection of lesions or growths during procedures (see CY 1998 PFS final rule at 62 FR 59048, 59082 for a more detailed explanation).
Prior to the enactment of section 122 of the CAA, section 1834(d)(2)(D) of the Act provided that if, during the course of a screening flexible sigmoidoscopy, a lesion or growth is detected which results in a biopsy or removal of the lesion or growth, payment under Medicare Part B shall not be made for the screening flexible sigmoidoscopy, but shall be made for the procedure classified as a flexible sigmoidoscopy with such biopsy or removal. Similarly, prior to the recent legislative change, section 1834(d)(3)(D) of the Act provided that if, during the course of a screening colonoscopy, a lesion or growth is detected that results in a biopsy or removal of the lesion or growth, payment under Medicare Part B shall not be made for the screening colonoscopy but shall be made for the procedure classified as a colonoscopy with such biopsy or removal. In these situations, Medicare pays for the flexible sigmoidoscopy and colonoscopy tests as diagnostic tests rather than as screening tests and the 100 percent payment rate for recommended preventive services under section 1833(a)(1)(Y) of the Act, as codified in our regulation at § 410.152(l)(5), has not applied. As such, beneficiaries currently are responsible for the usual coinsurance that applies to the services (20 or 25 percent of the cost of the services depending upon the setting).
Under section 1833(b) of the Act, before making payment under Medicare Part B for expenses incurred by a beneficiary for covered Part B services, beneficiaries must first meet the applicable deductible for the year. Section 4104 of the Affordable Care Act (that is, the Patient Protection and Affordable Care Act (Pub L. 111-148, March 23, 2010), and the Health Care and Education Reconciliation Act of 2010 (Pub. L. 111-152, March 30, 2010), collectively referred to as the "Affordable Care Act") amended section 1833(b)(1) of the Act to make the deductible inapplicable to expenses incurred for certain preventive services that are recommended with a grade of A or B by the USPSTF, including colorectal cancer screening tests as defined in section 1861(pp) of the Act. Section 4104 of the Affordable Care Act also added a sentence at the end of section 1833(b)(1) of the Act specifying that the exception to the deductible shall apply with respect to a colorectal cancer screening test regardless of the code that is billed for the establishment of a diagnosis as a result of the test, or for the removal of tissue or other matter or other procedure that is furnished in connection with, as a result of, and in the same clinical encounter as the screening test. Although amendments made by the Affordable Care Act addressed the applicability of the deductible in the case of a colorectal cancer screening test that involves biopsy or tissue removal, they did not alter the coinsurance provision in section 1833(a) of the Act for such procedures. Public commenters encouraged the agency to eliminate the coinsurance in these circumstances; Start Printed Page 39220 however, the agency found that statute did not provide for elimination of the coinsurance (75 FR 73170 at 73431).
Beneficiaries have continued to contact us noting their concern that a coinsurance percentage applies (20 or 25 percent depending upon the setting) under circumstances where they expected to receive only a colorectal screening test to which coinsurance does not apply. Instead, these beneficiaries received what Medicare considers to be a diagnostic procedure because, for example, polyps were discovered and removed during the procedure. Similarly, physicians have expressed concern about the reactions of beneficiaries when they are informed that they will be responsible for coinsurance if polyps are discovered and removed during a procedure that they had expected to be a screening procedure to which coinsurance does not apply.
Section 122 of the CAA addresses this coinsurance issue by successively reducing, over a period of years, the percentage amount of coinsurance for which the beneficiary is responsible. Ultimately, for services furnished on or after January 1, 2030, the coinsurance will be zero.
To implement the amendments made by section 122 of the CAA, we are proposing to modify our regulations to reflect the changes to Medicare statute. As amended, the statute effectively provides that, for services furnished on or after January 1, 2022, a flexible sigmoidoscopy or a colonoscopy can be considered a screening flexible sigmoidoscopy or a screening colonoscopy test even if an additional procedure is furnished to remove tissue or other matter during the screening test. Specifically, section 122(a)(3) of the CAA added a sentence to the end of section 1833(a) of the Act to include as colorectal screening tests described in section 1833(a)(1)(Y) of the Act, a colorectal cancer screening test, regardless of the code that is billed for the establishment of a diagnosis as a result of the test, or for the removal of tissue or other matter or other procedure that is furnished in connection with, as a result of, and in the same clinical encounter as the screening test. We note that only flexible screening sigmoidoscopies and screening colonoscopies are recognized currently as colorectal cancer screening tests that might involve removal of tissue or other matter. This new sentence added under section 1833(a) of the Act uses the same language that was used to amend the statute at section 1833(b)(1) of the Act and to broaden the scope of colorectal cancer screening tests to which a deductible does not apply. Section 122(b)(1) of the CAA then limits application of the 100 percent Medicare payment rate (that is, no beneficiary coinsurance) under section 1833(a)(1)(Y) of the Act for the additional colorectal cancer screening tests (those that are not screening tests "but for" the new sentence at the end of section 1833(a) of the Act) by making payment for them subject to a new section 1833(dd) of the Act. Section 1833(dd) of the Act provides for a series of increases in the Medicare payment rate percentage for those services over successive periods of years through CY 2029. Thereafter, section 1833(dd) of the Act has no effect, so payment for all colorectal cancer screening tests would be made at 100 percent under section 1833(a)(1)(Y) of the Act.
To codify the amendments made by section 122 of the CAA in our regulations, we are proposing to make two modifications to current regulations.
At § 410.37, we propose to modify our regulation where we define conditions for and limitations on coverage for colorectal cancer screening tests by adding a new paragraph (j). That paragraph would provide that, effective January 1, 2022, when a planned colorectal cancer screening test, that is, screening flexible sigmoidoscopy or screening colonoscopy test, requires a related procedure, including removal of tissue or other matter, furnished in connection with, as a result of, and in the same clinical encounter as the screening test, it is considered to be a colorectal cancer screening test.
At § 410.152(l)(5), we also propose to modify our regulation. Here we describe payment for colorectal cancer screening tests. Effective January 1, 2022, we propose to provide for an increase in the Medicare payment percentage that is phased in over time. As the Medicare payment percentage increases, the beneficiary coinsurance percentage decreases. We propose to revise § 410.152(l)(5) to provide that Medicare payment in a specified year is equal to a specified percent of the lesser of the actual charge for the service or the amount determined under the fee schedule that applies to the test. The phased in Medicare payment percentages for colorectal cancer screening services described in the proposed regulation at § 410.37(j) (and the corresponding reduction in coinsurance) are as follows:
- 80 percent payment for services furnished during CY 2022 (with coinsurance equal to 20 percent);
- 85 percent payment for services furnished during CY 2023 through CY 2026 (with coinsurance equal to 15 percent);
- 90 percent payment for services furnished during CY 2027 through CY 2029 (with coinsurance equal to 10 percent); and
- 100 percent payment for services furnished from CY 2030 onward (with coinsurance equal to zero percent).
Thus, between CYs 2022 and 2030, the coinsurance required of Medicare beneficiaries for planned colorectal cancer screening tests that result in additional procedures furnished in the same clinical encounter will be reduced over time from the current 20 or 25 percent to zero percent in CY 2030 and will remain at zero percent for these services furnished beginning in CY 2030 and thereafter.
J. Vaccine Administration Services: Comment Solicitation: Medicare Payments for Administering Preventive Vaccines
On January 31, 2020, under section 319 of the Public Health Service (PHS) Act (42 U.S.C. 247d), the Secretary of the Department of Health and Human Services (the Secretary) determined that a public health emergency (PHE) as a result of confirmed cases of 2019 Novel Coronavirus exists nationwide and has existed since January 27, 2020 (hereafter referred to as the PHE for COVID-19). The Secretary has since renewed this declaration for successive 90-day periods, the latest on April 15, 2021.
The PHE for COVID-19 has reinforced the important and positive impact that preventive vaccines can have on the health of Medicare beneficiaries and the broader public. At the time of publishing this proposed rule, the PHE for COVID-19 declaration is still in effect and the United States is in the middle of a national effort to vaccinate as many people against COVID-19 as quickly as possible. This national effort has at least temporarily altered the landscape for vaccines and vaccine administration by, for example, encouraging existing providers and suppliers to dramatically expand their vaccination capabilities and by encouraging new (and new types) of providers and suppliers to furnish vaccines.
Over the past several years, stakeholders have expressed concerns about the reduction in Medicare payment rates for the service to administer preventive vaccines covered by Medicare Part B under section 1861(s)(10) of the Act, including the influenza, pneumococcal, and hepatitis B virus (HBV) vaccines. In the last two PFS rulemaking cycles (that is, for CY 2020 and CY 2021), we have attempted Start Printed Page 39221 to address some of these concerns and these efforts are discussed in more detail below. However, CY 2021 payment rates for administration of these vaccines by suppliers including physicians, NPPs, and mass immunizers remain the same as in CY 2019: A national average rate of $16.94, which is geographically adjusted. In this section, we are seeking feedback on how we should update the payment rate for administration of these preventive vaccines under Medicare Part B.
1. Medicare Part B Payment for Vaccines
Under section 1861(s)(10) of the Act, Medicare Part B covers both the vaccine and its administration for the preventive vaccines specified—the influenza, pneumococcal, HBV, and COVID-19 vaccines. Under sections 1833(a)(1)(B) and (b)(1) of the Act, there is no applicable beneficiary coinsurance, and the annual Part B deductible does not apply for these vaccinations or the services to administer them. In CY 2021, payment for these vaccines is based on 95 percent of the Average Wholesale Price (AWP) for a particular vaccine product except where furnished in the settings for which payment is based on reasonable cost, such as a hospital outpatient department, rural health clinic (RHC), or federally qualified health center (FQHC). For example, for the 2020-2021 influenza season, payment limits for adult influenza vaccine products range from about $19 to $61 per adult dose. We note that most other preventive vaccines not specified for Medicare Part B coverage under section 1861(s)(10) of the Act, such as the shingles vaccine, are covered and paid for under Medicare Part D.
Section 3713 of the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) (Pub. L. 116-136) added the COVID-19 vaccine and its administration to section 1861(s)(10)(A) of the Act in the same subparagraph as the influenza and pneumococcal vaccines and their administration. To implement this section, we issued an interim final rule with comment period (November 4th COVID-19 IFC (85 FR 71145 through 71150)) which established that payments for COVID-19 vaccines and vaccine administration would be made in the same manner as payments for the influenza and pneumococcal vaccines. The IFC specifically amended §§ 414.707(a)(2)(iii) and 414.904(e)(1) to include the COVID-19 vaccine in the list of vaccines with payment limits calculated using 95 percent of the AWP (85 FR 71147). We note that Medicare does not pay providers and suppliers for the vaccine product when the federal government purchases it and gives it to the provider or suppliers for free, as has been the case for all COVID-19 vaccines as of the publication of this proposed rule.
We note that the vaccine administration services described under 1861(s)(10) of the Act are not technically valued or paid under the PFS, as they are not included within the statutory definition of physicians' services in section 1848(j)(3) of the Act. Despite this, we have historically based payment rates for the administration of these preventive vaccines by suppliers such as physicians, NPPs, and mass immunizers on an evaluation of the resource costs involved in furnishing the service, which is similar to the methodology that we use to establish payment rates for the PFS. We note further that we also assign a payment rate for administering these preventive vaccines under the Outpatient Prospective Payment System (OPPS), and those payment rates are for hospitals and home health agencies for preventive vaccine administration. Certain other types of providers and suppliers, such as RHCs, FQHCs and critical access hospitals (CAHs), are paid based on reasonable cost for vaccine administration. We also note that these payments are geographically adjusted based on the provider's wage index.
As discussed in the CY 2021 PFS proposed rule (85 CFR 50162), many stakeholders raised concerns about the reductions in payment rates for the preventive vaccine administration services that had occurred over the past several years. We generally have established payment rates for the three Healthcare Common Procedural Coding System (HCPCS) codes G0008, G0009, and G0010—which describe the services to administer an influenza, pneumococcal and HBV vaccines, respectively, based on a direct crosswalk to the PFS payment rate for CPT code 96372 (Therapeutic, prophylactic, or diagnostic injection (specify substance or drug); subcutaneous or intramuscular). Because we proposed and finalized reductions in valuation for that code for CY 2018, the payment rate for the vaccine administration codes was concurrently reduced. Further, because the reduction in RVUs for CPT code 96372 was significant enough to be required to be phased in over several years under section 1848(c)(7) of the Act, the reductions in overall valuation for the vaccine administration codes were likewise subject to reductions over several years. As noted in Table 21, the national payment rate for administering these preventive vaccines has declined more than 30 percent since 2015.
Start Printed Page 39222
We have attempted to address the reduction in payment rates for these vaccine administration HCPCS codes in the last two PFS rulemaking cycles. In the CY 2020 PFS final rule, we acknowledged that it is in the public interest to ensure appropriate resource costs are reflected in the valuation of the immunization administration services that are used to deliver these vaccines, and noted that we planned to review the valuations for these services in future rulemaking. For CY 2020, we maintained the CY 2019 national payment amount for immunization administration services described by HCPCS codes G0008, G0009 and G0010.
In the CY 2021 PFS proposed rule, we proposed to crosswalk G0008, G0009 and G0010 to CPT code 36000 (Introduction of needle or intracatheter, vein) (85 FR 50163). In the proposed rule, we noted that CPT code 36000 is a service with a similar clinical vignette, and that the additional clinical labor, supply, and equipment resources associated with furnishing CPT code 36000 were similar to costs associated with these vaccine administration codes. We also noted that this crosswalk would have resulted in payment rates for vaccine administration services at a rate that is approximately the same as the CY 2017 rate (as noted in Table 21) that was in place prior to the revaluation of CPT code 96372 (the original crosswalk code). In the CY 2021 PFS final rule, we did not finalize the proposed policy, and instead finalized a policy to maintain the CY 2019 payment amount for G0008, G0009 and G0010 (85 FR 84628). In the final rule, we also noted that we continued to seek additional information that specifically identifies the resource costs and inputs that should be considered to establish payment for vaccine administration services on a long-term basis.
As noted above, section 3713 of the CARES Act added the COVID-19 vaccine and its administration to the preventive vaccines covered under Medicare Part B under section 1861(s)(10)(A) of the Act in the same subparagraph as the influenza and pneumococcal vaccines and their administration. Section 3713 of the CARES Act allows us to implement the amendments made by that section through "program instruction or otherwise." In the November 4th COVID-19 IFC (85 FR 71147) implementing section 3713 of the CARES Act, we indicated that we would establish specific coding and payment rates for the COVID-19 vaccine and vaccine administration through technical direction to Medicare Administrative Contractors (MACs) and information posted publicly on CMS' website.
In December 2020, we publicly posted the applicable CPT codes for the Pfizer-BioNTech and Moderna COVID-19 vaccines and initial Medicare payment rates for administration of these vaccines upon the FDA's authorization of these vaccines. We announced an initial Medicare payment rate for COVID-19 vaccine administration of $28.39 to administer single-dose vaccines. For a COVID-19 vaccine requiring a series of two or more doses—for example, for both the Pfizer-BioNTech and Moderna products—we announced a payment rate for administration of the initial dose(s) of $16.94, which was based on the Medicare payment rate for administering the other preventive vaccines under section 1861(s)(10) of the Act. We also announced a payment rate for administering the second dose of $28.39, which was based on the payment rate that was proposed, but not finalized, for administration of the other preventive vaccines under section 1861(s)(10) of the Act in the CY 2021 PFS proposed rule, discussed in more detail above.
On March 15, 2021, we announced an increase in the payment rate for administering a COVID-19 vaccine to $40 per dose, effective for doses administered on or after March 15, 2021, which means the payment rate is $40 to administer a single dose product, and $40 each to administer the first and second dose in a two-dose regime ($80 total).
Start Printed Page 39223

As discussed above, payment rates for suppliers such as physicians, NPPs, and mass immunizers for administering the Part B covered preventive vaccines have generally been based on a direct crosswalk to CPT code 96372 (Therapeutic, prophylactic, or diagnostic injection (specify substance or drug); subcutaneous or intramuscular). This crosswalk code is paid under the PFS, and Medicare's process to value codes under the PFS relies in part on recommended resource inputs provided by the AMA RUC and steps to translate those recommended inputs into national RVUs.
In 2020, the RUC resubmitted its 2009 valuation recommendation for vaccine administration services described by CPT codes, including CPT codes 90460 (Administration of first vaccine or toxoid component through 18 years of age with counseling), 90471 (Administration of 1 vaccine), and 90473 (Administration of 1 nasal or oral vaccine). The AMA RUC also recently provided valuation recommendations for the CPT codes that describe the service to administer the COVID-19 vaccines.
As noted earlier, we also assign a payment rate for administering preventive vaccines under the OPPS by assigning an ambulatory payment classification (APC) to each service based on clinical and resource cost similarity to other services assigned to the APC. Geometric mean costs, which are generally used in establishing the prospective OPPS payments for each APC, are calculated using historical claims and cost report information. In CY 2021, CMS assigned HCPCS codes G0008, G0009 and G0010 to APC 5691 (level 1 drug administration), which has a national payment rate of $40 for CY 2021.
Our practice of setting payment rates for preventive vaccine administration services described by HCPCS codes G0008, G0009 and G0010 for physicians, NPPs, and mass immunizers by using the PFS approach (for example, a crosswalk to an existing CPT code) means that costs incorporated into the rate primarily reflect costs of furnishing the service in a physician office setting. It also means that the payment rate can be affected by other aspects of the PFS rate-setting methodology, such as the allocation of indirect PE, and broader changes to PFS codes and rates, including the multi-year phase-in of significant reductions in RVUs discussed earlier. We note that we have not historically collected or used information from other providers and suppliers, including pharmacies which are commonly enrolled as mass immunizers to furnish vaccines and vaccine administration services, for purposes of establishing a rate for these codes.
We are requesting feedback from stakeholders that would support the development of an accurate and stable payment rate for administration of the preventive vaccines described in section 1861(s)(10) of the Act for physicians, NPPs, mass immunizers and certain other providers and suppliers. We are interested in detailed feedback on the following questions, which we believe would assist us in establishing payment rates for these services that could be appropriate for use on a long-term basis.
- What are the different types of providers and suppliers that furnish preventive vaccines, and have these types of providers/suppliers changed as a result of the PHE for COVID-19? (We note that our claims data reflect the type of Medicare enrollment for those billing for the vaccine administration, but we are particularly interested in understanding additional, specific characteristics of the providers and suppliers that may not be distinguishable under the more general Medicare enrollment data.) We are also interested in whether different providers and suppliers furnish different aspects of the vaccine administration for the same beneficiary.
- What are the differences in incurred costs of furnishing flu, pneumonia and HBV vaccines compared to furnishing COVID-19 vaccines? Are there differences in the costs (per dose or otherwise) of furnishing a one-dose vaccine product vs. a two-dose vaccine product? Also, are there differences in cost of administering preventive vaccines furnished under the Part D benefit, such as the shingles vaccines, compared to those furnished under Part B?
- What are the resource costs that physicians, NPPs, mass immunizers and certain other suppliers incur when furnishing vaccines safely and effectively? We are interested in specific information on costs related to staffing/labor, infrastructure, patient onboarding/enrollment, vaccine storage and handling, vaccine procurement and coordination, supplies, CDC and state reporting requirements, patient counseling about safety and efficacy, and other costs we may not have considered. We are also interested in specific resource costs per vaccine dose within each cost category, if that is available.
- What are the impacts of the PHE for COVID-19 on resource costs incurred by vaccination providers, and do stakeholders envision that these impacts will continue after the PHE has ended? Following the end of the PHE, do you expect that the same types of vaccination providers and suppliers will continue to administer vaccines, or do you envision that this will change (if so, how, and what would be the primary factors driving the change)?
- As described previously, Medicare has generally relied on the PFS methodology for setting payment rates for HCPCS codes G0008, G0009 and Start Printed Page 39224 G0010. How should Medicare assess costs associated with furnishing these preventive vaccines outside of the physician office setting, such as in pharmacies, mass immunization sites, mobile vaccine clinics or other locations? In addition, we understand that there could be administrative burden associated with the routine collection of cost data to support more accurate rate-setting for suppliers that are vaccinating patients. Are there other ways to update and validate costs for a broader range of entities using existing data?
- Payment rates for vaccine administration currently vary by setting. For HCPCS codes G0008, G0009 and G0010, the CY 2021 national average payment rate for physicians, practitioners and other suppliers is $16.94, which is geographically adjusted, while for hospital outpatient departments it is $40. However, for COVID-19 vaccine administration, Medicare now pays $40 per administration in all settings, unless the vaccine in administered under certain circumstances in the beneficiary's home or residence (as discussed in more detail below). Should Medicare continue to pay differently for non-COVID-19 preventive vaccines furnished in certain settings or under certain conditions? If not, what factors contribute to higher costs for administration of non-COVID-19 vaccines that are not currently reflected in the Medicare payment rates?
- Should CMS use a different process to update the payment rates for administration of the preventive vaccines described in section 1861(s)(10) of the Act on an annual basis?
- In the last few years we have also crosswalked vaccine administration CPT codes 90460 (Administration of first vaccine or toxoid component through 18 years of age with counseling), 90461 (Administration of vaccine or toxoid component through 18 years of age with counseling), 90471 (Administration of 1 vaccine), 90472 (Administration of vaccine), 90473 (Administration of 1 nasal or oral vaccine), and 90474 (Administration of nasal or oral vaccine) to the same rate used by G0008, G0009 and G0010. How should Medicare address payment rates for these CPT codes under the PFS?
- Are there major differences between what Medicare pays physicians, NPPs and mass immunizers for non-COVID-19 preventive vaccine administration and what commercial insurers pay? To the extent possible we are also interested in feedback on specific rates used by other insurers.
2. Payment for COVID-19 Vaccine Administration in the Home
Effective June 8, 2021, we announced a new add-on payment with a national rate of $35.50 when a COVID-19 vaccine is administered in the beneficiary's home.[38] Under this new policy, providers and suppliers that administer a COVID-19 vaccine in a beneficiary's home under certain circumstances can bill Medicare for one of the existing COVID-19 vaccine administration CPT codes (0001A, 0002A, 0011A, 0012A, 0031A) along with HCPCS code M0201 (COVID-19 vaccine administration inside a patient's home; reported only once per individual home per date of service when only COVID-19 vaccine administration is performed at the patient's home). Providers and suppliers administering a COVID-19 vaccine in the home will be paid a national average payment $75.50 dollars per dose ($40 for COVID-19 vaccine administration and $35.50 for the additional payment for administration in the home, and both payments are geographically adjusted).
In establishing the additional payment for COVID-19 vaccine administration in the beneficiary's home, we also established certain conditions for the add-on payment described by HCPCS code M0201. More specifically, for purposes of this additional payment for administration of the COVID-19 vaccine in the beneficiary's home, we established that Medicare will make this payment when either of these situations applies:
- The patient has difficulty leaving the home to get the vaccine, which could mean any of these:
(1) They have a condition, due to an illness or injury, that restricts their ability to leave home without a supportive device or help from a paid or unpaid caregiver;
(2) They have a condition that makes them more susceptible to contracting a pandemic disease like COVID-19; or
(3) They are generally unable to leave the home, and if they do leave home, it requires a considerable and taxing effort;
- The patient is hard-to-reach because they have a disability or face clinical, socioeconomic, or geographical barriers to getting a COVID-19 vaccine in settings other than their home. These patients face challenges that significantly reduce their ability to get vaccinated outside the home, such as challenges with transportation, communication, or caregiving.
We also specified that payment is made for HCPCS code M0201 if the sole purpose of the visit is to administer the COVID-19 vaccine. However, Medicare will not pay the additional amount if the provider or supplier furnished another Medicare covered service in the same home on the same date.
For purposes of this add-on payment for in-home COVID-19 vaccine administration, we announced that a home can be a private residence temporary lodging (for example, a hotel or motel, campground, hostel, or homeless shelter), an apartment in an apartment complex or a unit in an assisted living facility or group home, or a patient's home that is made provider-based to a hospital during the PHE for COVID-19. As such, a home may be a domiciliary or rest home, meaning a facility, which provides room, board, and other personal assistance services (for example, an assisted living facility).
We also announced that the following locations are not considered to be the patient's home for purposes of the add-on payment for COVID-19 vaccine administration: Communal spaces of a multi-unit living arrangement; hospitals; Medicare SNFs, and Medicaid NFs, regardless of whether they are the patient's permanent residence; assisted living facilities participating in the CDC's Pharmacy Partnership for Long-Term Care Program when their residents are vaccinated through this program. We are clarifying that an institution is not considered to be a patient's home if the institution meets the requirements of sections 1861(e)(1), 1819(a)(1), or 1919(a)(1) of the Act, which includes hospitals and skilled nursing facilities, as well as most nursing facilities under Medicaid.[39]
Additionally, we established that assisted living facilities participating in the CDC Pharmacy Partnership for Long-Term Care Program partnership would not be eligible for this higher payment for COVID-19 vaccine administration in the home when their residents were vaccinated through this program.
In addition, the COVID-19 vaccine administration service must be furnished inside an individual's home. An individual unit in a multi-dwelling building is considered a home. For example, an individual apartment in an apartment complex or an individual bedroom inside an assisted living facility or group home is considered a home. We established that communal spaces of, or related to, congregate living arrangements (such as a communal area of an apartment or condominium complex, assisted living facility, group Start Printed Page 39225 home) are not considered a home for purposes of this add-on payment because multiple people could be vaccinated and monitored either simultaneously or in tandem in such communal spaces.
As noted in the code descriptor for HCPCS code M0201, this code can be billed only once per individual home per date of service. In situations where more than one Medicare beneficiary lives in the same individual home, the additional payment for COVID-19 vaccine administration in the home is limited to one time in that home on that day, while any additional COVID-19 vaccine administration services for other individuals in that same home would be paid at the generally applicable rate of approximately $40 without the additional in-home add-on payment amount.
We established the payment amount for HCPCS code M0201 for in-home vaccination to reflect the additional costs associated with administering the vaccine in the home, such as upfront administration costs like scheduling, the additional clinical time needed for post administration monitoring of a single patient, and public health reporting requirements. To identify an appropriate payment rate for HCPCS code M0201, we used the home health low utilization payment adjustment add-on factor for skilled nursing as a proxy for the increased resource costs, above those reflected in the base payment rate for COVID-19 vaccine administration, involved in arranging and furnishing COVID-19 vaccine administration services in the home. For home health services, we make a low utilization payment adjustment (LUPA) when, during a 30-day period of home health care (or prior to January 1, 2020, a 60-day episode of home health care) a patient receives minimal services (less visits than a predetermined threshold) and the home health agency is paid per visit rather than the full 30-day (previously 60-day) bundled payment amount (see 42 CFR 484.230). As stated in the CY 2008 HH PPS proposed rule, after the HH PPS went into effect we received comments and correspondence stating that the LUPA per-visit payment rates do not adequately account for the front-loading of costs in an episode. Commenters suggested that because of the small number of visits in a LUPA episode, HHAs have little opportunity to spread the costs of lengthy initial visits over a full episode (72 FR 25424). As such, under the Medicare home health payment system, LUPA add-on payments are made to account for the upfront fixed costs and prolonged visit lengths in a LUPA period/episode compared to those for non-LUPA periods/episodes. We believe the LUPA add-on factor for skilled nursing is an appropriate proxy for the upfront fixed costs and prolonged visit lengths that exemplify and constitute the increased resource costs involved in arranging and furnishing COVID-19 vaccine administration services in the home.
The CY 2021 LUPA add-on factor for skilled nursing is 1.8451, and we applied this to the base rate for COVID-19 vaccine administration of $40 per dose (effective March 15, 2021). This calculation results in a total proxy payment rate for in-home COVID-19 vaccine administration of approximately $74. Subtracting the $40 base rate for COVID-19 vaccine administration, which applies across most other settings, results in an additional proxy payment rate of roughly $34. To expedite access to this service and ensure consistency in payment rates for HCPCS code M0201 between health care professionals, other suppliers, and institutional providers, we established a payment rate that corresponds to the proxy we calculated based on the LUPA add-on factor using a reference to another proxy payment rate under the hospital OPPS. Specifically, we looked to APC payment amounts under the hospital OPPS that were similar to the $34 proxy amount and could be implemented with speed under the COVID-19 vaccine benefit (which relies on both institutional and professional claims processing systems). We identified New Technology APC 1494 under the hospital OPPS with a national payment rate of $35.50 as an appropriate reference payment amount for this service for most providers and suppliers, and established that amount as the national payment rate for HCPCS code M0201. That is, the national payment rate for HCPCS code M0201 is $35.50 for all providers and suppliers not paid reasonable cost.
In announcing the add-on payment for in-home COVID-19 vaccine administration, we noted that we established these policies on a "preliminary basis to ensure access to COVID-19 vaccines during the public health emergency" and that "we continue to evaluate the needs of Medicare patients and these policies, and will address them in the future, as needed".[40] We are using this proposed rule as a way to collect feedback on these policies and potential future changes.
- We are interested in feedback on our requirements, including the definition of the "home" and the types of clinical and non-clinical circumstances that make it difficult for a beneficiary to receive a COVID-19 vaccine outside the home. Do these requirements strike the appropriate balance of ensuring access to vaccines for vulnerable beneficiaries while also protecting against potential fraud? Should we maintain these requirements during the PHE as-is, and if not, what changes should we consider? Outside of the circumstances of the PHE that create a need for beneficiaries to be vaccinated as quickly and broadly as possible, under what circumstances do health care providers, suppliers, or others find particular need to vaccinate people at home rather than periodically in association with routine in-person visits?
- As noted, we established an add-on payment of $35.50, which is based on applying the LUPA add-on factor for skilled nursing to the national $40 payment rate for the base service as a proxy to reflect the additional resources involved in furnishing services in the home setting. We are interested in detailed feedback on the costs associated with furnishing COVID-19 vaccines in the home, and how these costs differ from costs of furnishing vaccines in traditional locations, such as a physician's office or mass immunization site.
- What other steps should we take related to program integrity and beneficiary protection with this new add-on payment for administering the COVID-19 vaccine in the home? What documentation should providers and suppliers that furnish vaccines in the home be required to maintain and/or provide?
We note that this add-on payment of $35.50 only applies when providers or suppliers furnish the COVID-19 vaccine in the beneficiary's home, and is not billable when providers and suppliers furnish a different preventive vaccine (influenza, pneumonia, HBV) in the home. We believe the additional payment is only appropriate for COVID-19 vaccines due to the unique circumstances of the PHE, as well as the upfront fixed costs and prolonged visit lengths that exemplify and constitute the increased resource costs involved in arranging and furnishing COVID-19 vaccine administration services in the home. However, we are interested in feedback on whether the same barriers that could prevent a beneficiary from obtaining a COVID-19 vaccine would also prevent them from obtaining other preventive vaccines, whether Medicare should make a similar add-on vaccine administration payment in those Start Printed Page 39226 circumstances, and whether the costs to furnish other preventive vaccines in the beneficiary's home would be consistent with the costs to furnish the COVID-19 vaccine.
3. Monoclonal Antibodies Used To Treat COVID-19
On November 10, 2020, the FDA issued an Emergency Use Authorization (EUA) for bamlanivimab monotherapy.[41] On November 21, 2020 the FDA issued an EUA for casirivimab and imdevimab, which are administered together.[42] On February 9, 2021, the FDA issued an EUA for bamlanivimab and etesevimab, which are also administered together.[43] On April 16, 2021, the FDA revoked the EUA for bamlanivimab monotherapy.[44] On May 26, 2021, the FDA issued an EUA for sotrovimab monotherapy.[45] On June 3, 2021, the FDA revised the EUA for casirivimab and imdevimab, which revised the dosing regimen from 2400mg (1200 mg of casirivimab and 1200 mg of imdevimab) to 1200mg (600 mg of casirivimab and 600 mg of imdevimab), authorized the addition of a new presentation consisting of a single vial of casirivimab and imdevimab co-formulated in a 1:1 ratio, and also authorized casirivimab and imdevimab to be administered together via subcutaneous injection in certain limited circumstances.[46] On June 24, 2021, the FDA issued an EUA for tocilizumab monotherapy.[47] Under the EUAs, for all of these products except for tocilizumab, they can be used for certain high-risk patients with mild-to-moderate COVID-19 with the goal of preventing further deterioration and hospitalization. Tocilizumab is authorized for hospitalized patients who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
When these products were authorized during the PHE for COVID-19, we made the determination to cover and pay for them under the COVID-19 vaccine benefit in section 1861(s)(10) of the Act. When we announced this approach, we also indicated that we would address "potential refinements to payment for administering monoclonal antibody products to treat COVID-19 through future notice-and-comment rulemaking".[48]
We make a separate payment for the products (when not given to the provider or supplier for free by the government) and for the service to administer them. We note that as of June 30, 2021, the monoclonal antibody products authorized by the FDA under an EUA include two products involving drugs administered together, casirivimab and imdevimab and bamlanivimab and etesevimab, the sotrovimab monotherapy, and the tocilizumab monotherapy. All four products may be administered through intravenous (IV) infusion, and casirivimab and imdevimab may be administered via subcutanoues injection in certain limited circumstances under the updated June 3rd EUA.
Initially, we established a national payment rate of $309.10 for the service to administer (through IV infusion only at the time) these products, which was based on one hour of infusion and post-infusion monitoring in the hospital outpatient setting. We note that while these products are typically infused over a period of roughly one hour, the EUA for casirivimab and imdevimab allows the product to be infused over a shorter time-period, such as 20 minutes, when appropriate. We note that, as of June 15, 2021, the EUAs require at least one hour of post infusion monitoring for all of the products available. On May 6, 2021, we increased the payment rate for administration to $450.00 and established a separate payment rate of $750.00 when a monoclonal antibody product used to treat COVID-19 is administered in a home or residence.[49]
The decision to cover and pay for monoclonal antibody products used to treat COVID-19 under the COVID-19 vaccine benefit prioritized access to these products during the COVID-19 pandemic by allowing almost all Medicare enrolled providers and suppliers, as permitted by state law and consistent with the terms of the EUA, to furnish and bill for administering these products across settings of care. Covering and paying for these services under the COVID-19 vaccine benefit also means that beneficiaries are not responsible for any cost sharing for the product or the service to administer it. We note that Medicare considers other monoclonal antibody products—that is, monoclonal antibody products used in the treatment of other health conditions—"biologicals" and pays for them based on the methodology in section 1847A of the Act when they are furnished in physician offices, ambulatory infusion clinics and under a similar methodology under the hospital OPPS. We also note that, for these care settings, we typically rely on the applicable AMA CPT codes to describe and pay for drug administration services performed by providers and suppliers.
As noted above, bamlanivimab monotherapy and casirivimab and imdevimab, administered together, were authorized in late 2020, we made the determination to cover and pay for them under the vaccine benefit in section 1861(s)(10) of the Act, and this decision prioritized beneficiary access for purposes of addressing the PHE for COVID-19. Since that time, the EUA for bamlanivimab monotherapy has been revoked, the EUA for casirivimab and imdevimab administered together has been revised to include a new presentation, a new dosing regimen, and a new route of administration (in certain limited circumstances), the sotrovimab monotherapy has been authorized and the tocilizumab monotherapy has been authorized. It is also becoming clear that, as more products enter the market, the federal government may not purchase them for distribution to providers and suppliers for free, as is the case with sotrovimab monotherapy and tocilizumab monotherapy. Given these fast-moving changes, we are seeking feedback on our approach to coverage and payment for COVID-19 monoclonal antibody products under the COVID-19 vaccine benefit. We are considering whether we should align payment and coverage for these products with our approach for other monoclonal antibody products following the end of the PHE. We believe that the context in which these products are furnished to beneficiaries after the end of the PHE may more closely resemble the circumstances under which similar drugs and biologics are ordinarily furnished, specifically to a more targeted patient population outside of a pandemic. Outside the context of the PHE, we believe treating these products like other drugs and biologics paid under section 1847A of the Act may better align Medicare coverage and payment policies for COVID-19 monoclonal antibody products with other monoclonal antibody products, which are purchased by providers and suppliers through Start Printed Page 39227 similar channels and administered using similar modalities. As noted above, coverage and payment for COVID-19 monoclonal antibodies under the COVID-19 vaccine benefit has meant that Medicare beneficiaries are not responsible for any cost-sharing, which is typically 20 percent of the allowed amount in most settings. We note that if Medicare were to pay for COVID-19 monoclonal antibody products under the methodologies in 1847A of the Act, it would mean that beneficiary co-insurance would apply, similar to how it applies to other drugs and biologics that are not paid for under a preventive vaccine benefit.
We also note that tocilizumab—typically sold under the brand name Actemra—was previously approved by the FDA for several indications.[50] As a result, during the PHE for COVID-19, Medicare has separate coding and payment rules for tocilizumab when it is furnished to patients with COVID-19 and in a manner consistent with the terms of the EUA, and for when tocilizumab is used for other clinical purposes. This may be confusing for hospital providers and we believe that treating these monoclonal antibody products like other drugs and biologics paid under section 1847A of the Act may help clarify these inconsistencies. We are interested in feedback on these issues.
We are also interested in additional feedback on the resource costs to administer COVID-19 monoclonal antibody products, such as costs associated with infrastructure, clinical labor, and equipment, including personal protective equipment. We recognize that administering monoclonal antibodies used to treat COVID-19 may be complex due the need to interact with beneficiaries that have active infections and manage the potential for spreading disease. We are interested in information on how the costs to furnish monoclonal antibodies used to treat COVID-19 compare with infusions of other complex biologics, and how the costs to furnish these products may be different when these products are administered in the home.
4. Summary
We have taken several steps to promote timely access to COVID-19 vaccines including monoclonal antibody products during the PHE for COVID-19. As explained above, we increased the payment rates we initially established for services to administer a COVID-19 injected vaccine and a COVID-19 infused or injected monoclonal antibody product. We also developed specific payment rates when these products are administered in the beneficiary's home. Taken together, these efforts signal our understanding of the importance of COVID-19 vaccines for the health of the individual beneficiary and the public. We also believe these efforts, and the PHE broadly, provide an opportunity to consider a more rational payment framework for the other preventive vaccines covered under Medicare Part B. We are encouraged by stakeholder engagement on these important issues and continue to seek information that reflects the resource costs that we should consider for vaccine administration services. We are interested in detailed feedback and verifiable data from the public to help inform whether we should consider making changes to payments for administering preventive vaccines, or develop separate payments for vaccine administration in the home.
We appreciate feedback from the public on these important issues regarding preventive vaccine administration, vaccine administration in the home, and monoclonal antibodies used to treat COVID-19.
K. Payment for Medical Nutrition Therapy Services and Related Services
Section 105 of the Medicare, Medicaid, and SCHIP Benefits Improvement and Protection Act of 2000 (BIPA) (Pub. L. 106-554, December 21, 2000) added section 1861(vv)(1) to the Act which provided Medicare coverage under Part B for Medical Nutrition Therapy (MNT) services when performed by registered dietitians and nutrition professionals pursuant to a referral from a physician.
Under section 1842(b)(18)(C) of the Act, registered dietitians and nutrition professionals are included in the list of NPPs that may bill Medicare and be paid directly for their services, effective January 1, 2002. To submit claims for MNT services, the registered dietitian or nutrition professional must enroll as such in accordance with our regulations at 42 CFR 414.64 and 424.510. Like other NPPs listed in section 1842(b)(18)(C) of the Act, registered dietitians and nutrition professionals who are employees or independent contractors of hospitals or physician groups may reassign their benefits to that hospital or physician group, as appropriate. The Medicare specialty code for "dietitian/nutritionist" is 71.
Under section 1833(a)(1)(T) of the Act, we were originally required to pay for MNT services at 80 percent of the lesser of the actual charge for the services or 85 percent of the amount determined under the PFS for the same services if the services had been furnished by a physician. We established payment regulations for MNT in our regulation at § 414.64 in the CY 2002 PFS final rule (66 FR 55278 through 55281 and 55332).
MNT services are defined as services that are furnished by a registered dietitian or nutrition professionals. These practitioners use three CPT® codes to bill for MNT assessment and intervention services with the referral of a physician. In cases where there is a second physician referral for MNT for the same patient within a calendar year (for example, based on a change in the patient's condition, diagnosis, or treatment regimen), the furnishing practitioner uses two other HCPCS codes to report these episodes. We have worked with stakeholders over the years to establish values for the services described by the five MNT codes.
The importance of MNT services for managing diabetes or renal disease, as well as the underutilization of the benefit by Medicare beneficiaries is discussed in this proposed rule at section III.H. More recently, stakeholders who are concerned about the low utilization rate for the services have requested that CMS make changes geared toward making MNT services more accessible to Medicare beneficiaries. These stakeholders believe the underutilization of MNT services is due to multiple factors. Some of these factors and our proposal to address them are discussed elsewhere in this rule (see section III.H.), including proposals to remove the requirement that the MNT referral be made by the "treating physician" and update the glomerular filtration rate (GFR) to reflect current medical practice. And, some factors are being considered here. First, stakeholders recommend that we modify the Medicare Claims Processing Manual (MCPM) to increase the visibility of MNT services by moving the provisions that address these services to appear near the provisions addressing other preventive services. (We note that MNT services are included in the definition of preventive services under section 1861(ddd)(3)(A) of the Act). Second, the stakeholders recommend that we revise our Medicare Benefit Policy Manual to address registered dietitians and nutrition professionals, and the MNT services they furnish, in a way that aligns with the provisions addressing other types of practitioners and the services they furnish. Start Printed Page 39228
We established the MNT regulations in the CY 2002 PFS final rule at § 410.130 through § 410.134 and § 414.64. There have since been two significant changes to payment for MNT services, which are discussed in more detail below: (1) We added MNT services to the Medicare telehealth services list and recognized that registered dietitians and nutrition professionals can furnish and bill for these services as distant site practitioners; and (2) section 4104 of the Affordable Care Act (ACA) amended the statute to remove application of the Medicare Part B deductible and coinsurance for MNT services effective January 1, 2011. In the CY 2006 PFS final rule (70 FR 70155 through 70157), we amended our regulation to add registered dietitians and nutrition professionals to the list of distant site practitioners for telehealth services at § 410.78(b)(2)(viii), and to add the three individual MNT services to the Medicare telehealth services list by adding "individual medical nutrition therapy" to § 414.65(a)(1). In the CY 2011 PFS final rule, we also added one of the group MNT codes (97804) to the Medicare telehealth services list (75 FR 73314 through 73315).
In the CY 2011 PFS final rule, (75 FR 73412 through 73430), we implemented the amendments made by section 4104 of the ACA, which were designed to remove financial barriers that may have prevented beneficiaries from obtaining certain preventive services. Section 4104 of the ACA amended section 1833(a)(1) of the Act by adding a new subparagraph (Y), which provides for Medicare Part B payment at 100 percent for preventive services described in section 1861(ddd)(3)(A) of the Act that are recommended with a grade of A or B by the United States Preventive Services Task Force (USPSTF); and, amended section 1833(b)(1) of the Act to specify that the annual Medicare Part B deductible does not apply to preventive services with a recommended grade of A or B by the USPSTF. Section 1861(ddd)(3) of the Act defines "preventive services" and includes MNT services as a preventive service through a cross references section 1861(ww)(2) of the Act. Additionally, section 4104 of the ACA amended section 1833(a)(1)(T) of the Act to specify that Medicare Part B payment is made at 100 percent (instead of 80 percent) of the lesser of the actual charge or 85 percent of the PFS payment amount for these services if they are recommended with an A or B rating by the USPSTF, thereby removing beneficiary coinsurance for these services. In the CY 2011 PFS final rule, we listed all preventive services and their recommended ratings from the USPSTF in Table 66 (66 FR 73420 through 73430), noting that all 5 MNT services received a grade of B from the USPSTF; and the last column in the table noted that the coinsurance and deductible are not applicable to these services beginning January 1, 2011. We codified the coinsurance exception for MNT services at § 410.152(l)(7) to indicate that Medicare Part B pays 100 percent of the Medicare payment amount, and the exception for the Medicare Part B deductible at § 410.160(b)(11).
At that time, the preventive services coinsurance and deductible changes were implemented through Change Request 7012 (Transmittal 864); however, we neglected to update the payment regulation for MNT services at § 414.64(a). As a result, we are now proposing to modify to the requirement at § 414.64(a) for payment of MNT services to reflect that MNT services, with their USPSTF recommended B rating, are paid at 100 percent of the lesser of the actual charges or 85 percent of the PFS amount.
Because the registered dietitian and nutrition professional are the only practitioners listed at section 1842(b)(18)(C) of the Act without a specific regulatory provision addressing them as a type of practitioner and specifying payment policies for their services, we are proposing to create a new section at § 410.72 to reflect these policies. We are proposing to include in the regulation at § 410.72 a cross reference to the regulation at § 410.134 that addresses the qualifications for registered dietitians and nutrition professionals. For covered services described at § 410.72(b), we are proposing as a condition of coverage to refer to medical nutrition therapy services as defined at § 410.130, and also to refer to the conditions for coverage of MNT services at § 410.132(a). Section 410.132(a) requires a referral for MNT services from a physician (an M.D. or D.O.), and that MNT services are personally performed by the registered dietitian or nutrition professional in a face-to-face encounter except when those services are furnished as a telehealth service as provided in § 410.78 of our regulations.
Because registered dietitians and nutrition professionals are also the primary specialty that furnishes diabetes self-management training (DSMT) services, we are proposing to include DSMT at § 410.72(b)(2) as an "other service" that registered dietitians and nutrition professionals can provide in cases where the registered dietitian or nutrition professional is a certified provider of DSMT services as specified at section 1861(qq)(2)(A) of the Act; and they have submitted necessary documentation to, and are accredited by, a CMS-approved accreditation organization, as specified in § 410.141(e) for DSMT services. We also propose to address in the regulation at § 410.72(b)(2) the current requirement that, as specified in the regulation at § 410.141(b)(1), DSMT services require a referral from the physician or qualified NPP (as defined in § 410.32(a)(2)) who is treating the beneficiary's diabetes condition. We also propose to specify in the regulation at § 410.72(b)(3) that MNT and DSMT services cannot be furnished together on the same date of service as detailed in the national coverage determination for MNT services (see https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?ncdid=252); and, that neither MNT nor DSMT services can be furnished incident to the professional services of a physician or other practitioner. For MNT services, we are proposing to clarify that MNT services cannot be provided incident to the services of a billing physician. As a distinct, stand-alone benefit under Medicare Part B at section 1861(s)(2)(V) of the Act, MNT services cannot be furnished incident to a physician's professional service that is separately specified at section 1861(s)(2)(A) of the Act. Further, if a physician also meets the qualifications to bill Medicare as a registered dietitian or nutrition professional (although not necessarily enrolled as one), they would have to personally provide any MNT services as explained above, meaning that those services could not be furnished by auxiliary personnel incident to their own professional services. For DSMT services, we are also proposing to clarify that DSMT services cannot be provided incident to the services of a billing physician or practitioner. DSMT is a distinct benefit under Medicare Part B, as specified in a stand-alone statutory provision at section 1861(s)(2)(S) of the Act. Approved DSMT entities are separately recognized programs, rather than individuals or practitioners, that provide DSMT services in accordance with their accreditation from a CMS-approved organization under § 410.142, indicating that the entity meets a set of quality standards described in § 410.144. Even when the DSMT services are billed by a physician or other practitioner, such as the DSMT certified provider, the physician or other practitioner could not provide Start Printed Page 39229 DSMT services directly, unless they themselves are also an approved DSMT entity. If a physician or practitioner is an approved entity, the DSMT services must be provided in accordance with the requirements to furnish such services. For these reasons, we are adding at § 410.72(b)(3)(ii) that neither MNT nor DSMT may be furnished and billed incident to the professional services of a physician or practitioner, where applicable.
As such, we are proposing to add at 410.72(d) that the registered dietitian or nutrition professional can be paid for their professional services only if those services have been personally performed by them. Section 1861(vv) of the Act clearly indicates that MNT services are only provided by registered dietitians and nutrition professionals; and this was reiterated at § 410.134 in the CY 2002 PFS final rule (66 FR 55331). In addition, in the CY 2002 PFS final rule, we established a regulation at § 410.132(a) that requires registered dietitians and nutrition professionals to provide MNT services and that those services consist of face-to-face nutritional assessments and interventions in accordance with nationally accepted dietary or nutritional protocols. Both of these provisions were codified in our regulations at §§ 410.132(a) and 410.134.
In the CY 2001 PFS final rule, we discussed that registered dietitians and nutrition professionals who are enrolled in Medicare could furnish services in various settings including private practices and outpatient hospitals, but that payment for MNT services would not be made when beneficiaries are inpatients in Part A stays in hospitals and skilled nursing facilities (SNFs) (66 FR 55279). We explained that our payment to hospitals and SNFs includes payment for MNT services. We established these regulations at § 414.64(c). We are proposing to add these rules to our regulation at § 410.72(c)(1) and (2), as on payment for services of registered dietitians and nutrition professionals when beneficiaries are inpatients of hospitals and SNFs. Also, in the CY 2001 PFS final rule, we finalized, in accordance with section 1861(s)(2)(V)(ii) of the Act, that there is no coverage for MNT services available for beneficiaries who are receiving maintenance dialysis for which payment is made under section 1881 of the Act, that is, services from an end-stage renal disease (ESRD) facility. This was codified at § 410.132(b). We are proposing to add this non-covered service to our regulation at § 410.72(c)(3) and note its cross reference to § 410.132(b).
In accordance with section 1842(b)(18)(B) of the Act, the registered dietitian or nutrition professional must accept assignment, meaning that they must accept the payment amount Medicare approves as payment in full and collect nothing from the beneficiaries for those services for which Medicare pays 100 percent of the Medicare approved amount or only collect the difference between the Medicare approved amount and the Medicare Part B payment in accordance with § 424.55. We are proposing to add at § 410.72(f) that the services of a registered dietitian or nutrition professional are provided on an assignment-related basis. Because Medicare pays 100 percent of the Medicare approved amount for MNT covered services, this means that beneficiaries cannot be billed any amount for MNT covered services. For other services, including DSMT, for which the Medicare Part B coinsurance percentage is 20 percent, a registered dietitian or nutrition professional must not collect amounts in excess of the limits specified in § 424.55 of our regulation, and if they do, they must refund the full amount of the impermissible charge to the beneficiary. Finally, we note that the proposed regulatory text for § 410.72(f) is consistent with the text in existing §§ 410.74(d)(2), 410.75(e)(2), 410.76(e)(2) and 410.77(d)(2). We are also considering whether alternate regulatory text that cross-refers to the assignment requirements in § 424.55 would provide additional clarity. Specifically, we are considering whether to specify restrictions at § 410.72(f) to specify that the services of a registered dietitian or nutrition professional are provided on an assignment-related basis, and the registered dietitian or nutrition professional may not charge a beneficiary in excess of the amounts permitted under 42 CFR 424.55. In addition, if a beneficiary has made payment for a service in excess of these limits, the registered dietitian or nutrition professional must refund the full amount of the impermissible charge to the beneficiary.
To ensure maximum consistency in our regulations, if we finalize the alternate regulatory text for § 410.72(f), we would also make corresponding revisions to §§ 410.74(d)(2), 410.75(e)(2), 410.76(e)(2) and 410.77(d)(2). We seek public comments on the clearest language to describe the assignment requirements.
We are seeking comment on our proposals.
III. Other Provisions of the Proposed Rule
A. Rural Health Clinics (RHCs) and Federally Qualified Health Centers (FQHCs)
1. Background
a. RHC and FQHC Payment Methodologies
As discussed in 42 CFR part 405, subpart X, RHC and FQHC visits generally are face-to-face encounters between a patient and one or more RHC or FQHC practitioners during which one or more RHC or FQHC qualifying services are furnished. RHC and FQHC practitioners are physicians, nurse practitioners (NPs), physician assistants (PA), certified nurse midwives (CNMs), clinical psychologists (CPs), and clinical social workers, and under certain conditions, a registered nurse or licensed practical nurse furnishing care to a homebound RHC or FQHC patient in an area with a shortage of home health agencies. A Transitional Care Management (TCM) service can also be an RHC or FQHC visit. In addition, a Diabetes Self-Management Training (DSMT) service or a Medical Nutrition Therapy (MNT) service furnished by a certified DSMT or MNT program may also be considered an FQHC visit. Only medically necessary medical, mental health, or qualified preventive health services that require the skill level of an RHC or FQHC practitioner are RHC or FQHC billable visits. Services furnished by auxiliary personnel (for example, nurses, medical assistants, or other clinical personnel acting under the supervision of the RHC or FQHC practitioner) are considered incident to the visit and are included in the per-visit payment.
RHCs generally are paid an all-inclusive rate (AIR) for all medically necessary medical and mental health services and qualified preventive health services furnished on the same day (with some exceptions). The AIR is subject to a payment limit, meaning that an RHC will not receive any payment beyond the specified limit amount. As of April 1, 2021, all RHCs are subject to a payment limit for the AIR, and this limit will be determined for each RHC in accordance with section 130 of the Consolidated Appropriations Act, 2021, described below.
FQHCs were paid under the same AIR methodology until October 1, 2014. Beginning that date, in accordance with section 1834(o) of the Act (as added by section 10501(i)(3) of the Affordable Start Printed Page 39230 Care Act), they began to transition to an FQHC PPS system in which they are paid based on the lesser of the FQHC PPS rate or their actual charges. The FQHC PPS rate is adjusted for geographic differences in the cost of services by the FQHC PPS geographic adjustment factor (GAF). The rate is increased by 34 percent when an FQHC furnishes care to a patient that is new to the FQHC, or to a beneficiary receiving an initial preventive physical examination (IPPE) or has an annual wellness visit (AWV).
Both the RHC AIR and FQHC PPS payment rates were designed to reflect the cost of all services and supplies that an RHC or FQHC furnishes to a patient in a single day. The rates are not adjusted for the complexity of the patient health care needs, the length of the visit, or the number or type of practitioners involved in the patient's care.
2. Payment Methodology for RHCs
a. Background
As we discussed previously, under Medicare Part B, payment to RHCs for services (defined in § 405.2411) furnished to beneficiaries is made on the basis of an all-inclusive payment methodology subject to a maximum payment per-visit (discussed in section III.A.3. of this proposed rule) and annual reconciliation. Our regulations, at § 405.2470 provides that RHCs are required to submit cost reports to allow the Medicare Administrative Contractor (MAC) to determine payment in accordance with 42 CFR part 405, subpart X, and instructions issued by CMS. The statutory payment requirements for RHC services are set forth at section 1833(a)(3) of the Act, (as amended by the Medicare Prescription Drug, Improvement, and Modernization Act of 2003[51] ), which states that RHCs are paid reasonable costs "* * * less the amount a provider may charge as described in clause of section 1866(a)(2)(A), but in no case may the payment exceed 80 percent of such costs. The beneficiary is responsible for the Medicare Part B deductible and coinsurance amounts. Section 1866(a)(2)(A)(ii) of the Act and implementing regulations at § 405.2410(b) establish beneficiary coinsurance at an amount not to exceed 20 percent of the clinic's reasonable charges for covered services.
We explain in § 405.2464(a) the AIR is determined by the MAC at the beginning of the cost reporting period. The MAC calculates the AIR that will apply for the upcoming cost reporting period for each RHC by dividing the estimated total allowable costs by estimated total visits for RHC services. The MAC also periodically reviews the AIR throughout the cost reporting period to assure that payments approximate actual allowable costs and visits and may adjust the rate. Productivity, payment limits, and other factors are also considered in the calculation. Allowable costs must be reasonable and necessary and may include practitioner compensation, overhead, equipment, space, supplies, personnel, and other costs incident to the delivery of RHC services (§ 405.2468).
Medicare payment for RHC services are ultimately determined at cost report settlement. That is, during the annual reconciliation as explained in § 405.2466, MACs determine the total reimbursement amount due the RHC for covered services furnished to Medicare beneficiaries based on the reporting period. The total reimbursement amount due is compared with total payments made to the RHC for the reporting period, and the difference constitutes the amount of the reconciliation. If the total reimbursement due the RHC exceeds the payments made for the reporting period, the MAC makes a lump-sum payment to the RHC to bring total payments into agreement with total reimbursement due the RHC. If the total payments made to an RHC for the reporting period exceed the total reimbursement due the RHC for the period, the MAC arranges with the RHC for repayment.
In the event a new RHC is in its initial reporting period, and the MAC does not have a cost report to set its AIR, the RHC provides the MAC an estimate of what it expects its costs to be for its initial reporting period. In the Provider Reimbursement Manual (Pub. 15-2), chapter 46, section 4600,[52] we explain that for an RHC's initial reporting period, the clinic completes the cost report's worksheets with estimates of costs and visits and other information required by the reports. The MAC uses these estimates to determine an interim rate of payment for the RHC. This interim rate may be adjusted throughout the reporting period. Following the end of the RHC's reporting period, the RHC is required to submit its worksheets, using data based on its actual experience for the reporting period. The AIR for the following year will then be based on the RHC's actual experience.
As discussed in Pub. 100-02, Chapter 13, section 80.2,[53] when RHCs are part of the same organization with more than one RHC, they may elect to file consolidated cost reports rather than individual cost reports. Under this type of reporting, each RHC in the organization need not file individual cost reports. Rather, the group of RHCs may file a single report that accumulates the costs and visits for all RHCs in the organization. In order to qualify for consolidation reporting, all RHCs in the group must be owned, leased, or through any other agreement, controlled by one organization.
3. RHC Payment Limit Per-Visit
a. Background
Prior to the Balanced Budget Act of 1997[54] (BBA), the payment methodology for an RHC depended on whether it was "provider-based" or "independent." Specifically, payment to provider-based RHCs for services furnished to Medicare beneficiaries was made on a reasonable cost basis by the provider's MAC in accordance with the regulations at 42 CFR part 413; whereas payment to independent RHCs for services furnished to Medicare beneficiaries was made on the basis of a uniform all-inclusive rate payment methodology in accordance with 42 CFR part 405, subpart X. In addition, payment to independent RHCs also was subject to a maximum payment per visit (also referred to as a "payment limit per-visit", "upper payment limit per-visit", or "cap") as set forth in section 1833(f) of the Act. This national statutory payment limit was set at $46 and was adjusted annually based on the Medicare Economic Index (MEI) described in section 1842(b)(3) of the Act.
Section 1833(f) of the Act was further amended by section 4205(a) of the BBA) (Pub. L. 105-33) to permit an exception to the national statutory payment limit for RHCs based in rural hospitals with less than 50 beds. Our guidance directed Medicare intermediaries to use the bed definition at § 412.105(b) and the rural definition at § 412.62(f)(1) to determine which RHCs are eligible for the exception. The hospital bed definition was based on available bed days and the rural definition was based on the Office of Management and Budget's Start Printed Page 39231 metropolitan statistical area (MSA) method.
Section 224 of the Medicare, Medicaid and SCHIP Benefits Improvement and Protection Act of 2000 (Appendix F of Consolidated Appropriations Act of 2001) (BIPA)[55] (Pub. L. 106-554, December 21, 2000) further amended section 1833(f) of the Act by expanding the eligibility criteria for receiving an exception to the national statutory payment limit for RHCs. Specifically, this section of BIPA extended the exemption to RHCs based in small, urban hospitals. Effective July 1, 2001, all hospitals of less than 50 beds were eligible to receive an exception from the per visit payment limit for their RHCs.
As discussed in Change Request 1958, Transmittal A-01-138 issued on December 6, 2001, following the implementation of the BBA provision, CMS announced an alternative bed size definition for very rural, sole community hospitals with seasonal fluctuations in patient census. The MAC reviews the number of beds twice a year to determine whether the provider-based RHC meets the exception, during the Desk Review process and during the interim rate process (that is, determining the RHC's AIR). The provider-based RHC continues to receive the exception until the hospital which they are affiliated with submits a cost report with more than 50 beds. However, in the May 8, 2020 Federal Register, in response to the PHE for COVID-19, we published the "Medicare and Medicaid Programs, Basic Health Program, and Exchanges; Additional Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency and Delay of Certain Reporting Requirements for the Skilled Nursing Facility Quality Reporting Program" interim final rule with comment period (85 FR 27550) (May 8, 2020 IFC). In the May 8, 2020 IFC, we implemented, on an interim basis, a change to the period of time used to determine the number of beds in a hospital at § 412.105(b) for purposes of determining which provider-based RHCs are subject to the payment limit (85 FR 27569). That is, for the duration of the PHE, we adopted an interim final policy to use the number of beds from the cost reporting period prior to the start of the PHE as the official hospital bed count for application of this policy. As such, RHCs with provider-based status that were exempt from the national statutory payment limit in the period prior to the effective date of the PHE (January 27, 2020) would continue to be exempt from the bed count requirement for the duration of the PHE for the COVID-19 pandemic, as defined at § 400.200, even if the hospital raised its bed count above 50. Once the PHE for COVID-19 ends, hospitals need to lower their bed count to less than 50 beds to utilize an RHC policy that has such a requirement.
b. Section 130 of the Consolidated Appropriations Act, 2021
Section 130 of the Consolidated Appropriations Act, 2021 (CAA 2021) (Pub. L. 116-260, December 27, 2020) updated section 1833(f) of the Act by restructuring the payment limits for RHCs beginning April 1, 2021. We note that section 2 of H.R. 1868 (Pub. L. 117-7), enacted April 14, 2021, provided a technical correction to section 1833(f) of the Act. The amendments made by this technical correction take effect as if included in the enactment of the Consolidated Appropriations Act of 2021 (Pub. L. 116-260).
Section 1833(f)(2) of the Act, as added by section 130 of the CAA 2021, states that beginning April 1, 2021, RHCs will begin to receive an increase in their payment limit per visit over an 8-year period, with a prescribed amount for each year from 2021 through 2028. Then, in a subsequent year, at the limit established for the previous year increased by the percentage increase in the MEI applicable to primary care services furnished as of the first of such subsequent year. This provision also subjects all new RHCs (including provider-based RHCs in a hospital with less than 50 beds and enrolled in Medicare after December 31, 2020) to the national statutory payment limit.
The national statutory payment limit for RHCs over an 8-year period is as follows:
- In 2021, after March 31, at $100 per visit;
- In 2022, at $113 per visit;
- In 2023, at $126 per visit;
- In 2024, at $139 per visit;
- In 2025, at $152 per visit;
- In 2026, at $165 per visit;
- In 2027, at $178 per visit; and
- In 2028, at $190 per visit.
Beginning April 1, 2021, provider-based RHCs that meet the qualifications in section 1833(f)(3)(B) of the Act, as added by section 130 of the CAA 2021 and amended by Public Law 117-7, are entitled to special payment rules, as described in section 1833(f)(3)(B) of the Act. That is, a provider-based RHC must meet the following criteria to have its payment limit established based on its per visit payment amount (or AIR):
- As of December 31, 2020, was in a hospital with less than 50 beds and after December 31, 2020 in a hospital that continues to have less than 50 beds (not taking into account any increase in the number of beds pursuant to a waiver during the PHE for COVID-19); and one of the following circumstances:
++ As of December 31, 2020, was enrolled in Medicare (including temporary enrollment during the PHE for COVID-19); or
++ Submitted an application for enrollment in Medicare (or a request for temporary enrollment during the PHE for COVID-19) that was received not later than December 31, 2020.
Specifically, beginning April 1, 2021, for provider-based RHCs that had a per visit payment amount (or AIR) established for services furnished in 2020, the payment limit per visit shall be set at an amount equal to the greater of: (1) The per visit payment amount applicable to such RHC for services furnished in 2020, increased by the percentage increase in the MEI applicable to primary care services furnished as of the first day of 2021; or (2) the national statutory payment limit for RHCs per visit. The details of the most recent MEI rebasing and revising is discussed in the CY 2011 PFS final rule with comment period (75 FR 73262). The MEI increase for an update year is based on historical data through the second quarter of the prior calendar year. For example, the 2021 update reflects data through the second quarter 2020. We note that the MEI percentage increase for CY 2021 is 1.4 percent, which reflects historical MEI data through the 2nd quarter 2020 and historical multifactor productivity (MFP) data through 2019. IGI is a nationally recognized economic and financial forecasting firm with which we contract to forecast the components of the MEI and other CMS market baskets, https://ihsmarkit.com/index.html.
In a subsequent year (that is, after 2021), the provider-based RHC's payment limit per visit shall be set at an amount equal to the greater of: (1) The payment limit per visit established for the previous year, increased by the percentage increase in the MEI applicable to primary care services furnished as of the first day of such subsequent year; or (2) the national statutory payment limit for RHCs. The proposed CY 2022 MEI update is 1.8 percent based on the IGI 1st quarter 2021 forecast of the MEI and productivity adjustment, which reflects historical MEI data through 4th quarter 2020 and historical MFP data through 2019. As is our general practice, we are Start Printed Page 39232 proposing that if more recent data become available after the publication of this proposed rule and before the publication of the final rule (for example, a more recent estimate of the MEI percentage increase or productivity adjustment), we would use such data, if appropriate, to determine the final CY 2022 MEI update.
For provider-based RHCs that meet certain requirements, but did not have a per visit payment amount (or AIR) established for services furnished in 2020, the payment limit per visit shall be at an amount equal to the greater of: (1) The per visit payment amount applicable to the provider-based RHC for services furnished in 2021; or (2) the national statutory payment limit for RHCs.
In a subsequent year (that is, after 2022), the provider-based RHCs payment limit per visit will be the greater of: (1) The payment limit per visit established for the previous year, increased by the percentage increase in MEI applicable to primary care services furnished as of the first day of such subsequent year; or (2) the national statutory payment limit for RHCs.
A provider-based RHC that meets the qualifications of section 1833(f)(3)(B) of the Act, as corrected by Public Law 117-7 will lose this designation if the hospital does not continue to have less than 50 beds, beyond the exemptions provided for the PHE for COVID-19. If this occurs, the provider-based RHC will be subject to the statutory payment limit per visit applicable for such year and not able to regain the specified provider-based payment limit.
Provider-based RHCs that are newly enrolled beginning January 1, 2021, and after are subject to the national statutory payment limit applicable for such year for RHCs.
c. Implementation of Section 130 of the Consolidated Appropriations Act, 2021
As we stated above, RHCs began to receive an increase in the national statutory payment limit over an 8-year period, with a prescribed amount for each year from 2021 through 2028. Prior to this legislation, the CY 2020 national statutory payment limit for RHCs was $86.31. Then for calendar year 2021, there are two sets of payment rules for RHCs. For the period before March 31, 2021, independent RHCs and provider-based RHCs that did not meet specified requirements were subject to the payment limit of $87.52 that CMS announced in Change Request 12035, Transmittal 10413 issued on October 29, 2020.[56] Provider-based RHCs that met specified requirements were not subject to a payment limit for the first quarter of calendar year 2021. However, beginning April 1, 2021, in accordance with section 130 of the CAA 2021, all RHCs are now subject to a payment limit. For example, beginning April 1, 2021 through December 31, 2021 the national statutory payment limit for RHCs is $100.00. To prepare for this change in payment limits during the calendar year, Change Request 12185, Transmittal 10679 was issued on March 16, 2021, to implement an increase in the RHC statutory payment limit per visit and establish the provider-based RHC payment limits per visit, which went in effect on April 1, 2021. We note, Change Request 12185, Transmittal 10679 was rescinded and replaced by Transmittal 10780 issued on May 4, 2021 to reflect the technical corrections in section 2 of H.R. 1868 (Pub. L. 117-7). We also note that this provision does not impact the way beneficiary coinsurance is calculated as described in § 405.2410(b)(1).
i. Specified Provider-Based RHCs
In section III.A.3.b. of this proposed rule, we discuss the qualifications specified in section 1833(f)(3)(B) of the Act, as amended by Public Law 117-7, that determine if a provider-based RHC is entitled to the special payment rules described in section 1833(f)(3)(A) of the Act. To determine if an RHC was in a hospital with less than 50 beds as of December 31, 2020, we will review each provider-based RHC using the existing bed count review process, as described above, to determine if this criterion is met. In addition, this process generally includes ongoing review by the MACs two times a year. The beds to be counted for purposes of this criterion are described in § 412.105(b), in accordance with existing policy.
In continuing with our existing policy and in accordance with section 1833(f)(3)(B)(i) of the Act which states that "as of December 31, 2020, was in a hospital with less than 50 beds and after such date such hospital continues to have less than 50 beds" an RHC will retain its specified provider-based status until the hospital which they are affiliated submits a cost report with more than 50 beds. An RHC will no longer retain its specified provider-based status nor be eligible for specified status in the future once the hospital which they are affiliated submits a cost report with more than 50 beds. However, in response to the PHE for COVID-19 and in accordance with section 1833(f)(3)(B)(I) of the Act, we will apply the policy that allows for increased hospital bed counts, as described in the May 8, 2020 IFC, for purposes of determining this bed count criterion for specified provider-based RHC status. That policy specifies that for the duration of the PHE, we will use the number of beds from the cost reporting period prior to the start of the PHE as the official hospital bed count. We note that the criteria specified in section 1833(f)(3)(B)(i) of the Act specifies "in a hospital with less than 50 beds" therefore, beginning April 1, 2021, we will apply the bed definition at § 412.105(b) exclusively.
Section 1833(f)(3)(B)(ii) of the Act, as added by section 2 of Public Law 117-7, requires that these specified provider-based RHCs as of December 31, 2020 are "enrolled under 1866(j) (including temporary enrollment during such emergency period for such emergency period)," or "submitted an application for enrollment under section 1866(j) of the Act (or a request for such a temporary enrollment for such emergency period) that was received not later than December 31, 2020." We propose that the RHC's effective date of enrollment (as established under existing regulations) would be used in our determination as to whether an RHC is enrolled under section 1866(j) of the Act as of December 31, 2020. In addition, with regard to an application for enrollment under section 1866(j) of the Act or a request for temporary enrollment, we propose to use the date an application or request was received to determine if the RHC met the qualification. RHCs that established temporary locations for the purpose of responding to the PHE for COVID-19, in accordance with their state pandemic response plan, are permitted to enroll and receive temporary Medicare billing privileges. When the PHE for COVID-19 ends, an RHC that had been temporarily enrolled under the flexibilities described above must submit a complete CMS-855 enrollment application in order to establish full Medicare billing privileges. Failure to do so will result in the deactivation of the RHC's temporary billing privileges. No payments can be made for services provided while the temporary billing privileges are deactivated. For RHCs enrolled through the temporary enrollment process that will need to submit a complete CMS-855 enrollment application, we propose, regardless of when the temporarily enrolled RHC is fully enrolled, that the RHC would be entitled to the special payment rules as long as it was Start Printed Page 39233 temporarily enrolled as of December 31, 2020 or a temporary enrollment request was received by December 31, 2020, and it meets the bed count requirement.
As stated above, section 1833(f)(3)(A) of the Act instructs Medicare to set payment limits per visit for these specified provider-based RHCs under certain payment rules. Specifically, beginning April 1, 2021, a payment limit per visit shall be set at an amount equal to the greater of: (1) The per visit payment amount applicable to such RHC for services furnished in 2020, increased by the percentage increase in the MEI applicable to primary care services furnished as of the first day of 2021; or (2) the statutory payment limit per visit as described in section 1833(f)(2) of the Act. For subsequent years, in accordance with section 1833(f)(3)(A)(ii) of the Act, that payment amount is increased by the percentage increase in the MEI or the statutory payment limit described in section 1833(f)(2) of the Act, whichever is greater.
We interpret the "per visit payment amount" to align with the interim rate process the MACs use in determining an RHC's AIR (discussed above in section III.A.2. of this proposed rule). That is, as explained in § 405.2464(a) the AIR is determined by the MAC using the most recently available cost report. Therefore, with regard to "services furnished in 2020" we interpret this to mean the period at which the services were furnished in 2020 and that costs for those services were reported. We understand that there may be more than one cost report that reports costs for services furnished in calendar year 2020. However, since section 130 of the CAA 2021 states that the "per visit payment amount" is to be increased by the CY 2021 MEI, if a provider has a cost reporting period that differs from a calendar year time-period then the MACs should use data based on the relevant cost report period ending in 2020.
Finally, we understand that certain RHCs file consolidated cost reports, as described above. For specified provider-based RHCs, existing RHCs that are independent, and existing RHCs that are in a hospital with greater than 50 beds, we will continue to use the parent RHCs' cost reports to determine the payment limit per visit (for multi-facility RHC systems), as consolidated cost reporting reduces the reporting burden and cost report preparation time for RHCs. Combining multiple individual RHC cost reports into a consolidated cost report allows RHCs to take advantage of administrative efficiencies and economies of scale that do not exist otherwise.
However, in accordance with section 1833(f)(2) of the Act, all new provider-based RHCs and independent RHCs enrolled, as of January 1, 2021, shall have a payment limit established at the national statutory payment limit for RHCs. Therefore, beginning with RHCs enrolled in Medicare as of January 1, 2021, we will no longer allow new RHCs to file consolidated cost reports.
ii. All Other RHCs
While there are criteria that allow for specified provider-based RHCs to be eligible for certain payment rules, all other RHCs are subject to payment limits as described in section 1833(f)(2) of the Act. While there may be new RHCs that are "in a hospital with less than 50 beds" and "enrolled under section 1866(j)", they will not have met these criteria by December 31, 2020. Thus, any new RHCs will also be subject to the national statutory payment limits as described in section 1833(f)(2) of the Act.
Though the payment limit is described, these RHCs will still have an AIR per visit determined based on their allowable costs for each year going forward. However, the payment limit that is established will be the maximum amount that an RHC will be paid by Medicare per visit. As discussed above, at the time of reconciliation, if an RHC's costs per visit are above the AIR, they will be paid an amount that reflects these additional costs, not to exceed the payment limit. If an RHC's costs per visit are below the AIR, then CMS will collect any overpayment for that visit. To implement this provision beginning April 1, 2021, CMS instructed the MACs to increase the payment limits to $100 per visit.
Although the payment limit per-visit as set forth in section 1833(f) of the Act has already been implemented in administrative instructions issued to the MACs in Change Request 12185, we are proposing revisions to § 405.2462 to reflect the provisions set forth in section 1833(f)(2) and (3) of the Act. We solicit comment on these revisions and on our proposals regarding the implementation of section 130 of the CAA 2021.
3. Payment for Attending Physician Services Furnished by RHCs or FQHCs to Hospice Patients
a. Background
In the Fiscal Year (FY) 2021 Hospice Payment Rate Update final rule (85 FR 47070) we explain that hospice care is a comprehensive, holistic approach to treatment that recognizes the impending death of a terminally ill individual and warrants a change in the focus from curative care to palliative care for relief of pain and for symptom management. Palliative care is at the core of hospice philosophy and care practices, and is a critical component of the Medicare hospice benefit. The goal of hospice care is to help terminally ill individuals continue life with minimal disruption to normal activities while remaining primarily in the home environment.
A hospice uses an interdisciplinary approach to deliver medical, nursing, social, psychological, emotional, and spiritual services through a collaboration of professionals and other caregivers, with the goal of making the beneficiary as physically and emotionally comfortable as possible. As referenced in our regulations at § 418.22(b)(1), to be eligible for Medicare hospice services, the patient's attending physician (if any) and the hospice medical director must certify that the individual is "terminally ill," as defined in section 1861(dd)(3)(A) of the Act and our regulations at § 418.3; that is, the individual's prognosis is for a life expectancy of 6 months or less if the terminal illness runs its normal course.
Section 1861(dd)(3)(B) of the Act defines the term "attending physician" to mean, with respect to an individual, the physician, the NP or PA who may be employed by a hospice program, whom the individual identifies as having the most significant role in the determination and delivery of medical care to the individual at the time the individual makes an election to receive hospice care.
As explained in Pub. 100-02, chapter 9, section 20.1,[57] the attending physician is a doctor of medicine or osteopathy who is legally authorized to practice medicine or surgery by the state in which he or she performs that function, an NP, or PA, and is identified by the individual, at the time he or she elects to receive hospice care, as having the most significant role in the determination and delivery of the individual's medical care. An NP is defined as a registered nurse who performs such services as legally authorized to perform (in the state in which the services are performed) in accordance with state law (or state regulatory mechanism provided by state law) and who meets training, education, and experience requirements described in § 410.75. A PA is defined as a professional who has graduated from an accredited PA educational program who Start Printed Page 39234 performs such services as he or she is legally authorized to perform (in the state in which the services are performed) in accordance with state law (or state regulatory mechanism provided by state law) and who meets the training, education, and experience requirements as the Secretary may prescribe. The PA qualifications for eligibility for furnishing services under the Medicare program can be found in the regulations at § 410.74 (c).
RHCs and FQHCs are not authorized under the statute to serve in the role of an attending physician. However, a physician, NP, or PA who works for an RHC or FQHC may provide hospice attending physician services during a time when they are not working for the RHC or FQHC (unless prohibited by their RHC or FQHC contract or employment agreement). These services would not be considered RHC or FQHC services, since they are not being provided by an RHC or FQHC practitioner during RHC or FQHC hours. The physician, NP, or PA would bill for services under Part B using their own provider number/NPI. In addition, any service provided to a hospice beneficiary by an RHC or FQHC practitioner must comply with Medicare prohibitions on commingling. Further information regarding commingling is available in Pub. 100-02, Chapter 13, section 100.[58]
b. Section 132 of the Consolidated Appropriations Act 2021
Section 132 of the CAA 2021 amended section 1834(o) of the Act and added a new section 1834(y) to the Act, to provide the authority for both FQHCs and RHCs, respectively, to receive payment for hospice attending physician services. Specifically, when a designated attending physician employed by or working under contract with an FQHC or RHC furnishes hospice attending physician services (as described in section 1812(d)(2)(A)(ii) of the Act) on or after January 1, 2022, the FQHC or RHC is eligible to receive payment under the FQHC PPS or RHC AIR, respectively.
Therefore, beginning January 1, 2022, a physician, NP, or PA who is employed by or working under contract with an RHC or FQHC may provide hospice attending physician services during a time when they are working for the RHC or FQHC. The RHC or FQHC would bill for these services as they would for any other qualified service to be paid the RHC AIR or the FQHC PPS rate, respectively. When the RHC/FQHC furnishes a hospice attending physician service that has a technical component, the provider furnishing the technical component would go to the hospice for payment as discussed in the Medicare Claims Processing Manual at https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c11.pdf.
We propose to codify the new statutory provisions as described in section 132 of the CAA 2021 in 42 CFR 405, subpart X, specifically:
- At § 405.2411, Scope of benefits, we are amending § 405.2411(b) to reflect that hospice attending physician services are covered when furnished during a patient's hospice election only when provided by an RHC/FQHC physician, NP, or PA designated by the patient at the time of hospice election as his or her attending physician and employed or under contract with the RHC or FQHC at the time the services are furnished.
- At § 405.2446, Scope of services, we are amending § 405.2446(c) to include that FQHC services are covered when they are hospice attending physician services furnished during a hospice election.
4. Concurrent Billing for Chronic Care Management Services (CCM) and Transitional Care Management (TCM) Services for RHCs and FQHCs
a. Background
In the CY 2013 PFS final rule (77 FR 68978 through 68994), Medicare payment for TCM services furnished by an RHC or FQHC practitioner was effective January 1, 2013, consistent with the effective date of payment for TCM services under the PFS. We adopted two CPT codes (99495 and 99496) to report physician or qualifying NPP care management services for a patient following a discharge from an inpatient hospital or SNF, an outpatient hospital stay for observation or partial hospitalization services, or partial hospitalization in a community mental health center. As a condition for receiving TCM payment, a face-to-face visit was required.
In the CY 2016 PFS final rule with comment period (80 FR 71080 through 71088), we finalized policies for payment of CCM services in RHCs and FQHCs. Payment for CCM services in RHCs and FQHCs was effective beginning on January 1, 2016, for RHCs and FQHCs that furnish a minimum of 20 minutes of qualifying CCM services during a calendar month to patients with multiple (two or more) chronic conditions that are expected to last at least 12 months or until the death of the patient, and that would place the patient at significant risk of death, acute exacerbation/decompensation, or functional decline. Payment was made for CCM services when CPT code 99490 was billed alone or with other payable services on an RHC or FQHC claim, and the rate was based on the PFS national average non-facility payment rate. The requirement that RHC or FQHC services be furnished face-to-face was waived for CCM services furnished to an RHC or FQHC patient because CCM describes non face-to-face services.
In the CY 2018 PFS final rule, (82 FR 53172 through 53180), we finalized payment for CCM, general Behavioral Health Integration (BHI), and the psychiatric collaborative care model (CoCM) services furnished by RHCs or FQHCs on or after January 1, 2018, described by HCPCS codes G0511 and G0512. HCPCS code G0511 is a General Care Management code for use by RHCs or FQHCs when at least 20 minutes of qualified CCM or general BHI services are furnished to a patient in a calendar month. HCPCS code G0512 is a psychiatric CoCM code for use by RHCs or FQHCs when at least 70 minutes of initial psychiatric CoCM services or 60 minutes of subsequent psychiatric CoCM services are furnished to a patient in a calendar month. The payment amount for HCPCS code G0511 is set at the average of the three national non-facility PFS payment rates for the CCM and general BHI codes and updated annually based on the PFS rates. The three codes are CPT code 99490 (20 minutes or more of CCM services), CPT code 99487 (60 minutes or more of complex CCM services), and CPT code 99484 (20 minutes or more of BHI services). The payment amount for HCPCS code G0512 is set at the average of the two national non-facility PFS payment rates for the CoCM codes and is updated annually based on the PFS rates. The two codes are CPT code 99492 (70 minutes or more of initial psychiatric CoCM services) and CPT code 99493 (60 minutes or more of subsequent psychiatric CoCM services).
In the CY 2019 PFS final rule (83 FR 59687), we finalized that effective January 1, 2019, the payment rate for HCPCS code G0511 (General Care Management Services) is set at the average of the national non-facility PFS payment rates for CPT codes 99490, 99487, 99484, and 99491.
In the CY 2020 PFS final rule with comment period (84 FR 62692), we added HCPCS code G2064 (30 minutes of PCM services furnished by physicians or NPPs) and G2065 (30 minutes or more of PCM services furnished by Start Printed Page 39235 clinical staff under the direct supervision of a physician or NPP) as a general care management service and included it in the calculation of HCPCS code G0511. Beginning January 1, 2021, the payment for HCPCS code G0511 is set at the average of the national non-facility PFS payment rates for CPT codes 99490, 99487, 99484, and 99491, and HCPCS codes G2064 and G2065, and is updated annually based on the PFS rates. Additional information on CCM requirements is available on the CMS Care Management web page[59] and on the CMS RHC[60] and FQHC[61] web pages.
Currently, RHCs and FQHCs may not bill for TCM services for a beneficiary if another practitioner or facility has already billed for CCM services for the same beneficiary during the same time-period.
b. Concurrent Billing for Chronic Care Management Services and TCM Services for RHCs and FQHCs
In the CY 2020 PFS final rule (84 FR 62687), we finalized a policy allowing suppliers paid under the PFS to concurrently bill care management codes that were previously restricted from being billed with TCM for services billed under the PFS. This included allowing concurrent billing of TCM with 14 HCPCS codes, as well as CPT codes 99490 and 99491, which describe CCM services furnished under the PFS. However, we did not extend this policy to care management services furnished in RHCs or FQHCs at that time.
Consistent with changes made in the CY 2020 PFS final rule for care management services billed under the PFS, for CY 2022, we are proposing to allow RHCs and FQHCs to bill for TCM and other care management services furnished for the same beneficiary during the same service period, provided that all requirements for billing each code are met. This would include the services described by HCPCS codes G0511 (General Care Management for RHCs and FQHCs only) and G0512 (Psychiatric CoCM code for RHCs and FQHCs only), which both describe a service period of one calendar month. We believe that when medically necessary, these services may complement each other rather than substantially overlapping or duplicating services since TCM services are furnished once within 30 days of a patient's discharge, whereas CCM services require a more comprehensive care management plan, care coordination and ongoing clinical care, and CoCM services describe care management services specifically for behavioral health conditions. We note that under this proposal, time and effort could not be counted more than once.
4. Proposed Conforming Technical Changes to 42 CFR 405.2466
In the November 6, 2020 Federal Register, we published the "Additional Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency" interim final rule with request for comment (85 FR 71145 through 71147) (hereinafter referred to as the November 6, 2020 IFC). In the November 6, 2020 IFC, we implemented section 3713 of the CARES Act (Pub. L 116-136, March 27, 2020), which established Medicare Part B coverage and payment for a COVID-19 vaccine and its administration.
As we discussed in that rule (85 FR 71147), section 3713 of the CARES Act added the COVID-19 vaccine and administration to section 1861(s)(10)(A) of the Act in the same subparagraph as the influenza and pneumococcal vaccines and their administration. Therefore, the Medicare allowed amount and billing processes for COVID-19 vaccinations are similar to those in place for influenza and pneumococcal vaccinations across provider/supplier settings. The amendments made to section 1861(s)(10)(A) of the Act were effective on the date of enactment, that is, March 27, 2020, and apply to a COVID-19 vaccine beginning on the date that such vaccine is licensed under section 351 of the PHS Act (42 U.S.C. 262). A list of vaccines and their effective dates are updated as they are available and located on the CMS website at https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/covid-19-vaccines-and-monoclonal-antibodies. Although there were regulations updated to reflect the changes set forth by the CARES Act, we inadvertently did not revise the specific regulation text that applies to RHCs and FQHCs.
Therefore, consistent with the changes described above, we are proposing to make conforming technical changes to the applicable RHC and FQHC regulations in 42 CFR part 405, subpart X, specifically:
- At § 405.2466, Annual reconciliation, we are proposing to amend paragraph (b)(1)(iv) to include the COVID-19 vaccine in the list of vaccines and their administration that would be paid at 100 percent of Medicare reasonable cost.
B. Rural Health Clinics (RHCs) and Federally Qualified Health Centers (FQHCs)—Telecommunications Technology
1. Revising the Definition of an RHC and FQHC Mental Health Visit
a. Payment Rules for RHC and FQHC Visits and for Medicare Telehealth Services
Section 1861(aa)(1) of the Act defines RHC services as physicians' services and such services and supplies that are furnished as an incident to a physician's professional service, and items and services as well as certain vaccines and their administration. It also includes services furnished by a PA, NP, clinical psychologist, or clinical social worker and services and supplies furnished as incident to these services as would otherwise be covered if furnished by a physician or incident to a physician's service. In the case of an RHC in an area with a home health agency shortage, part-time or intermittent nursing care and related medical supplies may be furnished by a registered professional nurse or licensed practical nurse to a homebound individual under certain conditions. Section 1861(aa)(3) of the Act defines FQHC services to include the specified RHC services and preventive services as well as required primary preventive health services.
As previously stated, RHC and FQHC visits are defined as medically-necessary, face-to-face encounters between a patient and an RHC or FQHC practitioner, during which time one or more RHC or FQHC qualifying services are furnished. Services furnished must be within the practitioner's state scope of practice, and only services that require the skill level of the RHC or FQHC practitioner are considered RHC or FQHC visits. The RHC and FQHC payment is based on the costs of all services, except in certain circumstances, such as vaccines and their administration.
RHCs are paid an all-inclusive rate (AIR) for medically-necessary primary health care services, and qualified preventive health services, furnished by an RHC practitioner. Medicare pays 80 percent of the RHC AIR, subject to a payment limit. Services furnished incident to an RHC professional service are included in the AIR and are not billed as a separate visit. The professional component of a procedure is usually a covered service, but is not a stand-alone billable visit. The costs of covered services provided incident to a Start Printed Page 39236 billable visit may be included on the RHC cost report.
FQHCs are paid 80 percent of the lesser of the FQHC's charge or the FQHC PPS payment rate. Except for grandfathered tribal FQHCs, the FQHC PPS payment rate reflects a base rate that is the same for all FQHCs, a geographic adjustment based on the location where services are furnished, and other applicable adjustments. The FQHC PPS rate was established based on the aggregate of FQHC total costs, and is updated yearly by the FQHC market basket.
Under the PFS, Medicare makes payment to professionals and other suppliers for physician's services, certain diagnostic tests, and some preventive services. Section 1834(m) of the Act specifies for Medicare telehealth services paid under the PFS, the payment amounts and circumstances under which Medicare makes payment for a discrete set of services, all of which must ordinarily be furnished in-person, when they are instead furnished using interactive, real-time telecommunication technology. When furnished under the telehealth rules, many of these specified Medicare telehealth services are still reported using codes that describe "face-to-face" services but are furnished using audio/video, real-time communication technology instead of in-person (82 FR 53006). Section 1834(m) of the Act also specifies conditions related to which professionals can be paid by Medicare for their professional services furnished via telehealth (referred to as distant site practitioners) and the originating site (both setting of care and geography) where a beneficiary is located while receiving telehealth services furnished remotely by the physician or practitioner through a telecommunications system. The regulation text at 42 CFR 410.78 describes a process for adding or deleting services to the list of Medicare telehealth services through the annual PFS rulemaking process and defines what technology may be used to furnish the service.
Under the permanent authority provided under section 1834(m)(4)(C)(ii) of the Act, RHCs and FQHCs, like hospitals, physician offices, and other sites, are authorized to serve as originating sites for eligible telehealth services. As defined in section 1834(m)(4)(C)(i) of the Act, the originating site is where the eligible telehealth individual is located at the time the service is furnished via a telecommunications system. As defined in section 1834(m)(4)(A) of the Act, the distant site is where the physician or practitioner is located at the time the service is provided via a telecommunications system. Originating sites are paid an originating site facility fee that is billed using HCPCS code Q3014 and is assigned a rate of $27.02 for CY 2021.
Section 3704 of the Coronavirus Aid, Relief, and Economic Security Act (the CARES Act) (Pub. L. 116-136, March 27, 2020) directs the Secretary to establish Medicare payment for telehealth services when RHCs and FQHCs serve as the distant site during the public health emergency (PHE) for COVID-19. Separately, section 3703 of the CARES Act expanded CMS' emergency waiver authority to allow for a waiver of any of the statutory telehealth payment requirements under section 1834(m) of the Act for telehealth services furnished during the PHE. Specifically, section 1834(m)(8)(B) of the Act, as added by the CARES Act, requires that the Secretary develop and implement payment methods for FQHCs and RHCs that serve as a distant site during the PHE for the COVID-19 pandemic. The payment methodology outlined in the CARES Act requires that rates shall be based on rates that are similar to the national average payment rates for comparable telehealth services under the Medicare PFS. CMS established rates based on the average amount for all PFS telehealth services on the telehealth list, weighted by volume. RHCs and FQHCs bill for these Medicare telehealth services using HCPCS code G2025 and the rate for CY 2021 is $99.45. The temporary authority under section 1834(m)(8) of the Act to pay RHCs and FQHCs for furnishing distant site Medicare telehealth services expires when the PHE for the COVID-19 pandemic is terminated. While they will continue to be able to serve as an originating site for Medicare telehealth services, the payment mechanism for the professional services of RHC and FQHC practitioners will be FQHC and RHC payments under the established methodology, that is the RHC AIR or the FQHC PPS.
b. Adoption of Telehealth Technologies for Mental Health Care
While not specific to RHC and FQHC telehealth services provided during the PHE, according to MedPAC's report, Telehealth in Medicare after the Coronavirus Public Health Emergency,[62] there were 8.4 million telehealth services paid under the PFS in April 2020, compared with 102,000 in February 2020. MedPAC also reported that during focus groups held in the summer of 2020, clinicians and beneficiaries supported continued access to telehealth visits with some combination of in-person visits. They cited benefits of telehealth, including improved access to care for those with physical impairments, increased convenience from not traveling to an office, and increased access to specialists outside of a local area. In their annual beneficiary survey, over 90 percent of respondents who had a telehealth visit reported being "somewhat" or "very satisfied" with their video or audio visit, and nearly two-thirds reported being "very satisfied."
Widespread use of telecommunications technology to furnish services during the PHE has illustrated interest within the medical community and among Medicare beneficiaries in furnishing and receiving care through the use of technology beyond the PHE. During the PHE for COVID-19 pandemic, RHCs and FQHCs, much like other provider types, have had to change how they furnish care in order to meet the needs of their patients, and use of the temporary authority to bill Medicare for PFS telehealth services has been widely utilized by RHCs and FQHCs during the PHE. This shift in how care is furnished has prompted us to reevaluate the regulations regarding visit requirements for encounters between an RHC or FQHC patient and an RHC or FQHC practitioner to ensure that they reflect contemporary medical practice.
Recently enacted legislation modified the circumstances under which Medicare makes payment for mental health services furnished via telehealth technology under the PFS following the PHE. Division CC, section 123 of the Consolidated Appropriations Act of 2021 (CAA) (Pub. L. 116-260, December 27, 2020) removed the domestic geographic originating site restrictions and added the home of the individual as a permissible originating site for telehealth services billed under the PFS when furnished for the purposes of diagnosis, evaluation, or treatment of a mental health disorder. This change correlates with a growing acceptance of the use of technology in the provision of mental health care. Clinicians furnishing telepsychiatry services at Massachusetts General Hospital Department of Psychiatry during the PHE observed several advantages of the virtual format for furnishing psychiatric services, noting that patients with psychiatric pathologies that interfere with their ability to leave home (for example, immobilizing depression, Start Printed Page 39237 anxiety, agoraphobia, and/or time-consuming obsessive-compulsive rituals) were able to access care more consistently since eliminating the need to travel to a psychiatry clinic can increase privacy, and therefore, decrease stigma-related barriers to treatment, potentially bringing care to many more patients in need, as well as enhanced ease of scheduling, decreased rate of no-shows, increased understanding of family and home dynamics, and protection for patients and practitioners with underlying health conditions.[63]
These findings are consistent with our analysis of Medicare claims data that indicate that use of interactive communication technology for mental health care is likely to continue to be in broad use beyond the circumstances of the pandemic. According to our analysis of Medicare Part B claims data for services furnished via Medicare telehealth under the PFS during the PHE, use of telehealth for many professional services spiked in utilization around April 2020 and diminished over time; however, utilization was still higher than it was prior to the PHE. In contrast, Medicare claims data suggests that for mental health services both permanently and temporarily added to the Medicare Telehealth list, subsequent to April 2020, the trend is toward maintaining a steady state of usage over time. Given this information, broad acceptance in the public and medical community, and the relatively stable Medicare utilization of services during the entire COVID-19 pandemic, we believe use of interactive communication technology in furnishing mental health care is becoming an established part of medical practice, very likely to persist well after the COVID-19 pandemic, and available across the country under Medicare statute for the range of professionals furnishing mental health care and paid under the PFS.
c. Revising the Definition of an RHC and FQHC Mental Health Visit
We believe beneficiaries receiving mental health services from RHC and FQHC practitioners should have the same access to mental health care delivered via telecommunications technology as beneficiaries receiving services from practitioners paid under the PFS. We also believe that disruptions in access to mental health care from trusted practitioners can be particularly problematic for Medicare beneficiaries, especially when it results in fragmented care. However, absent changes in the definition of mental health visits, RHCs and FQHCs would no longer be paid by Medicare for mental health care services delivered via telecommunications technology and would likely resume furnishing solely in-person, face-to-face mental health visits after the PHE, thereby removing the ability for beneficiaries to be able to receive these services from RHC/FQHC practitioners if furnished via interactive communication technology.
Because the definitions of RHC and FQHC services, as specified in sections 1861(aa)(1) and (3) of the Act, respectively, refer specifically to physicians' services, and services that would be physicians' services, but are instead furnished by certain other types of practitioners, we believe it would be consistent to align policies to provide access to services furnished by RHCs and FQHCs similar to PFS services, where appropriate and within statutory requirements. To ensure that beneficiaries can access services furnished by RHCs and FQHCs in a manner similar to mental health services under the PFS after the PHE, we believe it is appropriate to consider modifying our regulatory definition of a mental health visit to provide for remote access to RHC and FQHC services. Therefore, to avoid both the inequities in access to modes of care, and to avoid potentially problematic interruptions to care or the negative consequences of fragmented care, for CY 2022, we are proposing to revise the regulatory requirement that an RHC or FQHC mental health visit must be a face-to-face (that is, in person) encounter between an RHC or FQHC patient and an RHC or FQHC practitioner to also include encounters furnished through interactive, real-time telecommunications technology, but only when furnishing services for the purposes of diagnosis, evaluation, or treatment of a mental health disorder.
Additionally, similar to the discussion of proposals for mental health services furnished under the PFS, as described in section II.D. of this proposed rule, we believe that mental health telehealth services furnished via audio-only communications technology would increase access to care, especially in areas with poor broadband infrastructure and among patient populations that either are not capable of, or do not consent to, the use of devices that permit a two-way, audio/video interaction. Therefore, in order to align with proposals related to use of audio-only telecommunications technology to furnish similar mental health services under the PFS, we are proposing to allow RHCs and FQHCs to furnish mental health visits using audio-only interactions in cases where beneficiaries are not capable of, or do not consent to, the use of devices that permit a two-way, audio/video interaction. We note that the decision related to a service being furnished via telecommunications technology should be a patient-centered choice and that providers/practitioners should not force or impose services being furnished via telecommunications technology on beneficiaries who prefer to receive the services in-person. Additionally, some patients may prefer a hybrid whereby some mental health services are in person, but other times they are done using telecommunications technology. We believe that this decision should be based on the clinical judgment of the practitioner, in consideration of patient needs and preferences.
This proposed change would allow RHCs and FQHCs to report and be paid for mental health visits furnished via real-time, telecommunication technology in the same way they currently do when these services are furnished in-person. This proposed expansion of payable modes of mental health services furnished by RHCs and FQHCs corresponds with the expanded availability for professionals paid for Medicare Telehealth services under the PFS authorized by section 123 of the CAA and using the technology available for use for corollary services when paid under the PFS. This proposed revision would not allow RHCs or FQHCs to report visits furnished using asynchronous communications like email exchanges. Rather, RHCs and FQHCs would continue to report and be paid for furnishing medically necessary virtual communications services in accordance with the requirements for HCPCS code G0071 (83 FR 59686). Also, this proposed change would not allow RHCs and FQHCs to report Medicare telehealth services under section 1834(m) of the Act or be paid under the PFS since RHCs and FQHCs are not authorized to serve as distant site practitioners for Medicare telehealth services once the PHE for the COVID-19 pandemic has been terminated. In order to track utilization of mental health visits furnished using communication technology, we are proposing that RHCs and FQHCs would append the 95 modifier (Synchronous Telemedicine Service Rendered via Real-Time Interactive Audio and Video Telecommunications System) in instances where the service was furnished using audio-video communication technology or a new Start Printed Page 39238 service level modifier in cases where the service was furnished audio-only.
Additionally, we note that section 123 of the CAA also requires that there be an in-person service within 6 months prior to the furnishing of the telehealth service and at intervals thereafter as specified by the Secretary for mental health services furnished via Medicare telehealth under the PFS. We are seeking comment on whether we should consider a similar requirement for mental health services furnished by RHCs and FQHCs via telecommunications technology, or whether this requirement may be especially burdensome for beneficiaries receiving treatment at RHCs and FQHCs, particularly in rural areas. If we were to establish a similar requirement for RHC and FQHC mental health services, we could consider the proposal for Medicare telehealth services described in section II.D. of this proposed rule that there be an in-person service within 6 months prior to the furnishing of the telecommunications service and that an in-person service (without the use of telecommunications technology) be provided at least every 6 months while the beneficiary is receiving services furnished via telecommunications technology for diagnosis, evaluation, or treatment of mental health disorders, which would be documented in the patient's medical record, or whether we should defer to the clinical judgment of the practitioner on how often an in-person visit would be appropriate.
d. Regulatory Changes
We are proposing to revise the regulation at § 405.2463, to revise paragraph (a)(1)(i) to state that a mental health visit is a face-to-face (that is, in person) encounter (or, for mental health visits only, an encounter that meets the requirements under paragraph (b)(3)) between an RHC patient and an RHC practitioner. We are proposing to revise paragraph (b)(3) to define a mental health visit as a face-to-face encounter or an encounter where services are furnished using interactive, real-time, audio and video telecommunications technology or audio-only interactions in cases where beneficiaries are not capable of, or do not consent to, the use of devices that permit a two-way, audio/video interaction for the purposes of diagnosis, evaluation or treatment of a mental health disorder. We are also proposing to revise § 405.2469, FQHC supplemental payments, to revise paragraph (d) by adding that a supplemental payment required under this section is made to the FQHC when a covered face-to-face (that is, in-person) encounter or an encounter where services are furnished using interactive, real-time, telecommunications technology or audio-only interactions in cases where beneficiaries do not wish to use or do not have access to devices that permit a two-way, audio/video interaction for the purposes of diagnosis, evaluation or treatment of a mental health disorder occurs between a MA enrollee and a practitioner as set forth in § 405.2463.
C. Federally Qualified Health Centers (FQHCs) Payment for Tribal FQHCs—Comment Solicitation
1. Health Services to American Indians and Alaska Natives (AI/AN)
There is a special government-to-government relationship between the federal government and federally recognized tribes based on U.S. treaties, laws, Supreme Court decisions, Executive Orders and the U.S. Constitution. This government-to-government relationship forms the basis for federal health services to American Indians/Alaska Natives (AI/AN) in the U.S. In 1976, the Indian Health Care Improvement Act (IHCIA) (Pub. L. 94-437, September 30, 1976) amended the statute to permit payment by Medicare and Medicaid for services provided to AI/ANs in Indian Health Service (IHS) and tribal health care facilities that meet the applicable requirements. Under this authority, Medicare services to AI/ANs may be furnished by IHS operated facilities and programs and tribally-operated facilities and programs under Title I or Title V of the Indian Self Determination Education Assistance Act, as amended (ISDEAA) (Pub. L 93-638, January 4, 1975). According to the IHS Profile,[64] the IHS healthcare delivery system currently consists of 46 hospitals, with 24 of those hospitals operated by the IHS and 22 of them operated by tribes under the ISDEAA, as well as 492 health centers, 75 operated by IHS and 417 operated by tribes under the ISDEAA.
Payment rates for outpatient medical care (also referred to as outpatient hospital services) furnished by the IHS and tribal facilities is set annually by the IHS under the authority of sections 321(a) and 322(b) of the Public Health Service Act (the PHS Act) (42 U.S.C. 248 and 249(b)) (Pub. L. 83-568 (42 U.S.C. 2001(a)), and the IHCIA, based on the previous year cost reports from federal and tribal hospitals. The IHCIA provided the authority for CMS (then HCFA) to pay IHS and tribal facilities for its outpatient hospital services to Medicare eligible patients, using an outpatient per visit rate (also referred to as the Medicare all-inclusive payment rate (AIR).
2. Federally Qualified Health Centers (FQHCs) Prospective Payment System (PPS)
FQHCs were established in 1990 by section 4161 of the Omnibus Budget Reconciliation Act of 1990 (OBRA 90) (Pub. L. 101- 508, November 5, 1990), and were effective beginning on October 1, 1991. They are facilities that furnish services that are typically furnished in an outpatient clinic setting. There are many FQHCs operated by IHS and tribes. The statutory requirements that FQHCs must meet to furnish services to Medicare beneficiaries are in section 1861(aa)(4) of the Act. All FQHCs are subject to Medicare regulations at 42 CFR part 405, subpart X, and 42 CFR part 491. Based on these provisions, the following three types of organizations that are eligible to enroll in Medicare as FQHCs:
- Health Center Program grantees: Organizations receiving grants under section 330 of the PHS Act (42 U.S.C. 254b).
- Health Center Program "lookalikes": Organizations that have been identified by the Health Resources and Services Administration as meeting the requirements to receive a grant under section 330 of the PHS Act, but which do not receive section 330 grant funding.
- Outpatient health programs or facilities operated by a Tribe or tribal organization under the ISDEAA, or by an urban Indian organization receiving funds under Title V of the IHCIA.
FQHCs are also entities that were treated by the Secretary, for purposes of Medicare Part B, as a comprehensive federally funded health center as of January 1, 1990 (see section 1861(aa)(4)(C) of the Act). Section 1834 of the Act was amended in 2010 by section 10501(i)(3)(A) of the Affordable Care Act by adding a new subsection (o), "Development and Implementation of Prospective Payment System" for FQHCs. Section 1834(o)(1)(A) of the Act requires that the system include a process for appropriately describing the services furnished by FQHCs, and establish payment rates based on such descriptions of services, taking into account the type, intensity, and duration of services furnished by FQHCs. It also stated that the new system may include adjustments (such as geographic adjustments) as determined appropriate by the Secretary. Section 1833(a)(1)(Z) of the Act, as added by the Affordable Care Start Printed Page 39239 Act, requires that Medicare payment for FQHC services under section 1834(o) of the Act be 80 percent of the lesser of the actual charge or the PPS amount determined under section 1834(o) of the Act.
In accordance with the requirements in the statute, as amended by the Affordable Care Act, beginning on October 1, 2014, payment to FQHCs is based on the lesser of the national encounter-based FQHC PPS rate, or the FQHC's total charges, for primary health services and qualified preventive health services furnished to Medicare beneficiaries. The FQHC PPS rate is adjusted by the FQHC geographic adjustment factor (GAF), which is based on the Geographic Practice Cost Index used under the PFS. The FQHC PPS rate is also adjusted when the FQHC furnishes services to a patient that is new to the FQHC, and when the FQHC furnishes an IPPE or an AWV. Payment to the FQHC for a Medicare visit is the lesser of the FQHC's charges (as established by the G-code), or the PPS rate. The CY 2021 FQHC PPS rate is $176.45.
3. Grandfathered Tribal FQHCs
In the November 16, 2015 Federal Register, we published a final rule, entitled "Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2016 (referred to as CY 2016 PFS final rule). In that rule, we discuss the payment methodology and requirements finalized for grandfathered tribal FQHCs (80 FR 71089 through 71096). We stated that tribal facilities that met the conditions of § 413.65(m) on or before April 7, 2000, and had a change in their status on or after April 7, 2000, from IHS to tribal operation, or vice versa, or the realignment of a facility from one IHS or tribal hospital to another IHS or tribal hospital, such that the organization no longer met the Medicare Conditions of Participation (CoPs) for Medicare-participating hospitals at § 482.12, the "governing body" of the facility could nevertheless seek to become certified as a grandfathered tribal FQHC.
In CY 2016 PFS final rule, we explained that a different structure was needed to maintain access to care for AI/AN populations served by the hospitals and clinics impacted by the provider-based rules at § 413.65, while also ensuring that the tribal clinics are in compliance with our health and safety rules. We recognized that a tribal clinic billing under an IHS hospital's CMS Certification Number (CCN), without any additional administrative or clinical relationship with the IHS hospital, could put that hospital at risk for noncompliance with their CoPs because the clinic had a separate governing body although still provider-based. We explained that the FQHC program provided an alternative structure that met the needs of these tribal clinics and the populations they served, while also ensuring the IHS hospitals were not at risk of being cited for non-compliance with the requirements with their CoPs (80 FR 71090).
As stated in § 405.2462(d)(1) a "grandfathered tribal FQHC" is a FQHC that is operated by a tribe or tribal organization under the ISDEAA; was billing as if it were provider-based to an IHS hospital on or before April 7, 2000 and is not currently operating as a provider-based department of an IHS hospital. We refer to these tribal FQHCs as "grandfathered tribal FQHCs" to distinguish them from freestanding tribal FQHCs that are currently being paid the lesser of their charges or the adjusted national FQHC PPS rate, and from provider-based tribal clinics that may have begun operations subsequent to April 7, 2000. There are 7 "grandfathered tribal FQHCs".
Under the authority in section 1834(o) of the Act to include adjustments determined appropriate by the Secretary, we revised §§ 405.2462 and 405.2464 to pay these grandfathered tribal FQHCs on the Medicare outpatient per visit rate as set annually by the IHS, that is, the AIR and not the FQHC PPS payment rates (80 FR 71089). Payment rates for outpatient medical care (also referred to as outpatient hospital services) furnished by the IHS and tribal facilities is set annually by the IHS under the authority of sections 321(a) and 322(b) of the Public Health Service Act (the PHS Act) (42 U.S.C. 248 and 249(b)) (Pub. L. 83-568 (42 U.S.C. 2001(a)), and the IHCIA, based on the previous year cost reports from federal and tribal hospitals. The outpatient per visit rate is only applicable for those IHS or tribal facilities that meet the definition of a provider-based department as described at § 413.65(m), or a "grandfathered" tribal FQHC as described at § 405.2462(d)(1). There is an outpatient per visit AIR for Medicare visits in Alaska and a separate outpatient per visit AIR for Medicare visits in the lower 48 states. For CY 2021, the outpatient per visit rate for Medicare visits in Alaska is $662 and $414 in the lower 48 states (85 FR 86940). There are no grandfathered tribal FQHCs in Alaska because the tribes operate the hospitals, not IHS. We note that IHS does not operate any hospitals or facilities in Hawaii or the territories, and thus no rates are set, in those localities.
As we discussed in CY 2016 PFS final rule, the payment rate is not adjusted by the FQHC GAF; for new patients, annual wellness visits, or initial preventive physical examinations; or annually by the FQHC PPS market basket, as further adjustments would be unnecessary and/or duplicative of adjustments already made by IHS in deriving the rate. Comparatively, the FQHC PPS rate established by CMS is $176.45. The reimbursement is the lesser of the charges or the IHS AIR rate. We stated as part of the CY 2016 PFS final rule that we would monitor future costs and claims data of these tribal clinics and reconsider options as appropriate.
4. Paying all IHS- and Tribally-Operated Outpatient Clinics the AIR
CMS established a Tribal Technical Advisory Group (TTAG) in 2004 to provide advice and input to CMS on policy and program issues impacting AI/AN populations served by CMS programs. Although not a substitute for formal consultation with Tribal leaders, the TTAG enhances the government-to-government relationship and improves increased understanding between CMS and Tribes. The TTAG has subject specific subcommittees that meet on a regular basis in order to be more effective and perform in-depth analysis of Medicare, Medicaid, CHIP, and the Health Insurance Marketplace policies that have Tribal implications. The TTAG is comprised of 17 representatives: An elected Tribal leader, or an appointed representative from each of the 12 geographic areas of the IHS delivery system and a representative from each of the national Indian organizations headquartered in Washington DC—the National Indian Health Board, the National Congress of American Indians, and the Tribal Self-Governance Advisory Group. The American Recovery and Reinvestment Act of 2009 section 5006(e)(1), which became effective July 1, 2009, mandates that TTAG shall be maintained within CMS and added two new representative's positions: A representative and alternate from a national urban Indian health organization (National Council of Urban Indian Health) and a representative and alternate from the IHS.
The TTAG has requested[65] that CMS amend its Medicare regulations to make all IHS and tribally-operated outpatient Start Printed Page 39240 facilities eligible for payment at the IHS Medicare outpatient per visit rate/AIR. The TTAG explained that outpatient clinics, which are otherwise similar to grandfathered tribal FQHCs, are paid at different rates depending upon whether they meet the requirements as a "provider based facility," a "grandfathered tribal FQHC," a non-grandfathered tribal FQHC, or none of the above. They believe that the rates vary based on the Medicare regulatory definition, rather than the actual costs of the outpatient clinic. There are varying payment differentials among Medicare enrolled providers and suppliers under the authorities of the SSA. For example, Ambulatory Surgical Centers are paid differently than hospital outpatient departments; which are paid differently whether they're under the under the outpatient prospective payments system or a located in a critical access hospital.
The TTAG also questioned the need for grandfathered tribal FQHCs to file cost reports. Specifically, the TTAG stated that the FQHC cost reports have no relationship to the IHS Medicare outpatient per visit rate/AIR paid to grandfathered tribal FQHCs, as they use hospital cost reports in setting the rate. Therefore, they stated, the FQHCs should only need to file a cost report to the extent necessary to support payment for non-FQHC services that are reimbursed outside the Medicare outpatient per visit rate/AIR. We note that under section 1815(a) of the Act, providers participating in the Medicare program are required to submit financial and statistical information to achieve settlement of costs relating to health care services rendered to Medicare beneficiaries. Under the FQHC PPS, Medicare payment for FQHC services is the lesser of the FQHC PPS rate or the charges on the claim. In the establishment of the FQHC PPS, the statute does not exempt FQHCs from submitting cost reports. In addition, Medicare payments for the reasonable costs of the influenza and pneumococcal vaccines and their administration, allowable graduate medical education costs, and bad debts are determined and paid through the cost report. The FQHC market basket also uses information from the FQHC cost report to determine the cost share weights, which reflect the relative costs of input expenses that FQHCs face in order to provide FQHC services. Having a full picture of the costs of providing care by grandfathered FQHCs is important so that CMS can be sure that payments are adequate.
5. Comment Solicitation
We appreciate the TTAG's concerns with ensuring that CMS make appropriate payments among the clinics for similar services and the impact this has on tribal Medicare beneficiaries and ensuring that access to healthcare is available and equitable and we take these concerns seriously. However, we have insufficient information necessary to evaluate the costs and benefits of potential changes to these policies. Therefore, we would like to solicit comment on the TTAG's request for CMS to amend its Medicare regulations to make all IHS- and tribally-operated outpatient facilities/clinics eligible for payment at the Medicare outpatient per visit rate/AIR, regardless of whether they were owned, operated, or leased by IHS.
We seek information on the kinds of and number of facilities or clinics that could potentially enroll in Medicare as an FQHC, or are already an FQHC paid under the FQHC PPS, and if these clinics are freestanding or provider-based to expand on information provided by the IHS Profile. We seek information regarding the relative operating costs of IHS- and tribally-operated outpatient clinics compared to non-tribal FQHCs, stakeholder feedback and supporting evidence to address whether or why payment set at the IHS AIR would be more appropriate than payment rate under the FQHC PPS. Further, we seek comment on how the IHS AIR, which is based upon a limited number of hospital cost reports, relates to costs in such clinics and the kinds of services that the clinics furnish. Finally, we seek comment on the concerns that the AI/AN community may have on issues regarding access or inequity care in situations where a payment differential exists.
While, we have information on grandfathered tribal FQHCs and the outpatient hospital cost reports, we do not have any information specific to the composition of IHS and tribal facilities. For example, if the facility is not enrolled in Medicare as an FQHC or is not provider based to a hospital, is it a physician practice? It would be helpful to know how the facilities are organized and related. Are there other options for enrolling as different types of providers or suppliers?
As increasing the rate would increase payments from the Medicare Trust Fund, we are also seeking comment on the magnitude of that payment change and whether any program integrity concerns would be present with the increased payment. We also request comments on FQHC services that are paid through the cost report, like influenza, pneumococcal, and COVID-19 vaccinations and GME and how that impacts the request to not file cost reports. As stated above, having a full picture of the costs of providing care is important so that CMS can be sure that payments are adequate. Are these services included in the IHS/AIR?
We are also seeking input on other potential uses of the adjustment authority under section 1834(o)(1)(A) of the Act which provides that the FQHC PPS may include adjustments determined appropriate by the Secretary. For example, we could consider TTAG's request on the expansion of the payment policy finalized in the CY 2016 PFS final rule for grandfathered tribal FQHCs to all Tribally-operated outpatient clinics. Alternatively, we could develop a payment adjustment applicable to IHS- and tribally-operated outpatient clinics based on the cost differential reported in their cost reports when compared to non-IHS outpatient clinics, or non-provider-based clinics, if such differentials exist and would be interested in specific comments about appropriate adjustments to the FQHC PPS rate for clinics that are enrolled as FQHCs. We seek comment on other potential ways to determine whether the costs associated with furnishing services to AI/AN are uniquely greater than other clinics within the confines of the FQHC PPS outlined in section 1834(o)(1) of the Act.
D. Requiring Certain Manufacturers To Report Drug Pricing Information for Part B and Determination of ASP for Certain Self-Administered Drug Products
1. Requiring Certain Manufacturers To Report Drug Pricing Information for Part B (§§ 414.802 and 414.806)
a. Overview and Summary
Section 1927(b)(3)(A)(iii)(I) of the Act requires manufacturers with a Medicaid drug rebate agreement to report Average Sales Price (ASP) data as specified in section 1847A of the Act. Some manufacturers without Medicaid drug rebate agreements voluntarily submit ASP data for their single source drugs or biologicals that are payable under Part B; however, other manufacturers without Medicaid drug rebate agreements do not voluntarily submit such data. Without manufacturer reported ASP data, CMS cannot calculate the ASP payment limit, and consequently, payment is typically based on Wholesale Acquisition Cost (WAC).
Consistent with section 1847A(c)(3) of the Act and our regulations at Start Printed Page 39241 § 414.804(a)(2), the ASP is net of price concessions. However, consistent with the definition of WAC at section 1847A(c)(6)(B) of the Act, the WAC is not net of price concessions, and thus, is nearly always, and sometimes, significantly, higher than ASP. Drugs with payment allowances based on WAC may have greater "spreads" between acquisition costs and payment than drugs for which there is an ASP-based payment allowance, which, in turn, may: (1) Incent the use of the drug based on its spread rather than on purely clinical considerations; (2) result in increased payments under Medicare Part B; and (3) increase beneficiary cost sharing.
Section 401 of Division CC, Title IV of the CAA, 2021 (for the purposes of this section of this proposed rule, hereinafter is referred to as "section 401") amended section 1847A of the Act to add new section 1847A(f)(2) of the Act, which requires manufacturers without a Medicaid drug rebate agreement to report ASP information to CMS for calendar quarters beginning on January 1, 2022, for drugs or biologicals payable under Medicare Part B and described in sections 1842(o)(1)(C), (E), or (G) or 1881(b)(14)(B) of the Act, including items, services, supplies, and products that are payable under Part B as a drug or biological. Section 401(b)(2) also amended section 1847A(c)(6)(A) of the Act to permit the Secretary to exclude repackagers[66] from the definition of "manufacturer" for purposes of the ASP reporting requirement in section 1847A(f)(2) of the Act, if the Secretary determines appropriate.
Section 401(b)(1) also adds provisions to section 1847A of the Act addressing confidentiality, audit and verification provisions; civil money penalties for misrepresentation, late reporting, and reporting of false information; and increasing oversight and enforcement provisions. These provisions largely track the statutory provisions in section 1927(b) of the Act that apply to the reporting of ASP by manufacturers with Medicaid drug rebate agreements. Additionally, section 401(d) requires HHS Office of the Inspector General (OIG) to submit a report on the accuracy of ASP submissions to Congress by January 1, 2023.
Finally, section 401 amended section 1927(b) of the Act to clarify that for Part B ASP reporting, drugs would include items, services, supplies, and products that are payable under Medicare Part B as a drug or biological.
We are proposing regulatory changes to implement the new reporting requirements at 42 CFR, part 414, subpart J.
b. Reporting Requirements for Manufacturers Without a Medicaid Drug Rebate Agreement
Starting with calendar quarters beginning on January 1, 2022, manufacturers will be required to report ASP for drugs and biologicals payable under Medicare Part B consistent with the statutory requirements of section 1847A(f) of the Act, regardless of whether they have Medicaid drug rebate agreements. Our existing regulations at 42 CFR part 414, subpart J implement the ASP reporting requirements referenced in section 1847A(f)(1) of the Act, that is, the requirements of section 1927(b)(3) of the Act. Thus, the existing regulations at 42 CFR part 414, subpart J already set forth requirements for manufacturers with Medicaid drug rebate agreements to report their ASP information (and if required to make payment, WAC) each quarter.
Many manufacturers without Medicaid drug rebate agreements voluntarily submit ASP data consistent with these requirements. Whether obligated to report or voluntarily reporting, manufacturers are accustomed to the existing regulatory requirements at 42 CFR part 414 subpart J, and indeed, the methodology for reporting ASP reflected in these regulations does not currently distinguish between manufacturers with Medicaid drug rebate agreements and those without these agreements.
Because new section 1847A(f)(2) of the Act, as noted previously, largely parallels section 1927(b)(3) of the Act, and thus both manufacturers with Medicaid drug rebate agreements, as well as those without such agreements, will be subject to requirements already reflected in the existing regulations at subpart J, we do not believe it is necessary to propose substantial changes to the regulation text. For these reasons, our proposal to amend the regulations to reflect the new requirements of section 1847A(f)(2) of the Act seeks to preserve the status quo to the extent possible.
c. Definitions
As noted previously, the new section 1847A(f)(2) of the Act, as added by section 401(a), requires manufacturers without a Medicaid drug rebate agreement to report ASP information to CMS for calendar quarters beginning on January 1, 2022 for drugs or biologicals payable under Medicare Part B and described in sections 1842(o)(1)(C), (E), or (G) or 1881(b)(14)(B) of the Act, including items, services, supplies, and products that are payable under Part B as a drug or biological. Section 401 also made a conforming amendment to the ASP reporting requirements applicable to manufacturers with Medicaid drug rebate agreements at section 1927(b)(3)(A)(iii) of the Act to specify that those reporting requirements also apply to items, services, supplies, and products that are payable under Part B as a drug or biological.
To implement this change, we propose to amend the definition of the term "drug" at § 414.802 to mean a drug or biological, and includes an item, service, supply, or product that is payable under Medicare Part B as a drug or biological.
Section 1847A(c)(6)(A) of the Act incorporates the definition of manufacturer at section 1927(k)(5) of the Act, except that section 401(b)(2) permits the Secretary to exempt repackagers from the definition of manufacturer, as determined appropriate, for purposes of section 1847A(f)(2) of the Act. However, no such exemption is provided for manufacturers with Medicaid drug rebate agreements (see the definition of manufacturer at § 447.502). Consequently, the current ASP data reporting includes submissions by repackagers.
To confirm the Medicare Payment Advisory Commission's (MedPAC's) assertion in their June 2017 report (available at http://medpac.gov/docs/default-source/reports/jun17_ch2.pdf) that many repackagers currently do not report ASP data, and thus inform our consideration of whether we should propose to exclude repackagers from the definition of manufacturers for purposes of section 1847A(f)(2) of the Act, we conducted an analysis to estimate the proportion of repackaged products in our existing ASP data. If our existing ASP data do not contain an appreciable Start Printed Page 39242 proportion of repackaged products, it may be appropriate to exclude repackagers from the definition of manufacturer for this limited purpose. However, if repackaged products comprise an appreciable proportion of our existing ASP data, we would reasonably anticipate this trend to follow under the new requirements, and in such a scenario, it would not be appropriate to exclude repackagers from the definition of manufacturer for purposes of section 1847A(f)(2) of the Act because excluding their sales could distort the ASP.
To effectuate this analysis, we obtained a list of National Drug Codes (NDCs) of repackaged drugs from the United States Food and Drug Administration (FDA).[67] We also obtained a list of labeler codes for which the manufacturers have Medicaid drug rebate agreements.[68] We then performed a crosswalk both of these to our composite file of ASP data submissions to segregate our composite file of ASP data submissions into four categories:
(1) Repackaged products for which ASP data submissions were required (that is, manufacturers with Medicaid drug rebate agreements);
(2) Repackaged products for which ASP data submissions were voluntary (that is, for manufacturers without Medicaid drug rebate agreements);
(3) Non-repackaged products for which ASP data submissions were required; and
(4) Non-repackaged products for which ASP data submissions were voluntary.
We estimate that, of all 6319 products for which we currently receive ASP data submissions (the sum of categories (1)-(4) above), repackaged products accounted for 271 (4.29 percent) of these products. Additionally, repackaged products accounted for 137 (2.51 percent of) products for which ASP data submissions were required, and 134 (15.23 percent of) products for which ASP data were voluntarily submitted.
Additionally, we conducted another analysis to estimate: (1) The number of new ASP submissions we can expect as a result of the new requirements under section 401; and (2) the proportion of those submissions that involve repackaged products. To effectuate this analysis, we obtained a crosswalk of NDCs and Healthcare Common Procedure Coding System (HCPCS) codes that includes the NDCs and HCPCS codes of items for which ASP reporting is not currently required.[69] We supplemented this crosswalk by adding HCPCS codes with NDCs that are payable under Part B, but not already reflected in the crosswalk.[70] We then identified[71] and removed from the crosswalk all of the products contained in our composite file of ASP data submissions and those HCPCS codes that are non-covered under Medicare Part B. Adding the results of this analysis to the results of categories two and four from the prior analysis (that is, repackaged and non-repackaged products for which ASP submissions were voluntary), we estimate there will be 6994 total products for which manufacturers will now be required to submit ASP data. We then compared this number to the FDA's list of repackaged products in the previous analysis, and found that of the 6994 products for which manufacturers will be required to submit ASP data, 223 (3.19 percent) are repackaged products. Further, we estimate 6114 products for which their manufacturers did not previously (voluntarily) submit ASP data and will now be required to do so under the new reporting requirements of section 401. Of these, 89 (1.46 percent) are repackaged products.
These data do not persuade us that it is necessary to exempt repackagers from the new reporting requirements under section 401 at this time. Our current operational process to verify the accuracy of manufacturers' reported ASP data does not distinguish: (1) Products on the basis of repackaging, and (2) manufacturers who are required to report ASP data from those who do so voluntarily.
Each month, CMS reviews ASP data submissions at the NDC level (and for products without NDCs, the manufacturer's product code). Previously, we have not required manufacturers to identify which products are repackaged as part of these submissions. Exempting repackagers from the new requirements of section 1847A(f)(2) of the Act would significantly increase our administrative burden because we would have to undergo an additional quality check for each NDC from a different database for which data are submitted as part of our operational process to verify the accuracy of manufacturers' reported ASP data. Moreover, for products without NDCs, our ability to determine if these products are repackaged (without manufacturer attestation) to that effect is significantly limited. Finally, any such attestation would require a data source for us to verify the accuracy of the attestation, and no such data source currently exists.
These additional checks could, in turn, significantly increase the time it takes for us to calculate and display on our website the volume-weighted ASP payment limits. Additionally, we are concerned that exempting repackagers from the new reporting requirements could lead to a gap in ASP reporting, meaning that ASPs could be distorted to the extent that certain sales are carved out of the reporting requirement through the use of repackagers. Consequently, in order to maintain consistency and integrity of the ASP data for those manufacturers with and without Medicaid drug rebate agreements, we do not believe it is appropriate to exclude repackagers from the requirements of section 401 at this time. However, we may propose to exempt repackagers in the future, if warranted.
We solicit comment on this approach.
In summary, we propose to modify the definition of drug at § 414.802 to include any item, service, supply or product that is payable under Part B as a drug or biological. We are not proposing to exclude repackagers from the definition of manufacturer for purposes of the reporting requirements at section 1847A(f)(2) of the Act.
d. Civil Money Penalties
As amended by section 401(b), section 1847A(d)(4)(A) of the Act specifies the penalties associated with misrepresentations in the reporting of the manufacturer's ASP for a drug or biological. Consistent with our existing regulation at § 414.806, if the Secretary determines that a manufacturer has made a misrepresentation in the reporting of ASP data, a civil money penalty in an amount of up to $10,000 may be applied for each price misrepresentation and for each day in which the price misrepresentation was applied.
New sections 1847A(d)(4)(B) and (C) of the Act, as added by section 401(b), Start Printed Page 39243 apply civil money penalties for failure to report timely and accurate ASP data for manufacturers without Medicaid drug rebate agreements, consistent with the civil money penalties found at sections 1927(b)(3)(C)(i) and (ii) of the Act for manufacturers with Medicaid drug rebate agreements. Our current regulations at § 414.806 refer to section 1927(b)(3)(C) of the Act, as amended by section 303(i)(4) of the Medicare Prescription Drug, Improvement, and Modernization Act (MMA) of 2003 (Pub. L. 108-173, December 8, 2003), as specifying the penalties associated with a manufacturer's failure to submit timely information or the submission of false information.
We propose to amend § 414.806 to reflect the new provisions specifying penalties for manufacturers without Medicaid drug rebate agreements and to provide some technical changes to streamline the regulations text. Specifically, we propose to do the following:
- Add paragraph (a), labeled as "Misrepresentation", moving the existing regulatory language at § 414.806 specific to misrepresentation to this paragraph;
- Remove the sentence which reads, "If the Secretary determines that a manufacturer has made a misrepresentation in the reporting of ASP data, a civil money penalty in an amount of up to $10,000 may be applied for each price misrepresentation and for each day in which the price misrepresentation was applied," since the previous sentence in the regulations text already references the statutory provision for this language;
- Add paragraph (b), labeled as "Failure to provide timely information or the submission of false information";
- Add paragraph (b)(1) to clarify that the existing language at § 414.806 regarding civil money penalties for failure to submit timely information or for the submission of false information applies to manufacturers with a Medicaid drug rebate agreement;
- Remove the phrase "as amended by section 303(i)(4) of the MMA"; and
- Add paragraph (b)(2) to reflect new sections 1847A(d)(4)(B) and (C) of the Act regarding civil money penalties for failure to submit timely information or for the submission of false information for manufacturers without a Medicaid drug rebate agreement.
We welcome comments on these proposals.
e. Summary of all Proposals
In summary, to implement the new reporting requirements for manufacturers without Medicaid drug rebate agreements, we are proposing to modify:
- The definition of drug at § 414.802; and
- The regulations describing civil money penalties at § 414.806.
We welcome comments on these proposals.
2. Determination of ASP for Certain Self-Administered Drug Products (§ 414.904)
a. Background
Drugs and biologicals payable under Medicare Part B fall into three general categories: those furnished incident to a physician's services (hereinafter referred to as "incident to") (section 1861(s)(2) of the Act), those administered via a covered item of durable medical equipment (DME) (section 1861(s)(6) of the Act), and others as specified by statute (for example, certain vaccines described in sections 1861(10)(A) and (B) of the Act). Payment limits for most drugs and biologicals separately payable under Medicare Part B are determined using the methodology in section 1847A of the Act, and in many cases, payment is based on the Average Sales Price (ASP) plus a statutorily mandated 6 percent add-on. Most drugs payable under Part B are paid under the "incident to" benefit under section 1861(s)(2) of the Act, which includes drugs and biologicals not usually self-administered by the patient.
Paragraphs (4)(A) and (6) of sections 1847A(b) of the Act require that the Medicare Part B payment amount for a single-source drug or biological be determined using all of the NDCs assigned to it. Section 1847A(b)(5) of the Act further states that the payment limit shall be determined without regard to any special packaging, labeling, or identifiers on the dosage form or product or package. In 2007, CMS issued a program instruction (available at https://www.cms.gov/Medicare/Coding/MedHCPCSGenInfo/Downloads/051807_coding_annoucement.pdf), as permitted under section 1847A(c)(5)(C) of the Act, stating that the payment limit for a single source drug or biological will be based on the pricing information for products produced or distributed under the applicable FDA approval (such as a New Drug Application (NDA) or Biologics License Application (BLA)). Therefore, all versions of a single source drug or biological product (or NDCs) marketed under the same FDA approval number (for example, NDA or BLA, including supplements) are considered the same drug or biological, for payments made under section 1847A of the Act and are crosswalked to the same billing and payment code. This means that a self-administered version marketed under the same FDA approval is subject to the ASP reporting requirements and is not excluded from the payment limit calculation, even though Medicare does not make separate Part B payment for it. This is consistent with our longstanding policy on the scope of the ASP reporting requirements. (Please see our final rule titled, "Medicare Program; Revisions to Payment Policies, Five-Year Review of Work Relative Value Units, Changes to the Practice Expense Methodology Under the Physician Fee Schedule, and Other Changes to Payment Under Part B; Revisions to the Payment Policies of Ambulance Services Under the Fee Schedule for Ambulance Services; and Ambulance Inflation Factor Update for CY 2007," published in the December 1, 2006 Federal Register (71 FR 69675)). The price of a drug or biological product that may be administered by the patient (that is, self-administered) may differ from versions that are administered incident to a physician's service, which may affect the ASP-based payment limit for drug or biological product's billing and payment code.
The HHS OIG conducted studies[72] [73] of payment-limit calculations for certain drugs paid under section 1847A of the Act. The OIG identified two highly utilized biological products for which there are both Part-B-covered (versions administered incident to a physician's service) and non-covered versions (those identified to be self-administered) for which the NDCs were marketed under the same FDA approval number. OIG's studies found that when the ASPs of the self-administered versions are included in the payment limit calculation, the resulting payment limit is substantially higher than if the ASPs of only the incident-to versions had been included.
The OIG studies concluded that as a result, Medicare payment amounts were inflated, causing the program and its beneficiaries to pay an additional $366 million from 2014 through 2016 and $497 million from 2017 through 2018. They recommended that legislative changes be made to provide CMS the flexibility to determine when certain versions of a drug identified to be self-administered should be included in ASP payment limit calculations.
Section 405 of Division CC, Title IV of the CAA, 2021, amended section 1847A of the Act by redesignating Start Printed Page 39244 existing subsection (g) as subsection (h) and adding new subsection (g), which describes the Medicare Part B ASP payment-limit adjustment for certain drugs and biological products for which NDCs have been identified by the OIG to be self-administered and not covered under Medicare Part B. The new section 1847A(g)(1) of the Act directs OIG to conduct periodic studies to identify NDCs for drug or biological products that are identified to be self-administered for which payment may not be made under Part B pursuant to section 1861(s)(2) of the Act and that OIG determines should be excluded from the determination of the payment amount under section 1847A of the Act.
New section 1847A(g)(2) of the Act specifies that if the OIG identifies an NDC under section 1847A(g)(1) of the Act, it must inform the Secretary at such times as the Secretary may specify. Then the Secretary shall, to the extent appropriate, apply as the payment limit for the applicable billing and payment code the lesser of: (1) The payment allowance that would be determined under section 1847A of the Act if the NDC for the identified drug or biological product were excluded from the calculation; or (2) the payment limit otherwise determined under section 1847A of the Act without application of section 1847A(g) of the Act. In other words, the Medicare payment limit for a drug or biological product's billing and payment code in these circumstances would be the lesser of the payment limit determined including the NDCs identified to be self-administered and the payment limit determined after excluding the NDCs identified to be self-administered (hereinafter referred to as the "lesser-of payment methodology").
Although section 1847A(g)(1) of the Act provides us with discretion in whether to apply the lesser-of methodology to billing and payment codes that include self-administered versions identified by the OIG (because we are directed to apply the methodology to the extent deemed appropriate), new section 1847A(g)(3) of the Act, requires the application of the lesser-of methodology to the two billing and payment codes identified in the OIG's July 2020 report titled, "Loophole in Drug Payment Rule Continues To Cost Medicare and Beneficiaries Hundreds of Millions of Dollars," (available at https://oig.hhs.gov/oei/reports/OEI-BL-20-00100.asp) (hereinafter referred to as "OIG's July 2020 report")) beginning July 1, 2021. To meet the implementation date required by this provision, we applied the lesser-of methodology to the payment limit calculations for the billing and payment codes representing Cimzia® (certolizumab pegol) and Orencia® (abatacept), details on these calculations are described in this section. In a memorandum providing supplemental information on the OIG July 2020 report, the OIG provided specific NDCs that the report identified: 00003-2188-11, 00003-2188-51, 00003-2814-11, 00003-2818-11, 50474-0710-79, 50474-0710-81. The lesser-of methodology was applied to these billing and payment codes for the July 2021 ASP Drug Pricing Files and crosswalks along with program instructions in a change request (CR) at https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2021-asp-drug-pricing-files.
We propose to codify the new requirements of section 1847A(g) of the Act at § 414.904. Our proposals described in the next section specify when the application of the lesser-of methodology would be appropriate, describe how we will apply the lesser-of payment methodology to billing and payment codes that OIG has identified pursuant to studies described in section 1847A(g)(1) of the Act, and codify the approach we used for the certolizumab pegol and abatacept billing and payment codes.
b. Identification of Billing and Payment Codes to Which the Lesser-of Policy Will Be Applied
As noted previously, section 1847A(g)(1) of the Act directs OIG to conduct periodic studies to identify NDCs for drug or biological products that are self-administered and for which payment is not made under Part B. Section 1847A(g)(2) of the Act specifies that if OIG makes an identification under section 1847A(g)(1) of the Act, OIG informs CMS at such times as we may specify, and in such an event, we apply the lesser-of methodology to the extent deemed appropriate. We propose that when the OIG conducts a periodic study, OIG informs us at the time the study becomes are publicly available. CMS will obtain the NDCs identified by the OIG study described in section 1847A(g)(1) of the Act. However, if the specific NDCs are not available in the OIG study report, we will request OIG provide documentation of the identified NDCs to CMS.
To allow operational time for assessment and application of the lesser-of methodology, we believe it is reasonable that the application of the lesser-of methodology be reflected beginning in the ASP pricing file two quarters following the OIG study publication. For example, if the OIG study becomes available to the public in the first quarter of the calendar year, the lesser-of methodology would be applied to the payment limit calculation of the applicable billing and payment code in the third quarter ASP pricing file (in other words, the July ASP pricing file) and each quarter thereafter.
c. Calculation of Payment Allowance Using the Lesser-of Payment Methodology
Sections 1847A(g)(2) and (g)(3) of the Act set forth the lesser-of payment methodology for applicable billing and payment codes with NDCs for certain drug or biological products identified by the OIG as self-administered products for which payment may not be made under this part because such products are not covered under section 1861(s)(2) of the Act. In this section, we describe how we propose to apply the lesser-of methodology. We propose to codify this methodology, which we currently use for the billing and payment codes that describe certolizumab pegol and abatacept, and which we propose to use for billing and payment codes for which OIG identifies a drug or biological product with NDCs identified to be self-administered as described in section 1847A(g)(1) of the Act.
The ASP payment limit calculation is described in section 1847A(b)(6) of the Act and codified at § 414.904(b)(2)(ii) and (c)(2)(ii), which specifies that for a billing and payment code, the volume-weighted average of the average sales prices reported by the manufacturer is determined by:
- Computing the sum of the products (for each NDC assigned to such drug products) of:
++ The manufacturer's average sales price determined by the Secretary without dividing such price by the total number of billing units for the NDC for the billing and payment code; and
++ The total number of units sold; and
- Dividing the sum determined under (A) by the sum of by the sum of the products (for each NDC assigned to such drug products) of
++ The total number of units specified sold; and
++ The total number of billing units for the NDC for the billing and payment code.
When applying the lesser-of methodology described in 1847A(g)(2) and (g)(3) of the Act, we propose to make two calculations as described in section 1847A(b)(6) of the Act: (1) The ASP payment limit for the billing and payment code, excluding the NDCs that have been identified by the OIG study (that is, excluding the ASPs for those NDCs as well as the units of such NDCs Start Printed Page 39245 sold in the quarter); and (2) the ASP payment limit for the billing and payment code, including such NDCs' ASPs and units sold. The calculation resulting in the lower payment limit will be used as the payment limit for the applicable billing and payment code for that quarter's ASP pricing files. We propose to apply the lesser-of methodology to the billing and payment codes containing OIG-identified products each quarter when determining ASP payment limits.
New section 1847A(g) of the Act does not change ASP reporting requirements, and consistent with section 1847A(f)(1) of the Act and, beginning January 1, 2022, section 1847A(f)(2) of the Act, manufacturers must continue to report ASP data for all NDCs of the drug or biological product. Under new section 1847A(g) of the Act, ASP data for all NDCs under the same FDA approval application (for example, NDA or BLA, including any supplements) are required to carry out the lesser-of calculations for the purposes of determining the payment limit for the billing and payment code. Even if the resulting payment limit does not reflect the ASPs or units sold of self-administered versions of a product identified by the OIG, the manufacturer must continue to report those versions' ASPs and units sold to the Secretary.
The implementation of the lesser-of methodology is not expected to be associated with substantial administrative costs. We plan to incorporate methodology in the current operational process that is used to determine ASP payment limits each quarter. The OIG found that Medicare and its beneficiaries would have saved a combined $497 million on certolizumab pegol and abatacept over 2 years (2017-2018) if such a methodology had been in place.
d. Exceptions
We further propose that the application of the lesser-of methodology is deemed appropriate in all cases in which OIG identifies a drug or biological product in a periodic study described in section 1847A(g)(1) of the Act and made publicly available, unless the drug or biological product is in short supply.[74] As stated in the OIG's July 2020 report, CMS expressed concern about potential impact on beneficiary access if certain versions identified to be self-administered were excluded from the ASP payment limit calculation. Because of potential for drug shortages that may affect patient care, beneficiary and provider access, and drug prices for providers, we would consider it not appropriate to apply the lesser-of methodology when a product is in short supply. Similar to the average manufacturer price (AMP) price substitution provision in section 1847A(d)(3)(C) of the Act (codified in § 414.904(d)(3)), we propose to add § 414.904(d)(4)(ii) to specify that we will not apply the lesser-of methodology (that is, we will determine the payment allowance including all NDCs of the drug or biological product) if the drug and dosage form(s) represented by the billing and payment code are reported by the Drug Shortage list established under section 506E of the Federal Food, Drug, and Cosmetic Act (FFDCA) at the time that ASP payment limits are being finalized for the next quarter. However, we propose that this exception to the application of the lesser-of methodology would not apply in the case of the billing and payment codes for certolizumab pegol and abatacept because section 1847A(g)(3) of the Act does not provide us with the same discretion as section 1847A(g)(2) of the Act. Thus, for these applicable billing and payment codes we will always apply the lesser-of methodology. We recognize that NDCs identified by an OIG study described in section 1847A(g)(1) or (g)(3) of the Act may change, for example, because of a manufacturer change. In the event that the manufacturer of an OIG-identified product simply redesignates the NDC for its product, we believe the new NDC also would meet the same criteria defined in the OIG study. In this circumstance, we expect that the product labeling would not contain substantial changes regarding the redesignated NDC. Therefore, we propose to add § 414.904(d)(4)(iv) to codify the application of the lesser-of methodology such that the manufacturer-reported pricing data associated with redesignated NDCs will be used in the lesser-of methodology in the same way as the original OIG-identified NDC.
Once an OIG study identifies self-administered versions of a drug or biological product, there may be subsequent FDA approvals of other products with the same active ingredient, such as new syringe sizes, new types of injector syringes, generic formulations, biosimilar biological products, or interchangeable biological products. For example, this would include the situation in which the current manufacturer of certolizumab pegol or abatacept obtains a supplemental FDA approval for a new version of the product. Similarly, this would also include the situation in which another manufacturer gains FDA approval of a product with the same active ingredient as an OIG-identified self-administered version. We believe that provisions at new section 1847A(g) of the Act would require a new OIG study as described in section 1847A(g)(1) of the Act in order for us to apply the lesser-of methodology to the drug or biological product.
e. Summary
In summary, to implement new section 1847A(g) of the Act, we are proposing to:
- Add § 414.904(d)(4) to codify the lesser-of payment methodology and define when the application of the lesser-of methodology would first be reflected in the ASP pricing file following the OIG study publication; and
- Describe the lesser of methodology at § 414.904(d)(4)(iv).
- Describe exceptions to application of the lesser-of methodology at § 414.904(d)(4)(ii).
- Clarify application of the lesser-of methodology for billing and payment code described under section 1847A(g)(3) of the Act at § 414.904(d)(4)(iii).
- Describe the application of the lesser-of methodology to redesignated NDCs of those identified in the OIG studies at § 414.904(d)(4)(v).
We welcome comments on these proposals.
E. Medicare Part B Payment for Drugs Approved Through the Pathway Established Under Section 505(b)(2) of the Federal Food, Drug, & Cosmetic Act
1. Background
For most drugs that are payable under Medicare Part B, payment-limit amounts are determined using the methodology in section 1847A of the Act. In many cases, the payment-limit amount is based on the Average Sales Price (ASP) plus a statutorily mandated 6 percent add-on. Additionally, small molecule drugs payable under Medicare Part B using the methodology in section 1847A of the Act fall into two broad, mutually exclusive categories: (1) Multiple source drugs, and (2) single source drugs. These terms are defined in sections 1847A(c)(6)(C) and (D) of the Act, respectively. Start Printed Page 39246
In most cases, the distinction between multiple source drugs and single source drugs is straightforward. We published program instructions in 2007 (available at https://www.cms.gov/Medicare/Coding/MedHCPCSGenInfo/Downloads/051807_coding_annoucement.pdf) that address how these distinctions are made. However, a subset of drugs that are approved by the FDA under New Drug Applications (NDAs) are approved through the pathway established under section 505(b)(2) of the FFDCA (Pub. L. 75-717, June 25, 1938) (hereinafter referred to as "section 505(b)(2) drug products"). For section 505(b)(2) drug products, the distinction between multiple source drugs and single source drugs can be less straightforward.
The drug approval pathway established under section 505(b)(2) of the FFDCA (hereinafter referred to as "the section 505(b)(2) pathway") provides an avenue for applications that contain full reports of investigations of safety and effectiveness, where at least some of the information needed for an approval comes from studies not conducted by or for the applicant, and for which the applicant has not obtained a right of reference or use.[75] An application submitted under the section 505(b)(2) pathway (hereinafter referred to as a "section 505(b)(2) application") may rely either on the FDA's findings of safety, effectiveness, or both, for an already-FDA-approved drug product or on published literature, provided that: (1) Such reliance is scientifically justified, and (2) the section 505(b)(2) application complies with applicable statutory and regulatory requirements, including, but not limited to, patent certification, if appropriate. Unlike a generic drug product approved under an Abbreviated New Drug Application (ANDA), a section 505(b)(2) drug product is not required to have the same FDA-approved labeling as the labeling for the already-FDA-approved drug product(s) upon which the section 505(b)(2) application relied. (For more information, see the FDA's May 2019 guidance titled, "Determining Whether to Submit an ANDA or a 505(b)(2) Application," available at https://www.fda.gov/media/124848/download.)
The number of section 505(b)(2) drug products approved each year has been growing, from about 40 per year from 2011 to 2016, to about 60 to 70 per year from 2017 to 2020. Approximately 10 to 20 percent of these section 505(b)(2) drug products are payable under Medicare Part B. Of these, some section 505(b)(2) drug products share substantial portions of the FDA-approved labeling with the approved drug product(s) upon which the section 505(b)(2) application relied, for example prescribing information on safety, efficacy, and pharmacokinetics. In some cases, the section 505(b)(2) drug product even shares substantial portions of labeling with generic drug products that are payable under Part B as multiple source drugs. Medicare Part B claims data from 2020 indicate that spending for some of these section 505(b)(2) drug products (that is, those that could be assigned to a multiple source drug code under the framework described below, but are instead currently assigned to a single source drug code) is substantially greater than that for the corresponding generic drug products assigned to a multiple source drug code. One example is a sterile injectable drug that was first approved as a lyophilized powder for reconstitution in a vial and later was approved through the section 505(b)(2) pathway as a concentrated liquid in a vial. Another example is a drug available as a lyophilized powder for reconstitution in a vial that was then approved through the section 505(b)(2) pathway as a ready-to-use intravenous (IV) solution in a bag. Analysis of 2020 claims data for the separately coded section 505(b)(2) drug product (that is, the ready-to-use IV solution) shows that Medicare spending per service unit was approximately eight times that of the corresponding products in the multiple source drug code. Moreover, in the July 2021 ASP Pricing File (available at https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2021-asp-drug-pricing-files), the payment limit for the section 505(b)(2) drug product is 17.2 times the payment limit for the multiple source code, when adjusted for the different dose descriptors of each code. In another example, there were approximately 7.54 million allowed service units, representing approximately $1.38 million of allowed charges, for a multiple source drug code, but for the separately coded section 505(b)(2) drug product, over the same time-period there were approximately 1.08 million allowed service units, representing approximately $2.13 million in allowed charges. Calculating the allowed charges per allowed service unit, each service unit of the section 505(b)(2) drug product cost Medicare 10.78 times that of the corresponding products assigned to the multiple source drug code, costing Medicare an additional $1.93 million. In the July 2021 ASP Pricing File, the payment limit for the section 505(b)(2) drug product is 21.3 times the payment limit for the multiple source code.
Based on these observed data points, we are planning an additional analysis of spending on section 505(b)(2) drug products and potential savings to Medicare and Medicare beneficiaries that may be realized if certain section 505(b)(2) drug products were to be assigned to multiple source drug codes based on the framework described in section 3 of this preamble.
2. CY 2021 Proposal
In the CY 2021 PFS proposed rule, we proposed to codify our long-standing approach to determine whether a section 505(b)(2) drug product is described by an existing multiple source drug code, or if the section 505(b)(2) drug product would be assigned to a single source drug code. In that proposal, we explained generally how information about the section 505(b)(2) drug product's active ingredient(s), drug product name (this refers to nomenclature of the drug product as found in the United States Pharmacopeia—National Formulary (USP-NF) and nomenclature as found in title of the FDA-approved labeling), and description; labeling information; and ordering (prescribing) and clinical use would factor into a determination. Commenters on our proposal in the CY 2021 PFS proposed rule (primarily manufacturers) stated that the proposal conflicted with both the Medicare statute and the FDA's therapeutic equivalence (TE) ratings,[76 77] and would impair access for patients, underpay providers, and stifle innovation. Several commenters from beneficiary advocate and provider organizations generally repeated the same points, although some commenters expressed support for curbing drug prices, particularly if the proposal did not affect patient access. Several commenters appeared to take a middle ground that conditionally supported the proposals, particularly if more detail could be provided and if effects on patient access were considered. Several commenters supported the proposals without conditions. Several commenters expressed that we should provide more Start Printed Page 39247 detail about the decision framework and the determination process.
Some commenters on the CY 2021 PFS proposed rule requested that we provide more details about the process by which certain section 505(b)(2) drug products would be assigned to multiple source drug codes. Commenters requested that we include more detail on how factors described in the CY 2021 PFS proposal, (for example, differences in the active ingredient and labeling) may be interpreted and which drug products might be affected. Commenters also requested that we provide the public more time to assess a more detailed proposal as well as an opportunity, such as through future rulemaking, for public input both on the proposal and on decisions about specific drug products.
Several commenters stated that if we move forward with the CY 2021 proposal, we should exclude products with "meaningful differences" from the policy and encouraged us to continue an approach "that allows for innovation, competition, and ultimately more therapeutic choices for Medicare beneficiaries." We recognize that some section 505(b)(2) drug products have clear differences in factors such as safety, efficacy, or pharmacokinetics, which would not result in the assignment of the product to the existing multiple source drug code. The framework discussed in the next section would address situations in which a section 505(b)(2) drug product is not described by an existing multiple source drug code, and therefore, would not be assigned to the existing multiple source drug code.
In response to commenters' requesting more detail about our proposed approach and to delay finalizing a decision, we did not finalize our proposals in the CY 2021 PFS proposed rule regarding section 505(b)(2) drug products. We stated that the delay would allow time for CMS to further consider this issue. As part of our further consideration, we are soliciting comment on a more detailed framework (hereinafter referred to as "the framework") for determining when a section 505(b)(2) drug product is a multiple source drug under section 1847A(c)(6)(C) of the Act.
The framework is consistent with program instruction published in 2007, which addressed how we would assign "single source drugs" and "biological products" using a multi-step process. However, this program instruction did not expressly address how we would assign multiple source drugs. The program instruction uses the term "drug" at the billing and payment code level when discussing single source drugs in the same way that the discussion in this preamble uses the term "drug" in reference to multiple source drugs. Development of standards for identifying multiple source drugs (that is, the framework) would add to the 2007 program instruction and provide detail about an approach to Medicare Part B payment for section 505(b)(2) drug products.
The framework described in the next section aims to build off the current CMS policy for assigning drug products to billing and payment codes by describing detailed standards for determining whether a section 505(b)(2) drug product corresponds to an existing multiple source drug code. We are not proposing to adopt the framework at this time. Rather, we are seeking comment on the framework to inform future policy making.
3. The Framework
The framework is a determination process to identify when section 505(b)(2) drug products without an FDA TE rating to an existing drug product payable under Part B correspond to an existing multiple source drug code for the purpose of payment under Medicare Part B. The framework would provide additional detail about the decision-making process and increase transparency about potential determinations resulting from the framework.
The first portion of the framework would compare certain qualities of the section 505(b)(2) drug product with drug products already assigned to an existing multiple source drug code.[78] This includes comparison of the: (1) Active ingredient(s); (2) dosage form (if part of the drug product name); (3) salt form; and (4) other ingredients in the drug product formulation. The drug product assessment could result in a match or non-match designation. Section 505(b)(2) drug products receiving a match designation in the first portion of the framework would continue to a verification step. This step would compare the pharmacokinetic and clinical studies of the section 505(b)(2) drug product's FDA-approved labeling with those of the drug products already assigned to an existing multiple source code. Finally, a determination would be made as to whether the section 505(b)(2) drug product could be assigned to the existing multiple source code.
For full details on the framework, please see https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.
We are soliciting comment on:
- The framework and how it aligns with the statutory definitions of single source and multiple source drugs in section 1847A(c)(6)(C) and (D) of the Act, respectively;
- How the framework distinguishes situations in which a section 505(b)(2) drug product is not described by an existing multiple source drug code; and
- The potential impacts of the framework on Medicare beneficiaries, the government, and other stakeholders.
F. Appropriate Use Criteria for Advanced Diagnostic Imaging
Section 218(b) of the Protecting Access to Medicare Act (Pub. L. 113-93, April 1, 2014) (PAMA) amended Title XVIII of the Act to add section 1834(q) of the Act directing us to establish a program to promote the use of appropriate use criteria (AUC) for advanced diagnostic imaging services. We have taken steps to implement this program over several years, and codified the AUC program in our regulations at 42 CFR 414.94. In CY 2020, we began conducting an educational and operations testing period for the claims-based reporting of AUC consultation information, which has been extended through CY 2021.
The CY 2016 PFS final rule with comment period (80 FR 70886) addressed the initial component of the new Medicare AUC program, specifying applicable AUC. In the CY 2016 PFS final rule with comment period, we established an evidence-based process and transparency requirements for the development of AUC, defined provider-led entities (PLEs) and established the process by which PLEs may become qualified to develop, modify or endorse AUC. The first list of qualified PLEs was posted on the CMS website at the end of June 2016 at which time their AUC libraries became specified applicable AUC for purposes of section 1834(q)(2)(A) of the Act.
The CY 2017 PFS final rule (81 FR 80170) addressed the second component of this program, specification of qualified clinical decision support mechanisms (CDSMs). In the CY 2017 PFS final rule, we defined CDSM, identified the requirements CDSMs must meet for qualification, including preliminary qualification for mechanisms documenting how and when each requirement is reasonably Start Printed Page 39248 expected to be met, and established a process by which CDSMs may become qualified. We also defined applicable payment systems under this program, specified the first list of priority clinical areas, and identified exceptions to the requirement that ordering professionals consult specified applicable AUC when ordering applicable imaging services. The first list of qualified CDSMs was posted on the CMS website in July 2017.
The CY 2018 PFS final rule (82 FR 53190) addressed the third component of this program, the consultation and reporting requirements. In the CY 2018 PFS final rule, we established the start date of January 1, 2020 for the Medicare AUC program for advanced diagnostic imaging services. Specifically, for services ordered on and after January 1, 2020, we established that ordering professionals must consult specified applicable AUC using a qualified CDSM when ordering applicable imaging services, and furnishing professionals must report AUC consultation information on the Medicare claim. We further specified that the AUC program will begin on January 1, 2020 with a year-long educational and operations testing period during which time AUC consultation information is expected to be reported on claims, but claims would not be denied for failure to include proper AUC consultation information. We also established a voluntary period from July 2018 through the end of 2019 that ordering professionals who are ready to participate in the AUC program may consult specified applicable AUC through qualified CDSMs and communicate the results to furnishing professionals; and furnishing professionals who are ready to do so may report AUC consultation information on the claim at https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10481.pdf.
Additionally, to incentivize early use of qualified CDSMs to consult AUC, we established in the CY 2018 Updates to the Quality Payment Program; and Quality Payment Program: Extreme and Uncontrollable Circumstances Policy for the Transition Year final rule with comment period and interim final rule (hereinafter "CY 2018 Quality Payment Program final rule"), a high-weight improvement activity for ordering professionals who consult specified AUC using a qualified CDSM for the Merit-based Incentive Payment System (MIPS) performance period that began January 1, 2018 (82 FR 54193).
In the CY 2019 PFS final rule (83 FR 59452), we made further additions and clarifications to the AUC program requirements. We added independent diagnostic testing facility (IDTF) to the definition of applicable settings under § 414.94(b). We also clarified that the furnishing professionals (including provider or supplier entities furnishing advanced diagnostic imaging services in an applicable setting, paid for under an applicable payment system) are required to report AUC consultation information on the claims as specified under § 414.94(k). We established significant hardship exception criteria and process under § 414.94(i)(3) to be specific to the AUC program and independent of other Medicare programs. We specified under § 414.94(j)(2) that when delegated by the ordering professional, clinical staff under the direction of the ordering professional may perform the AUC consultation with a qualified CDSM. Finally, we announced our intention to use G-codes and modifiers to report AUC consultation information on the Medicare claims. In 2020, in response to the Public Health Emergency (PHE) for the Coronavirus Disease 2019 (COVID-19) (PHE for COVID-19), the educational and operations testing period was extended through CY 2021.
1. Background
AUC present information in a manner that links a specific clinical condition or presentation; one or more services; and an assessment of the appropriateness of the service(s). Evidence-based AUC for imaging can assist clinicians in selecting the imaging study that is most likely to improve health outcomes for patients based on their individual clinical presentation. For purposes of this program, AUC is a set or library of individual AUC. Each individual criterion is an evidence-based guideline for a particular clinical scenario based on a patient presenting symptoms or condition.
AUC need to be integrated as seamlessly as possible into the clinical workflow. CDSMs are the electronic portals through which clinicians access the AUC during the patient workup. They can be standalone applications that require direct entry of patient information, but may be more effective when they are integrated into electronic health records (EHRs). Ideally, practitioners would interact directly with the CDSM through their primary user interface, thus minimizing interruption to the clinical workflow.
2. Statutory Authority
Section 218(b) of the PAMA added a new section 1834(q) of the Act entitled, "Recognizing Appropriate Use Criteria for Certain Imaging Services," which directed the Secretary to establish a program to promote the use of AUC. Section 1834(q)(4) of the Act requires ordering professionals to consult with specified applicable AUC through a qualified CDSM for applicable imaging services furnished in an applicable setting and paid for under an applicable payment system; and payment for such service may only be made if the claim for the service includes information about the ordering professional's consultation of specified applicable AUC through a qualified CDSM.
3. Discussion of Statutory Requirements
There are four major components of the AUC program under section 1834(q) of the Act, and each component has its own implementation date: (1) Establishment of AUC by November 15, 2015 (section 1834(q)(2) of the Act); (2) identification of mechanisms for consultation with AUC by April 1, 2016 (section 1834(q)(3) of the Act); (3) AUC consultation by ordering professionals, and reporting on AUC consultation by January 1, 2017 (section 1834(q)(4) of the Act); and (4) annual identification of outlier ordering professionals for services furnished after January 1, 2017 (section 1834(q)(5) of the Act). We did not identify mechanisms for consultation by April 1, 2016. Therefore, we did not require ordering professionals to consult CDSMs or furnishing professionals to report information on the consultation by the January 1, 2017 date.
a. Establishment of AUC
In the CY 2016 PFS final rule with comment period, we addressed the first component of the Medicare AUC program under section 1834(q)(2) of the Act—the requirements and process for establishment and specification of applicable AUC, along with relevant aspects of the definitions under section 1834(q)(1) of the Act. This included defining the term "provider-led entity" and finalizing requirements for the rigorous, evidence-based process by which a PLE would develop AUC, upon which qualification is based, as provided in section 1834(q)(2)(B) of the Act and in the CY 2016 PFS final rule with comment period. Using this process, once a PLE is qualified by us, the AUC that are developed, modified or endorsed by the qualified PLE are considered to be specified applicable AUC under section 1834(q)(2)(A) of the Act. We defined PLE to include national professional medical societies, health systems, hospitals, clinical practices and collaborations of such entities such as the High Value Healthcare Collaborative or the National Comprehensive Cancer Network. Start Printed Page 39249 Qualified PLEs may collaborate with third parties that they believe add value to their development of AUC, provided such collaboration is transparent. We expect qualified PLEs to have sufficient infrastructure, resources, and the relevant experience to develop and maintain AUC according to the rigorous, transparent, and evidence-based processes detailed in the CY 2016 PFS final rule with comment period.
In the same rule, we established a timeline and process under § 414.94(c)(2) for PLEs to apply to become qualified. Qualified PLEs are listed at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Appropriate-Use-Criteria-Program/PLE.html (OMB Control Number 0938-1288).
b. Mechanism for AUC Consultation
In the CY 2017 PFS final rule, we addressed the second major component of the Medicare AUC program—the specification of qualified CDSMs for use by ordering professionals for consultation with specified applicable AUC under section 1834(q)(3) of the Act, along with relevant aspects of the definitions under section 1834(q)(1) of the Act. This included defining the term CDSM and finalizing functionality requirements of mechanisms, upon which qualification is based, as provided in section 1834(q)(3)(B) of the Act and in the CY 2017 PFS final rule. We defined CDSM as an interactive, electronic tool for use by clinicians that communicates AUC information to the user and assists them in making the most appropriate treatment decision for a patient's specific clinical condition. Tools may be modules within or available through certified EHR technology (as defined in section 1848(o)(4) of the Act) or private sector mechanisms independent from certified EHR technology or a mechanism established by the Secretary.
In the CY 2017 PFS final rule, we established a timeline and process in § 414.94(g)(2) for CDSM developers to apply to have their CDSMs qualified. Qualified CDSMs are listed at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Appropriate-Use-Criteria-Program/CDSM.html (OMB Control Number 0938-1315).
c. AUC Consultation and Reporting
In the CY 2018 PFS final rule, we addressed the third major component of the Medicare AUC program—consultation with applicable AUC by the ordering professional and reporting of such consultations under section 1834(q)(4) of the Act. We established a January 1, 2020 effective date for the AUC consultation and reporting requirements for this program. We also established a voluntary period during which early adopters could begin reporting limited consultation information on Medicare claims from July 2018 through December 2019. During the voluntary period, there is no requirement for ordering professionals to consult AUC or furnishing professionals to report information related to the consultation. On January 1, 2020, the program began with an educational and operations testing period and during this time, we have continued to pay claims whether or not they correctly include AUC consultation information. Ordering professionals must consult specified applicable AUC through qualified CDSMs for applicable imaging services furnished in an applicable setting, paid for under an applicable payment system and ordered on or after January 1, 2020; and furnishing professionals must report the AUC consultation information on the Medicare claim for these services ordered on or after January 1, 2020.
Consistent with section 1834(q)(4)(B) of the Act, we also established that the following information must be reported on Medicare claims for advanced diagnostic imaging services as specified in section 1834(q)(1)(C) of the Act and defined in § 414.94(b), furnished in an applicable setting as defined in section 1834(q)(1)(D) of the Act, paid for under an applicable payment system as defined in section 1834(q)(4)(D) of the Act, and ordered on or after January 1, 2020: (1) The qualified CDSM consulted by the ordering professional; (2) whether the service ordered would or would not adhere to specified applicable AUC, or whether the specified applicable AUC consulted was not applicable to the service ordered; and (3) the NPI of the ordering professional (if different from the furnishing professional).
Section 1834(q)(4)(C) of the Act provides for exceptions to the AUC consultation and reporting requirements in the case of: A service ordered for an individual with an emergency medical condition, a service ordered for an inpatient and for which payment is made under Medicare Part A, and a service ordered by an ordering professional for whom the Secretary determines that consultation with applicable AUC would result in a significant hardship. In the CY 2017 PFS final rule, we adopted a regulation at § 414.94(h)(1)(i) to specify the circumstances under which AUC consultation and reporting requirements are not applicable and in the CY 2019 PFS final rule, we updated the significant hardship exception criteria to be specific to the AUC program and independent of other programs. An ordering professional experiencing any of the following when ordering an advanced diagnostic imaging service is not required to consult AUC using a qualified CDSM, and the claim for the applicable imaging service is not required to include AUC consultation information. Significant hardship exceptions under § 414.94(i)(3) include: Insufficient internet access; EHR or CDSM vendor issues; or extreme and uncontrollable circumstances.
We remind readers that, consistent with section 1834(q)(4)(A) of the Act, ordering professionals must consult AUC for every applicable imaging service furnished in an applicable setting and paid under an applicable payment system unless a statutory exception applies.
Section 1834(q)(4)(D) of the Act specifies the applicable payment systems for which AUC consultation and reporting requirements apply. In the CY 2017 PFS final rule, we defined applicable payment system to reflect the statutory requirements in § 414.94(b) as: (1) The PFS established under section 1848(b) of the Act; (2) the PPS for hospital outpatient department services under section 1833(t) of the Act; and (3) the ambulatory surgical center payment system under section 1833(i) of the Act.
Section 1834(q)(1)(D) of the Act specifies the applicable settings in which AUC consultation and reporting requirements apply: A physician's office, a hospital outpatient department (including an emergency department), an ambulatory surgical center, and any other "provider-led outpatient setting determined appropriate by the Secretary." In the CY 2017 PFS final rule, we added this definition to § 414.94(b). As noted above, we expanded that definition to add an IDTF in the CY 2019 PFS final rule.
d. Identification of Outliers
The fourth component of the Medicare AUC program is specified in section 1834(q)(5) of the Act, Identification of Outlier Ordering Professionals. The identification of outlier ordering professionals under this paragraph facilitates a prior authorization requirement that applies for outlier professionals beginning January 1, 2020, as specified under section 1834(q)(6) of the Act. Because we established a start date of January 1, 2020 for AUC consultation and reporting requirements, we did not identify any outlier ordering professionals by that date. As such, Start Printed Page 39250 implementation of the prior authorization component is delayed. However, we did finalize in the CY 2017 PFS final rule the first list of priority clinical areas to guide identification of outlier ordering professionals as follows:
- Coronary artery disease (suspected or diagnosed).
- Suspected pulmonary embolism.
- Headache (traumatic and non-traumatic).
- Hip pain.
- Low back pain.
- Shoulder pain (to include suspected rotator cuff injury).
- Cancer of the lung (primary or metastatic, suspected or diagnosed).
- Cervical or neck pain.
We will use future rulemaking to establish the methodology for the identification of outlier ordering professionals who would eventually be subject to a prior authorization process when ordering advanced diagnostic imaging services.
4. Proposals for Continuing Implementation
a. Proposed Clarification of AUC Program Scope
i. Modified Orders
Updates or modifications to orders for advanced diagnostic imaging services may be warranted in certain situations once the beneficiary is under the care of the furnishing professional. Unless they are also serving as the ordering professional, furnishing professionals may not consult AUC on behalf of or in place of the ordering professional. The Medicare Benefit Policy Manual (BPM) (Pub. L. 100-02) addresses situations where the furnishing professional performs imaging services that differ from ordered services in chapter 15, sections 80.6.1-4. These BPM sections on modified orders state that when an interpreting physician determines that a different or additional imaging service not included on the order should be performed, the interpreting physician or testing facility generally may not perform the test until a new order from the treating physician/practitioner has been received. If the treating physician/practitioner cannot be reached to change or obtain a new order, the interpreting physician or testing facility may furnish the additional imaging service under the following circumstances, as documented in the patient's medical record: The treating physician/practitioner could not be reached, the ordered test is performed and an additional diagnostic test is medically necessary because of the abnormal result of that test, delaying performance of the additional test would have an adverse effect on the patient's care, the result of the additional test is communicated to and used by the treating physician/practitioner in the patient's treatment, and the interpreting physician/practitioner documents in the report the reasons for the additional testing.
When the furnishing professional performs additional imaging services not reflected on the order under these circumstances, we do not believe it would be appropriate to consider them to be acting as an ordering professional such that an AUC consultation would be needed. Instead, we believe the furnishing professional in these situations is the interpreting physician/practitioner who is exercising their professional judgment to provide the ordering professional with additional diagnostic test results for use in managing the patient's care. Additionally, they are doing so only because, after performing the ordered test and determining that additional testing is expedient given the results of that test, the ordering professional cannot be reached to request a modified or additional order. Given the conditions under which these additional imaging services are performed, we propose that when the furnishing professional for an advanced diagnostic imaging service performs one or more additional services under the circumstances described in chapter 15, section 80.6.2-4 of the BPM, neither the ordering professional nor the furnishing professional are required to consult AUC for the additional service(s). In these situations, the AUC consultation information from the original order is to be reported on the claim line for the additional service(s). Where the furnishing professional modifies the order for an advanced diagnostic imaging service without obtaining a new order from the ordering professional, the AUC consultation information provided by the ordering professional with the original order should be reflected on the Medicare claim to demonstrate that the requisite AUC consultation occurred. Because the BPM instructions state that the interpreting physician or testing facility generally may not perform a modified or new test until a new order from the treating physician/practitioner has been received, we expect situations where AUC consultations do not occur for new or modified orders to be infrequent.
ii. Extreme and Uncontrollable Circumstances Hardship Exception
In the CY 2019 PFS final rule, we describe extreme and uncontrollable circumstances to include disasters, natural or man-made, that have a significant negative impact on healthcare operations, area infrastructure or communication systems. We also explain these may include areas where events occur that have been designated by FEMA as a major disaster or a public health emergency declared by the Secretary. To further clarify, these circumstances are events that are entirely outside the control of the ordering professional that prevent the ordering professional from consulting AUC through a qualified CDSM. We believe the hardship criteria under this program are similar to other programs such as the Promoting Interoperability performance category of the Merit-based Incentive Payment System (MIPS), particularly the flexibility that is given to clinicians to identify what they consider to be extreme and uncontrollable circumstances.
The PHE for COVID-19 has been in effect since January 27, 2020. Stakeholders have described challenges in continuing to prepare for the payment penalty phase of the AUC program due to resource reallocation resulting from the PHE. Some stakeholders have explained that all health technology projects unrelated to the PHE were halted, including projects that impact establishing or updating health IT systems that enable AUC consultation through qualified CDSMs. Stakeholders have also indicated that human resources were reallocated to focus on responding to the PHE. Additionally, we recognize that practitioners have been heavily impacted in their own practice of medicine to respond to the PHE and provide treatment to patients which may have prevented them from focusing on and participating in the educational and operations testing period to prepare for the payment penalty phase. While we are continuing to move forward in implementing the AUC program, we want to assure stakeholders that they may attest to a significant hardship under the AUC program due to extreme and uncontrollable circumstances due to the PHE for COVID-19, and such an attestation may be used as needed by ordering practitioners throughout the PHE. Furthermore, as the AUC program progresses into the payment penalty phase, self-attestation for a significant hardship exception will continue to be available for ordering professionals experiencing extreme and uncontrollable circumstances due to the PHE. We also recognize that ordering professionals may experience significant Start Printed Page 39251 hardships related to or resulting from the PHE that extend beyond the date the PHE expires and note that AUC program exceptions will continue to be available for such significant hardships as defined at § 414.94(i)(3).
b. Claims Processing
As we move ahead to implement the payment penalty phase of this program, we must address additional operational and administrative issues. We explain these issues here, and our assessments and proposals for addressing them. We are soliciting comments on whether additional scenarios require our consideration, and whether the proposed solutions adequately address issues raised by stakeholders. We are soliciting any additional information stakeholders may offer to assist us in developing claims processing system edits or other measures to ensure that only appropriate claims are subject to AUC claims processing edits. The AUC program will be fully implemented when we have the necessary edits established in the claims processing system and we begin using those edits to deny Medicare claims that fail to report the required AUC consultation information. Therefore, we need to find workable solutions that allow the AUC program to accurately pay and deny claims using the information available on Medicare claims, while working within the limitations of the Medicare claims processing system. The identification of claims that are or are not subject to the Medicare AUC Program must be precise to avoid inadvertently denying claims that should be paid. Because implementation of this program establishes edits for advanced diagnostic imaging claims, the inadvertent denial of claims would disproportionately impact radiologists, hospital outpatient departments and freestanding imaging centers. Also, as we have noted previously, the AUC program is unique in that the burden of consulting AUC and providing AUC consultation information to the furnishing professional falls on the ordering professional, yet the claims that are denied for failing to report AUC consultation information are for services furnished and billed by the professionals and facilities that furnish advance diagnostic imaging.
Two main Medicare claim types are subject to claims processing edits in the AUC program. These are the CMS-1500 and its electronic equivalent (referred to here as the practitioner claim) submitted by physicians and practitioners, ASCs, and IDTFs, and the UB-04, also called the CMS-1450, (referred to here as the institutional claim) submitted by hospital outpatient departments and on-campus and off-campus provider-based departments. These claim types differ in the data elements they contain; therefore, claims processing edits will not be identical across claim types.
We have already issued partial claims processing instructions (CR11268, Transmittal 2404)[79] to support the educational and operations testing period. We established HCPCS Level III G-codes for furnishing professionals to report which CDSM was consulted. We also established HCPCS modifiers for furnishing professionals to report adherence, non-adherence and not applicable AUC consultation responses on the same claim line as the corresponding G-code. Both G-codes and modifiers are applicable to practitioner and institutional claims. We established additional HCPCS modifiers for furnishing professionals to report situations in which the ordering professional is not required to consult AUC. These modifiers are reported on the same claim line as the code for the advanced diagnostic imaging procedure since a G-code would not be reported. We also established a procedure code list that identifies the advanced diagnostic imaging codes that are subject to the AUC program. Based on a review of CY 2020 Medicare claims (noting for readers that during this year the AUC program was only in the education and operations testing phase with no payment penalties), we estimate between 9-10 percent of all claims subject to the AUC program reported information sufficient to be considered compliant with the program, which means that 90-91 percent of claims would not be considered compliant with AUC program requirements. In other words, if the claims processing systems edits had been in place for the payment penalty phase, only 9-10 percent of claims subject to the AUC program would have been paid as opposed to being denied or rejected. An additional 6-7 percent of claims subject to the AUC program included some relevant information, which demonstrates an awareness of the AUC program among these billing entities; but the claims did not include all of the necessary AUC consultation information that will ultimately be required for the claim to be paid.
i. Ordering Professional NPI
There are locations on both the practitioner and institutional claim types to report the NPI of the ordering professional. The institutional claim uses the K3 segment and the practitioner claim uses the referring professional field. However, to fully implement the AUC program, we must establish a claims processing edit to require these fields to be populated on all advanced diagnostic imaging claims subject to the AUC program.
In addition, there currently are situations in which multiple advanced diagnostic imaging services ordered by more than one ordering professional may be reported on a single claim. This would not be workable for purposes of reporting AUC consultation information because the referring professional field is reported at the claim-level and not at the claim line- or service-level for professional claims. Therefore, the furnishing professional will need to submit separate claims for the services ordered by each referring or ordering professional. In other words, only one ordering professional can be reported per claim.
ii. Critical Access Hospitals
As discussed in the CY 2018 PFS final rule with comment period (82 FR 53192), advanced diagnostic imaging services furnished in an outpatient department of a critical access hospital (CAH) are not subject to the AUC program because, in accordance with section 1833(q)(1)(D) of the Act, a CAH is not an applicable setting under the program. Therefore, we must identify these advanced diagnostic imaging services and allow them to bypass the AUC program claims processing edits. For institutional claims, we intend to apply the AUC program claims processing edits to type of bill 13x, which is used only for outpatient hospital settings. CAHs submit outpatient claims using type of bill 85x, rather than type of bill 13x.
In the CY 2019 PFS final rule (83 FR 59694), we further explained that because section 1834(q)(4)(B) of the Act clearly includes all claims paid under applicable payment systems without exclusion, the claims from both furnishing professionals and facilities must include AUC consultation information. We revised our regulation at § 414.94(k) to specify that AUC consultation information must be reported on Medicare claims for advanced diagnostic imaging services furnished in an applicable setting and paid under an applicable payment system. Prior to this revision, § 414.94(k) required furnishing professionals to report AUC consultation on the claim, without also specifying that facility claims must include the AUC consultation Start Printed Page 39252 information. In the CY 2019 PFS final rule, we explained that the AUC consultation information would be included on the practitioner's claim for the professional component (PC) of the service and on the provider's or supplier's claim for the facility portion or technical component (TC) of the service. Under § 414.94(k), the requirement to report AUC consultation information on the claim applies to both the PC and TC of the imaging services that are furnished in an applicable setting and paid under an applicable payment system. Section 1834(q)(4)(B) of the Act further specifies that the requirement to report AUC consultation information is specific to claims for advanced diagnostic imaging services furnished in an applicable setting and paid under an applicable payment system. We believe that all claims for advanced diagnostic imaging services, both the PC and TC, must include the AUC consultation information when they are furnished both in an applicable setting and paid under an applicable payment system. However, if advanced diagnostic imaging services are not entirely furnished in an applicable setting, we believe that neither the PC nor TC claim should be required to include AUC consultation information. This ensures consistent application of the AUC consultation requirements across claims submitted for advanced diagnostic imaging services even when the PC and TC components of the service are furnished by different furnishing professionals. As such, we propose that claims submitted by physicians or practitioners for the PC of an advanced diagnostic imaging service when the TC was not furnished in an applicable setting would not be subject to the AUC program since the setting where the TC of the imaging service is furnished is not subject to the AUC program consultation and reporting requirements. If a physician or practitioner submits a claim for the PC of an advanced imaging service for which the TC was performed as an outpatient CAH service, there currently is not a systems-based way for us to recognize that the TC of the service was furnished by a CAH. Place of service codes reported on practitioner claims are not specific enough. We have not yet identified a way to segregate these claims and automatically allow them to bypass AUC program claims processing edits. Therefore, as discussed below, we propose to establish a separate HCPCS modifier that will be used to identify practitioner claims for advanced diagnostic imaging services that are not subject to the AUC program and that are not otherwise identified using the other AUC program modifiers designated to identify specific situations where the claims are not subject to the AUC program.
iii. Maryland Total Cost of Care Model
Section 1834(q)(4)(D) of the Act specifies that the applicable payment systems for which AUC consultation and reporting requirements apply are the PFS, the hospital OPPS and the ambulatory surgical center payment system. We define applicable payment system consistent with statute at § 414.94(b) and, as noted above, require AUC consultation information to be reported on Medicare claims for advanced diagnostic imaging services, both the PC and TC, furnished in an applicable setting and paid under an applicable payment system at § 414.94(k). Section 1834(q)(4)(B) of the Act specifies that the requirement to report AUC consultation information is specific to claims for advanced diagnostic imaging services furnished in an applicable setting and paid under an applicable payment system. We believe that all claims for the advanced diagnostic imaging services, both the PC and TC, must include the AUC consultation information when they are furnished both in an applicable setting and paid under an applicable payment system. Therefore, if both the PC and TC for advanced diagnostic imaging services are not paid under an applicable payment system, neither the PC nor TC claim is required to include AUC consultation information. This ensures consistent application of the AUC consultation requirements across claims submitted for advanced diagnostic imaging services even when the PC and TC components of the service are furnished by different furnishing professionals. Similar to claims for the PC of services for which the TC is furnished outside of an applicable setting, and because both practitioner and institutional claims are subject to the AUC program as discussed above, when the practitioner or institutional claim for the advanced imaging service is not subject to the AUC program (for example, payment is not made under an applicable payment system), the corresponding practitioner or institutional claim for the same imaging service is also not subject to the AUC program.
Stakeholders alerted CMS to concerns about whether advanced diagnostic imaging services furnished in hospitals participating in the Maryland Total Cost of Care Model would be subject to the AUC program. We appreciate that this has been brought to our attention and we seek comments on other models. Advanced diagnostic imaging services furnished in outpatient departments of Maryland hospitals that participate in the Hospital Payment Program within the Maryland Total Cost of Care Model are not subject to the AUC program because these services are not paid under an applicable payment system (Maryland hospitals that receive payments under the Hospital Payment Program within the Maryland Total Cost of Care Model are not paid under the OPPS). Because these services are not subject to the AUC program requirements when furnished in a hospital paid under the Hospital Payment Program within the Maryland Total Cost of Care Model, as opposed to an applicable payment system, we propose that the PCs of these advanced diagnostic imaging services, when billed separately, are also not required to include AUC consultation information. We believe we can identify all institutional claims from a hospital that is paid under the Hospital Payment Program within the Maryland Total Cost of Care Model based on their CMS Certification Number (CCN) and allow those claims to bypass AUC program claims processing edits. We understand that when the TC and PC of advanced diagnostic imaging services are billed separately, the professional claim must identify in box 32 the location where the TC of the imaging service was furnished to the patient. Therefore, we believe we will have the ability to identify situations in which the imaging service was furnished in a hospital that is paid under the Hospital Payment Program within the Maryland Total Cost of Care Model and exclude those claims from being subject to AUC program claims processing edits. We believe this can be accomplished by using the CCN and will continue to work to determine if a list of CCNs can be used as the source of our edits in addition to determining the frequency that the list will be updated.
Note that advanced diagnostic imaging services furnished in applicable settings in the state of Maryland and paid under an applicable payment system are subject to the AUC program—the above discussion applies only to the outpatient departments of hospitals that are paid under the Hospital Payment Program within the Maryland Total Cost of Care Model.
iv. Inpatients Converted to Outpatients
While uncommon, there are situations in which a beneficiary's hospital inpatient status is changed to outpatient. Certain criteria must be met for this to occur and, if met, condition Start Printed Page 39253 code 44 (inpatient admission changed to outpatient) is appended to the institutional claim (https://www.cms.gov/regulations-and-guidance/guidance/transmittals/downloads/r299cp.pdf). We propose to allow institutional claims with condition code 44 to bypass AUC claims processing edits. We make this proposal because, at the time advanced diagnostic imaging services were ordered and furnished, they were ordered for and furnished to a beneficiary who was in inpatient status. As such, the AUC consultation requirement would not have applied at that time. We believe that any professional claims would include place of service code 21 (inpatient hospital) since the expectation, until just prior to discharge, would be that the patient is in an inpatient status. We expect less than half of one percent of claims will include condition code 44.
v. Deny or Return Claims That Fail AUC Claims Processing Edits
As discussed above, claims that do not properly include AUC consultation information will not be paid once we fully implement the AUC claims processing edits. We are considering whether claims that do not pass the AUC claims processing edits, and therefore will not be paid, should be initially returned to the health care provider so they can be corrected and resubmitted, or should be denied so they can be appealed. On one hand, we expect there will be some errors in reporting AUC consultation information on claims, especially early on, and health care providers might find it helpful to have the opportunity to correct claims. However, there may be situations in which the health care provider would prefer the claim be denied so they have an earlier opportunity to appeal. We are requesting comments to help us better understand which path would be most appropriate once we fully implement the AUC program claims edits. Additionally, we are requesting comments on whether the payment penalty phase should begin first with returning claims and then transition to denying claims after a period of time, which may be helpful to furnishing professionals and facilities as they become more proficient in submitting claims under the AUC program.
vi. Medicare as a Secondary Payer
We understand based on feedback from stakeholders that, in some EHRs, the primary payer information is readily available and known to the ordering professional; however, secondary payer information typically is not available. Additionally, it is possible that when Medicare is the secondary payer that no Medicare payment would be made at all after the primary payer makes payment. Medicare is reported as the secondary payer for approximately 1.5 percent of advanced diagnostic imaging services that are subject to the AUC program. Because the secondary payer information for a patient generally is not available to the ordering professional, and because no Medicare payment may be involved at all when Medicare is the secondary payer, we propose to exclude claims that identify Medicare as the secondary payer from application of the AUC consultation and reporting requirements. Specifically, we propose to allow claims that identify Medicare as the secondary payer (using block 1 or the electronic equivalent of the practitioner claims and using FL 50/51 or the electronic equivalent of institutional claims) to bypass the AUC program claims processing edits.
vii. Date of Service and Date of Order
We will specify a start date for the AUC program claims processing edits to take effect. Medicare claims include a date of service but do not allow for the date of an imaging order to be recorded. Because we cannot identify the order date for an advanced imaging service based on claims, we propose that the AUC program claims processing edits for the payment penalty phase will be applicable for advanced imaging services furnished on or after the effective date of the claims edits. For imaging services ordered prior to, but furnished on or after the effective date of the AUC program claims processing edits, the furnishing professional would apply the separate HCPCS modifier discussed in section III.F.4.b.ii. (Critical Access Hospitals) of this proposed rule to indicate that the claim is not subject to the AUC claims processing edits.
viii. HCPCS Modifiers
We established two primary sets of HCPCS modifiers for this program. One set is to be included on the same claim line as the G-code identifying the CDSM that was consulted, and reports whether the imaging service adheres to the AUC (modifier ME), does not adhere to the AUC (modifier MF), or the qualified CDSM does not contain AUC that applies to the order (modifier MG). We intend for these modifiers to continue to be used when the program enters the payment penalty phase. Additionally, reporting of these modifiers should be limited to one per qualified CDSM G-code since these modifiers are mutually exclusive.
The second set of HCPCS modifiers is available for use when the ordering professional does not consult a qualified CDSM. On these claims, providers would not add a G-code for a CDSM because a consultation did not take place, and the HCPCS modifier would be included on the same line as the procedure code for the advanced diagnostic imaging service that was furnished. These HCPCS modifiers include the three that were created to describe significant hardship exceptions (insufficient internet access (modifier MB), EHR or CDSM vendor issues (modifier MC) and extreme and uncontrollable circumstances (modifier MD)). Additionally, section 1834(q)(4)(C) of the Act includes an exception for services ordered for an individual with an emergency medical condition and modifier MA is available to identify claims for patients with a suspected or confirmed emergency medical condition. This set of codes is mutually exclusive and we expect only one to be reported per procedure code-level claim line.
Modifier QQ was created for use during the voluntary period, before more detailed modifiers and codes were created, to indicate that an ordering professional consulted a qualified CDSM for the service and related data was provided to the furnishing professional. The descriptor for this code explains that the ordering professional consulted a qualified CDSM for this service and the related information was provided to the furnishing professional. Modifier QQ continues to be available for use through the educational and operations testing period, but we intend to end the use of that modifier and not carry it forward into the payment penalty phase since we have established and will require the use of distinct modifiers to communicate specific AUC consultation information.
Modifier MH was created for use during the educational and operations testing phase to identify claims for which AUC consultation information was not provided to the furnishing professional and furnishing facility. When the AUC program enters the payment penalty phase, we will no longer have a need for this modifier because claims will be required to include AUC consultation information or indicate a reason the information is not required in order to avoid AUC program claims processing edits. Beginning for services furnished on and after the effective date of the AUC program claims processing edits, we propose to redefine modifier MH to describe situations in which the Start Printed Page 39254 ordering professional is not required to consult AUC and the claim is not required to report AUC consultation information. For example, we would repurpose modifier MH to be used in the scenarios described in sections III.F.4.b.ii. (Critical Access Hospitals), III.F.4.b.iii (Maryland Total Cost of Care Model) if other options to identify claims are not feasible, and III.F.4.b.vii. (Date of Service and Date of Order) of this proposed rule as those scenarios would fall outside the scope of the AUC program requirements.
ix. Additional Claims Processing Information
Section 1834(q)(1)(D) of the Act specifies the applicable settings for the AUC program as a physician's office, a hospital outpatient department (including an emergency department), and ambulatory surgical center and any other provider-led outpatient setting determined appropriate by the Secretary. As discussed in the CY 2019 PFS final rule (83 FR 59690 and 59691), we added IDTFs to the definition of applicable setting at § 414.94(b) to the three applicable settings specified in statute because it is a provider-led outpatient setting in which advanced diagnostic imaging services are furnished by licensed, certified nonphysician personnel under appropriate physician supervision. To identify these settings through the Medicare claims system we evaluated type of bill and place of service codes to identify those aligned with applicable settings under the AUC program. For institutional claims, we propose to limit AUC program claims processing edits to apply only to type of bill 13x (hospital outpatient). This claim type code encompasses the hospital outpatient department and the emergency department which represent all applicable settings under the program that would bill Medicare using institutional claims. For practitioner claims, we propose to limit the edits to claims with place of service codes 11 (office), 15 (mobile unit), 19 (off campus outpatient hospital), 22 (on campus outpatient hospital), 23 (emergency room) and 24 (ASC). These place of service codes should encompass all applicable settings under the AUC program as defined at § 414.94(b). Because these type of bill and place of service codes reflect the applicable settings within which advanced diagnostic imaging services must be furnished to be subject to the AUC program requirements, we believe setting these parameters will allow us to more accurately pay claims while avoiding the need for other types of professionals and facilities to append modifiers to their claims.
x. Claims Processing Summary
We have presented above some of the scenarios that CMS and stakeholders have identified as being potentially challenging or impracticable for application of the AUC program claims processing edits for purposes of the payment penalty phase. We request feedback on whether additional scenarios require consideration and whether the proposed claims processing solutions will adequately address the issues raised. We also request feedback on areas that stakeholders believe need more education to inform our ongoing outreach and education efforts. While much of the discussion is about identifying claims that are not subject to the AUC program, we note that physicians and other practitioners, or providers submitting claims for advanced imaging services that are not subject to the AUC program can voluntarily report AUC consultation information. We intend to allow those claims to process through the system. We request commenters to provide additional information to assist us in developing edits that ensure only appropriate claims are subject to AUC claims processing edits.
c. Timing of Payment Penalties
We have previously announced in August 2020, via the CMS AUC website, that the education and operations testing period of the AUC program would be extended through 2021 and the payment penalty phase would begin in January 2022. However, given the many complexities around the scope and application of AUC program claims processing edits, we believe that notice and comment rulemaking is the most appropriate means for us to discuss the implementation and claims processing issues, the start date of the payment penalty phase, and to obtain stakeholder feedback before subsequently finalizing a course of action in the final rule. This process will help ensure that we will appropriately identify claims for denial when the payment penalty phase of the program begins. In addition, we acknowledge the circumstances of physicians and other practitioners, and providers, due to the PHE for COVID-19 and that additional time may be needed to prepare for the payment penalty phase given the challenges and practice disruptions they have experienced while responding to the PHE.
The earliest that our claims processing system can begin screening claims using the AUC program claims processing edits for the payment penalty phase is October 2022. This is because it would not be possible for us to finalize implementation and claims processing plans in this final rule (typically published on or before November 1) and make those decisions effective any earlier than the 3rd calendar quarter of 2022. Implementing the types of claims processing edits necessary for this program generally requires a long lead time. However, we note that an effective date for the claims processing edits in October 2022 may be misaligned with typical annual updates to the systems used by the health care providers that are subject to the AUC program such as EHR, CDSM or claims submission systems. Therefore, we believe the earliest practicable effective date for the AUC program claims processing edits and payment penalty phase is January 1, 2023.
While the above date takes into account technical system and programming concerns, it does not expressly take into the account the impact that the PHE for COVID-19 has had, and may yet have, on practitioners, providers and beneficiaries. Therefore, we are proposing a flexible effective date for AUC program claims processing edits and payment penalty phase to begin the later of January 1, 2023, or the January 1 that follows the declared end of the PHE for COVID-19.
We acknowledge that the AUC program has been significantly delayed. We seek public comment on this proposal for the payment penalty phase to begin, and whether we have appropriately taken into account the PHE for COVID-19 and other factors. We recognize that some practitioners and institutions have already invested in qualified CDSMs, while others have had to redirect their resources during the PHE. We seek information from the public on the state of readiness of practitioners, facilities, and EHR and CDSM vendors.
5. Summary
In summary, we are providing clarifications and proposals around the scope of the AUC program specifically pertaining to updates or modifications to orders for advanced diagnostic imaging services and the extreme and uncontrollable circumstances significant hardship exception. We are also proposing several claims processing solutions to ensure accurate identification of claims that are and are not subject to the AUC program requirements. These proposals address special circumstances related to: Services furnished by a CAH, services paid under the Maryland Total Cost of Start Printed Page 39255 Care Model, inpatients converted to outpatients, situations when Medicare is the secondary payer, and imaging services ordered prior to the payment penalty phase but furnished on or after the start of the payment penalty phase. We also discuss identifying the ordering professional on practitioner claims for the imaging service and request feedback on whether it is more appropriate to deny or return claims that fail AUC claims processing edits. We are also proposing to begin the AUC claims processing systems edits and payment penalty phase of the program on the later of January 1, 2023, or the January 1 of the year after the year in which the PHE for COVID-19 ends. We invite the public to submit comments on these clarifications and proposals.
We will continue to post information on our website for this program, accessible at www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Appropriate-Use-Criteria-Program/index.html.
G. Removal of Selected National Coverage Determinations
CMS periodically identifies and removes National Coverage Determinations (NCDs) that no longer contain clinically pertinent and current information, in other words those items and services that no longer reflect current medical practice, or that involve items or services that are used infrequently by beneficiaries. Clinical science and technology evolves and items and services that were once considered state-of-the-art or cutting edge and experimental may be established as reasonable and necessary for Medicare beneficiaries or replaced by more beneficial technologies or clinical paradigms.
In the CY 2021 PFS final rule (85 FR 84472), we established rulemaking as an appropriate vehicle for receiving public comment on removing outdated NCDs, replacing the prior subregulatory administrative process used on two occasions in 2013 and 2015. Using rulemaking under section 1871(a)(2) of the Act allows us to consider removal of several NCDs at once as compared to the public comment process established in section 1862(l) of the Act, to be used in making and reconsidering individual NCDs.
Eliminating an NCD that provides national coverage for items and services means that the item or service will no longer be automatically covered by Medicare (42 CFR 405.1060). Instead, the initial coverage determinations for those items and services will be made by local Medicare Administrative Contractors (MACs). On the other hand, removing an NCD that bars coverage for an item or service under title XVIII (that is, national noncoverage NCD), allows MACs to cover the item or service if the MAC determines that such action is appropriate under the statute. Removing a national non-coverage NCD may permit more immediate access to technologies that may now be beneficial for some uses. As the scientific community continues to conduct research, which produces new evidence, the evidence base we previously reviewed may have evolved to support other policy conclusions.
In the CY 2021 PFS final rule, we did not establish an exclusive list of criteria that we would use for identifying and evaluating NCDs for removal. Instead, based on recommendations in public comments, and to be more flexible and nimble, we added considerations to the six factors established in 2013 to guide our decision making process. In addition to the six factors listed below, we also consider the general age of an NCD, changes in medical practice/standard of care, the pace of medical technology development since the last determination, and availability and quality of clinical evidence and information to support removal of an NCD. We would consider proposing the removal of an NCD if:
- We believe that allowing local contractor discretion to make a coverage decision better serves the needs of the Medicare program and its beneficiaries.
- The technology is generally acknowledged to be obsolete and is no longer marketed.
- In the case of a noncoverage NCD based on the experimental status of an item or service, the item or service in the NCD is no longer considered experimental.
- The NCD has been superseded by subsequent Medicare policy.
- The national policy does not meet the definition of an "NCD" as defined in sections 1862(l) or 1869(f) of the Act.
- The benefit category determination is no longer consistent with a category in the statute.
When we evaluate particular NCDs for removal, we take into account information gathered from stakeholders, the claims data for those items and services, and factors such as whether there may be documentation requirements within the NCD that are outdated and create a barrier to coverage. The rulemaking process provides an opportunity to consider public input before the NCD would be removed. We could decide to retain those NCDs after considering public comments.
In Table 23, we list the NCDs that we propose to remove. In addition to conducting an internal review to identify appropriate NCDs for removal, we receive removal requests from a variety of external stakeholders, such as medical specialty societies, device manufacturers, beneficiaries, physicians and providers, and other interested individuals. Additionally, sometimes topics are brought to our attention by the MAC medical directors. Also, we received comments to the NCD Removal proposal in response to the CY 2021 PFS proposed rule suggesting another seven NCDs for CMS to consider removing. After reviewing those comments and considering other available evidence and information, we are proposing to remove one of those seven NCDs in this rulemaking cycle. We have opened a national coverage analysis (NCA) using the NCD process for one and believe the other five NCDs should be retained.
We solicit comment on the two NCDs discussed in Table 23, as well as comments recommending other NCDs for CMS to consider for removal in a future rulemaking or through the NCD process.

The following outlines each NCD and provides a summary of the rationale for removal. Each of the current NCDs below is available in the Medicare National Coverage Determinations Manual located at https://www.cms.gov/ Start Printed Page 39256 Regulations-and-Guidance/Guidance/Manuals/internet-Only-Manuals-IOMs-Items/CMS014961.
1. NCD 180.2 Enteral and Parenteral Nutritional Therapy (July 11, 1984)
- Circumstances/Factor: We believe that allowing local contractor discretion to make a coverage decision better serves the needs of the Medicare program and its beneficiaries.
- Rationale: External stakeholders suggested that portions of this NCD are outdated. Enteral nutrition is the delivery of food to a patient with a functioning gastrointestinal tract who, due to pathology to, or non-function of the structures that normally permit food to reach the digestive tract, cannot maintain weight and strength. Enteral nutrition is provided through a nasogastric, jejunostomy, or gastrostomy tube. Parenteral nutrition is provided intravenously to the patient with pathology of the alimentary tract severe enough, that it does not allow for absorption of sufficient nutrients. This NCD does not provide as a matter of course, for pharmacy prepared parental solutions, which would increase patient safety. It also unnecessarily adds to patient and provider burden as it requires repeated reviews of medical necessity for those individuals who need enteral or parenteral nutrition services as a result of chronic diseases that affect the ability to eat or to digest/absorb nutrition. Local contractors have proposed LCDs that, if finalized, would provide parenteral and enteral nutrition coverage for certain Medicare beneficiaries. Therefore, we believe that removing this NCD would better serve the needs of the Medicare program and its beneficiaries.
2. NCD 220.6 Positron Emission Tomography (PET) Scans (September 3, 2013)
- Circumstances/Factor: We believe that allowing local contractor discretion to make a coverage decision better serves the needs of the Medicare program and its beneficiaries.
- Rationale: External stakeholders suggested this NCD may be outdated. NCD 220.6 established broad national non-coverage for non-oncologic indications of PET and was established in 2000. Thus we required that every non-oncologic indication for PET must have its own NCD in order to receive coverage. In 2013, we reconsidered the NCD to allow coverage for diagnostic PET imaging for oncologic uses not already determined by an NCD, to be made at the discretion of local Medicare administrative contractors (MACs), due to "various improvements in the technical, regulatory and professional aspects of PET imaging for diagnosis." Since the 2013 reconsideration, new non-oncologic PET agents have been approved by the FDA and multiple professional medical societies have published guidelines relevant to appropriate use of these agents. We believe that local contractor discretion provides an immediate avenue to potential coverage in appropriate candidates for non-oncologic indications. Therefore, we are proposing to eliminate subsection 220.6 to remove the broad national bar to coverage of PET scans for non-oncologic indications, thus allowing local Medicare contractors to make a coverage determination under section 1862(a)(1)(A) of the Act for beneficiaries. We believe this framework better serves the needs of the Medicare program and its beneficiaries. For clarity, we are not proposing to change any other subsections of 220.6. Thus, the NCDs listed at 220.6.1 through 220.6.20 would not be changed by this proposal.
In summary, we solicit comment on the proposal to remove the two NCDs, as well as comments recommending other NCDs for CMS to consider for future removal. We request commenters include a rationale to support their comments. We will use the public comments to help inform our decision to take one of three actions on the three NCDs proposed for removal:
- Remove the NCD, as proposed, allowing for coverage to be determined by the MACs.
- Retain the current policy as an NCD.
- Reconsider the NCD by opening a National Coverage Analysis. Comments suggesting that the NCD should be revised, rather than eliminated, should include new evidence that was not previously available at the time of the original NCD or at the time the NCD was last reconsidered, in order to support a change in national coverage.
H. Pulmonary Rehabilitation, Cardiac Rehabilitation and Intensive Cardiac Rehabilitation
Conditions of coverage for pulmonary rehabilitation (PR), cardiac rehabilitation (CR) and intensive cardiac rehabilitation (ICR) are codified at 42 CFR 410.47 and 410.49. We are proposing revisions to the PR and CR/ICR regulations to emphasize that though one program treats a respiratory disease and one treats cardiac conditions, both types of programs aim to improve quality of life for their participants using similar methods. Because many components are shared between PR and CR/ICR, we strive to ensure consistency in the regulatory language used for these therapeutic programs. Additionally, we are proposing to more closely conform the PR and CR regulations by removing a PR requirement, and to add COVID-19 as a covered condition for PR for certain beneficiaries. As discussed by Fleg and colleagues (2020),[80] CR and PR continue to be severely underutilized despite clear benefits on clinical and patient-centered outcomes. In fact Million Hearts® 2022, a national initiative co-led by the Centers for Disease Control and Prevention (CDC) and CMS to prevent 1 million heart attacks and strokes within 5 years, has incorporated a goal for increasing CR utilization. Million Hearts® worked with CR professionals to set a goal of 70 percent CR participation for eligible patients.[81] With these proposals to improve accuracy and consistency of the regulatory language specifying Medicare conditions of coverage for PR and CR/ICR, we hope to assist programs to better understand the PR and CR/ICR conditions of coverage.
1. Statutory Authority
Section 144(a) of the Medicare Improvements for Patients and Providers Act of 2008 (Pub. L. 110-275, July 15, 2008) (MIPPA) amended Title XVIII to add new section 1861(eee) of the Act to provide coverage of CR and ICR under Medicare part B, as well as new section 1861(fff) to provide coverage of PR under Medicare part B. The statute specified certain conditions for coverage of these services and an effective date of January 1, 2010. Conditions of coverage for PR, CR and ICR consistent with the statutory provisions of section 144(a) of the MIPPA were codified in §§ 410.47 and 410.49 respectively through the CY 2010 PFS final rule with comment period (74 FR 61872 through 61886 and 62002 through 62003 (PR) 62004 through 62005 (CR/ICR)).
2. Background
Under § 410.47(b), Medicare part B covers PR for beneficiaries with moderate to very severe chronic obstructive pulmonary disease (COPD) Start Printed Page 39257 (defined as GOLD classification II, III and IV), when referred by the physician treating the chronic respiratory disease and allows additional medical indications to be established through a national coverage determination (NCD). We have not expanded coverage of PR further using the NCD process.
The conditions of coverage for CR and ICR set forth in MIPPA were codified in § 410.49 through the CY 2010 PFS final rule with comment period. In 2014, we expanded coverage of CR through the NCD process (NCD 20.10.1, Cardiac Rehabilitation Programs for Chronic Heart Failure (Pub. 100-03) to beneficiaries with stable, chronic heart failure. Section 51004 of the Bipartisan Budget Act (Pub. L. 115-123, February 9, 2018) (BBA of 2018), amended section 1861(eee)(4)(B) of the Act to expand coverage of ICR to include patients with stable, chronic heart failure. Section 410.49 was updated to codify this expansion through the CY 2020 PFS final rule (84 FR 62897 through 62899 and 63188).
Under § 410.49(b), Medicare part B covers CR and ICR for beneficiaries who have experienced one or more of the following: (1) An acute myocardial infarction within the preceding 12 months; (2) a coronary artery bypass surgery; (3) current stable angina pectoris; (4) heart valve repair or replacement; (5) percutaneous transluminal coronary angioplasty (PTCA) or coronary stenting; (6) a heart or heart-lung transplant; (7) stable, chronic heart failure defined as patients with left ventricular ejection fraction of 35 percent or less and New York Heart Association (NYHA) class II to IV symptoms despite being on optimal heart failure therapy for at least 6 weeks, on or after February 18, 2014 for cardiac rehabilitation and on or after February 9, 2018 for intensive cardiac rehabilitation; or (8) other cardiac conditions as specified through an NCD. The NCD process may also be used to specify non-coverage of a cardiac condition for ICR if coverage is not supported by clinical evidence.
As set forth in statute, PR, CR and ICR are programs furnishing physician-supervised items and services that may be furnished in a physician's office or hospital outpatient setting or in other settings determined appropriate by the Secretary.[82] When items and services are furnished under these programs, a physician must be immediately available and accessible for medical consultation and medical emergencies. PR, CR and ICR programs must include: Physician-prescribed exercise, psychosocial assessment, outcomes assessment, cardiac risk factor modification (for CR/ICR) and education or training (for PR), and individualized treatment plans (ITPs) established, reviewed and signed by a physician every 30 days. The statute also includes physician requirements for PR and CR/ICR programs. Namely, section 1861(eee)(5) of the Act requires that the Secretary establish standards to ensure that a physician with expertise in the management of individuals with cardiac pathophysiology is responsible for the CR/ICR program and that such physician, in consultation with appropriate staff, is involved substantially in directing the progress of individual in the program. Section 1861(fff)(3) of the Act similarly requires the Secretary establish standards that ensure that a physician with expertise in the management of individuals with respiratory pathophysiology is responsible for the PR program and, in consultation with appropriate staff, is involved substantially in directing the progress of individual in the program. We established physician standards for PR at § 410.47 and for CR/ICR at § 410.49.
Under the statute, PR and CR/ICR programs include individualized treatment that is furnished under a written plan established, reviewed, and signed by a physician every 30 days. We codified this requirement in §§ 410.47 and 410.49 by defining and describing the ITP which must be established, reviewed, and signed by a physician every 30 days. Because the statute requires a plan to be established, reviewed, and signed by a physician every 30 days, we cannot alter this requirement.
Stakeholders have indicated to us that it is very challenging for a program to fulfill these tasks on each patient's first day of PR or CR/ICR. Stakeholders have also expressed concerns that there is not separate and additional payment for medical directors or other physicians to develop and sign the ITPs. In response to these concerns, we note that the medical director and any staff physician(s) working in the PR or CR/ICR program who is involved in the patient's care and has knowledge related to the patient's condition, or the patient's treating and/or referring physician, may establish, review and sign ITPs. When appropriate and when all billing requirements are met, a separately billable evaluation and management (E/M) service may be furnished by the medical director or other PR or CR/ICR staff physician(s) working in the program in connection with establishing and signing the ITP on or before the first day of PR or CR/ICR. Additionally, physicians treating patients for their cardiovascular or respiratory conditions, but who are not staff of the PR or CR/ICR programs, are not precluded from developing and signing ITPs for their patients before they begin PR or CR/ICR programs. While the CY 2010 PFS final rule for PR (74 FR at 61883) stated that the PR physician must review and sign the ITP prior to initiation of PR even if the plan was developed by a different physician, we recognize that this imposes greater burden and may potentially delay treatment. ITPs developed and signed on or before the first day of PR by a physician who is treating the patient's respiratory condition outside of the PR program will not require an additional signature from the PR medical director (or any other physician working in the program) on or before the first day of PR. Similarly, ITPs developed and signed on or before the first day of CR/ICR by a physician outside of the CR/ICR program treating the patient's cardiovascular condition, do not require an additional signature from the CR/ICR medical director (or other physician working in the program) on or before the first day of CR/ICR. The PR and CR/ICR medical director and other appropriate staff would review these ITPs on or before the first day services are furnished. The medical director or other physician working in the program, in consultation with staff, may revise the ITP as needed to ensure the plan is appropriately individualized, regardless of which physician establishes and signs the plan.
3. Proposed Revisions
As described above, PR and CR/ICR programs are subject to many of the same statutory requirements. Despite the consistency in requirements set forth in statute, we recognize that some of the conditions of coverage codified in regulation are not identical across both programs. We are proposing conforming changes to the regulatory text for both PR and CR/ICR to establish consistency in terminology, definitions and requirements where appropriate which will result in clearer and more streamlined regulatory text. We are also proposing to adjust the regulatory structure of § 410.47 to align with § 410.49. The proposed revisions will also enable stakeholders with interest in both PR and CR/ICR programs to more easily compare requirements and implement programs. Start Printed Page 39258
a. Definitions
We are proposing revisions to six PR definitions at § 410.47(a), including individualized treatment plan, medical director, outcomes assessment, physician-prescribed exercise, psychosocial assessment and supervising physician; and revisions to three CR/ICR definitions at § 410.49(a), including medical director, outcomes assessment, and physician-prescribed exercise. Specifically, the proposed revisions to the PR definitions of ITP, psychosocial assessment and supervising physician align with the definitions of the same terms for CR/ICR. The proposed revisions to the PR definition of physician-prescribed exercise align with the definition of physician-prescribed exercise for CR/ICR and also include revisions to provide examples of physical activities appropriate to the patient population (which were relocated from the PR components section (previously § 410.47(c)). Similar revisions are proposed for the CR/ICR definition of physician-prescribed exercise. We are proposing to modify language in the PR definition of medical director to align with the CR/ICR definition of medical director to more specifically describe the role of the PR medical director. We are proposing conforming changes to the CR/ICR definition of medical director. Proposed revisions to the PR and CR/ICR definitions of outcomes assessment remove and revise redundant and unnecessary language. Also, we are proposing to clearly state that outcome assessments may be performed by either the physician or the PR or CR/ICR program staff and that all results of these evaluations performed by program staff must be considered by the physician in the development and/or review of ITPs. These proposals are consistent with descriptions provided in the CY 2010 PFS proposed rule (74 FR at 33608, 33613) which state that PR and CR/ICR staff must provide outcomes assessments to the physician and serve to clearly communicate the important supportive role program staff may play to the physicians of these rehabilitation programs. The proposed conforming changes are designed to more accurately define the existing terms and ensure consistency in definitions used for the same terms across PR and CR/ICR programs. We chose to largely maintain the CR/ICR regulatory text and align the PR regulatory text with CR/ICR based on stakeholder feedback and questions regarding the PR requirements. Aligning PR with CR/ICR, as opposed to aligning CR/ICR with PR requirements, better addresses stakeholder feedback and improves consistency in terminology, definitions and descriptions of conditions of coverage. With the proposed revisions and increased consistency, we also aim to improve program efficiency in implementing the conditions of coverage.
b. Covered Conditions
The definition for PR at § 410.47(a) specifies that PR is a physician-supervised program for COPD and certain other chronic respiratory diseases. The CDC uses the term post-COVID conditions to describe health issues that persist more than 4 weeks after first being infected with the causative virus[83] indicating that this timeframe provides a rough approximation of effects that occur beyond the acute period. Similarly, the National Institute for Health and Care Excellence (NICE), the Scottish Intercollegiate Guidelines Network (SIGN) and the Royal College of General Practitioners (RCGP) have jointly used 4 weeks to differentiate the acute symptoms of COVID from `long COVID,' the signs and symptoms that continue or develop after acute COVID-19.[84] Based on the information from the CDC, NICE, SIGN and RCGP, we consider COVID-19 to be chronic when symptoms persist for more than 4 weeks. Symptoms include dyspnea, depression and anxiety which can impair physical function and cause incapacitation.[85 86] We are proposing to cover PR for Medicare beneficiaries who have been diagnosed with severe manifestations of COVID-19, defined as requiring hospitalization in the ICU or otherwise, and who experience continuing symptomatology, including respiratory dysfunction, for at least 4 weeks post discharge.
Management of COVID-19 post-acute syndrome is an evolving issue in the health of our beneficiaries. We recognize that there is limited evidence available assessing the benefits that PR may provide for patients who were diagnosed with COVID-19. However, early research and consensus statements emphasize the restorative role that PR will likely play in the patient recovering from COVID-19.[87 88] We are soliciting comments regarding the appropriateness of the coverage criteria for PR for beneficiaries diagnosed with COVID-19, including both the characteristics of the patients for whom PR is covered and the timing of their symptoms as presented above.
c. Components
We are proposing revisions to the description of each of the five PR components under § 410.47(b)(2) (previously § 410.47(c)). Proposed revisions to the descriptions of physician prescribed exercise, psychosocial assessment and outcomes assessment include removing language already used in the definition of each term or references to the definitions in § 410.47(a). The inclusion of already established definition language is redundant and therefore unnecessary. Proposed revisions to the education or training component more concisely explain, but do not change, the existing requirements for meeting this component. Proposed revisions to the description of the ITP align with the description used for the CR/ICR ITP. As noted in the section above, we largely align the PR regulatory text with CR/ICR to better address stakeholder feedback and improve consistency in terminology, definitions and descriptions of conditions of coverage to assist in improving program efficiency in implementing the conditions of coverage.
d. Settings
We are proposing minor edits to align the PR setting text in § 410.47(b)(3)(i) (previously § 410.47(d)(1)) with the CR/ICR setting text and reorganize this section to move and update, consistent with the corresponding CR/ICR section, the requirement that all settings must have a physician immediately available and accessible for medical consultations and emergencies.
e. Physician Standards
We are proposing revisions to align regulatory text regarding the standards for the PR medical director and the supervising physician found at Start Printed Page 39259 § 410.47(c) and (d) (previously § 410.47(e)) with the corresponding CR/ICR medical director and supervising physician text and minor conforming changes to CR/ICR language § 410.49(d) and (e). These revisions will not only align similar requirements for PR and CR/ICR programs, but also more accurately describe the roles and responsibilities of physicians in PR programs, and thereby address stakeholder feedback requesting more specificity around the roles and standards for the physicians involved in PR programs. Specifically, we are proposing to replace the existing PR "physician standards" section with two separate sections. The first, entitled "medical director standards" delineates requirements for the PR medical director, and the second, "supervising physician standards" delineates requirements for physicians fulfilling the supervising physician role when PR items and services are furnished. These revisions also include removing language that is redundant to the definition for medical director already set forth in § 410.47(a) and the requirement that a physician have "direct patient contact related to the periodic review of his or her treatment plan." We are proposing to remove the direct patient contact language because this requirement is overly burdensome and unnecessary since a physician is already required to, in consultation with staff, review patient ITPs every 30 days. Direct physician-patient contact can be written into an ITP for patients who require such attention; however, it is not necessary for every patient and the need for it should instead be specified by the clinician. Furthermore, while we believe direct physician-patient contact within the PR program every 30 days is not necessary for every PR patient, we note that patients are seen by PR staff and their progress is tracked at each session where staff are able to identify the need for direct physician-patient contact as appropriate. Additionally, patients participating in PR generally continue to have ongoing interactions with their treating physicians outside of PR. Because the need for direct physician-patient contact is individualized and patients continue to engage with their treating physicians outside of PR, we are proposing to remove the requirement for direct physician-patient contact within the PR program every 30 days. We are requesting public comment on whether removing the regulatory requirement for direct physician-patient contact every 30 days would be potentially detrimental to PR patients by eliminating a critical physician interaction, or if necessary interactions are already occurring outside of the PR program at appropriate intervals as determined by a physician treating the patient for his or her respiratory condition.
These proposed revisions and clearer delineations of the roles and standards for the PR medical director and, separately, the supervising physician, are important to address stakeholder feedback and reduce burden on PR programs, physicians and patients while ensuring treatment is truly individualized as directed by statute. As these proposed revisions, more accurately describe and delineate the roles and standards for the medical director and the supervising physician, please note that the PR or CR/ICR medical director may serve as a supervising physician if he or she also meets the requirements for a supervising physician. Two different physicians are not necessarily required, as long as the definitions and descriptions in §§ 410.47 and 410.49 are met.
f. Limitations
We are proposing conforming changes to § 410.47(e) (previously § 410.47(f)) and § 410.49(f) to improve clarity of these sections and more closely align the descriptions for session duration, number of sessions covered and time-period over which sessions must be provided.
4. Summary
To improve consistency and accuracy across PR and CR/ICR conditions of coverage, we are proposing largely conforming changes throughout §§ 410.47 and 410.49. We are also proposing to add coverage of PR for beneficiaries who were hospitalized with a COVID-19 diagnosis and experience persistent symptoms, including respiratory dysfunction, for least 4 weeks after hospital discharge and to remove a PR program requirement that is overly burdensome and unnecessary for all PR patients which was also not expressly required in statute. We believe these proposals result in clearer and more streamlined regulatory text and better assist stakeholders in understanding and implementing PR, CR and ICR programs. We look forward to public comments on our proposals, in particular our proposals to remove the PR direct physician-patient contact requirement and to add coverage of PR for beneficiaries who were hospitalized with a COVID-19 diagnosis and experience persistent symptoms, including respiratory dysfunction, for at least 4 weeks after hospital discharge.
I. Medical Nutrition Therapy
Medical nutrition therapy became a distinct Medicare benefit under section 1861(s)(2) of the Act pursuant to section 105 of the Medicare, Medicaid, and SCHIP Benefits Improvement Protection Act of 2000 (BIPA). Medicare beneficiaries with diabetes or renal disease can receive individualized medical nutrition therapy (MNT) provided by a registered dietitian or nutrition professional, pursuant to a referral by a physician (as defined in section 1861(r)(1) of the Act), with no cost to the beneficiary. Currently, 42 CFR 410.132(c), further requires that the referral must be made by the treating physician. The treating physician was defined as the primary care physician or specialist, coordinating care for the beneficiary with diabetes or renal disease. The regulation also specifically defines renal disease as including chronic renal insufficiency based on glomerular filtration rate (GFR) eligibility criteria.
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Kidney Foundation and Academy of Nutrition and Dietetics support MNT for adults with chronic kidney disease (CKD). The National Kidney Foundation and the Academy of Nutrition and Dietetics' Clinical Practice Guideline on Nutrition in Chronic Kidney Disease[89] acknowledges that the goals of MNT are to optimize nutritional status, and to minimize risks imposed by comorbid conditions and alterations in metabolism on the progression of kidney disease and on adverse clinical outcomes. The authors recognize that patients with CKD have changing needs according to their disease stage and they recommended MNT for each stage of CKD.
In addition, evidence supports the use of MNT as a component of quality diabetes care, including its integration into the medical management of diabetes. Nutrition therapy that includes the development of an eating plan designed to improve blood glucose, blood pressure, and lipid profiles is important in the management of diabetes and can lower the risk of cardiovascular disease, coronary heart disease, and stroke. Despite these findings and endorsement by leading clinical societies, including the American Diabetes Association, American College of Cardiology and the Start Printed Page 39260 National Kidney Foundation, less than 1 percent of the estimated 14 million eligible Medicare beneficiaries have accessed MNT.
Over the years, we have heard from several stakeholder groups requesting that we update the MNT regulations to improve beneficiary access. In this proposed rule, we provide background on the MNT services, discuss the MNT regulation revisions, and make proposals to implement these modifications. We are proposing to make changes to the treating physician requirements and update the chronic renal insufficiency GFR criteria in order to improve access and utilization of the MNT benefit. The statute expressly requires the order of a physician; therefore, we are unable to extend referral privileges to NPPs.
1. Background: MNT
MNT is defined in sections 1861(s)(2)(V) and 1861(vv)(1) of the Act and codified in 42 CFR 410.130 (definitions), § 410.132 (MNT), and § 410.134 (provider qualifications).
a. Definitions (§ 410.130)
In 42 CFR subpart G, we define the following definitions that apply to MNT at § 410.130:
- Chronic renal insufficiency.
- Diabetes.
- Episode of care.
- Medical nutrition therapy services.
- Physician.
- Renal disease.
- Treating physician.
b. Medical Nutrition Therapy (§ 410.132)
In § 410.132(a), we outline the conditions for coverage of MNT services. That is, Medicare Part B pays for MNT services provided by a registered dietitian or nutrition professional as defined in § 410.134 when the beneficiary is referred for the service by the treating physician. Services covered consist of face-to-face nutritional assessments and interventions in accordance with nationally-accepted dietary or nutritional protocols. The regulation contains an exception that permits MNT services to be provided as telehealth services under § 410.78.
In § 410.132(b), we outline the limitations on coverage of MNT services. First, the MNT services based on a diagnosis of renal disease as described in 42 CFR subpart G are not covered for beneficiaries receiving maintenance dialysis for which payment is made under section 1881 of the Act. Also, a beneficiary may only receive the maximum number of hours covered under the DSMT benefit for both DSMT and MNT during the initial DSMT training period unless additional hours are determined to be medically necessary under the national coverage determination (NCD) process. In years when the beneficiary is eligible for MNT and follow-up DSMT, Medicare will cover the maximum number of hours covered under MNT unless additional hours are determined to be medically necessary under the NCD process. Under the current MNT NCD (NCD 180.1), Medicare covers 3 hours of MNT the initial year of referral and up to 2 hours of MNT for subsequent years. In addition, if a beneficiary has both diabetes and renal disease, Medicare will cover the maximum number of hours covered under the renal MNT benefit in one episode of care unless he or she is receiving initial DSMT services, in which case the beneficiary would receive whichever is greater. Finally, an exception to the maximum number of hours described here may be made when the treating physician determines that there is a change of diagnosis, medical condition, or treatment regimen related to diabetes or renal disease that requires a change in MNT during an episode of care.
At § 410.132(c), we discuss that a referral may only be made by the treating physician when the beneficiary has been diagnosed with diabetes or renal disease as defined in 42 CFR subpart G with documentation maintained by the referring physician in the beneficiary's medical record. We also note that referrals must be made for each episode of care and any additional assessments or interventions required by a change of diagnosis, medical condition, or treatment regimen during an episode of care.
c. Provider Qualifications (§ 410.134)
For Medicare Part B coverage of MNT, only a registered dietitian or nutrition professional may provide the services. At § 410.134, we define registered dietitian or nutrition professional as an individual who, on or after December 22, 2000: (1) Holds a bachelor's or higher degree granted by a regionally accredited college or university in the United States (or an equivalent foreign degree) with completion of the academic requirements of a program in nutrition or dietetics accredited by an appropriate national accreditation organization recognized for this purpose; (2) has completed at least 900 hours of supervised dietetics practice under the supervision of a registered dietitian or nutrition professional; and (3) is licensed or certified as a dietitian or nutrition professional by the state in which the services are performed. In a state that does not provide for licensure or certification, the individual will be deemed to have met this requirement if he or she is recognized as a registered dietitian by the Commission on Dietetic Registration or its successor organization. However, a dietitian or nutritionist licensed or certified in a state as of December 21, 2000 is not required to hold a bachelor's or higher degree granted by a regionally accredited college or university in the United States (or an equivalent foreign degree) with completion of the academic requirements of a program in nutrition or dietetics accredited by an appropriate national accreditation organization recognized for this purpose; (2) and need not complete at least 900 hours of supervised dietetics practice under the supervision of a registered dietitian or nutrition professional. In addition, a registered dietitian in good standing, as recognized by the Commission of Dietetic Registration or its successor organization, is deemed to have met these requirements.
2. Proposal for MNT Revisions
a. Removal of the Treating Physician Restriction
For CY 2022, we are proposing to revise the regulations at §§ 410.130 and 410.132. Sections 1861(s)(2)(V) and 1861(vv)(1)) of the Act define MNT services as nutritional diagnostic, therapy, and counseling services for the purpose of disease management which are furnished by a registered dietitian or nutrition professional pursuant to a referral by a physician (either an M.D. or D.O.) (as defined in section 1861(r)(1) of the Act). The current regulation further provides that Medicare pays for MNT services when the beneficiary is referred for the service by the treating physician, which is defined as the primary care physician or specialist coordinating care for the beneficiary with diabetes or renal disease. As discussed above in section III.I.2. of this proposed rule and codified at § 410.132(c), we required referrals only by the treating physician when the beneficiary has been diagnosed with diabetes or a renal disease, with documentation maintained by the referring physician in the beneficiary's medical record. In the CY 2002 PFS final rule (66 FR 55246, November 1, 2001), we believed the treating physician requirement was necessary to ensure coordination of care by the primary care physician or specialist for beneficiaries with chronic diseases in order to assure quality (66 FR 55277). Start Printed Page 39261 This relatively narrow definition, however, is now believed to have contributed to the low uptake of referrals to MNT services, although we note that few studies have examined MNT use.
We are proposing to eliminate the requirement that the referral be made by the treating physician and, consistent with the language of the statute, require MNT services to be pursuant to a referral by a physician (as defined in section 1861(r)(1) of the Act) at § 410.130 and § 410.132. It would be reasonable for any physician to refer a beneficiary to MNT. The treating physician restriction is no longer necessary to expect care to be coordinated. Care coordination between the hospital or post-acute care provider and the primary care provider is the goal and a standard of care in today's medical environment. We have worked to improve, through various efforts, the exchange of patient information between healthcare settings, and that a patient's healthcare information follows them after discharge from a hospital or post-acute care provider. Such improved transitions of care and exchange of information helps to assure that Medicare beneficiaries will continue to receive quality services. We are proposing to delete the term treating and the definition of treating physician, as there is a separate definition for physician within this provision. Therefore, we are not proposing any change to Medicare's definition of treating physician and the deletion of treating physician only applies to this provision.
b. Update the GFR Eligibility Criteria for Patients With CKD
We are proposing to revise the regulations at § 410.130. Section 1861(s)(2)(V) of the Act states that MNT services are available to beneficiaries with diabetes or a renal disease. In 2001, we established the definition of chronic renal insufficiency for the purpose of the MNT benefit using definitions from the Institute of Medicine report, "The Role of Nutrition in Maintaining Health in the Nation's Elderly."[90] The definitions and staging of chronic kidney disease have evolved since the release of the report and stakeholders have noted that our definition does not reflect current medical practice. Therefore, we are proposing to update the GFR eligibility criteria so that it aligns with up to date accepted standards for CKD stage III through stage V, specifically GFR 15-59 mL/min/1.73m2. The accepted CKD staging system separates stage III into two parts: Stage III-a; and Stage III-b. Stage III-a is GFR 45-59. The existing regulatory upper limit of 50 is mid stage III-a and does not meet the widely accepted standard of when a person is diagnosed with moderate kidney disease. The NIDDK and National Kidney Foundation's staging of CKD align with the proposed change in GFR criteria.[91 92]
3. Proposed Regulatory Text Changes
We are proposing to make changes to the treating physician requirements and GFR eligibility criteria outlined in § § 410.130 and 410.132, consistent with statutory limitations. We propose to revise § 410.130 (definitions) and § 410.132 (MNT) by: (1) Revising the chronic renal insufficiency definition; (2) striking the treating physician definition; and (3) revising conditions for coverage of MNT services, limitations on coverage of MNT services, and referrals.
(1) Definition of Chronic Renal Insufficiency
We propose to revise § 410.130 by revising the chronic renal insufficiency definition by removing the GFR eligibility criteria of 13—50 ml/min/1.73m2 and replacing with 15—59 ml/min/1.73m2.
(2) Definition of Treating Physician
We propose to revise § 410.130 by removing the definition of treating physician.
(3) Proposed Changes to Conditions for Coverage of MNT Services, Limitations on Coverage of MNT Services, and Referrals
At § 410.132, we are proposing to revise conditions for coverage of MNT services, limitations on coverage of MNT services, and referrals by removing the terms "the" and "treating," and replacing them with "a," at paragraphs (a), (b)(5), and (c). In paragraph (c), we are also proposing to strike the term, "maintained," and replace it with the term, "noted."
4. Summary
The MNT services may help reduce illnesses and improve quality of life for people with diabetes or renal disease. We believe the proposed changes to the treating physician requirements and GFR eligibility criteria are in the best interest of the Medicare program and its beneficiaries. The physician requirement change will increase the capacity and availability of physicians who can refer beneficiaries to MNT, which would alleviate some of the demand on primary care physicians as the usual source to perform this particular function. We note that stakeholders have contacted CMS and suggested such flexibility in the past. We recognize that MNT is not a highly utilized service and we believe these revisions will allow for Medicare patients to gain greater access to MNT services. We look forward to receiving public comment on these proposals.
J. Medicare Shared Savings Program
On March 23, 2010, the Patient Protection and Affordable Care Act (Pub. L. 111-148) was enacted, followed by enactment of the Health Care and Education Reconciliation Act of 2010 (Pub. L. 111-152) on March 30, 2010, which amended certain provisions of the Patient Protection and Affordable Care Act (hereinafter collectively referred to as "the Affordable Care Act"). Section 3022 of the Affordable Care Act amended Title XVIII of the Act (42 U.S.C. 1395 et seq.) by adding section 1899 to the Act to establish the Medicare Shared Savings Program (Shared Savings Program) to facilitate coordination and cooperation among healthcare providers to improve the quality of care for Medicare fee-for-service (FFS) beneficiaries and reduce the rate of growth in expenditures under Medicare Parts A and B. (See 42 U.S.C. 1395jjj.) Eligible groups of providers and suppliers, including physicians, hospitals, and other healthcare providers, may participate in the Shared Savings Program by forming or participating in an Accountable Care Organization (ACO). Under the Shared Savings Program, providers of services and suppliers that participate in an ACO continue to receive traditional Medicare FFS payments under Parts A and B, but the ACO may be eligible to receive a shared savings payment if it meets specified quality and savings requirements.
Section 1899 of the Act has been amended through subsequent legislation. The requirements for assignment of Medicare FFS beneficiaries to ACOs participating under the program were amended by the 21st Century Cures Act (the CURES Act) (Pub. L. 114-255, December 13, 2016). The Bipartisan Budget Act of 2018 (Pub. L. 115-123, February 9, 2018), further Start Printed Page 39262 amended section 1899 of the Act to provide for the following: Expanded use of telehealth services by physicians or practitioners participating in an applicable ACO to furnish services to prospectively assigned beneficiaries, greater flexibility in the assignment of Medicare FFS beneficiaries to ACOs by allowing ACOs in tracks under retrospective beneficiary assignment a choice of prospective assignment for the agreement period; permitting Medicare FFS beneficiaries to voluntarily identify an ACO professional as their primary care provider and requiring that such beneficiaries be notified of the ability to make and change such identification, and mandating that any such voluntary identification will supersede claims-based assignment; and allowing ACOs under certain two-sided models to establish CMS-approved beneficiary incentive programs.
The Shared Savings Program regulations are codified at 42 CFR part 425. The final rule establishing the Shared Savings Program appeared in the November 2, 2011 Federal Register (Medicare Program; Medicare Shared Savings Program: Accountable Care Organizations; final rule (76 FR 67802) (hereinafter referred to as the "November 2011 final rule")). A subsequent major update to the program rules appeared in the June 9, 2015 Federal Register (Medicare Program; Medicare Shared Savings Program: Accountable Care Organizations; final rule (80 FR 32692) (hereinafter referred to as the "June 2015 final rule")). The final rule entitled, "Medicare Program; Medicare Shared Savings Program; Accountable Care Organizations—Revised Benchmark Rebasing Methodology, Facilitating Transition to Performance-Based Risk, and Administrative Finality of Financial Calculations," which addressed changes related to the program's financial benchmark methodology, appeared in the June 10, 2016 Federal Register (81 FR 37950) (hereinafter referred to as the "June 2016 final rule"). A final rule, "Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program—Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program—Accountable Care Organizations—Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act", appeared in the November 23, 2018 Federal Register (83 FR 59452) (hereinafter referred to as the "November 2018 final rule" or the "CY 2019 PFS final rule"). In the November 2018 final rule, we finalized a voluntary 6-month extension for existing ACOs whose participation agreements would otherwise expire on December 31, 2018; allowed beneficiaries greater flexibility in designating their primary care provider and in the use of that designation for purposes of assigning the beneficiary to an ACO if the clinician they align with is participating in an ACO; revised the definition of primary care services used in beneficiary assignment; provided relief for ACOs and their clinicians impacted by extreme and uncontrollable circumstances in performance year 2018 and subsequent years; established a new Certified Electronic Health Record Technology (CEHRT) use threshold requirement; and reduced the Shared Savings Program quality measure set from 31 to 23 measures (83 FR 59940 through 59990 and 59707 through 59715).
A final rule redesigning the Shared Savings Program appeared in the December 31, 2018 Federal Register (Medicare Program: Medicare Shared Savings Program; Accountable Care Organizations—Pathways to Success and Uncontrollable Circumstances Policies for Performance Year 2017; final rule) (83 FR 67816) (hereinafter referred to as the "December 2018 final rule"). In the December 2018 final rule, we finalized a number of policies for the Shared Savings Program, including a redesign of the participation options available under the program to encourage ACOs to transition to two-sided models; new tools to support coordination of care across settings and strengthen beneficiary engagement; and revisions to ensure rigorous benchmarking.
In the interim final rule with comment period (IFC) entitled "Medicare and Medicaid Programs; Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency", which was effective on the March 31, 2020 date of display and appeared in the April 6, 2020 Federal Register (85 FR 19230) (hereinafter referred to as the "March 31, 2020 COVID-19 IFC"), we removed the restriction which prevented the application of the Shared Savings Program extreme and uncontrollable circumstances policy for disasters that occur during the quality reporting period if the reporting period is extended, to offer relief under the Shared Savings Program to all ACOs that may be unable to completely and accurately report quality data for 2019 due to the Public Health Emergency (PHE) for COVID-19 (85 FR 19267 and 19268).
In the IFC entitled "Medicare and Medicaid Programs; Basic Health Program, and Exchanges; Additional Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency and Delay of Certain Reporting Requirements for the Skilled Nursing Facility Quality Reporting Program" which was effective on May 8, 2020, and appeared in the May 8, 2020 Federal Register (85 FR 27573 through 27587) (hereinafter referred to as the "May 8, 2020 COVID-19 IFC"), we modified Shared Savings Program policies to: (1) Allow ACOs whose current agreement periods expire on December 31, 2020, the option to extend their existing agreement period by 1-year, and allow ACOs in the BASIC track's glide path the option to elect to maintain their current level of participation for performance year 2021; (2) adjust program calculations to remove payment amounts for episodes of care for treatment of COVID-19; and (3) expand the definition of primary care services for purposes of determining beneficiary assignment to include telehealth codes for virtual check-ins, e-visits, and telephonic communication. We also clarified the applicability of the program's extreme and uncontrollable circumstances policy to mitigate shared losses for the period of the PHE for COVID-19 starting in January 2020.
We have also made use of the annual CY PFS rules to address quality reporting for the Shared Savings Program and certain other issues. Refer to the CY 2020 PFS proposed rule for a summary of policies finalized in prior PFS rules (84 FR 40705). In the CY 2021 PFS final rule, we finalized new Shared Savings Program quality reporting requirements that align with the Alternative Payment Model (APM) Performance Pathway (APP) under the Quality Payment Program and revised the quality performance standard for performance years beginning on or after January 1, 2021, to reduce reporting burden and focus on patient outcomes. We also finalized a policy that waived the requirement that ACOs administer the Consumer Assessment of Healthcare Providers and Systems (CAHPS) for ACOs survey for performance year 2020. In addition, we finalized updates to the definition of primary care services used Start Printed Page 39263 for beneficiary assignment, and policies to reduce burden associated with repayment mechanisms. In the CY 2021 PFS final rule, we also finalized the Shared Savings Program provisions included in the March 31, 2020 COVID-19 IFC and the May 8, 2020 COVID-19 IFC, with several modifications in response to public comments received.
Policies applicable to Shared Savings Program ACOs for purposes of reporting for other programs have also continued to evolve based on changes in the statute. The Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) (Pub. L. 114-10, April 16, 2015) established the Quality Payment Program. In the CY 2017 Quality Payment Program final rule with comment period (81 FR 77008), we established regulations for the Merit-Based Incentive Payment System (MIPS) and Advanced APMs and related policies applicable to eligible clinicians who participate in APMs, including the Shared Savings Program.
As a general summary, in this proposed rule, we are proposing to:
- Clarify the Application of the CAHPS for MIPS Survey sampling policies, including the CAHPS for MIPS minimum sampling thresholds, for Shared Savings Program ACOs.
- Amend the reporting requirements under the APM Performance Pathway (APP) for performance year 2022 and performance year 2023.
++ Solicit comments on addressing health disparities and promoting health equity.
++ Solicit comments on the feasibility of TIN level reporting and sampling for eCQMs/MIPS CQMs.
++ Solicit comments on reporting options for specialist providers within an ACO.
++ Update the APM Performance Pathway (APP) measure set to remove the Risk-Standardized, All-Cause Unplanned Admissions for Multiple Chronic Conditions (MCC) for ACOs and replace it with the Risk Standardized, All-Cause Unplanned Admissions for Multiple Chronic Conditions for MIPS.
- Amend the quality performance standard for performance year 2023 by freezing the quality performance standard at the 30th percentile MIPS Quality performance category score.
++ Solicit comments on publicly displaying prior year performance scores that equate to the 30th or 40th percentile MIPS Quality performance category scores.
- Revise the extreme and uncontrollable circumstances policy to align with the proposal to freeze the quality performance standard at the 30th percentile MIPS Quality performance category score for performance year 2023.
- Update the definition of primary care services used in beneficiary assignment at § 425.400(c).
- Revise the repayment mechanism arrangement policy in the following manner:
++ To reduce the percentages used in the existing methodology for determining the repayment mechanism amount and to specify the number of assigned beneficiaries used as a multiplier in the calculations, such that the ACO's repayment mechanism amount would be calculated as the lesser of the following: (1) One-half percent of the total per capita Medicare Parts A and B FFS expenditures for the ACO's assigned beneficiaries, based on expenditures and the number of assigned beneficiaries for the most recent calendar year for which 12 months of data are available; or (2) 1 percent of the total Medicare Parts A and B FFS revenue of its ACO participants, based on revenue for the most recent calendar year for which 12 months of data are available, and based on the ACO's number of assigned beneficiaries for the most recent calendar year for which 12 months of data are available.
++ To specify how we identify the number of assigned beneficiaries used in the repayment mechanism amount calculation and the annual repayment mechanism amount recalculation.
++ To allow a one-time opportunity for certain ACOs that established a repayment mechanism to support their participation in a two-sided model beginning on July 1, 2019, January 1, 2020, or January 1, 2021, to elect to decrease the amount of their existing repayment mechanisms.
++ To revise the threshold for determining whether an increase in the repayment mechanism amount is required.
- Streamline the application process by revising requirements concerning the disclosure of prior participation in the Shared Savings Program by the ACO, ACO participants, and ACO providers/suppliers, in light of other requirements that consider an ACO's prior participation.
- Reduce the frequency and circumstances under which ACOs submit sample ACO participant agreements and executed ACO participant agreements to CMS.
- Amend the beneficiary notification requirement as it applies to ACOs under prospective assignment and ACOs under preliminary prospective assignment with retrospective reconciliation.
- Solicit comments on considerations related to the use of regional FFS expenditures in the Shared Savings Program's benchmarking methodology.
1. Quality and Other Reporting Requirements
a. Background
Section 1899(b)(3)(C) of the Act states that the Secretary shall establish quality performance standards to assess the quality of care furnished by ACOs and seek to improve the quality of care furnished by ACOs over time by specifying higher standards, new measures, or both. As we stated in the November 2011 final rule establishing the Shared Savings Program (76 FR 67872), our principal goal in selecting quality measures for ACOs has been to identify measures of success in the delivery of high-quality health care at the individual and population levels, with a focus on outcomes. In the November 2011 final rule, we adopted a quality measure set spanning four domains: Patient experience of care, care coordination/patient safety, preventative health, and at-risk population (76 FR 67872 through 67891). We subsequently updated the measures comprising the quality performance measure set for the Shared Savings Program through rulemaking in the CY 2015, 2016, 2017, and 2019 PFS final rules (79 FR 67907 through 67920, 80 FR 71263 through 71268, 81 FR 80484 through 80489, and 83 FR 59707 through 59715 respectively).
Between performance years 2017 (the first performance year under MIPS) and 2020, eligible clinicians who were participating in an ACO and who were subject to MIPS (MIPS eligible clinicians) were scored under the APM scoring standard under MIPS (81 FR 77260). These clinicians include any MIPS eligible clinicians who were participating in an ACO in a track, or payment model within a track (Track 1 and Levels A through D of the BASIC track) of the Shared Savings Program that is not an Advanced APM, as well as those MIPS eligible clinicians participating in an ACO in a track, or payment model within a track (Track 2, Level E of the BASIC track, and the ENHANCED track, or the Medicare ACO Track 1+ Model (Track 1+ Model)) that is an Advanced APM, but who do not become Qualifying APM Participants (QPs) as specified in § 414.1425, and are not otherwise excluded from MIPS.
In the CY 2021 PFS final rule, CMS finalized modifications to the Shared Savings Program quality reporting Start Printed Page 39264 requirements and quality performance standard for PY 2021 and subsequent performance years (85 FR 84720 through 84736). For performance year 2021 and subsequent years, ACOs are required to report quality data via the APP. In addition, CMS finalized a phase-in approach to the new Shared Savings Program quality performance standard that ACOs must achieve in order to be eligible to share in savings or avoid maximum losses. This phase-in allows for a gradual increase of the quality performance standard from a quality performance score that is equivalent to or higher than the 30th percentile across all MIPS Quality performance category scores in performance years 2021 and 2022 to a quality performance score that is equivalent to or higher than the 40th percentile across all MIPS Quality performance category scores in performance year 2023 and subsequent years.
b. Clarification of the Application of CAHPS for MIPS Sampling Policies to Shared Savings Program ACOs
In the CY 2021 PFS final rule (85 FR 84722), we finalized that beginning in performance year (PY) 2021, Shared Savings Program Accountable Care Organizations (ACOs) are required to report quality data via the Alternative Payment Model (APM) Performance Pathway (APP). As part of the APP, ACOs are required to administer the CAHPS for MIPS survey (85 FR 84730 through 84732).
In the CY 2021 PFS final rule, we noted, in response to public comments, that the CAHPS for MIPS survey uses the same survey instrument to assess the same patient experience domains (or Summary Survey Measures (SSMs)) as the CAHPS for ACO survey. We noted that both the CAHPS for MIPS and the CAHPS for ACOs survey use the same shortened, streamlined version of the survey that we implemented for both CAHPS for ACOs and CAHPS for MIPS in 2018, reflecting efforts by CMS to reduce the number of questions. Moreover, in 2019, the two programs used identical survey instruments.
As discussed in the CY 2021 PFS final rule, we conducted analyses to assess the impact of aligning CAHPS scoring and benchmarking using 2019 CAHPS for ACOs and CAHPS for MIPS data. The results of these analyses indicate that scoring ACOs using the MIPS methodology resulted in ACOs having a similar distribution of quality points as MIPS groups. This distribution was wider than the distribution of quality points using the ACO scoring methodology largely due to differences across the two programs in the approach to benchmarking (85 FR 84731).
In addition, we clarified that beneficiaries assigned to an ACO or MIPS group, who are eligible for the CAHPS for MIPS or CAHPS for ACOs survey, are randomly selected for inclusion in the sample. Samples are drawn at the ACO level for CAHPS for ACOs and at the TIN level for MIPS groups. Therefore, each ACO or MIPS group sample is representative of the ACO or group population.
We stated that due to the alignment of CAHPS for ACOs with CAHPS for MIPS, we will use the benchmarking and scoring methodology for CAHPS for MIPS to assess ACOs' performance on the CAHPS survey measures. We explained that a single set of benchmarks will be calculated using data from all applicable CAHPS for MIPS reporters. We score the CAHPS for MIPS survey as one quality measure, which is a different scoring approach from the Shared Savings Program quality scoring methodology, which scored the 10 CAHPS for ACOs SSMs in one patient/caregiver experience quality domain. As described in the CY 2017 Quality Payment Program final rule (81 FR 77284), each scored SSM has an individual benchmark and is scored individually and compared against the benchmark to establish the number of points earned. The CAHPS score is the average number of points across scored SSMs.
As stated in the CY 2021 PFS final rule (85 FR 84731), eligible beneficiaries assigned to an ACO or MIPS group are randomly selected to be included in the sample for the CAHPS for ACOs or CAHPS for MIPS survey. In the CY 2021 PFS final rule, we explained that the target sample size for CAHPS samples for all participating ACOs, groups, and virtual groups is 860; for ACOs, groups, and virtual groups with 860 or more survey-eligible patients, a random sample of 860 patients is drawn. We also noted that groups and virtual groups with fewer than 860 survey-eligible patients are eligible to participate in the CAHPS for MIPS if they meet the minimum sampling thresholds for CAHPS for MIPS:
- Large groups or virtual groups with 100 or more eligible clinicians: 416 eligible patients.
- Medium groups or virtual groups with 25-99 eligible clinicians: 255 eligible patients.
- Small groups or virtual groups with 2-24 eligible clinicians: 125 eligible patients.
These minimum sampling thresholds are necessary to ensure that groups have an adequate sample size to ensure that the survey responses will be representative of the care furnished by the clinicians in the group. Groups that do not have an adequate sample size would be at risk for not receiving enough survey responses to be representative of the care provided.
In the CY 2021 PFS final rule, we stated that we will continue to draw the CAHPS survey samples for Shared Savings Program ACOs administering the CAHPS for MIPS survey at the Shared Savings Program ACO level, with a target sample size of 860 going forward. Although we did not specifically state in the CY 2021 PFS final rule that the MIPS minimum sampling thresholds would also apply to ACOs participating in the Shared Savings Program, we want to clarify that they do apply for performance year 2021 and subsequent years. As explained in the CY 2021 PFS final rule, under the APP we are replacing the CAHPS for ACOs that was previously used in the Shared Savings Program with the CAHPS for MIPS. Because our intent in including the CAHPS for MIPS in the APP was to align reporting requirements under the Shared Savings Program with MIPS, we believe that the discussion surrounding the CAHPS for MIPS minimum sampling thresholds for groups and virtual groups can be reasonably understood to indicate that the CAHPS for MIPS minimum sampling thresholds would also apply to Shared Savings Program ACOs. We note that we received stakeholder feedback after the publication of the CY 2021 PFS final rule asking whether the CAHPS for MIPS minimum sampling thresholds would also apply to Shared Savings Program ACOs. From the feedback received, we determined that it was necessary to clarify that the minimum sampling threshold will apply.
As discussed previously in this section, minimum sampling thresholds are necessary to ensure that ACOs have an adequate sample size to ensure that the survey responses will be representative of the care furnished by the ACO clinicians. In addition, we do not want ACOs to be required to contract with a vendor to administer the survey if there is a high risk that the ACO will not have a sufficient sample size to generate a response rate for the survey that will be sufficient to reliably calculate a score for the CAHPS for MIPS survey. Aligning the minimum sampling thresholds for ACOs with the CAHPS for MIPS minimum sampling thresholds allows for consistency across all entities reporting the CAHPS for MIPS. Furthermore, we believe applying the CAHPS for MIPS minimum Start Printed Page 39265 sampling thresholds does not negatively impact Shared Savings Program ACOs because, as discussed below, only a few ACOs would potentially be impacted by these minimum sampling thresholds.
Based on the analysis of proxy data from 2020, nearly all ACOs will fall into the large size classification; that is, they will have 100 or more eligible clinicians that have assigned their billing to TINs participating in the ACO. To quantify the actual number of eligible clinicians associated with each ACO, we used the latest available reassignment and claims data from an internal file that is regularly created twice each performance year to identify the number of individual providers (NPIs) associated with each ACO's participant TINs. We conducted an analysis with proxy ACO sampling frames from 2020 and 44 ACOs fell into the medium size category of 25-99 eligible clinicians, and no ACOs were determined to have fewer than 24 eligible clinicians. Based on this analysis, we estimate that few ACOs would not be able to administer the CAHPS for MIPS due to sample size. All ACOs classified as medium-sized had more than 860 beneficiaries eligible for sampling. However, based on our analysis, one large-sized ACO would not have been able to administer the CAHPS survey for PY 2020, if we had required ACOs to administer a CAHPS for MIPS survey in performance year 2020 and these sampling rules had applied at that time because the sample size requirements would not have been met. Two additional large-sized ACOs were close to the minimum sampling threshold and would have been at risk for not being able to administer the CAHPS for MIPS survey for performance year 2020. We note that in both cases, these ACOs would have been eligible for CAHPS sampling based on their counts of assigned, quality-eligible[93] beneficiaries with 2 visits during the performance year; however, a large proportion (over 50 percent) of the beneficiaries assigned to these ACOs were residing in nursing homes and institutionalized beneficiaries are excluded from CAHPS for MIPS sampling.
Given that the minimum sampling sizes are set to ensure that groups or ACOs receive enough responses to be representative of the care their clinicians provide, we believe it is important that we should not burden ACOs that fall below the thresholds with the cost of hiring a vendor and fielding a CAHPS for MIPS survey that may not produce enough responses to calculate the CAHPS for MIPS score. Accordingly, we will inform any ACO that is at risk of falling below the minimum sampling threshold that it may not have enough beneficiaries to field a CAHPS for MIPS survey prior to the deadline for contracting with a CAHPS for MIPS survey vendor. An ACO that does not meet the minimum sampling threshold to administer the survey will not receive a score for the CAHPS for MIPS survey under the APP. When an ACO fails to meet the sampling threshold and is unable to administer the survey, the ACO's measure set will be scored accordingly, and the number of measures included in the calculation of the ACO's quality performance score will be reduced from 10 to 9 measures or from 6 to 5 measures in the APP for PY 2021. This means that the denominator used to calculate the quality score will be lower, such that an ACO that falls below the minimum threshold will not be penalized for its inability to administer a CAHPS for MIPS survey.
We seek comment on this clarification that the CAHPS for MIPS Minimum Sampling Thresholds also apply to Shared Savings Program ACOs.
In section IV.A.3.d. of this proposed rule, we discuss proposals related to the CAHPS for MIPS survey. In section IV.A.3.d, the term "performance period" is used to describe the time-period over which quality performance is assessed under MIPS, which is a full calendar year (January 1 through December 31) (except as otherwise specified for administrative claims-based measures in the MIPS final list of quality measures). In contrast, the Shared Savings Program uses the term "performance year" to describe each period for which ACOs' quality performance is assessed. For performance year 2021 and subsequent performance years, the relevant period is also the full calendar year. Therefore, while the terminology used in the Shared Savings Program and MIPS differs, the period of time for which quality performance is assessed under the APP is the same for both programs.
c. Amending the Reporting Requirements Under the APM Performance Pathway for Performance Years 2022 and 2023
In the CY 2021 PFS final rule, we finalized a change to the quality reporting requirements for purposes of the Shared Savings Program (85 FR 84720 through 84734). Effective for performance year 2021 and subsequent performance years, Shared Savings Program ACOs are required to report quality data via the APP. The quality reporting requirements under the Shared Savings Program align with the requirements that apply under the APP under the Quality Payment Program. Under this new approach, ACOs only need to report one set of quality metrics via the APP to satisfy the quality reporting requirements under both the Shared Savings Program and the MIPS. The quality measures reported via the APP for purposes of the MIPS Quality performance category will also be used to determine the quality performance of the ACO for purposes of determining eligibility for shared savings and calculating shared losses, where applicable. We refer readers to Table 40 of the CY 2021 PFS final rule (85 FR 84733) for a list of the measures included in the final APP measure set for performance year 2021.
Under the policies adopted in the CY 2021 PFS final rule:
- For performance year 2021, ACOs are required to report quality data via the APP, and can choose to actively report either the 10 measures under the CMS Web Interface or the 3 eCQM/MIPS CQM measures. In addition, ACOs are required to field the CAHPS for MIPS survey, and CMS will calculate 2 measures using administrative claims data.
- For performance year 2022 and subsequent performance years, ACOs are required to actively report quality data on the 3 eCQM/MIPS CQM measures via the APP. In addition, ACOs are required to field the CAHPS for MIPS survey, and CMS will calculate two measures using administrative claims data. All 6 measures will be included in the calculation of the ACO's quality performance score for purposes of the Shared Savings Program.
Our initial proposal in the CY 2021 PFS proposed rule included the removal of the CMS Web Interface collection type and a requirement that ACOs report quality data via the eCQM/MIPS CQM collection type starting in PY 2021. Public comments on our proposal expressed concerns about moving ACOs away from a collection type under which they report quality data on a sample of their assigned Medicare beneficiary population to a collection type that requires ACOs to report quality data on a broader, all-payer population.
For example, we received public comments expressing concerns about the increased burden of reporting eCQM/MIPS CQM measures, as ACOs would be responsible for aggregating the data across multiple ACO participant Taxpayer Identification Numbers (TINs) and submitting this data to CMS. In Start Printed Page 39266 addition, commenters expressed concerns about the increased cost of modifying existing electronic health record (EHR) technology, obtaining new EHR interfaces and aggregation tools, and updating performance dashboards. Also, there was concern that vendors and developers would need additional lead time to update and test systems, train staff and configure tools and measurement algorithms to aggregate data at an ACO level, in order to handle the wave of new entities reporting using eCQM/MIPS CQM measures.
In the CY 2021 PFS final rule (85 FR 84730), we noted that while the three eCQM/MIPS CQM measures are based on all payer data, we believe they are appropriate for assessing the quality of care furnished by ACOs, as required by section 1899(b)(3) of the Act. These measures focus on the management of chronic health conditions that are a high priority and have high prevalence among Medicare beneficiaries. To the extent that these conditions are also prevalent among other populations of patients that receive services from the eligible clinicians participating in an ACO, we believe it is relevant to consider the quality of care that is furnished by ACO participants across all of their patients as part of assessing the overall quality of care furnished by the ACO. We also noted that measuring care delivery to all patients is appropriate because improving care processes and practices is expected to improve care for all patients (for example, improvements to an electronic health record would be expected to improve care for all patients, not just Medicare patients). Additionally, we explained that CMS would not want ACOs participating in the Shared Savings Program to improve care for Medicare beneficiaries by reducing care quality for non-Medicare beneficiaries. Thus, looking at the overall quality of care furnished to all patients is consistent with the goal of improving care furnished by ACOs, by ensuring that care delivery is improving across all patients, rather than encouraging ACOs to focus disproportionately on improving measure performance for Medicare beneficiaries.
However, in light of the concerns raised during the public comment period for the CY 2021 PFS proposed rule, in the CY 2021 PFS final rule, we decided to extend the use of the CMS Web Interface as a collection type under the APP for performance year 2021. We believed that this additional year would allow ACOs the time needed to make the necessary changes to begin reporting quality data via eCQMs/MIPS CQMs.
Since the CY 2021 PFS final rule was issued, stakeholders have continued to express concerns about requiring ACOs to report eCQMs/MIPS CQMs via the APP, due to the cost of purchasing and implementing a system wide infrastructure to aggregate data from multiple ACO participant TINs and varying EHR systems. We note that for performance years beginning on or after January 1, 2019, ACOs are required to certify that they meet the CEHRT use requirements as specified at § 425.506(f). Specifically, ACOs in a track that:
- Does not meet the financial risk standard to be an Advanced APM must certify that the percentage of eligible clinicians participating in the ACO that use CEHRT to document and communicate clinical care to their patients or other health care providers meets or exceeds 50 percent; or
- Meets the financial risk standard to be an Advanced APM must certify that the percentage of eligible clinicians participating in the ACO that use CEHRT to document and communicate clinical care to their patients or other health care providers meets or exceeds the threshold established under § 414.1415(a)(1)(i).
We define CEHRT for purposes of the Shared Savings Program at § 425.20 and the term has the same meaning as provided under § 414.1305 for purposes of the Quality Payment Program. For 2019 and subsequent years, CEHRT is defined to mean EHR technology that meets the 2015 Edition Base EHR definition and that has been certified to the 2015 Edition health IT certification criteria necessary to report on applicable objectives and measures specified for the MIPS Promoting Interoperability performance category and includes clinical quality measure certification criteria that support the calculation and reporting of clinical quality measures that can be electronically accepted by CMS. Health IT certified to clinical quality measure certification criteria can help to support ACOs' efforts to meet quality measure reporting requirements.
According to a recent National Association of Accountable Care Organizations (NAACOS) survey[94] regarding the readiness of ACOs to report eCQM/MIPS CQM data, NAACOS noted that 77 percent of respondents indicated they do not have the infrastructure in place to aggregate data on behalf of their ACO participant TINs on quality performance across all payers starting in 2022. On average, an ACO has 36 ACO participant TINs and the largest Shared Savings Program ACO has 436 ACO participant TINs. The NAACOS survey also noted that almost 40 percent of ACOs have more than 15 EHR systems. Additionally, stakeholders have raised privacy and other concerns about reporting eCQMs/MIPS CQMs on all-payer populations, rather than a sample of assigned Medicare beneficiaries, as required for the CMS web interface measures. These concerns focus on perceived HIPAA Privacy Rule limitations on sharing protected health information (PHI) for non-Medicare beneficiaries with an ACO.
Furthermore, we have heard concerns from ACOs that are acting as business associates of their health care provider ACO participants regarding their ability to update their business associate agreements (BAAs) to include the PHI of patients who are not covered by Medicare. Stakeholders have indicated that current agreements may only address sharing the PHI of Medicare beneficiaries. Therefore, they have raised concerns that reporting all payer eCQMs would violate their BAAs as well as the HIPAA Privacy Rule business associate requirements at 45 CFR 164.502(a) and 164.504(e).
To report eCQMs successfully, health care providers must adhere to the requirements identified by the CMS quality program in which they intend to participate. For purposes of reporting eCQMs/MIPS CQMs under MIPS, clinicians are expressly required under § 414.1340(a) to submit data on the applicable percentage of patients that meet the measure's denominator criteria, regardless of payer. Under § 414.1380(b)(1)(i)(B)(1)(iii), failure to meet this requirement may result in the clinician receiving zero points for the measure, which may adversely impact their MIPS final score and payment adjustment. As such, we believe the disclosure of all-payer data to CMS as required by § 414.1340(a) would be permitted by the HIPAA Privacy Rule under the provision that permits disclosures of PHI as "required by law."[95] Under this provision, a HIPAA covered entity, or its business associate when authorized by its BAA, may use or disclose PHI to the extent that such use or disclosure is required by law and the use or disclosure complies with and is limited to the relevant requirements of such law. We note that the HIPAA Privacy Rule minimum necessary Start Printed Page 39267 standard does not apply to uses or disclosures that are required by law.[96]
Furthermore, the HIPAA Privacy Rule generally permits a covered entity to disclose PHI to a business associate and to allow a business associate to create, receive, maintain, or transmit PHI on its behalf, provided that the parties have a BAA that meets the requirements of 45 CFR 164.504(e) and permits the business associate to use or disclose PHI only as permitted or required by its BAA or as required by law. The BAA must, among other things, establish the permitted and required uses and disclosures of PHI by the business associate. ACO providers and suppliers that are MIPS eligible clinicians will need to review and update any relevant BAAs as necessary to include the disclosure of all-payer data, in addition to data for Medicare beneficiaries to the ACO. We believe that ACO providers/suppliers should be able to update those agreements, in consultation with their legal counsel as necessary, to reflect the need to share data for patients covered by all payers with the ACO, in order to permit the ACO to completely and accurately report data on eCQM/MIPS CQM measures consistent with the MIPS reporting requirements.
In addition, we want to correct a statement from the CY 2021 PFS final rule (85 FR 84730). In that final rule, we provided an example of how an ACO could aggregate eCQM measure data. In this example, we stated that an ACO could, on behalf of its ACO participants, combine the results from all the ACO participant TIN QRDA 3 files, by adding numerators, denominators, etc. and create an aggregate QRDA 3 file (or other compliant file format) and submit as an ACO to CMS. However, this example did not take into account the potential for duplicate patients for a given measure across the ACO participant TINs within an ACO. It also did not take into account that two of the three eCQMs require that the most recent blood pressure or HgbA1c be captured to assess performance for those measures. Accordingly, we want to clarify that an ACO that submits eCQM quality data to CMS must de-duplicate the patient level measures data across its ACO providers/suppliers to ensure that the aggregated QRDA 3 file that is submitted to CMS incorporates only quality data that meets the intent of the measure.
Based on the feedback we received, we are convinced that ACOs and their ACO participants, Health IT vendors, and developers need additional time to prepare for reporting all-payer eCQM/MIPS CQM measures. We believe the updates we are proposing to the reporting requirements under the APP are responsive to stakeholder requests to delay the requirement that ACOs report all-payer eCQM/MIPS CQM measures, while still providing incentives for ACOs that are ready report to eCQM/MIPS CQM measures. As discussed in section IV.A.3.d.(1)(d) of this proposed rule, we are proposing to extend the CMS Web Interface as a collection type for the Quality Payment Program for PY 2022 for MIPS Groups, Virtual groups, and Shared Savings Program ACOs reporting under the APP. For PY 2023, we are proposing that the CMS Web Interface would be a collection type under the APP only for Shared Savings Program ACOs. Accordingly, we are proposing to modify the quality measure set that must be reported by Shared Savings Program ACOs under the APP, as discussed in this section and section IV.A.3.c.(2)(a) of this proposed rule.
To further address stakeholder feedback about ACOs' readiness to report all-payer measures, and in particular the concerns regarding aggregation of eCQM/MIPS CQM data across multiple ACO participant TINs using multiple different electronic health record (EHR) technology, while also providing incentives for ACOs to take the steps necessary to report all-payer measures, we are proposing that:
- For performance year 2022: An ACO would be required to report on either:
++ The ten CMS Web Interface measures and administer a CAHPS for MIPS survey and CMS would calculate the two claims based measures included under the APP, or
++ The three eCQM/MIPS CQM measures and administer a CAHPS for MIPS survey and CMS would calculate the two claims based measures included under the APP. If an ACO selects this option, meets the data completeness requirement at § 414.1340 and the case minimum requirement at § 414.1380 for all three eCQM/MIPS CQM measures, and achieves a quality performance score equivalent to or higher than the 30th percentile of the performance benchmark on at least one measure in the APP measure set, the ACO would meet the quality performance standard used to determine eligibility for shared savings and to avoid maximum shared losses, if applicable, for that performance year. We believe that allowing ACOs that report eCQM/MIPS CQM measures to meet the quality performance standard if they achieve a score that is equivalent to or higher than the 30th percentile benchmark on one measure in the APP measure set would provide an incentive to ACOs to report the eCQM/MIPS CQM measures, while allowing them time to gauge their performance on the eCQM/MIPS CQM measures before full reporting of these measures is required beginning in PY 2024. If an ACO chooses this option, its performance on all three eCQM/MIPS CQM measures would be used for purposes of MIPS scoring under the APP. If an ACO decides to report both the ten CMS Web Interface measures and the three eCQM/MIPS CQM measures, it will receive the higher of the two quality scores for purposes of the MIPS Quality performance category.
Please note, as indicated in Tables 25 and 40, three of the CMS Web Interface measures (Statin Therapy for the Prevention and Treatment of Cardiovascular Disease (Quality ID# 438); Depression Remission at Twelve Months (Quality ID# 370), and Preventive Care and Screening: Tobacco Cessation: Screening and Cessation Intervention (Quality ID# 236)) do not have benchmarks for performance year 2022, and therefore, will not be scored. However, these measures are required to be reported in order to complete the CMS Web Interface dataset. Based on the ACO's chosen reporting option, either 6 (three eCQMs/MIPS CQMs + two claims based measures + CAHPS for MIPs Survey measure) or 10 measures (seven CMS Web Interface measures + two claims based measures + CAHPS for MIPS Survey measure) will be included in the calculation of the ACO's quality performance score.
If an ACO does not report any of the ten CMS Web Interface measures or any of the three eCQM/MIPS CQM measures and does not administer a CAHPS for MIPS survey under the APP, the ACO will not meet the quality performance standard.
- For performance year 2023: The ACO would be required to report on either:
++ The ten CMS Web Interface measures, at least one eCQM/MIPS CQM measure, and administer a CAHPS for MIPS survey and CMS would calculate the two claims-based measures included under the APP or
++ The three eCQM/MIPS CQM measures and administer a CAHPS for MIPS survey and CMS would calculate the two claims based measures included under the APP. If an ACO selects this option, meets the data completeness requirement at § 414.1340 and the case minimum requirement at § 414.1380 for all three eCQM/MIPS CQM measures, and achieves a quality performance score equivalent to or higher than the 30th percentile of the performance benchmark on at least one measure in Start Printed Page 39268 the APP measure set, the ACO would meet the quality performance standard used to determine eligibility for shared savings and to avoid maximum shared losses, if applicable, for that performance year. If an ACO chooses this option, its performance on all three eCQM/MIPS CQM measures would be used for purposes of MIPS scoring under the APP. If an ACO decides to report both the ten CMS Web Interface measures and the three eCQM/MIPS CQM measures, it will receive the higher of the two quality scores for purposes of the MIPS Quality performance category.
If an ACO does not report at least one eCQM/MIPS CQM measure in the APP measure set, the ACO would not meet the quality performance standard.
- For performance year 2024 and subsequent performance years: The ACO would be required to report the three eCQM/MIPS CQM measures and administer a CAHPS for MIPS survey and CMS would calculate the two claims based measures included under the APP. If an ACO does not report any of the three eCQM/MIPS CQM measures and does not administer a CAHPS for MIPS survey under the APP, the ACO would not meet the quality performance standard.
Finally, for the first performance year of an ACO's first agreement period under the Shared Savings Program, if the ACO meets MIPS data completeness and case minimum requirements we are proposing that the ACO would meet the quality performance standard, if:
- For performance year 2022. The ACO reports the ten CMS Web Interface measures or the three eCQM/MIPS CQM measures and administers a CAHPS for MIPS survey under the APP.
- For performance year 2023. The ACO reports the ten CMS Web Interface measures and at least one eCQM/MIPS CQM measure or reports the three eCQM/MIPS CQM measures, and administers a CAHPS for MIPS survey under the APP.
- For performance year 2024 and subsequent performance years. The ACO reports on the three eCQM/MIPS CQM measures and administers a CAHPS for MIPS survey under the APP.
The proposed changes are summarized in Table 24. We are proposing changes to the regulation at § 425.512(a) to reflect these changes to the quality reporting requirements for performance years 2022 and 2023. We note that as part of these proposed changes, we are also proposing to update the provision at § 425.512(a)(2), which applies to new ACOs that are in the first performance year of their first agreement under the Shared Savings Program and are able to meet the quality performance standard under the Shared Savings if they completely and accurately report all required measures via the APP, to reflect the proposed changes to the quality reporting requirements for performance years 2022 and 2023.
Start Printed Page 39269

We seek comment on these proposed updates to the reporting requirements under the APP for performance year 2022 and subsequent years. In addition, we are seeking comment on whether we should extend the CMS Web Interface collection type for more than the 2 years proposed above. We believe the proposed 2-year extension would provide sufficient time to allow ACOs and their ACO participants to take the necessary steps to address the concerns raised by stakeholders, but are interested in hearing if stakeholders believe additional time would be needed to enable ACOs and their ACO participants to prepare for eCQM/MIPS CQM reporting.
(1) Solicitation of Comments on Addressing Health Disparities and Promoting Health Equity
We note that we continue to believe the move to eCQM/MIPS CQM measures is the appropriate next step for ACO quality measurement. For many years, ACOs have only reported on a sample of their assigned Medicare beneficiary population, as the CMS Web Interface Start Printed Page 39270 only requires that ACOs report on 248 consecutively ranked beneficiaries for each measure in the CMS Web Interface measure set. As we move forward with reporting under the APP and increasing the quality performance standard as described above, we believe that looking at the overall quality of care furnished to all patients is consistent with the goal of improving care furnished by ACOs by ensuring that care delivery is improving across all patients, rather than encouraging ACOs to focus disproportionately on improving measure performance for Medicare beneficiaries. We also believe that assessing Shared Savings Program ACO quality performance on a broader population can have a positive impact on the quality of care for all groups, including Medicare beneficiaries. We also expect the transition to all-payer eCQM/MIPS CQM measures will help to address health disparities and promote health equity by promoting a single standard of care across all patients receiving care from practices participating in Shared Savings Program ACOs regardless of location or racial/ethnic group. However, we are seeking comments and recommendations on how ACOs can utilize their resources to ensure that patients, regardless of racial/ethnic group, geographic location and/or income status, have access to equal care and how ACOs can improve the quality of care provided to certain communities, while addressing the disparities that currently exist in healthcare. We are also seeking comments and recommendations on how we can encourage health care providers serving vulnerable populations to participate in ACOs and other value-based care initiatives, including whether any adjustments should be made to quality measure benchmarks to take into account ACOs serving vulnerable populations.
(2) Solicitation of Comments on Feasibility of TIN Level Reporting and Sampling for eCQMs/MIPS CQMs
To assist ACOs in reporting eCQM/MIPS CQM measures and to address concerns regarding data aggregation across multiple TINs with multiple different EHR systems, we are seeking comment on allowing ACO providers/suppliers to submit eCQMs/MIPS CQM measures to CMS at the ACO participant TIN level. CMS would then calculate/aggregate the TIN level quality data to create an ACO level score. For example, CMS could calculate the average of the decile scores for each measure for each TIN within the ACO to create an aggregate measure level score for the ACO. Alternatively, CMS could calculate an ACO-level numerator for each measure (sum of performance met across TINs within the ACO) and an ACO-level denominator (sum of the met and performance not met across TINs within the ACO), then divide the two—numerator/denominator × 100—to obtain the ACO-level performance rate. We seek comment on these two potential approaches as well as any other suggested approaches to the aggregation of ACO participant TIN level quality data at the ACO level.
While we continue to believe that reporting on all-payer data is important to improve the quality of care furnished to all patients, including Medicare beneficiaries, we have heard from stakeholders that CMS should allow ACOs to report the eCQM/MIPS CQM measures for a smaller, more defined beneficiary population rather than the all-payer population, to serve as an intermediary step to reporting on all patients. Thus, while we believe that the move to all-payer measures is the next step in quality reporting, we acknowledge that the denominator of patients for a given quality measure for an ACO may be significantly higher, depending on ACO size and composition, than for MIPS groups. Therefore, we seek comment on how stakeholders would envision CMS determining an appropriate beneficiary population. For example, should ACOs report on a small sample size similar to the sample size for the CMS Web Interface? Should CMS broaden the beneficiary sample to include all assigned beneficiaries that meet the denominator for a given measure? Should CMS provide ACOs with a bigger sample size, larger than the size that has historically been used for CMS Web Interface but smaller than all the assigned beneficiaries that meet the denominator for a given measure? Or should CMS develop ACO-level eCQM/MIPS CQM measure sampling specifications? We seek comment on whether CMS should create a specific sampling methodology for ACOs, alternate sampling methodologies that could be used, as well as phase-in and tiered implementation strategies.
(3) Comment Solicitation for Reporting Options for Specialist Providers Within an ACO
We have also heard from stakeholders that the population health/primary care focused measures in the APP are not applicable for specialist providers within an ACO. In order to address measure applicability for specialist providers, we are seeking comment on allowing ACO participant TINs to report either the eCQM/MIPS CQM measures in the APP measure set at the TIN level or the applicable MIPS Value Pathways, including how APP and MIPS Value Pathway data reported at the ACO participant TIN level could be aggregated in order to assess ACO quality performance. In addition, we seek input on the role specialists play in ACOs and what specialty measures in the current eCQM or MIPS CQM measure set should be considered for inclusion in the Shared Savings Program quality measure set in future performance years. Alternatively, how could the existing APP measure set be used or modified to reinforce the role of specialists in ACO population health strategies?
(4) Updates to the APM Performance Pathway (APP) Measure Set
In section IV.A.3.(c) of this proposed rule, we are proposing to replace the Risk-Standardized, All-Cause Unplanned Admissions for Multiple Chronic Conditions for ACOs (MCC for ACOs measure) with the Risk Standardized, All-Cause Unplanned Admissions for Multiple Chronic Conditions for MIPS (MCC for MIPS measure) for performance year 2022. We are proposing to remove the MCC for ACOs measure from the APP measure set in order to reduce the potential for confusion around performance scores and feedback for MIPS eligible clinicians who might otherwise have been scored on both measures with differing results. This proposed change would continue the transition towards alignment of the quality measures reported by MIPS eligible clinicians who are not participants in APMs, such as the Shared Savings Program, and those who are, as discussed in the CY 2021 PFS final rule (85 FR 84720). For a detailed description of this proposal refer to section IV.A.3.(c) of this proposed rule.
By removing the MCC for ACOs measure and aligning the quality measure set for the Shared Savings Program with MIPS, we would have the opportunity to align quality measurement between CMS programs. In addition, given that the Hospital-Wide, 30-day, All-Cause Unplanned Readmission (HWR) Rate for MIPS Eligible Clinician Groups measure calculated as part of the APP looks at an ACO's all Medicare population rather than just the ACO's assigned beneficiary population, we believe the proposal to move to the MCC for MIPS measure would be consistent with the approach under the APP of assessing, measuring and improving quality of care across a broader population of patients. Further details on the specifications for the MCC Start Printed Page 39271 for MIPS measure can be found in Table A-5 in Appendix A of this proposed rule.
Please see Table 25 for the proposed APP measure set that would be reported by Shared Savings Program ACOs for PY 2022 and subsequent performance years.

d. Shared Savings Program Quality Performance Standard
(1) Proposal To Freeze the Quality Performance Standard at the 30th Percentile of All MIPS Quality Performance Category Scores for Performance Year 2023
The quality performance standard is the minimum performance level ACOs must achieve in order to be eligible to share in any savings earned, avoid maximum shared losses under certain payment tracks, and avoid quality-related compliance actions. As noted above, in the CY 2021 PFS final rule we finalized a gradual phase in of the revised quality performance standard. Specifically, an ACO would meet the quality performance standard if: Start Printed Page 39272
- For performance years 2021 and 2022, the ACO achieves a quality performance score that is equivalent to or higher than the 30th percentile across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring; and
- For performance year 2023 and subsequent performance years, the ACO achieves a quality performance score that is equivalent to or higher than the 40th percentile across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring (85 FR 84735).
We finalized this phase-in approach to address the concerns raised by commenters about the limited time for ACOs to gain familiarity with the new quality reporting requirements under the APP and potential challenges in meeting the new quality performance standard, as well as concerns regarding the shift from a domain-based scoring approach to the original proposal to require an ACO to achieve an overall quality score equivalent to the 40th percentile across all MIPS quality performance category scores starting in PY 2021. In conjunction with the decision to phase-in the quality performance standard, we also adopted a phase-in of the reporting requirements under the APP for Shared Savings Program ACOs, as described previously.
In the CY 2021 PFS final rule, we also discussed the potential impact of the final policies on ACO quality performance. We projected that, absent an improvement in quality performance by ACOs, roughly 1-in-5 ACOs, or approximately 20 percent of ACOs, could fall below the 40th percentile MIPS Quality performance category score by performance year 2023, and would not be eligible to share in savings or would owe maximum shared losses, if applicable (85 FR 85007 through 85008). For the CY 2021 rulemaking we conducted analysis, in order to understand better how well ACOs might perform once the CMS Web Interface is no longer an available collection type. The analysis simulated ACO performance on eCQM or MIPS CQM measures, using 2018 and 2019 quality data submitted via the CMS Web Interface. Based on the analysis of the 2018 and 2019 data there were two differing estimates of the number of ACOs that would not meet the quality performance standard. The estimated percent of Shared Savings Program ACOs falling below the 40th percentile MIPS Quality performance category score was 6.5 percent based on a simulation using 2018 data and 22.9 percent based on a simulation using 2019 data.
In the CY 2021 PFS final rule, we indicated that we would continue to monitor emerging performance to determine the impact of a measured increase to the quality performance standard. We stated that we might revisit the policy in future rulemaking in order to promote an attainable standard and degree of improvement based on initial performance under the new methodology (85 FR 85008). If our proposal to extend the availability of the CMS Web Interface as a reporting mechanism under the APP is finalized, performance year 2024 would be the first year that all ACOs would be required to report all three eCQM/MIPS CQM measures and would have no option to report data via the CMS Web Interface, with data submission beginning in 2025. However, we have heard from some ACOs that they are beginning to update their systems and workflows to further develop their capacity for reporting on the eCQM/MIPS CQM measure set in performance year 2022. These ACOs are gearing up for all-payer reporting and are performing self-tests in order to understand their performance on the 3 eCQM/MIPS CQM measures. It is also important to note that some ACOs will likely report on eCQM/MIPS CQM measures beginning with the 2021 performance year. Therefore, there is an opportunity for ACOs to gain some familiarity with meeting the requirements for these measures starting in performance year 2021. Even with all of these contingencies in place and our proposals to phase-in reporting of the eCQM/MIPS CQM measures, we still recognize that transitioning to eCQM/MIPS CQM quality data reporting and aggregation may come with unforeseen data collection and/or system operational issues. Therefore, we have concluded that it would be appropriate to freeze the quality performance at the 30th percentile MIPS Quality performance category score for an additional year; and to raise the quality performance standard in conjunction with the transition to reporting of the three eCQM/MIPS CQMs measures by all ACOs in PY 2024. Although this increase in the quality performance standard to the 40th percentile would coincide with the first full year of eCQM/MIPS CQM quality reporting, we believe our proposal to extend the CMS Web Interface for an additional 2 years and to allow for a gradual phase in of reporting the three eCQMs/MIPS CQMs is responsive to stakeholder concerns related to the transition to eCQM/MIPS CQM measures and the need for data aggregation and would provide time for both ACOs and EHR vendors to put in place processes and systems, such that ACOs will be well positioned to report eCQM/MIPS CQMs by performance year 2024.
As discussed earlier in this proposed rule, as part of this gradual phase-in to full reporting of eCQMs/MIPS CQMs, we are proposing to include incentives to encourage early adoption of full eCQM/MIPS CQM reporting prior to performance year 2024. As part of the phase-in, and in order to transition ACOs to reporting all-payer eCQM/MIPS CQM measures, for performance year 2023 we would require an ACO to report at least one eCQM/MIPS CQM measure (that meets data completeness and case minimum requirements) in addition to the CMS Web Interface measures in order to meet the quality performance standard. In addition, we are also proposing for both performance year 2022 and performance year 2023 that ACOs that elect to report all three eCQM/MIPS CQM measures and meet the data completeness requirement and case minimum requirement for all three measures would meet the quality performance standard if they achieve a quality performance score equivalent to or higher than the 30th percentile of the performance benchmark on at least one measure in the APP measure set.
We believe that our proposal to freeze the quality performance standard at the 30th percentile for an additional year, is consistent with the requirement in the statute that CMS increase the quality performance standard overtime. There are two ways to increase the quality performance standard: (1) By increasing the threshold for the quality performance standard, and (2) by moving to all payer measure populations that ACOs are required to report for purposes of Shared Savings Program quality. In this proposed rule, we are proposing to do both by requiring that ACOs begin the transition to reporting all-payer measures before increasing the quality performance standard starting in performance year 2024.
Therefore, we propose to freeze the quality performance standard at the 30th percentile MIPS Quality performance category score for PY 2023, and to establish incentives to encourage ACOs to begin the transition to eCQM/MIPS CQM measure reporting in PY 2022 and PY 2022. This would mean that for all Shared Savings Program ACOs, CMS would designate the quality performance standard as the ACO reporting via the APP established under § 414.1367 of this chapter and for:
- Performance year 2022, if an ACO reports: Start Printed Page 39273
++ The 10 CMS Web Interface measures and achieves a quality performance score that is equivalent to or higher than the 30th percentile across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring, or
++ The three eCQM/MIPS CQM measures, meeting the data completeness requirement at § 414.1340 of this chapter and case minimum requirement at § 414.1380 of this chapter for all three measures, and achieves a quality performance score equivalent to or higher than the 30th percentile of the performance benchmark on at least one measure in the APP measure set.
If the ACO does not report any of the 10 CMS Web Interface measures or any of the three eCQM/MIPS CQM measures and does not administer a CAHPS for MIPS survey, the ACO would not meet the quality performance standard.
- Performance year 2023, if an ACO reports:
++ The 10 CMS Web Interface measures and at least one eCQM/MIPS CQM measure, and achieves a quality performance score that is equivalent to or higher than the 30th percentile across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring, or
++ The three eCQM/MIPS CQM measures, meeting the data completeness requirement at § 414.1340 of this chapter and case minimum requirement at § 414.1380 of this chapter for all three measures, and achieves a quality performance score equivalent to or higher than the 30th percentile of the performance benchmark on at least one measure in the APP measure set.
If the ACO does not report at least one eCQM/MIPS CQM, the ACO would not meet the quality performance standard.
Our proposal to extend the CMS Web Interface collection type and phase-in the reporting of the eCQMs/MIPS CQMs provides the transition time that stakeholders have requested in order to be ready to submit aggregated data on eCQMs/MIPS CQMs. We believe that the transition to the all-payer eCQM/MIPS CQM measures is the future of Shared Savings Program quality performance assessment and that ACOs are well-positioned to impact the quality of care across an all-payer population not just the Medicare population given their redesigned care processes and quality improvement activities. Ultimately, we believe that the transition time afforded ACOs by extending the availability of the CMS Web Interface as a collection type, in conjunction with the incentives to encourage early adoption of eCQM/MIPS CQM reporting, should allow ACOs to prepare for full reporting of eCQMs/MIPS CQMs as well as the incremental increase in the quality performance standard to the 40th percentile of MIPS Quality performance category score in PY 2024. Accordingly, we are proposing that for PY 2024 and all subsequent performance years, CMS would designate the quality performance standard for all Shared Savings Program ACOs, with the exception of ACOs in the first performance year of their first agreement period under the Shared Savings Program, as the ACO reporting quality data via the APP established under § 414.1367 according to the method of submission established by CMS and achieving a quality performance score that is equivalent to or higher than the 40th percentile across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring. We also propose to revise the regulation at § 425.512 to reflect the extended phase-in of the ACO quality performance standard.
As we explained in the CY 2021 PFS final rule, this approach to phasing in the new, higher quality performance standard is consistent with the statutory requirement in section 1899(b)(3)(C) of the Act, which requires the Secretary to establish quality performance standards to assess the quality of care furnished by ACOs and to seek to improve the quality of care furnished by ACOs over time by specifying higher standards, new measures or both for purposes of assessing such quality of care. We believe that even though we are proposing to freeze the quality performance standard at the 30th percentile MIPS Quality performance category score for an additional year, we will still be holding ACOs to a higher standard than the previous quality standard, which merely required ACOs to achieve the 30th percentile relative to national benchmarks on one measure in each domain. We recognize the change from the CMS Web Interface collection type to the eCQM or MIPS CQM collection type, coupled with this higher quality performance standard, adds complexity for ACOs as they may need to utilize new approaches to combining data across EHR systems to allow for a new data submission type, as well as aggregating ACO participant data for submission to CMS. However, we believe that with this proposal to delay the increase in the quality performance standard, coupled with the proposal to extend the CMS Web Interface through PY2023, with incentives for e early adoption of eCQM/MIPS CQM reporting, ACOs will have ample time to prepare for the transition to full eCQM/MIPS CQM reporting in PY 2024 and the incremental increase in the quality performance standard to the 40th percentile MIPS Quality performance category score. We also believe this proposed timeline for phasing in the new quality performance requirements under the Shared Savings Program would signal to ACOs, EHR vendors, and other stakeholders that eCQM/MIPS CQM reporting is the path forward for the Shared Savings Program and clearly establish the standard that ACOs would need to achieve in order to be eligible to share in maximum savings and avoid owing the maximum shared losses, if applicable.
We also considered the possibility of extending the freeze of the Shared Savings Program quality performance standard at the 30th percentile MIPS Quality performance category score for performance year 2024. This alternative would delay the incremental increase in the quality performance standard until all ACOs have at least one year of experience in reporting data for all three eCQM/MIPS CQM measures. This delay would allow ACOs additional time to gain experience reporting on the eCQM/MIPS CQM measures and also provide CMS with more information on ACO performance on all-payer measures and the ability of ACOs to aggregate data across multiple EHR systems and multiple practices, in order to inform the quality performance standard in outlying years. However, for the reasons described previously, we believe the timeline we are proposing for phasing in the new quality reporting and quality performance requirements will provide ample time for ACOs to prepare to meet these new requirements while also satisfying the statutory requirement that we seek to improve the quality of care furnished by ACOs over time.
We seek comment on our proposal to freeze the Shared Savings Program quality performance standard at the 30th percentile across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring for PY 2023 and to increase the quality performance standard to the 40th percentile across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring starting in PY 2024. In addition, we seek comment on the alternative of freezing the Shared Savings Program quality performance standard at the 30th percentile across all MIPS Quality Start Printed Page 39274 performance category scores, excluding entities/providers eligible for facility-based scoring for PYs 2023 and 2024.
(2) Comment Solicitation on Publicly Displaying Prior Year Performance Scores That Equate to the 30th or 40th Percentile Across MIPS Quality Performance Category Scores
Stakeholders have expressed concerns regarding the lack of information on the level of quality performance that would equate to the 30th or 40th percentile MIPS Quality performance category score and that would enable an ACO to be eligible to share in savings or to avoid maximum shared losses, if applicable. These stakeholders have expressed concern that these data are not publicly available prior to the start of a performance year and that they do not believe that ACOs have a way of determining what quality score they would need to achieve to meet the quality performance standard. For a given performance year, the 30th or 40th percentile MIPS Quality performance category score is calculated based on the distribution across all MIPS Quality performance category scores, excluding entities/providers eligible for scoring for facility-based scoring, only once MIPS final scoring is complete.
Therefore, there is no information that can be provided prior to or during the performance year. However, we note that for performance year 2018 the MIPS Quality performance category score at the 30th percentile was equivalent to 83.9 and the MIPS Quality performance category score at the 40th percentile was equivalent to 93.3. For performance year 2019 the MIPS Quality performance category score at 30th percentile was equivalent to 87.9 and the MIPS Quality performance category score at the 40th percentile was equivalent to 95.7.
We seek comment on whether publicly displaying prior year performance scores that equate to the 30th or 40th MIPS Quality performance category scores would help to address ACOs' concerns regarding the lack of advance information regarding the quality performance score they must meet in order to satisfy the quality performance standard under the Shared Savings Program. We also seek comment on other ways we could address these concerns.
e. Revisions to the Extreme and Uncontrollable Circumstances Policy
In the CY 2021 PFS final rule (85 FR 84744 through 84747), we updated the extreme and uncontrollable circumstances policy for performance year 2021 and subsequent performance years to align with the gradual phase in of the revised quality performance standard. Specifically, we finalized that for:
- PY 2021 and PY 2022, the minimum quality performance score for an ACO affected by an extreme and uncontrollable circumstance during the performance year, including the applicable quality data reporting period for the performance year, will be set equal to the 30th percentile MIPS Quality performance category score. If the ACO is able to report quality data and meets the MIPS data completeness and case minimum requirements, we will use the higher of the ACO's quality performance score or the 30th percentile MIPS Quality performance category score. If an ACO is unable to report quality data and meet the MIPS Quality data completeness and case minimum requirements due to an extreme and uncontrollable circumstance, we will apply the 30th percentile MIPS Quality performance category score.
- PY 2023, the minimum quality performance score for an ACO affected by an extreme and uncontrollable circumstance during the performance year, including the applicable quality data reporting period for the performance year, will be set equal to the 40th percentile MIPS Quality performance category score. If the ACO is able to report quality data and meets the MIPS data completeness and case minimum requirements, we will use the higher of the ACO's quality performance score or the 40th percentile MIPS Quality performance category score. If an ACO is unable to report quality data and meet the MIPS Quality data completeness and case minimum requirements due to an extreme and uncontrollable circumstance, we will apply the 40th percentile MIPS Quality performance category score (85 FR 84746).
As discussed in section III.J.1.d. of this proposed rule, we are proposing to make changes to the quality performance standard for Shared Savings Program ACOs by freezing the quality performance standard at the 30th percentile for PY 2023. Therefore, we are also proposing to update the extreme and uncontrollable circumstances policy under the Shared Savings Program consistent with the proposal in section III.J.1.d. of this proposed rule. Specifically, we propose to set the minimum quality performance score for an ACO affected by an extreme and uncontrollable circumstance during performance year 2023, including the applicable quality data reporting period for the performance year, to equal the 30th percentile MIPS Quality performance category score across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring, for the relevant performance year.
For performance years 2021 and 2022, if the ACO is able to report quality data via the APP and meets the MIPS data completeness and case minimum requirements, we would use the higher of the ACO's MIPS Quality performance category score or the 30th percentile MIPS Quality performance category score. If the ACO is unable to report quality data and meet the MIPS Quality data completeness and case minimum requirements due to an extreme and uncontrollable circumstance, we would apply the 30th percentile MIPS Quality performance category score.
For performance year 2023, if the ACO is able to report quality data via the APP, including at least one eCQM/MIPS CQM measure, and meets data completeness and case minimum requirements, CMS will use the higher of the ACO's quality performance score or the equivalent of the 30th percentile MIPS Quality performance category score. If the ACO is unable to report quality data and meet the MIPS Quality data completeness and case minimum requirements due to an extreme and uncontrollable circumstance, we would apply the 30th percentile MIPS Quality performance category score.
Similarly, we propose that for performance year 2024 and subsequent years, the minimum quality performance score for an ACO affected by an extreme and uncontrollable circumstance during the performance year, including the applicable quality data reporting period for the performance year, would be set equal to the 40th percentile MIPS Quality performance category score across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring, for the relevant performance year. If the ACO is able to report quality data via the APP and meets the MIPS data completeness and case minimum requirements, we would use the higher of the ACO's MIPS Quality performance category score or the 40th percentile MIPS Quality performance category score. If the ACO is unable to report quality data and meet the MIPS Quality data completeness and case minimum requirements due to an extreme and uncontrollable circumstance, we would apply the 40th percentile MIPS Quality performance category score. We believe these proposed updates are appropriate to align with the proposed changes to the quality performance standard in section III.J.1.d. of this proposed rule, and would also allow impacted ACOs to be Start Printed Page 39275 eligible to share in savings at their maximum sharing rate or to avoid maximum shared losses, if applicable. We also propose to make conforming changes to the Shared Savings Program regulations at § 425.512(b) to reflect these proposed revisions to the extreme and uncontrollable circumstances policy.
We seek comment on these proposed revisions to the extreme and uncontrollable circumstances policy.
2. Revisions to the Definition of Primary Care Services Used in Shared Savings Program Beneficiary Assignment
a. Background
Section 1899(c)(1) of the Act, as amended by the CURES Act and the Bipartisan Budget Act of 2018, provides that for performance years beginning on or after January 1, 2019, the Secretary shall assign beneficiaries to an ACO based on their utilization of primary care services provided by a physician who is an ACO professional and all services furnished by Rural Health Clinics (RHCs) and Federally Qualified Health Centers (FQHCs). However, the statute does not specify a list of services considered to be primary care services for purposes of beneficiary assignment.
In the November 2011 final rule (76 FR 67853), we established the initial list of services, identified by Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding System (HCPCS) codes, that we considered to be primary care services. In that final rule, we indicated that we intended to monitor CPT and HCPCS codes and would consider making changes to the definition of primary care services to add or delete codes used to identify primary care services, if there were sufficient evidence that revisions were warranted. We have updated the list of primary care service codes in subsequent rulemaking to reflect additions or modifications to the codes that have been recognized for payment under the Medicare PFS and to incorporate other changes to the definition of primary care services for purposes of the Shared Savings Program.
In the June 2015 final rule (80 FR 32746 through 32748), we expanded the definition of primary care services to include two transitional care management (TCM) codes (CPT codes 99495 and 99496), and one chronic care management (CCM) code (CPT code 99490). As discussed in the final rule, the TCM codes were established to pay a patient's physician or practitioner to coordinate the patient's care in the 30 days following a hospital or SNF stay. Including these codes in the definition of primary care services reflects our belief that the work of community physicians and practitioners in managing a patient's care following discharge from a hospital or nursing facility (NF) to ensure better continuity of care for these patients and help reduce avoidable readmissions is a key aspect of primary care.
In the CY 2016 PFS final rule (80 FR 71270 through 71273), we revised the definition of primary care services to exclude services billed under CPT codes 99304 through 99318, containing the place of service 31 modifier specifying that the service was furnished in a skilled nursing facility (SNF). We also revised the definition of primary care services to include claims submitted by Electing Teaching Amendment (ETA) hospitals.
In the CY 2018 PFS final rule (82 FR 53212 and 53213), we revised the definition of primary care services to include three additional CCM service codes, 99487, 99489, and G0506, and four behavioral health integration (BHI) service codes, G0502, G0503, G0504 and G0507.
We further revised the definition of primary care services in the November 2018 final rule (also referred to as the CY 2019 PFS final rule) (83 FR 59964 through 59968), by adding new codes to the definition of primary care services (CPT codes 99497, 99498, 96160, 96161, 99354, and 99355, and HCPCS codes G0444, G0442, and G0443), and by revising how we determine whether services identified by CPT codes 99304 through 99318 were furnished in a SNF.
In the May 8, 2020 COVID-19 IFC (85 FR 27582 through 27586), we revised the definition of primary care services for purposes of beneficiary assignment for the performance year starting on January 1, 2020, and for any subsequent performance year that starts during the COVID-19 PHE defined in § 400.200, to include the following additions specified in § 425.400(c)(2): (1) HCPCS code G2010 (remote evaluation of patient video/images) and HCPCS code G2012 (virtual check-in); (2) CPT codes 99421, 99422 and 99423 (online digital evaluation and management service (e-visit)); and (3) CPT codes 99441, 99442, and 99443 (telephone evaluation and management services).
In the CY 2021 PFS final rule (85 FR 84786 through 84793), we finalized the additional primary care service codes adopted in the May 8, 2020 COVID-19 IFC with modifications to allow these codes to be used in determining beneficiary assignment when the assignment window (as defined at § 425.20) for a benchmark or performance year includes any months during the PHE for COVID-19 defined in § 400.200, and to apply these additional primary care service codes to all months of the assignment window, when the assignment window includes any month(s) during the PHE for COVID-19.
In the CY 2021 PFS final rule (85 FR 84748 through 84755), we expanded the definition of primary care services for purposes of determining beneficiary assignment to include: Online digital E/M CPT codes 99421, 99422, and 99423; assessment of and care planning for patients with cognitive impairment CPT code 99483; chronic care management code CPT code 99491; exclusion of advance care planning CPT code 99497 and the add-on code 99498 when billed in an inpatient care setting; remote evaluation of patient video/images HCPCS codes G2010; virtual check-in HCPCS code G2012; non-complex chronic care management HCPCS code G2058 and its replacement CPT code 99439; principal care management HCPCS codes G2064 and G2065; and psychiatric collaborative care model HCPCS code G2214. In this same final rule (85 FR 84755 through 84756), we finalized revisions to the existing exclusion for professional services billed under CPT codes 99304 through 99318 that are furnished in a SNF to include services reported on an FQHC or RHC claim that includes CPT codes 99304 through 99318, when those services are furnished in a SNF.
For performance years beginning on January 1, 2021, and subsequent performance years, we defined primary care services in § 425.400(c)(1)(v) for purposes of assigning beneficiaries to ACOs under § 425.402 as the set of services identified by the following HCPCS/CPT codes:
CPT codes:
(1) 96160 and 96161 (codes for administration of health risk assessment).
(2) 99201 through 99215 (codes for office or other outpatient visit for the evaluation and management of a patient).
(3) 99304 through 99318 (codes for professional services furnished in a nursing facility; professional services or services reported on an FQHC or RHC claim identified by these codes are excluded when furnished in a SNF).
(4) 99319 through 99340 (codes for patient domiciliary, rest home, or custodial care visit).
(5) 99341 through 99350 (codes for evaluation and management services furnished in a patient's home for claims identified by place of service modifier 12). Start Printed Page 39276
(6) 99354 and 99355 (add-on codes, for prolonged evaluation and management or psychotherapy services beyond the typical service time of the primary procedure; when the base code is also a primary care service code under § 425.400(c)(1)(v)).
(7) 99421, 99422, and 99423 (codes for online digital evaluation and management).
(8) 99439 (code for non-complex chronic care management).
(9) 99483 (code for assessment of and care planning for patients with cognitive impairment).
(10) 99484, 99492, 99493 and 99494 (codes for behavioral health integration services).
(11) 99487, 99489, 99490 and 99491 (codes for chronic care management).
(12) 99495 and 99496 (codes for transitional care management services).
(13) 99497 and 99498 (codes for advance care planning; services identified by these codes furnished in an inpatient setting are excluded).
HCPCS codes:
(1) G0402 (code for the Welcome to Medicare visit).
(2) G0438 and G0439 (codes for the annual wellness visits).
(3) G0442 (code for alcohol misuse screening service).
(4) G0443 (code for alcohol misuse counseling service).
(5) G0444 (code for annual depression screening service).
(6) G0463 (code for services furnished in Electing Teaching Amendment hospitals).
(7) G0506 (code for chronic care management).
(8) G2010 (code for the remote evaluation of patient video/images).
(9) G2012 (code for virtual check-in).
(10) G2058 (code for non-complex chronic care management).
(11) G2064 and G2065 (codes for principal care management services).
(12) G2214 (code for psychiatric collaborative care model).
b. Proposed Revisions
(1) HCPCS and CPT Codes Used in Assignment
Based on feedback from ACOs and our further review of the HCPCS and CPT codes currently recognized for payment under the PFS, we believe it would be appropriate to amend the definition of primary care services used in the Shared Savings Program assignment methodology to include certain additional codes and make other technical changes to the definition of primary care services, for use in determining beneficiary assignment for the performance year starting on January 1, 2022, and subsequent performance years.
We propose to revise the definition of primary care services in the Shared Savings Program regulations to include the following additions: (1) Chronic Care Management (CCM) CPT code 99X21, if finalized through the CY 2022 PFS rulemaking; (2) Principal Care Management (PCM) CPT codes 99X22, 99X23, 99X24, and 99X25, if finalized through the CY 2022 PFS rulemaking; (3) Prolonged office or other outpatient evaluation and management (E/M) service HCPCS code G2212; and (4) Communication Technology-Based Service (CTBS) HCPCS code G2252, if payment for this code is made permanent through the CY 2022 PFS rulemaking. The following provides additional information about the CPT codes and HCPCS codes that we are proposing to add to the definition of primary care services used in assignment:
- Chronic Care Management (CCM) CPT code 99X21. For CY 2022, the American Medical Association (AMA) CPT Editorial Panel created a new CPT code that describes CCM services furnished by clinical staff under the supervision of a physician or nonphysician practitioner (NPP) who can bill E/M services, and CCM services personally furnished by a physician or NPP. Elsewhere in this proposed rule, we propose valuation of CPT code 99X21 (Chronic care management services with the following required elements: Multiple (two or more) chronic conditions expected to last at least 12 months, or until the death of the patient; chronic conditions that place the patient at significant risk of death, acute exacerbation/decompensation, or functional decline; comprehensive care plan established, implemented, revised, or monitored.; each additional 30 minutes by a physician or other qualified health care professional, per calendar month).
In earlier rulemaking, we finalized the inclusion of CCM CPT codes 99487, 99489, 99490, and 99491 (codes for chronic care management) in the definition of primary care services for the Shared Savings Program. Refer to the June 2015 final rule (80 FR 32746 through 32748), CY 2018 PFS final rule (82 FR 53212 through 53213), and CY 2021 PFS final rule (85 FR 84749 through 84750 and 84754). "Non-complex" CCM services (CPT codes 99490 and 99491), and "complex" CCM services (CPT codes 99487 and 99489) share a common set of service elements, including the following: (1) Initiating visit, (2) structured recording of patient information using certified electronic health record technology (EHR), (3) 24/7 access to physicians or other qualified health care professionals or clinical staff and continuity of care, (4) comprehensive care management including systematic assessment of the patient's medical, functional, and psychosocial needs, (5) comprehensive care plan including a comprehensive care plan for all health issues with particular focus on the chronic conditions being managed, and (6) management of care transitions. They differ in the amount of clinical staff service time provided, the involvement and work of the billing practitioner, and the extent of care planning performed.
The CCM services that will be furnished under the new CPT code 99X21 are similar to the CCM services that are billed under the exiting CCM codes that are included in the Shared Savings Program's current definition of primary care services, which includes CCM CPT codes 99487, 99489, 99490, 99491 and HCPCS code G0506. Because the Shared Savings Program's definition of primary care services includes other CCM CPT codes and HCPCS codes, we believe it would be appropriate to include HCPCS code 99X21, if finalized through the CY 2022 PFS rulemaking, in the definition of primary care services under § 425.400(c) for the performance year starting on January 1, 2022, and subsequent performance years.
- Principal Care Management (PCM) services CPT Codes 99X22, 99X23, 99X24, and 99X25. The AMA CPT Editorial Panel has created the following new CPT codes that describe PCM services furnished by clinical staff under the supervision of a physician or NPP who can bill E/M services, and PCM services personally furnished by a physician or NPP:
++ 99X22 (Principal care management services, for a single high-risk disease, with the following required elements: One complex chronic condition expected to last at least 3 months, and which places the patient at significant risk of hospitalization, acute exacerbation/decompensation, functional decline, or death, the condition requires development, monitoring, or revision of disease-specific care plan, the condition requires frequent adjustments in the medication regimen, and/or the management of the condition is unusually complex due to comorbidities; ongoing communication and care coordination between relevant practitioners furnishing care; first 30 minutes provided personally by a physician or other qualified health care professional, per calendar month). Start Printed Page 39277
++ 99X23 (Principal care management services, for a single high-risk disease, with the following required elements: One complex chronic condition expected to last at least 3 months, and which places the patient at significant risk of hospitalization, acute exacerbation/decompensation, functional decline, or death; the condition requires development, monitoring, or revision of disease-specific care plan, the condition requires frequent adjustments in the medication regimen, and/or the management of the condition is unusually complex due to comorbidities; ongoing communication and care coordination between relevant practitioners furnishing care; additional 30 minutes provided personally by a physician or other qualified health care professional, per calendar month).
++ 99X24 (Principal care management services, for a single high-risk disease, with the following required elements: One complex chronic condition expected to last at least 3 months, and which places the patient at significant risk of hospitalization, acute exacerbation/decompensation, functional decline, or death; the condition requires development, monitoring, or revision of disease-specific care plan; the condition requires frequent adjustments in the medication regimen, and/or the management of the condition is unusually complex due to comorbidities; ongoing communication and care coordination between relevant practitioners furnishing care; first 30 minutes of clinical staff time directed by physician or other qualified health care professional, per calendar month.).
++ 99X25 (Principal care management services, for a single high-risk disease, with the following required elements: One complex chronic condition expected to last at least 3 months, and which places the patient at significant risk of hospitalization, acute exacerbation/decompensation, functional decline, or death; the condition requires development, monitoring, or revision of disease-specific care plan; the condition requires frequent adjustments in the medication regimen, and/or the management of the condition is unusually complex due to comorbidities; ongoing communication and care coordination between relevant practitioners furnishing care; each additional 30 minutes of clinical staff time directed by a physician or other qualified health care professional, per calendar month).
Because the Shared Savings Program's definition of primary care services already includes the temporary HCPCS codes G2064 and G2065 that will be replaced by the permanent CPT codes 99X22 and 99X24, and CPT codes 99X23 and 99X25 represent the same services furnished for a greater length of time, we believe it would be appropriate to propose to include CPT code 99X22, 99X23, 99X24, and 99X25, if finalized through the CY 2022 PFS rulemaking, in the definition of primary care services under § 425.400(c) for the performance year starting on January 1, 2022, and subsequent performance years. Although the temporary HCPCS codes G2064 and G2065 will be replaced by the permanent CPT codes, the Shared Savings Program will retain the temporary HCPCS codes in the definition of primary care services used for assignment, to be used in conducting beneficiary assignment for benchmark years.
- Prolonged office or other outpatient evaluation and management (E/M) service HCPCS code G2212: In the CY 2021 PFS final rule (85 FR 84573 through 84574), CMS finalized a new HCPCS code G2212 (Prolonged office or other outpatient evaluation and management service(s) beyond the maximum required time of the primary procedure which has been selected using total time on the date of the primary service; each additional 15 minutes by the physician or qualified healthcare professional, with or without direct patient contact (List separately in addition to CPT codes 99205, 99215 for office or other outpatient evaluation and management services) (Do not report G2212 on the same date of service as 99354, 99355, 99358, 99359, 99415, 99416). (Do not report G2212 for any time unit less than 15 minutes)) to be used when billing Medicare for prolonged office/outpatient evaluation and management (E/M) visits instead of CPT code 99417, starting in 2021. We stated our belief that the creation of HCPCS code G2212 will serve to resolve the potential differences between Medicare and other interpretations of CPT rules, and better address questions about the required times and what time may be counted toward the required time to report prolonged office/outpatient E/M visits (see the CY 2020 PFS final rule for a more detailed discussion of this issue, (84 FR 62849 through 62850)).
The current definition of primary care services used in the Shared Savings Program assignment methodology includes CPT codes 99205 and 99215 (codes for office or other outpatient visit for the evaluation and management of a patient). Because HCPCS code G2212 is defined as an add-on code for those office/outpatient E/M services, representing the same underlying services being furnished for a longer period of time, we believe it would be appropriate to propose to include HCPCS code G2212 in the definition of primary care services under § 425.400(c) for the performance year starting on January 1, 2022, and subsequent performance years.
- Communication Technology-Based Service (CTBS) HCPCS code G2252: In the CY 2021 PFS final rule (85 FR 84536), CMS established additional coding and payment for services delivered via synchronous communication technology, which can include audio-only communication on an interim basis for CY 2021. We stated our belief that establishing payment for a longer service (11-20 minutes) on an interim basis would support access to care for beneficiaries who may be reluctant to return to in-person visits unless absolutely necessary, and allow us to consider whether this policy should be adopted on a permanent basis. Therefore, for CY 2021, on an interim basis, we established HCPCS code G2252 (Brief communication technology-based service, e.g., virtual check-in, by a physician or other qualified health care professional who can report evaluation and management services, provided to an established patient, not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment; 11-20 minutes of medical discussion). Elsewhere in this proposed rule, we are proposing to pay for this service on a permanent basis starting January 1, 2022.
HCPCS code G2252 is similar to G2012 (Brief communication technology-based service, e.g., virtual check-in, by a physician or other qualified health care professional who can report evaluation and management services, provided to an established patient, not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment; 5-10 minutes of medical discussion), but allows for an extended period of medical discussion. Because G2012 is already included the definition of primary care services at § 425.400(c), we believe including G2252 in the Shared Savings Program definition of primary care services used for assignment, beginning with performance year 2022, would result in more accurate assignment of beneficiaries based on where they receive the plurality of their Start Printed Page 39278 primary care services. Accordingly, we propose to include HCPCS code G2252 in the definition of primary care services under § 425.400(c) for the performance year starting on January 1, 2022, and subsequent performance years, if payment for the code is made permanent through the CY 2022 PFS rulemaking.
We propose to specify a revised definition of primary care services in a new provision of the Shared Savings Program regulations at § 425.400(c)(1)(vi) to include the list of HCPCS and CPT codes specified in § 425.400(c)(1)(v) with the proposed additional CPT codes 99X21, 99X22, 99X23, 99X24, and 99X25, and HCPCS codes G2212 and G2252, if finalized through the CY 2022 PFS rulemaking, as applicable. We propose the new provision at § 425.400(c)(1)(vi) would be applicable for use in determining beneficiary assignment for the performance year starting on January 1, 2022, and subsequent performance years. Further, we propose technical modifications to the introductory text in § 425.400(c)(1)(v) to specify the applicability of this provision for determining beneficiary assignment for the performance year starting on January 1, 2021.
(2) Extending the Applicability of the Expanded Definition of Primary Care Services in Response to the COVID-19 PHE
As previously described in this section III.J.2.a. of this proposed rule, in the May 8, 2020 COVID-19 IFC (85 FR 27582 through 27586), we adopted an expanded definition of primary care services for purposes of beneficiary assignment to reflect services furnished during the COVID-19 PHE. This expanded definition was finalized with modifications in the CY 2021 PFS final rule (85 FR 84785 through 84793). According to § 425.400(c)(2), when the assignment window (as defined in § 425.20) for a benchmark or performance year includes any month(s) during the COVID-19 PHE defined in § 400.200, in determining beneficiary assignment, we use the primary care service codes identified in § 425.400(c)(1), and additional primary care service codes as follows:
CPT codes:
(1) 99421, 99422, and 99423 (codes for online digital evaluation and management services).
(2) 99441, 99442, and 99443 (codes for telephone evaluation and management services).
HCPCS codes:
(1) G2010 (code for the remote evaluation of patient video/images).
(2) G2012 (code for virtual check-in).
These additional primary care services are applicable to all months of the assignment window, when the assignment window includes any month(s) during the COVID-19 PHE defined in § 400.200.
In the CY 2021 PFS final rule (85 FR 84748 through 84755), we updated the definition of primary care services under § 425.400(c) permanently for purposes of determining beneficiary assignment under § 425.402 for the performance year starting on January 1, 2021, and subsequent performance years, so that the following codes would not be linked to the duration of the PHE for COVID-19: (1) HCPCS code G2010 (remote evaluation of patient video/images) and HCPCS code G2012 (virtual check-in); (2) CPT codes 99421, 99422 and 99423 (online digital evaluation and management service (e-visit)).
In the CY 2021 PFS final rule, we noted that we did not consider including CPT codes 99441, 99442, and 99443 in the definition of primary care services at § 425.400(c) on a permanent basis (85 FR 84751). Telephone E/M services CPT codes 99441 (Telephone evaluation and management service by a physician or other qualified health care professional who may report evaluation and management services provided to an established patient, parent, or guardian not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment; 5-10 minutes of medical discussion); 99442 (Telephone evaluation and management service by a physician or other qualified health care professional who may report evaluation and management services provided to an established patient, parent, or guardian not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment; 11-20 minutes of medical discussion); and 99443 (Telephone evaluation and management service by a physician or other qualified health care professional who may report evaluation and management services provided to an established patient, parent, or guardian not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment; 21-30 minutes of medical discussion) are non-covered services when not provided during the PHE for COVID-19, as defined in § 400.200, and so could not be included in the definition of primary care services for purposes of assignment outside the context of the PHE.
In section II.D. of this proposed rule, we are proposing to revise the timeframe for services added on a temporary basis to the Medicare telehealth services list to allow additional time for stakeholders to perform an adequate analysis of those services for consideration in determining whether to include them on the Medicare telehealth services list on a permanent basis.
In order to remain consistent with Medicare FFS payment policies, we propose to revise our existing definition of primary care services for purposes of beneficiary assignment in order to include CPT codes 99441, 99442, and 99443 until they are no longer payable under the Medicare FFS payment policies as specified under section 1834(m) of the Act and §§ 410.78 and 414.65. We propose to specify this modification by revising § 425.400(c)(2)(i)(A)(2) to include an exception to the applicability of the expanded definition of primary care services, to extend the timeframe for use of CPT codes 99441, 99442, and 99443, and making conforming revisions to paragraphs (c)(2)(i) and (c)(2)(ii).
(3) Incorporation of Replacement Codes Into the Definition of Primary Care Services To Reflect Current Coding
In the June 2015 final rule (80 FR 32746 through 32748), we established a policy under which we make any revisions to the definition of primary care services for purposes of beneficiary assignment through the annual PFS rulemaking process. We established this policy in order to promote flexibility for the Shared Savings Program and to allow the definition of primary care services used for assignment in the Shared Savings Program to respond quickly to HCPCS/CPT coding changes made in the annual PFS rulemaking process. Accordingly, as part of the PFS rulemaking process, we periodically update the definition of primary care services used for assignment to include additional codes that we designate as primary care services for purposes of the Shared Savings Program, including new HCPCS/CPT codes or revenue codes and any subsequently modified or replacement codes.
On a routine basis, the CPT Editorial Panel may delete existing CPT codes and replace them with new CPT codes. In addition, one use of HCPCS G-codes is to identify professional healthcare procedures and services that may not have assigned CPT codes. Thus, the CPT Editorial Panel may also create new CPT Start Printed Page 39279 codes to replace these temporary HCPCS codes.
Currently, there may be a period of time between the issuance of a replacement code and the effective date of the final rule that incorporates the replacement code into the definition of primary care services, when the replacement code is not captured in the Shared Savings Program assignment methodology. Therefore, we are proposing to incorporate into the definition of primary care services a permanent CPT code when it directly replaces another CPT code or a temporary HCPCS code (for example, a G-code) that is already included in the definition of primary care services for purposes of determining beneficiary assignment under the Shared Savings Program. In general, we would expect to determine that a code is a direct replacement for another code based either on its having a substantially similar code description or the relevant discussion in CMS rulemaking establishing payment for the replacement code. This proposed approach would help to ensure the appropriate identification of primary care services used in the Shared Savings Program's assignment methodology by allowing for the immediate inclusion of replacement CPT codes in the determination of beneficiary assignment and lead to continuity in the assignment of beneficiaries receiving those services based on current coding. This continuity would improve predictability for ACOs, while also increasing the consistency of care coordination for their assigned beneficiaries.
We further propose that such replacement codes would be incorporated into the definition of the primary care services for purposes of determining beneficiary assignment for the performance year starting on January 1, 2022, and subsequent performance years, when the assignment window for a benchmark or performance year (as defined in § 425.20) includes any day on or after the effective date of the replacement code for payment purposes under FFS Medicare. For ACOs under preliminary prospective assignment with retrospective reconciliation, CMS assigns beneficiaries in a preliminary manner at the beginning of a performance year and quarterly based on the most recent 12 months of data available. For final assignment for a 12-month benchmark year or performance year, the assignment window is the 12-month calendar year that corresponds to the performance year or benchmark year. Under this proposal, a replacement CPT code that becomes effective during a 12-month initial, quarterly, or final assignment window would be included in the definition of primary care services used to determine beneficiary assignment for the applicable performance year or benchmark year. For ACOs under prospective assignment, claims-based beneficiary assignment is determined prospectively at the beginning of each benchmark and performance year based on the beneficiary's use of primary care services in the most recent 12 months for which data are available, based on an offset assignment window before the start of the benchmark or performance year. Under this proposal, a replacement CPT code that becomes effective during the offset assignment window would be included in the definition of primary care services used to determine beneficiary assignment for the applicable performance year or benchmark year.
We anticipate that we would continue to undergo periodic notice and comment rulemaking, through the annual PFS rulemaking, to amend the list of CPT codes and HCPCS codes that make up the definition of primary care services used for assignment in the Shared Savings Program to codify the applicable replacement CPT codes.
As discussed in section III.J.2.b.(1) of this proposed rule, we propose to incorporate the revised definition of primary care services used for assignment in a new provision of the Shared Savings Program regulations at § 425.400(c)(1)(vi), applicable for use in determining beneficiary assignment for the performance year starting on January 1, 2022, and subsequent performance years. As part of this revised definition, we propose to incorporate a provision in paragraph (c)(1)(vi)(C), specifying that the primary care service codes for purposes of assigning beneficiaries include a CPT code identified by CMS that directly replaces a CPT code specified in § 425.400(c)(1)(vi)(A) or a HCPCS code specified in § 425.400(c)(1)(vi)(B), when the assignment window (as defined in § 425.20) for a benchmark or performance year includes any day on or after the effective date of the replacement code for payment purposes under FFS Medicare.
We seek comment on these proposed changes to the definition of primary care services used for assigning beneficiaries to Shared Savings Program ACOs for the performance year starting on January 1, 2022, and subsequent performance years. We also welcome comments on any other existing HCPCS or CPT codes, and new HCPCS or CPT codes proposed elsewhere in this proposed rule, that we should consider adding to the definition of primary care services for purposes of assignment in future rulemaking.
3. Repayment Mechanisms
a. Background
An ACO that will participate in a two-sided model must demonstrate that it has established an adequate repayment mechanism to provide CMS assurance of its ability to repay shared losses for which the ACO may be liable upon reconciliation for each performance year. The requirements for an ACO to establish and maintain an adequate repayment mechanism are described in § 425.204(f), and we have provided additional program guidance on repayment mechanism arrangements.[97] We established the repayment mechanism requirements through earlier rulemaking,[98] and recently modified the repayment mechanism requirements in the December 2018 final rule (83 FR 67928 through 67938) and the CY 2021 PFS final rule (85 FR 84756 through 84763).
According to § 425.204(f)(4)(ii), for a BASIC track or ENHANCED track ACO, the repayment mechanism amount must be equal to the lesser of the following: (1) 1 percent of the total per capita Medicare Parts A and B FFS expenditures for the ACO's assigned beneficiaries, based on expenditures for the most recent calendar year for which 12 months of data are available; or (2) 2 percent of the total Medicare Parts A and B FFS revenue of its ACO participants, based on revenue for the most recent calendar year for which 12 months of data are available. As discussed in the December 2018 final rule (83 FR 67866), this approach allows CMS to use the same sources of revenue and expenditure data during the program's annual application cycle to estimate the ACO's repayment mechanism amount and to determine the ACO's participation options according to whether the ACO is categorized as a low revenue ACO or high revenue ACO.
As specified under § 425.204(f)(4)(iii), for agreement periods beginning on or Start Printed Page 39280 after July 1, 2019, CMS recalculates the ACO's repayment mechanism amount before the second and each subsequent performance year in the agreement period based on the certified ACO participant list for the relevant performance year. We require an increase in the repayment mechanism amount if the recalculated repayment mechanism amount exceeds the existing repayment mechanism amount by at least 50 percent or $1,000,000, whichever is the lesser value. Under § 425.204(f)(4)(iii), an ACO cannot decrease the amount of its repayment mechanism during its agreement period as a result of changes in its composition.
As discussed in prior rulemaking, program stakeholders have continued to identify the repayment mechanism requirement as a potential barrier for some ACOs to enter into performance-based risk tracks, particularly small, physician-only and rural ACOs that may lack access to the capital that is needed to establish a repayment mechanism with a large dollar amount (see for example, 83 FR 67929).
The design of the current repayment mechanism amount calculation, which is based on a percentage of expenditures for the ACO's assigned beneficiaries or a percentage of ACO participant revenue, seeks to approximate a percentage of the ACO's maximum possible shared losses, according to the loss recoupment limits (also referred to as the loss sharing limits) applicable to ACOs under two-sided models. Comparing the calculations for determining repayment mechanism amounts to the calculations for determining the loss sharing limits indicates that repayment mechanisms cover approximately 25 percent of estimated maximum possible losses for ACOs in the BASIC track (determined by dividing 1 percent, the percentage used in the repayment mechanism amount calculation under § 425.204(f)(4)(ii)(A), by 4 percent, the percentage of the benchmark-based loss sharing limit under Level E of the BASIC track under § 425.605(d)(1)(v)(D)(2)), and 7 percent of estimated maximum possible losses for ACOs in the ENHANCED track (determined by dividing 1 percent, the percentage used in the repayment mechanism amount calculation under § 425.204(f)(4)(ii)(A), by 15 percent, the percentage of the benchmark-based loss sharing limit under the ENHANCED track under § 425.610(g)). Based on operational experience, we have found that the repayment mechanism amounts for most ACOs are much larger than needed to cover actual losses, as repayment mechanism amount calculations have been based on a percentage of an amount that approximates the ACO's loss sharing limit (which is as high as 15 percent of updated benchmark expenditures in the ENHANCED track),[99] and actual historical shared losses have been much lower than the loss sharing limit, averaging 0.96 percent of the ACO's benchmark. Some ACOs have been required to establish repayment mechanisms with amounts that are 9 times greater than their actual shared losses. Additionally, of the 35 times ACOs have owed shared losses, as determined based on reconciliation for the Shared Savings Program's first performance year concluding on December 31, 2013, through performance years (or a performance period) in 2019, only one ACO has neglected to repay CMS timely, and most ACOs chose to repay shared losses without the use of their repayment mechanism arrangements. For the one ACO that did not repay CMS, we were able to recoup more than half of the shared losses owed using the ACO's repayment mechanism, and the remaining debt was referred to the Department of Treasury for collection.
Considering this experience, which suggests there may be low risk to the Shared Savings Program by allowing lower repayment mechanism amounts, and the potential reduction in burden on ACOs by lower repayment mechanism amounts, we believe it is appropriate to modify the approach to calculating repayment mechanism amounts. We believe reducing the required amounts of repayment mechanisms may allow ACOs to use these funds to improve patient care and coordination and reduce a potential barrier to entry into performance-based risk models.
In this section of this proposed rule, we discuss four proposed policy changes regarding required repayment mechanism amounts. Under the first policy, we would modify the methodology for calculating repayment mechanism amounts to reduce the required amounts. Second, we would specify how we identify the number of assigned beneficiaries used in the repayment mechanism amount calculation and the annual repayment mechanism amount recalculation. Third, we would permit eligible ACOs that established a repayment mechanism to support their participation in a two-sided model beginning on July 1, 2019, January 1, 2020, or January 1, 2021, to elect to reduce the amount of their existing repayment mechanisms if their recalculated repayment mechanism amount for performance year 2022 is lower than their existing repayment mechanism amount. Fourth, we would modify the threshold for determining whether an ACO is required to increase its repayment mechanism amount during its ACO's agreement period.
b. Proposed Revisions
(1) Repayment Mechanism Amount Calculations
We considered two options for modifying the calculation of repayment mechanism amounts to result in lower amounts: (1) Reducing the percentages used in the existing repayment mechanism amount calculations specified in § 425.204(f)(4)(ii); or (2) revising the methodology to use a per beneficiary dollar amount estimation methodology. In evaluating these options, we considered the potential impact on low revenue ACOs and high revenue ACOs, as defined according to § 425.20. We also considered a balance of factors, including whether to retain an approach similar to the existing methodology or to use an alternative approach that could simplify the repayment mechanism amount calculation to make it more predictable. We also considered the magnitude of potential decreases in the repayment mechanism amounts under each option. We propose the first option, to reduce the percentages used in the existing repayment mechanism amount calculations, but we are seeking comment on the second, alternative option we considered. We propose to lower the repayment mechanism Start Printed Page 39281 amounts by reducing the percentages used in our current methodology, under which we calculate the repayment mechanism amount as the lesser of the following: (1) 1 percent of the total per capita Medicare Parts A and B FFS expenditures for the ACO's assigned beneficiaries, based on expenditures for the most recent calendar year for which 12 months of data are available; or (2) 2 percent of the total Medicare Parts A and B FFS revenue of its ACO participants, based on revenue for the most recent calendar year for which 12 months of data are available. Specifically, we propose to calculate the amount as the lesser of the following: (1) One-half (0.5) percent of the total per capita Medicare Parts A and B FFS expenditures for the ACO's assigned beneficiaries, based on expenditures for the most recent calendar year for which 12 months of data are available; or (2) 1 percent of the total Medicare Parts A and B FFS revenue of its ACO participants, based on revenue for the most recent calendar year for which 12 months of data are available.
Under this proposal, ACOs would receive a 50 percent decrease in their repayment mechanism amounts compared to the current methodology. These amounts would offer lower repayment mechanism amounts for ACOs, while still reserving what we believe to be a reasonable amount in the event CMS uses an ACO's repayment mechanism funds to support recoupment of shared losses. Our review of data for ACOs under a two-sided model revealed that if this repayment mechanism amount calculation method were in place for PY 2021, the mean repayment mechanism savings would be $297,665 for low revenue ACOs and $2.31 million for high revenue ACOs; the minimum repayment mechanism savings would be $27,030 for low revenue ACOs and $78,106 for high revenue ACOs; and the maximum repayment mechanism savings would be $1.97 million for low revenue ACOs and $11.70 million for high revenue ACOs.
A second, alternative option we considered would be to estimate the repayment mechanism amount using a per beneficiary dollar amount that would be based on a percentage of actual historical median per capita shared losses for Shared Savings Program ACOs, multiplied by an estimate of the size of the ACO's assigned population as identified during the annual application or annual change request cycle. In considering this option, we analyzed data from the 35 instances when Shared Savings Program ACOs in two-sided models have ever incurred shared losses, defined as performance year expenditures above the ACO's benchmark by an amount equal to or greater than the ACO's minimum loss rate. Using data from actual historical shared losses, we determined median per beneficiary shared losses were $100.90 and calculated per beneficiary dollar amounts projected to cover 5 to 25 percent of shared losses for ACOs, as illustrated in Table 26.

Under this second, alternative option, we considered using separate per beneficiary dollar amounts, for low revenue ACOs and high revenue ACOs. We believe using two separate percentages is supported for a number of reasons. Compared to high revenue ACOs, low revenue ACOs are likely to have a lower loss sharing limit in the BASIC track (determined as a percentage of ACO participant revenue not to exceed a percentage of the ACO's updated benchmark), under which eligible low revenue ACOs may participate for up to two agreement periods. Historically, low revenue ACOs have owed shared losses less often and have had lower amounts of per beneficiary shared losses compared to high revenue ACOs. Additionally, we believe high revenue ACOs, which tend to include institutional providers and are typically larger and better capitalized, are likely better financially prepared to secure a higher amount in their repayment mechanism than low revenue ACOs, which tend to be smaller and have less capital. For low revenue ACOs, to cover 10 percent of median actual historical shared losses, rounding to the nearest $1 increment, we considered requiring a repayment mechanism amount equal to $10 per beneficiary. For high revenue ACOs, to cover 20 percent of median actual historical shared losses we considered requiring $20 per beneficiary (refer to Table 26). These amounts would offer a lower repayment mechanism amount for 99 percent of low and high revenue ACOs with existing repayment mechanisms, while still reserving what we believe to be a reasonable amount in the event CMS uses an ACO's repayment mechanism funds to support recoupment of shared losses. Our review of data for ACOs in a two-sided model revealed that if this repayment mechanism amount calculation method were in place for PY 2021,the mean repayment mechanism savings would be $410,682 for low revenue ACOs and $3.84 million for high revenue ACOs; the minimum repayment mechanism savings would be $6,513 for low revenue ACOs and $120,491 for high revenue ACOs; and the maximum repayment mechanism savings would be $3.45 million for low revenue ACOs and $19.73 million for high revenue ACOs.
We believe there are a number of advantages to the option under which we would calculate repayment Start Printed Page 39282 mechanism amounts using per beneficiary dollar amounts for low revenue ACOs and high revenue ACOs. For one, low revenue ACOs would receive additional relief through lower repayment mechanism amounts, relative to high revenue ACOs, under this approach. We believe this is appropriate considering the previously described factors: The lower potential loss liability for low revenue ACOs; historically, low revenue ACOs have incurred shared losses less often and have had lower per beneficiary shared losses compared to high revenue ACOs; and low revenue ACOs tend to be less well capitalized and may face potential barriers to establishing repayment mechanisms. Second, this approach aligns with the existing repayment mechanism amount calculation methodology, which tends to require proportionally higher amounts for high revenue ACOs, that tend to have higher average total expenditures for ACO assigned beneficiaries and higher total ACO participant revenue, compared to low revenue ACOs. Third, an approach that uses a per beneficiary dollar amount would simplify the method to calculate the repayment mechanism amount, compared to the existing methodology, and may help ACOs better project repayment mechanism amounts prior to entering two-sided models, either at the point of application to a new agreement period or during the ACO's agreement period within the BASIC track's glide path as ACOs transition from a one-sided model to a two-sided model. Lastly, this approach would lower the mean repayment mechanism amount for ACOs more than the reduction that would occur under our proposal to lower the percentages used in the existing amount calculation methodology.
However, we have significant concerns with an approach that uses a per beneficiary dollar amount that is applied based on whether an ACO is determined to be a low revenue ACO or a high revenue ACO, which if unresolved outweigh the potential benefits of the approach. For one, there would be a significant repayment mechanism amount difference for ACOs near the 35 percent threshold that differentiates low revenue ACOs and high revenue ACOs, and this difference in repayment mechanism amount may not correlate to covering a significant additional increase in risk.
Second, the determination of whether an ACO is a low revenue ACO or high revenue ACO can change during the application cycle and between performance years within an agreement period. Although changes in ACO composition have the potential to affect required amounts determined under the existing repayment mechanism amount calculation methodology, we believe ACO composition changes could result in a greater magnitude of change in the repayment mechanism amount under an approach that applies a $10 per beneficiary amount for low revenue ACOs and a $20 per beneficiary amount for high revenue ACOs.
For ACOs establishing a repayment mechanism under the per beneficiary dollar amount approach, a change in revenue determination in later stages of the application cycle or change request cycle would delay calculation of an ACO's final repayment mechanism amount. In turn, this could delay when the ACO could submit finalized repayment mechanism documentation to demonstrate it meets the repayment mechanism requirement for entering a two-sided model. We are also concerned that ACOs whose revenue determinations change from low revenue to high revenue would face a substantial increase in the required repayment mechanism amount which they could find challenging to finance. However, based on our operational experience there have been relatively few cases where an ACO's revenue determination changes during the later stages of the application review period or change request cycle.
During an ACO's agreement period, a change in the ACO's revenue determination may cause significant fluctuation in an ACO's repayment mechanism amount under an approach that calculates the repayment mechanism amount using a per beneficiary dollar amount based on whether an ACO is determined to be a low revenue ACO or a high revenue ACO. Based on our operational experience, however, few ACOs entering agreement periods beginning on July 1, 2019, and in subsequent years, have experienced a change in revenue determination during their agreement period. Section 425.600(e) specifies an approach to addressing the circumstance where an ACO that entered an agreement period under Level E of the BASIC track because it was low revenue and experienced with performance-based risk Medicare ACO initiatives, becomes high revenue during its agreement period. This approach requires the ACO to take corrective action to meet the definition of low revenue ACO, or CMS takes compliance action as specified in §§ 425.216 and 425.218, which may include termination of the participation agreement. Further, in the absence of a policy to permit decreases in the repayment mechanism amount during the ACO's agreement period, ACOs that establish a repayment mechanism based on a high revenue ACO determination and are subsequently determined to be a low revenue ACO would need to maintain a relatively higher repayment mechanism amount for the duration of their 5-year agreement period.
To resolve these concerns, we considered using a single per beneficiary dollar amount for all ACOs, based on the values described in Table 26. However, we were unable to identify a single per beneficiary dollar amount that would account for historically higher per beneficiary shared losses owed by high revenue ACOs, while resulting in lower repayment mechanism amounts compared to the existing repayment mechanism calculation approach for most low revenue ACOs. Specifically, the dollar amount that would allow for relatively lower repayment mechanism amounts for all ACOs would be $8 per beneficiary, to cover 7.5 percent of median actual historical shared losses, rounding to the nearest $1 increment, which we believe is too low for high revenue ACOs. A higher per beneficiary dollar amount, such as $15, to cover 15 percent of median actual historical shared losses, rounding to the nearest $1 increment, would be relatively disadvantageous to approximately 20 percent of low revenue ACOs.
Both our proposal and the second, alternative option would lower repayment mechanism amounts and would reduce the amount available to CMS to support repayment of shared losses. However, we believe this risk to CMS is mitigated for a number of reasons. As noted previously in this section of this proposed rule, in our analysis of repayment mechanism amounts compared to actual historical shared losses, we believe the lower amounts would continue to provide CMS with reasonable assurance of an ACO's ability to repay shared losses. Further, as discussed in earlier rulemaking (85 FR 50249), the Shared Savings Program's existing policies require ACOs to pay shared losses, in full, within 90 days of written notification from CMS of the amount owed (according to §§ 425.605(e)(3), 425.606(h)(3), 425.610(h)(3)). ACOs have an interest in fully paying the amount of shared losses owed within the 90-day payment window to remain in compliance with the Shared Savings Program's requirements and avoid compliance actions including involuntary termination from the program. CMS may terminate an ACO's participation agreement for reasons Start Printed Page 39283 including, but not limited to, non-compliance with requirements in 42 CFR part 425 (§ 425.218(b)(1)), such as failure to repay shared losses owed according to the program's regulations and may take pre-termination actions as described in § 425.216(a). Under § 425.221(b)(2)(ii)(B), an ACO under a two-sided model whose participation agreement is terminated by CMS under § 425.218 is liable for a pro-rated share of any shared losses determined for the performance year during which the termination becomes effective. ACOs must also timely repay shared losses owed to avoid accruing interest on any unpaid amounts and to avoid referral of an unpaid debt to the Department of Treasury for collection. Based on our operational experience, nearly all ACOs fully repay shared losses without use of their repayment mechanism arrangement. ACOs will continue to have the option to secure a repayment mechanism at an amount greater than the CMS required amount, if they feel that is appropriate to prepare their ACO to repay all shared losses.
Furthermore, we believe that reduced repayment mechanism amounts could reduce costs for ACOs in fees charged by financial institutions for letters of credit and by insurance companies for surety bonds, although we would not anticipate a significant reduction in fees charged by banks or credit unions for establishing and maintaining escrow accounts. For example, reducing the required repayment mechanism amount of a given ACO by $1 million, could reduce the cost of obtaining a letter of credit or surety bond by roughly 1 or 2 percent, in this example resulting from $10,000 or $20,000 in reduced fees for the ACO. We estimate that such relief, in total for all participating ACOs, could be worth $2 to $4 million annually under the proposed approach (assuming a reduction of approximately $196 million in repayment mechanism amounts, in aggregate) and $3 to $6 million annually under the second, alternative option (assuming a reduction of approximately $322 million in repayment mechanism amounts, in aggregate).
In light of these considerations, we propose to revise the regulations in § 425.204(f)(4)(ii) to reduce by one-half the percentages used in the methodology for calculating repayment mechanism amounts for ACOs in a two-sided model of the BASIC track or the ENHANCED track. We propose to revise the percentage specified in § 425.204(f)(4)(ii)(A), for calculating an amount based on expenditures for the ACO's assigned beneficiaries, from 1 percent to one-half percent. We propose to revise the percentage specified in § 425.204(f)(4)(ii)(B), for calculating an amount based on ACO participant revenue, from 2 percent to 1 percent. Under this proposed approach for calculating repayment mechanism amounts for ACOs in a two-sided model of the BASIC track or the ENHANCED track, the repayment mechanism amount would be equal to the lesser of the following: (1) One-half percent of the total per capita Medicare Parts A and B FFS expenditures for the ACO's assigned beneficiaries, based on expenditures for the most recent calendar year for which 12 months of data are available; or (2) 1 percent of the total Medicare Parts A and B FFS revenue of its ACO participants, based on revenue for the most recent calendar year for which 12 months of data are available.
We seek comment on this proposal and the second, alternative option for calculating repayment mechanism amounts using a per beneficiary dollar amount, based on a percentage of actual historical median per capita shared losses for Shared Savings Program ACOs, multiplied by an estimate of the size of the ACO's assigned population as identified during the annual application or annual change request cycle. We seek comment on applying different per beneficiary dollar amounts for low revenue ACOs and high revenue ACOs under this alternative approach. We welcome comments to address the dollar amounts projected to cover the percentage of median actual historical shared losses that would be an appropriate basis for low revenue ACOs (such as $10) and high revenue ACOs (such as $20) under this methodology. We also seek comment on approaches for addressing our concerns about changes in revenue determinations significantly affecting an ACO's repayment mechanism amount, such as applying a single per beneficiary dollar amount to all ACOs. We also note that if we were to adopt such an approach, we would need to address with greater specificity factors including: (1) How we would identify the population of assigned beneficiaries that would be used in the calculation as a multiplier for the per beneficiary dollar amount; and (2) the frequency with which we would consider modifications to the per beneficiary dollar amount. We welcome comments on these considerations.
We propose that these modifications would be effective and applicable on January 1, 2022. We note that the Shared Savings Program's application cycle (for new, renewing and re-entering ACOs) and change request cycle (for ACOs within an agreement period) for the performance year beginning on January 1, 2022 occurs between spring and fall 2021. During this timeframe, ACOs preparing to enter two-sided models for performance year 2022 are awaiting the final repayment mechanism amount for establishing a repayment mechanism, and ACOs within two-sided models are awaiting the determination of whether their repayment mechanism amount must be increased in accordance with § 425.204(f)(4)(iii) (as discussed in section III.J.3.b.(4) of this proposed rule). If the proposed modifications to the repayment mechanism amount calculation methodology described in this section of this proposed rule are finalized, and effective and applicable on January 1, 2022, we would communicate to ACOs their final repayment mechanism amounts after the issuance of the final rule. We are committed to ensuring that ACOs do not overfund their repayment mechanism arrangements according to the existing methodology if we finalize the proposed revisions to reduce repayment mechanism amounts.
(2) Population of Assigned Beneficiaries Used in Calculating and Recalculating Repayment Mechanism Amounts
We propose to amend the regulations at §§ 425.204(f)(4)(ii) and 425.204(f)(4)(iii) to specify how we identify the number of assigned beneficiaries used in calculating and recalculating the repayment mechanism amount (respectively). For context, we first describe our current approach for calculating repayment mechanism amounts under § 425.204(f)(4)(ii) (for ACOs establishing a repayment mechanism to support their participation under a two-sided model) and under § 425.204(f)(4)(iii) (the annual recalculation to determine if an ACO is required to increase the amount of its repayment mechanism).
In accordance with § 425.204(f)(4)(ii), for ACOs in a two-sided model of the BASIC track, or the ENHANCED track, the repayment mechanism amount must be equal to the lesser of the following: (1) 1 percent of the total per capita Medicare Parts A and B fee-for-service expenditures for the ACO's assigned beneficiaries, based on expenditures for the most recent calendar year for which 12 months of data are available (hereinafter referred to as an expenditure-based amount); or (2) 2 percent of the total Medicare Parts A and B fee-for-service revenue of its ACO participants, based on revenue for the most recent calendar year for which 12 months of data are available (hereinafter referred to as a revenue-based amount). Start Printed Page 39284
Currently, we use the following steps to calculate the expenditure-based amount specified in § 425.204(f)(4)(ii)(A), which is a percentage of the total per capita Medicare Parts A and B FFS expenditures for the ACO's assigned beneficiaries, based on expenditures for the most recent calendar year for which 12 months of data are available (referred to below as the "relevant historical calendar year"):
- Step 1: Identify the beneficiaries that would have been assigned to the ACO for the relevant historical calendar year (determined based on the ACO participant list for the upcoming performance year submitted by the ACO for CMS' review during the application cycle or change request cycle, referred to below as the "ACO participant list for the upcoming performance year") and multiply the number of such beneficiaries by an assignment growth factor to account for expected growth in assignment.
- Step 2: Determine estimated per capita FFS expenditures by calculating the total per capita Medicare Parts A and B FFS expenditures incurred during the relevant historical calendar year by the beneficiaries identified in step 1, and dividing that amount by the total number of beneficiaries identified in step 1 before the assignment growth factor is applied; and multiplying the resulting per capita FFS expenditure amount by a dollar trend factor to account for expected growth in Medicare FFS expenditures.
- Step 3: Calculate the product of the number of assigned beneficiaries determined according to step 1, and the estimated per capita FFS expenditures determined according to step 2.
- Step 4: Calculate the repayment mechanism amount by multiplying the amount determined in step 3 by the applicable percentage (currently 1 percent).
We currently use the following steps in calculating the revenue-based amount specified in § 425.204(f)(4)(ii)(B), which is based on revenue for the most recent calendar year for which 12 months of data are available (referred to below as the "relevant historical calendar year"):
- Step 1: Identify the beneficiaries that would have been assigned to the ACO for the relevant historical calendar year (determined based on the ACO participant list for the upcoming performance year) and multiply the number of such beneficiaries by an assignment growth factor.
- Step 2: Using the ACO participant list for the upcoming performance year, determine the estimated per capita FFS revenues of ACO participants by calculating ACO participants' total Medicare Parts A and B FFS revenue based on claims for services furnished to any beneficiary by ACO participants during the relevant historical calendar year, and dividing the dollar amount by the total number of assigned beneficiaries identified in step 1 before the assignment growth factor is applied;[100] and multiplying the resulting number by a dollar trend factor to account for expected growth in Medicare FFS revenue.
- Step 3: Calculate the product of the number of assigned beneficiaries determined according to step 1, and the estimated per capita FFS revenues of ACO participants determined according to step 2.
- Step 4: Calculate the repayment mechanism amount by multiplying the amount determined in step 3 by the applicable percentage (currently 2 percent).
Regardless of the ACO's selected assignment methodology, within step 1 of the expenditure-based and revenue-based repayment mechanism amount calculations, CMS uses an assigned beneficiary population identified based on preliminary prospective assignment with retrospective reconciliation as described in § 425.400(a)(2). This ensures that the assignment window used to determine assigned beneficiaries aligns with the relevant historical calendar year used to calculate expenditures and revenue used in step 2 of the expenditure-based amount and revenue-based amount calculation.
We believe there are several important reasons for using historical data for determining the assigned beneficiary population, Medicare Parts A and B FFS expenditures for the ACO's assigned beneficiaries, and ACO participants' Medicare Parts A and B FFS revenue. For one, this approach ensures CMS' timely determination of final repayment amount estimates for ACOs required to establish a repayment mechanism arrangement prior to the start of a new agreement period under a two-sided model, or prior to start of the upcoming performance year under a two-sided model (for ACOs transitioning from a one-sided to a two-sided model along the BASIC track's glide path). Second, under this approach, the data used to determine repayment mechanism amounts is consistent with the data used in making other determinations during the application cycle and annual change request cycle, including determination of whether an ACO is categorized as a low revenue ACO or high revenue ACO.
In accordance with § 425.204(f)(4)(iii), for agreement periods beginning on or after July 1, 2019, CMS recalculates the ACO's repayment mechanism amount before the second and each subsequent performance year in the agreement period in accordance with § 425.204(f), based on the certified ACO participant list for the relevant performance year. Currently, in recalculating ACOs' repayment mechanism amounts we use the same approach to calculating the expenditure-based amount and revenue-based amount in accordance with § 425.204(f)(4)(ii), as previously described in this section. That is, in recalculating the repayment amount we determine the assigned beneficiary population, Medicare Parts A and B FFS expenditures for the ACO's assigned beneficiaries, and ACO participants' Medicare Parts A and B FFS revenue, for the most recent calendar year for which 12 months of data are available.
We propose to modify the methodology for the annual repayment mechanism amount recalculation. Specifically, we propose to determine the number of assigned beneficiaries that is used as a multiplier in step 3 of the expenditure-based amount and revenue-based amount calculations, based on more recently available assignment data, rather than using a population projected to be assigned to the ACO based on historical data (that is, for the most recent calendar year for which 12 months of data are available). In determining the number of beneficiaries used as a multiplier in the recalculation estimate, we propose to determine the size of the ACO's assigned population based on the number of beneficiaries assigned to the ACO at the beginning of the performance year, as specified under § 425.400(a)(2)(i) (for ACOs under preliminary prospective assignment with retrospective reconciliation) or paragraph (a)(3)(i) (for ACOs under prospective assignment). This population of assigned beneficiaries is specified in the ACO's initial assignment list report for the performance year. For all ACOs, this population is identified based on an assignment window that is offset from the calendar year (that is, from October 1 through September 30 prior to the start of the performance year), and which is the basis for determining prospective assignment for the performance year. Under the proposed approach that uses more recent Start Printed Page 39285 assignment data in determining the recalculation estimate, we would not apply an assignment growth factor as a multiplier for the population size since we would no longer be using historical data to project the size of the ACO's assigned population. We believe this proposed approach would help ensure the recalculated repayment mechanism amounts account for an ACO's composition as reflected in the size of its assigned population for the performance year for which the recalculated amount relates, and thereby provide more accurate recalculated amounts.
Under this proposed approach, we anticipate performing the annual recalculation of the repayment mechanism amounts shortly before or shortly after the start of the new performance year. CMS will perform the recalculation of the repayment mechanism once the initial assignment list report is available, which is typically delivered to ACOs in the early winter (around mid-December), prior to the start of the relevant future performance year. We also note that under the existing approach and the proposed approach to determining the assigned population used as a multiplier in the annual recalculation of the repayment mechanism amounts, the effects on ACO's amounts are varied, resulting in relatively higher or lower amounts depending on the change in the size of the population.
In annually recalculating the repayment mechanism amount under this proposed approach, we would follow the previously described steps for calculating the expenditure-based amount and revenue-based amount, with the exception of the number of beneficiaries used as a multiplier in step 3 of the calculations. In step 3 of the expenditure-based amount calculation, we would calculate the product of the total number of assigned beneficiaries specified in the ACO's initial assignment list report for the relevant future performance year, and the estimated per capita FFS expenditures determined for the relevant historical calendar year (determined according to step 2). In step 3 of the revenue-based amount calculation, we would calculate the product of the total number of assigned beneficiaries specified within the ACO's initial assignment list report for the relevant future performance year, and the estimated per capita FFS revenues of ACO participants determined for the relevant historical calendar year (determined according to step 2).
Several examples illustrate the calculation and recalculation of the repayment mechanism amounts under the proposals. First, for an ACO applying to enter a two-sided model for an agreement period beginning on January 1, 2022, we will calculate the repayment mechanism amount during the application cycle which occurs during CY 2021. During this time, CY 2020 is the most recent calendar year for which 12 months of data are available, and is the relevant historical calendar year for purposes of calculating the repayment mechanism amount. As described in this illustration, the proposed approach to identifying the assigned beneficiary population, Medicare Parts A and B FFS expenditures for the ACO's assigned beneficiaries, and ACO participants' Medicare Parts A and B FFS revenue used within these calculations is consistent with our current operational approach.
In step 1 of the expenditure-based amount and revenue-based amount calculations, we would identify the beneficiaries that would have been assigned to the ACO for CY 2020, determined based on the ACO participant list for PY 2022 submitted with the ACO's application, and determined using preliminary prospective assignment with retrospective reconciliation. That is, we would determine assignment based on the 12-month assignment window from January 1, 2020, through December 31, 2020.[101] We would multiply the number of such beneficiaries by an assignment growth factor.
In step 2 of the expenditure-based amount calculation, we would calculate total Medicare Parts A and B FFS expenditures incurred in CY 2020 by the beneficiaries determined under step 1 to be assigned to the ACO for CY 2020. In step 2 of the revenue-based amount calculation, we would calculate ACO participants' total Medicare Parts A and B FFS revenue, based on claims for services furnished to any beneficiary by ACO participants during CY 2020. We would determine the estimated per capita FFS expenditures, and the estimated per capita FFS revenues of ACO participants, by dividing the CY 2020 dollar amounts by the number of assigned beneficiaries for CY 2020 (determined in accordance with step 1) before the assignment growth factor is applied. We would multiply the resulting numbers by a dollar trend factor.
In step 3 of the expenditure-based amount calculation, the number of assigned beneficiaries for CY 2020 would be multiplied by the estimated per capita FFS expenditures determined for CY 2020 in accordance with step 2. In step 3 of the revenue-based amount calculation, the number of assigned beneficiaries for CY 2020 would be multiplied by the estimated per capita Medicare FFS revenues of ACO participants determined for CY 2020 in accordance with step 2.
In step 4, we would calculate the repayment mechanism amount by multiplying the amount determined in step 3 by the applicable percentage. Currently, that is 1 percent under the expenditure-based amount calculation, and 2 percent under the revenue-based amount calculation. Under the proposals described in section III.J.3.b.(1) of this proposed rule, the applicable percentages would be one-half percent under the expenditure-based amount calculation, and 1 percent under the revenue-based amount calculation.
Our second example illustrates how we would perform the annual recalculation of the repayment mechanism amount for performance year (PY) 2022.
In step 1 of both the expenditure-based amount and revenue-based amount calculations, we use a similar method for identifying the CY 2020 assigned population as described in the first example. That is, we would identify the beneficiaries that would have been assigned to the ACO for CY 2020, determined based on the ACO's certified ACO participant list for PY 2022, and determined using preliminary prospective assignment with retrospective reconciliation. Again, we would determine assignment based on the 12-month assignment window from January 1, 2020, through December 31, 2020.
In step 2 of the expenditure-based amount calculation, we would calculate total Medicare Parts A and B FFS expenditures incurred in CY 2020 by the beneficiaries determined under step 1 to be assigned to the ACO for CY 2020. In step 2 of the revenue-based amount calculation, we would calculate ACO participants' total Medicare Parts A and B FFS revenue, based on claims for services furnished to any beneficiary by ACO participants during CY 2020, using the ACO's certified ACO participant list for PY 2022. We would determine the estimated per capita FFS expenditures, and the estimated per capita FFS revenues of ACO participants, by Start Printed Page 39286 dividing the CY 2020 dollar amounts by the number of assigned beneficiaries for CY 2020 (determined in accordance with step 1).
In step 3, we would identify the total number of assigned beneficiaries specified within the ACO's initial assignment list report for PY 2022. This population of assigned beneficiaries would be specified in the ACO's initial assignment list report for PY 2022, and would be the population identified based on the assignment window from October 1, 2019 through September 30, 2020, and which would be the basis for determining prospective assignment for PY 2022. Assignment would be determined based on the ACO's certified ACO participant list for PY 2022. In step 3 of the expenditure-based amount calculation, the number of assigned beneficiaries for PY 2022 would be multiplied by the estimated per capita FFS expenditures determined for CY 2020 in accordance with step 2. In step 3 of the revenue-based amount calculation, the number of assigned beneficiaries for PY 2022 would be multiplied by the estimated per capita FFS revenues determined for CY 2020 in accordance with step 2.
In step 4, we would recalculate the repayment mechanism amount by multiplying the amount determined in step 3 by the applicable percentage. Currently, that is 1 percent under the expenditure-based amount calculation, and 2 percent under the revenue-based amount calculation. Under the proposals described in section III.J.3.b.(1) of this proposed rule, the applicable percentages would be one-half percent under the expenditure-based amount calculation, and 1 percent under the revenue-based amount calculation.
We propose to modify § 425.204(f)(4)(ii) to more clearly specify the assigned population used as a multiplier in calculating the repayment mechanism amount. Under the existing regulation text at § 425.204(f)(4)(ii)(A), the potential repayment mechanism amount is a specified percentage of total per capita Medicare Parts A and B fee-for-service expenditures "for the ACO's assigned beneficiaries, based on expenditures for the most recent calendar year for which 12 months of data are available." We propose to amend paragraph (f)(4)(ii)(A) to refer to a specified percentage of total per capita Medicare Parts A and B fee-for-service expenditures "for the ACO's assigned beneficiaries, based on expenditures and the number of assigned beneficiaries for the most recent calendar year for which 12 months of data are available" (emphasis added to reflect revised text).
Under the existing regulation text at § 425.204(f)(4)(ii)(B), the potential repayment mechanism amount is a specified percentage of total Medicare Parts A and B fee-for-service revenue "of its ACO participants, based on revenue for the most recent calendar year for which 12 months of data are available." We propose to amend paragraph (f)(4)(ii)(B) to refer to a specified percentage of total Medicare Parts A and B fee-for-service revenue "of its ACO participants, based on revenue for the most recent calendar year for which 12 months of data are available, and based on the ACO's number of assigned beneficiaries for the most recent calendar year for which 12 months of data are available" (emphasis added to reflect revised text).
We also propose technical and conforming changes to the introductory text of § 425.204(f)(4)(iii). We propose to remove as unnecessary and irrelevant the text specifying that the provision applies for agreement periods beginning on or after July 1, 2019. We propose to revise the introductory text for clarity to specify that CMS would recalculate the ACO's repayment mechanism amount "for" the second and each subsequent performance year in the agreement period, rather than "before" the second and each subsequent performance year in the agreement period. We propose to make a conforming change to the introductory text of § 425.204(f)(4)(iii) to specify that CMS' recalculation of the ACO's repayment mechanism amount would be in accordance with § 425.204(f)(4)(ii) based on the certified ACO participant list for the relevant performance year, "except that the number of assigned beneficiaries used in the calculations would be the number of beneficiaries assigned to the ACO at the beginning of the relevant performance year under § 425.400(a)(2)(i) (for ACOs under preliminary prospective assignment with retrospective reconciliation) or § 425.400(a)(3)(i) (for ACOs under prospective assignment)."
We propose that these modifications would be effective and applicable on January 1, 2022. If finalized as proposed, these policies would be used in determining required repayment mechanism amounts for ACOs establishing a repayment mechanism arrangement to support their participation in a two-sided model beginning with performance year 2022, and in subsequent performance years, and in determining recalculated repayment mechanism amounts for performance year 2022 and subsequent performance years, as well as the determination that an eligible ACO has a one-time opportunity to decrease the amount of its repayment mechanism amount as described in section III.J.3.b.(3) of this proposed rule.
(3) Optional One-Time Repayment Mechanism Decrease for Eligible ACOs
In connection with the proposal for lowering the repayment mechanism amounts, described in section III.J.3.b.(1) of this proposed rule, we are proposing to allow certain ACOs a one-time opportunity to decrease the amount of their repayment mechanisms. The purpose of this proposal is to let any ACO that established a repayment mechanism to support its participation in a two-sided model beginning on July 1, 2019, January 1, 2020, or January 1, 2021, to decrease its repayment mechanism amount before it seeks to renew its agreement under the new proposed policy, which if finalized, would otherwise be the first opportunity for the ACO to reduce its repayment mechanism amount. Along these lines, the one-time decrease would also avoid unnecessary burden that could result if ACOs seek to terminate their participation agreements early and apply to re-enter the program in order to reduce their required repayment mechanism amounts.
As discussed in section III.J.3.b.(1) of this proposed rule, if the proposed modifications to the repayment mechanism amount calculation methodology are finalized, and effective and applicable on January 1, 2022, we would ensure that the revised methodology would be used in determining repayment mechanism amounts for ACOs establishing a repayment mechanism to support their participation in a two-sided model beginning with performance 2022. Therefore, ACOs entering a two-sided model for an agreement period beginning on January 1, 2022, and ACOs with an earlier start date participating in the BASIC track's glide path and entering a two-sided model starting on January 1, 2022, would have established repayment mechanism amounts determined according to the proposed amount calculation methodology, if finalized. Therefore, we would not consider such ACOs eligible for the proposed one-time opportunity to decrease the amount of their repayment mechanism.
Under this proposal, an eligible ACO that established a repayment mechanism to support its participation in a two-sided model beginning on July 1, 2019, January 1, 2020, or January 1, 2021, may elect to decrease the amount Start Printed Page 39287 of its repayment mechanism if the recalculated repayment mechanism amount for performance year 2022 is less than the existing repayment mechanism amount. To determine if an ACO is eligible to lower its repayment mechanism amount, we propose to compare the ACO's existing repayment mechanism amount with the recalculated amount of the ACO's repayment mechanism based on its certified ACO participant list for performance year 2022, calculated in accordance with § 425.204(f)(4)(iii) (including any modifications finalized to the recalculation methodology which would be effective and applicable January 1, 2022, as discussed in this proposed rule). If the recalculated repayment mechanism amount for performance year 2022 is less than the existing repayment mechanism amount, the ACO would be eligible to decrease the amount of its repayment mechanism to the recalculated amount. Under this approach, we would permit a one-time decrease in the repayment mechanism amount even for relatively small differences in dollar amounts.
We propose that CMS would notify the ACO in writing that the ACO may elect to decrease the amount of its repayment mechanism. If this proposal is finalized, we anticipate that we would notify an ACO of its opportunity to reduce its repayment mechanism amount after the start of performance year 2022. We also propose that an ACO must submit such election, and revised repayment mechanism documentation, in a form and manner and by a deadline specified by CMS. We expect that the deadline for submitting the election and revised repayment documentation would be 30 days from the date of the written notice from CMS, although we recognize that there may be circumstances that necessitate a longer timeframe. CMS would review the revised repayment mechanism documentation and may reject the election if the repayment mechanism documentation does not comply with the requirements of § 425.204(f).
We propose to amend § 425.204 to add paragraph (f)(4)(v) to establish the policy and relevant procedure that would allow eligible ACOs that established a repayment mechanism to support their participation in a two-sided model beginning on July 1, 2019, January 1, 2020, or January 1, 2021, to elect to lower the amount of their repayment mechanism arrangements.
(4) Threshold for Increasing Repayment Mechanism Amounts
In accordance with § 425.204(f)(4)(iii), for agreement periods beginning on or after July 1, 2019, CMS recalculates the ACO's repayment mechanism amount before the second and each subsequent performance year in the agreement period based on the certified ACO participant list for the relevant performance year. If the recalculated repayment mechanism amount exceeds the existing repayment mechanism amount by at least 50 percent or $1,000,000, whichever is the lesser value, CMS notifies the ACO in writing that the amount of its repayment mechanism must be increased to the recalculated repayment mechanism amount. Within 90 days after receipt of such written notice from CMS, the ACO must submit for CMS approval documentation that the amount of its repayment mechanism has been increased to the amount specified by CMS.
In establishing the annual repayment mechanism amount recalculation policy in earlier rulemaking (83 FR 67930), we explained the purpose of this approach was to address changes in the ACO's composition of ACO participant TINs and the individuals who bill through the participant TINs over the course of an agreement period and to ensure the adequacy of an ACO's repayment mechanism. In establishing the annual recalculation policy (83 FR 67932), we explained that a threshold of 50 percent or $1,000,000 would likely require an increased repayment mechanism amount only for ACOs that had the largest changes in their estimated repayment mechanism value (the top 5 to 10 percent of ACOs). We believed this approach would minimize an ACO's administrative burden and financial institution fees while adjusting for meaningful changes in repayment mechanism amounts that would help protect the Medicare Trust Funds.
We continue to believe that the annual repayment mechanism amount recalculation serves an important function in identifying the need for repayment mechanism increases when an ACO's composition changes. Such changes could result in higher expenditures for the ACO's assigned beneficiaries, higher ACO participant revenue, or a larger assigned beneficiary population. Each of these changes could increase the amount of potential shared losses for an ACO under a two-sided model.
Based on our operational experience with the recalculation policy, we have found that ACOs whose recalculated repayment mechanism amount is at least 50 percent higher than their existing amount, but less than $1,000,000 more, tend to be low revenue ACOs with relatively smaller existing repayment mechanism amounts, typically less than $300,000. Further, based on our operational experience and input from ACOs and other program stakeholders, modifications to repayment mechanism arrangements to revise the amount are burdensome for ACOs. These modifications are time consuming to arrange, and can result in additional fees charged by financial institutions for ACOs to modify their arrangements, in addition to requiring ACOs to set aside additional funds (such as with escrow accounts). We believe the burden for these ACOs to increase their repayment mechanism amounts is disproportional to the benefit to CMS in the availability of additional repayment mechanism arrangement funds to support repayment of losses.
Further, we believe it is timely to revisit the amount increase thresholds under the repayment mechanism amount recalculation policy in light of our proposal described in section III.J.3.b.(1) of this proposed rule to reduce the amounts required for repayment mechanism arrangements. If we finalize our proposal to reduce by one-half the repayment mechanism amounts, the 50 percent threshold of the amount recalculation provision would be proportionally lower, and the burden for these ACOs to increase their repayment mechanism amounts would be even more disproportional to the benefit to CMS.
We believe that requiring an increase in the repayment mechanism amount if the recalculated amount for the performance year is at least $1,000,000 greater than the existing amount balances our interest in ensuring the repayment mechanism amount accounts for significant changes in an ACO's composition during its agreement period, while avoiding burdensome repayment mechanism modifications for relatively small dollar amounts. Therefore, we propose to amend the regulations at § 425.204(f)(4)(iii)(A) to remove the 50 percent threshold from the annual repayment mechanism increase threshold, such that if the recalculated repayment mechanism amount exceeds the existing repayment mechanism amount by at least $1,000,000, CMS would notify the ACO in writing that the amount of its repayment mechanism must be increased to the recalculated repayment mechanism amount. We anticipate this approach would reduce the number of ACOs required to annually increase their repayment mechanism amounts and would further simplify the repayment mechanism amount calculations. Start Printed Page 39288
We propose that this modification would be effective and applicable on January 1, 2022. If finalized as proposed, the revised threshold would be used in determining required repayment mechanism increases for performance year 2022, and subsequent performance years.
4. Reducing Shared Savings Program Application Burden
a. Background
In order to participate in the Shared Savings Program, a prospective ACO must submit an application and certify that it satisfies the eligibility and other requirements of the Shared Savings Program, including regulatory requirements to disclose prior participation. Under § 425.204(b), an ACO must disclose in its Shared Savings Program application whether the ACO, its ACO participants, or its ACO providers/suppliers have participated in the Shared Savings Program under the same or a different name or is related to or affiliated with another Shared Savings Program ACO. The ACO must also disclose in the application whether the related participation agreement was terminated (voluntarily or involuntarily), the cause for prior termination, and what safeguards are in place to enable the ACO, ACO participant, or ACO provider/supplier to participate in the program for the full term of the participation agreement. We refer to both of these disclosure requirements as the "prior participation disclosure requirement."
Our application evaluation criteria for renewing ACOs and re-entering ACOs are designed to prevent ACOs with a history of poor performance or a history of noncompliance with the Shared Savings Program regulations from participating in the program. Under § 425.224(b), we determine whether to approve an application based on an evaluation of several criteria, including the following: (1) Whether the ACO has a history of noncompliance with the program's requirements, including a failure to meet the quality performance standard; (2) the ACO's history of financial performance; (3) whether an ACO under a two-sided model failed to repay shared losses owed to the program; and (4) whether the ACO has demonstrated in its application that it has corrected the deficiencies that caused it to perform poorly or to be terminated.
Additionally, under § 425.204(c)(6), all applicants, including initial, renewing, and re-entering applicants, must submit as part of the application process and upon request by CMS, documents demonstrating that their ACO participants, ACO providers/suppliers, and other individuals or entities performing functions or services related to ACO activities are required to comply with the requirements of the Shared Savings Program. Such documents must include sample or form agreements and the first and signature pages of each executed ACO participant agreement. We may request all pages of an executed ACO participant agreement to confirm that it conforms to the sample form agreement submitted by the ACO. The ACO is also required to certify that each of its ACO participant agreements meet all Shared Savings Program requirements in 42 CFR part 425.
Under § 425.116(c), we also require an ACO to submit an executed ACO participant agreement for each participant at the time of its initial application, participation agreement renewal process, and when making additions to its list of ACO participants in accordance with § 425.118. The agreements may be submitted in the form and manner specified under § 425.204(c)(6) or as otherwise specified by CMS.
b. Proposed Revisions
In conducting Shared Savings Program application reviews, we have found that the document submission requirements in §§ 425.204(b) and (c)(6), and 425.116(c) substantially increase applicant burden without lending significant value to our review of an organization's application to confirm that the ACO meets the eligibility requirements for participation. We therefore propose to revise §§ 425.204(b) and (c)(6), and § 425.116(c) to reduce applicant burden.
First, we propose to modify § 425.204(b) so that the prior participation disclosure requirement is prescribed only at the request of CMS during the application process—rather than as a mandatory submission with the ACO's initial or renewal application. Under this proposal, we would continue review of an ACO's history of compliance with the Shared Savings Program regulations and the ACO's quality and financial performance results in accordance with § 425.224(b), at CMS' request.
Second, we propose to modify § 425.204(c)(6) to remove provisions requiring an ACO to submit sample ACO participant agreements during the application process. Under this proposal, sample ACO participant agreements and the first and signature pages of each executed ACO participant agreement would need to be submitted during the application process only if requested by CMS, rather than as a mandatory submission with the ACO's initial or renewal application. The ACO must still certify that the all of its ACO participant agreements comply with the regulatory requirements of the Shared Savings Program. CMS would retain the discretion to request ACO participant agreement documentation at any time during an agreement period.
Third, we propose to modify § 425.116(c) to remove provisions requiring an ACO to submit an executed ACO participant agreement for each ACO participant at the time of its initial application or participation agreement renewal process. We would retain the requirement that an ACO must submit an executed ACO participant agreement for each ACO participant that it requests to add to its list of ACO participants. We believe these three proposals will collectively reduce the administrative and programmatic burden for ACOs significantly without sacrificing program integrity and reinforce that ACOs are responsible for ensuring their ACO participant agreements meet Shared Savings Program requirements.
(1) Prior Participation Requirement (§ 425.204(b))
We propose to modify § 425.204(b) so that the prior participation disclosure requirement is prescribed only at the request of CMS—rather than as a mandatory submission with the ACO's initial or renewal application. During the application cycle and for the purposes of evaluating program eligibility, CMS already determines prior participation for initial and re-entering ACO applicants by reviewing ACO- and ACO participant-level information. We screen all ACO applicants, initial ACOs and re-entering ACOs, to determine if they have participated in the Shared Savings Program, including if their prior participation agreement was terminated early (voluntarily or involuntarily). We also identify initial ACOs as re-entering ACOs if greater than 50 percent of their ACO participants were included on the ACO participant list under § 425.118, of the same ACO in any of the 5 most recent performance years prior to the agreement start date (§ 425.20), in order to hold these ACOs accountable for their ACO participants' experience with the program.
Additionally, all ACO participants and ACO providers/suppliers undergo a rigorous screening process during the application cycle (and throughout the agreement period, if approved to participate in the program) to ensure Start Printed Page 39289 they meet certain program requirements. CMS' screening processes are protective of the program and provide CMS with eligibility information about individual ACO participants including: Medicare-enrollment status (§ 425.20); program integrity history (§ 425.305(a)); any participation in other Medicare shared savings initiatives (§ 425.114); and participation in other Shared Savings Program ACOs, including whether the ACO participant submitted claims used in beneficiary assignment (§ 425.306). These robust application screening processes for ACO participants and ACO providers/suppliers provides necessary information about ACOs and individual ACO participants.
We propose to revise § 425.204(b) to provide that, upon request by CMS during the application cycle, the ACO must submit information regarding prior participation in the Shared Savings Program by the ACO, its ACO participants, or its ACO providers/suppliers, including such information as may be necessary for CMS to determine whether to approve an ACO's application in accordance with § 425.224(b). Under this proposal, and to ensure future compliance, we may request additional information from an ACO concerning its prior participation or the prior participation of their ACO participants or its ACO providers/suppliers. In that case, we would require the ACO to include in its response assurances describing how they will remain in compliance with program requirements—particularly as to the quality performance standard and financial performance—while completing the full term of the participation agreement. Thus, with the robust evaluation criteria of § 425.224(b) for renewing and re-entering ACOs and the application screening processes for ACO participants and ACO providers/suppliers, we believe we can effectively evaluate an ACO's prior participation and determine its suitability to participate in the program without requiring ACOs to self-identify prior participation under § 425.204(b), including the cause of termination (if any), and what safeguards have been put into place.
(2) Submission of Sample Agreements (§ 425.204(c)(6))
We propose to revise § 425.204(c)(6) to require an ACO to submit sample or form ACO participant agreement documents during the application cycle only upon request. We review sample agreements to ensure they contain the language required under § 425.116. However, it is ultimately the ACO's responsibility to ensure that all of its ACO participant agreements comply with the Shared Savings Program requirements. We have concerns that CMS review of sample participant agreements gave the incorrect impression that CMS had determined that an agreement met all regulatory requirements.
We believe that removing the requirement at § 425.204(c)(6) to submit sample agreements reduces administrative burden on both ACOs and CMS in the submission and reviewing of sample agreements. Under our proposal, we would retain the ability to request ACO sample participant agreements during the application cycle and at any point during an agreement period. Although we would not expect to routinely request during the application cycle that an ACO submit copies of ACO participant agreement documentation, it could be particularly useful in the case of ACOs that have a history of noncompliance with § 425.116 or other program requirements.
We would retain the requirement in § 425.204(c)(6) that the ACO must certify that each of its ACO participant agreements comply with the requirements of the Shared Savings Program. We believe this modification to § 425.204(c)(6) more clearly prescribes that the ACO is ultimately responsible for compliance with all program requirements.
(3) Submission of Executed Participant Agreements (§ 425.116(c))
Lastly, we propose to modify § 425.116(c) to remove language requiring an ACO to submit an executed ACO participant agreement for each ACO participant at the time of its initial application and during the participation agreement renewal process. The submission of agreements at the time of initial application will be governed by § 425.204(c)(6) and does not need to be addressed in § 425.116(c). Moreover, unless there have been amendments to an ACO participant agreement, we would not need to collect for a second time executed ACO participant agreements with ACO participants who are actively participating in an ACO at the time it is applying to renew its participation agreement with the program. In our experience, neither ACOs nor their ACO participants have frequently raised concerns about continuing participation with an ACO into a new agreement period, nor notified CMS of changes to ACO participant agreements. An ACO must notify CMS within 30 days after the termination of an ACO participant agreement in accordance with § 425.118(b)(2).
We would retain the remainder of § 425.116(c), which requires ACOs to submit ACO participant agreements when requesting additions to their ACO participant lists in accordance with § 425.118 and specifies that the agreements may be submitted in the form and manner specified under § 425.204(c)(6). We note that although ACOs may request additions to an ACO participant list at specified times during a performance year, all approved ACO participant list additions become effective on January 1 of the following performance year (§ 425.118(b)(1)(ii)).We continue to find value in reviewing executed ACO participant agreements in these circumstances. ACO participant additions may take the form of an initial applicant or renewing ACO submitting proposed ACO participants (that may or may not have participated with another ACO), or a currently participating ACO adding proposed participants (that may or may not be participating with another ACO) to their ACO participant list. Collecting executed agreements (which may include collecting only the first and signature page(s) under § 425.204(c)(6)) for additions to an ACO's participant list provides CMS with evidence that the ACO and the participant are each aware of the agreement and are participating together in the Shared Savings Program. Should CMS need to review executed participant agreements other than when ACOs are adding to their list of ACO participants, CMS can request them at that time under proposed § 425.206(c)(6) or under its audit authority in accordance with § 425.314.
5. Beneficiary Information Notices for ACOs With Prospective Assignment
a. Background
To ensure full transparency between Shared Savings Program ACOs and the beneficiaries they serve, § 425.312(a)(1) provides that an ACO must ensure that Medicare FFS beneficiaries are notified about all of the following: (1) That its ACO providers/suppliers are participating in the Shared Savings Program; (2) the beneficiary's opportunity to decline claims data sharing; and (3) the beneficiary's ability to, and the process by which, he or she may identify or change identification of the individual he or she designated as their primary clinician for purposes of voluntary alignment. Under § 425.312(a)(2)(i), we require this information to be furnished by an ACO participant posting signs in its facilities Start Printed Page 39290 and, in settings in which beneficiaries receive primary care services, making standardized written notices available upon request.
In the December 2018 final rule, we specified at § 425.312(a)(2)(ii) that, during the performance year beginning on July 1, 2019 and each subsequent performance year, the information must also be furnished by an ACO or ACO participant providing each beneficiary with a standardized written notice prior to or at the first primary care visit of the performance year in the form and manner specified by CMS. While we continued to encourage ACO participants to distribute the notice to beneficiaries at the point of care to address any beneficiary questions or concerns, the flexibility was granted so that an ACO or its ACO participants could distribute beneficiary notifications through electronic transmission (such as email) or mail. We note that, regardless of the method of notification used, CMS may review evidence related to the dissemination of the beneficiary information notice at any time under its audit authority in accordance with § 425.314.
In the December 2018 final rule, we finalized requirements to further strengthen the beneficiary notification requirements. Specifically, we made changes to permit an ACO (not just its ACO participants) to disseminate the beneficiary information notice to beneficiaries, to require the notice to be provided prior to or at the first primary care visit of each performance year, and to permit the distribution of the notice through electronic transmission (such as email) or mail. We believe the modifications made to the beneficiary notification requirements in the December 2018 final rule help empower beneficiary choice, support beneficiary engagement, improve transparency, and ensure that beneficiaries are informed about the program and how it may affect their care and the use of their data. In making the decision to provide a CMS-approved template, we aimed to make the notification a comprehensive resource that compiled information about the program and what participation in the program means for beneficiary care. In addition, we believed that the availability of CMS-approved beneficiary notification templates would mitigate the potential for administrative and operational burden on providers.
b. Proposed Revisions
In considering the several different iterations of the beneficiary notice requirement over the history of the program,[102] we have concluded that the current requirement to provide beneficiary notifications prior to or at the first primary care visit of the performance year is overly broad with respect to ACOs that have selected the prospective assignment methodology. Such ACOs are currently required to provide the beneficiary notice to beneficiaries who will never be assigned to the ACO for the performance year.
As noted earlier, the intention of the beneficiary notification is to empower beneficiaries, encourage beneficiary engagement, and improve transparency. For an ACO participating under the prospective assignment methodology, described in § 425.400(a)(3), all of the ACO's beneficiaries are assigned at the beginning of the performance year. Under § 425.704(d)(1)(ii), such ACOs may request beneficiary identifiable claims data only for FFS beneficiaries that appear on the ACOs' prospective assignment list at the beginning of the performance year and who have not opted out of data sharing. Beneficiaries who are not assigned to an ACO that has selected prospective assignment will never be assigned to the ACO during the relevant performance year and will not be subject to data sharing with the ACO. In short, such beneficiaries have no need to receive any information about the Shared Savings Program at the beginning of a performance year. Therefore, we believe that it would cause unnecessary confusion for beneficiaries to receive the notice if they are not prospectively assigned to an ACO because the notice describes details that will not apply to them (for example, information on data sharing and the SNF 3-day rule waiver).
In contrast, for ACOs under preliminarily prospective assignment with retrospective reconciliation, the preliminary prospective assignment list provided to the ACO at the beginning of the performance year does not include all FFS beneficiaries who may ultimately be assigned to the ACO. As such, we continue to believe all FFS beneficiaries receiving primary care services from ACO providers and/or suppliers should receive the notice. This ensures that all beneficiaries ultimately assigned to the ACO would be informed of their right to decline data sharing.
We propose to amend § 425.312(a)(2) to set forth different beneficiary notification obligations depending on the assignment methodology selected by the ACO. Specifically, we propose at § 425.312(a)(2)(ii) to provide that, in the case of an ACO that has selected preliminary prospective assignment, the ACO or ACO participant must provide the standardized written beneficiary notice to each fee-for-service beneficiary prior to or at the first primary care visit of the performance year. We propose to add at § 425.312(a)(2)(iii) that, in the case of an ACO that has selected prospective assignment, the ACO or ACO participant must provide the standardized written notice to each prospectively assigned beneficiary prior to or at the first primary care visit of the performance year.
We continue to believe that the requirement to provide the beneficiary information notice is important to empowering beneficiaries and providing important information about their care, but we also understand that the current requirement of disseminating the beneficiary information notice annually may have the potential to be overly burdensome to ACOs and/or their ACO participants. We seek comment from stakeholders on whether we should modify the frequency with which the beneficiary information notice must be furnished, for example, by reducing the frequency of the existing requirement from annually to once per agreement period. We expect that ACOs would be required to provide the notice to their FFS beneficiaries, based on assignment methodology, including any beneficiaries who seek care from ACO providers/suppliers throughout the agreement period. ACOs would also be responsible for issuing the beneficiary information notice during subsequent agreement periods, reminding assigned beneficiaries of their participation in the Shared Savings Program. Beneficiaries would continue to be able to modify their decision on whether to allow data sharing at any point. While we have received feedback from program stakeholders regarding the current annual requirement being too frequent, potentially confusing beneficiaries, and increasing burden on ACOs, reducing the frequency to once per agreement period may ultimately be too infrequent, given the many changes a beneficiary may experience with their health and life in general in that span of time. It is our goal to provide the notifications in a way that will continue to empower and inform beneficiaries without overwhelming or confusing them with Start Printed Page 39291 information. We encourage stakeholders to provide feedback on this suggestion, as well as other suggestions they may have in the spirit of burden reduction with regard to the beneficiary notification requirement as well as transparency and beneficiary engagement.
6. Seeking Comment on Considerations Related to the Use of Regional FFS Expenditures in Establishing, Adjusting, Updating, and Resetting the ACO's Historical Benchmark
a. Background on the Shared Savings Program Benchmarking Methodology
Section 1899(d)(1)(B)(ii) of the Act addresses how ACO benchmarks are to be established and updated under the Shared Savings Program. This provision specifies that the Secretary shall estimate a benchmark for each agreement period for each ACO using the most recent available 3 years of per beneficiary expenditures for Parts A and B services for Medicare FFS beneficiaries assigned to the ACO. This benchmark shall be adjusted for beneficiary characteristics and such other factors as the Secretary determines appropriate and updated by the projected absolute amount of growth in national per capita expenditures for Parts A and B services under the original Medicare FFS program, as estimated by the Secretary. The benchmark shall be reset at the start of each agreement period. In addition to the statutory benchmarking methodology established in section 1899(d) of the Act, section 1899(i)(3) of the Act grants the Secretary the authority to use other payment models, including payment models that would use alternative benchmarking methodologies, if the Secretary determines that doing so would improve the quality and efficiency of items and services furnished under the Medicare program and that the alternative methodology would result in program expenditures equal to or lower than those that would result under the statutory payment model.
In the November 2011 final rule establishing the Shared Savings Program, we adopted policies for establishing, updating, and resetting the benchmark at § 425.602. The Shared Savings Program's regulations have since evolved to include different benchmarking methodologies, including modifications to § 425.602, and the addition of separate benchmarking policies for ACOs entering a second or subsequent agreement period at § 425.603. Benchmarking policies applicable to all ACOs in agreement periods beginning on July 1, 2019, and in subsequent years, are specified in § 425.601. We refer readers to discussions of the benchmark calculations in earlier rulemaking for details on the development of the current policies (see November 2011 final rule, 76 FR 67909 through 67927; June 2015 final rule, 80 FR 32785 through 32796; June 2016 final rule, 81 FR 37953 through 37991; and December 2018 final rule, 83 FR 68005 through 68030).
For details on the benchmarking calculations, we refer readers to the regulations at 42 CFR part 425, subpart G, as well as the Medicare Shared Savings Program, Shared Savings and Losses and Assignment Methodology Specifications (version #9, February 2021), available at https://www.cms.gov/files/document/medicare-shared-savings-program-shared-savings-and-losses-and-assignment-methodology-specifications.pdf-0.
In the following discussion, we summarize select aspects of the Shared Savings Program's benchmarking methodology and related concerns that have been expressed by ACOs and other stakeholders. We specify some considerations based on our initial analyses of these issues, and seek comment on considerations that may inform future policy developments. However, we note that we are still in the process of monitoring program calculations based on the initial performance years of experience under the new participation options and program modifications that were adopted as part of the Pathways to Success rulemaking and are applicable for ACOs in agreement periods beginning on July 1, 2019, and in subsequent years, including changes to the benchmarking methodology (finalized in the December 2018 final rule (83 FR 67816)). In addition, we are also monitoring the impact of any anomalies in Medicare FFS expenditures and healthcare utilization by Medicare FFS beneficiaries resulting from the COVID-19 Public Health Emergency, which we anticipate could further inform our considerations of future modifications to Shared Savings Program benchmarking policies (see for example, discussion in the CY 2021 PFS final rule, 85 FR 84770 through 84785).
b. Request for Comment on Calculation of the Regional Adjustment and Blended National-Regional Growth Rates for Trending and Updating the Benchmark
In calculating the historical benchmark, CMS uses historical expenditures for the ACO's assigned beneficiaries, as well as factors based on regional FFS expenditures, factors based on national FFS expenditures, and factors based on a blend of national and regional FFS expenditures. As we have described in earlier rulemaking, incorporating regional expenditures into benchmark calculations makes the ACO's cost target more independent of its historical expenditures and more reflective of FFS spending in its region (see for example, 81 FR 37950, 37951 and 37955). We have also acknowledged in earlier rulemaking that the incorporation of factors based on regional FFS expenditures into ACO benchmarks will have varying effects on ACOs depending on each organization's individual circumstances (see for example, 81 FR 37950, 37954 through 37957, and 81 FR 37975 through 37977; and 83 FR 67816, 68017 and 68026).
In accordance with § 425.601(a)(8), CMS adjusts historical benchmark expenditures by Medicare enrollment type (ESRD, disabled, aged/dual eligible, aged/non-dual eligible) by a percentage of the difference between the average per capita expenditure amount for the ACO's regional service area and the ACO's historical benchmark amount (referred to herein as the "regional adjustment"). The percentage that is applied in calculating the regional adjustment is determined in accordance with § 425.601(f) and depends on whether the ACO has lower or higher spending compared to the ACO's regional service area and the agreement period for which the ACO is subject to the regional adjustment, according to the phase-in schedule of the applicable weights. CMS caps the per capita dollar amount of the regional adjustment for each Medicare enrollment type at a dollar amount equal to ±5 percent of national per capita expenditures for Parts A and B services under the original Medicare FFS program in benchmark year (BY) 3 for assignable beneficiaries (as defined in § 425.20) in that Medicare enrollment type identified for the 12-month calendar year corresponding to BY3.
In accordance with § 425.601(a)(5), in establishing and resetting an ACO's benchmark, CMS trends forward expenditures for each benchmark year (BY1 and BY2) to BY3 dollars using a blend of national and regional growth rates, making separate calculations for each Medicare enrollment type. Similarly, in accordance with § 425.601(b), CMS updates the historical benchmark annually for each year of the agreement period using a blend of national and regional growth rates between BY3 and the performance year. As described in the December 2018 final rule (83 FR 68024 through 68030), we Start Printed Page 39292 used our statutory authority under section 1899(i)(3) of the Act to adopt this policy under which we update the historical benchmark using a blend of national and regional growth rates, rather than the projected absolute amount of growth in national per capita expenditures for Parts A and B services under the original Medicare FFS program as required under section 1899(d)(1)(B)(ii) of the Act. CMS accounts for an ACO's penetration in its region when calculating the national-regional blended growth rates, by placing a higher weight on the national component of the blend and a lower weight on the regional component as the ACO's penetration in its region increases.
In determining regional FFS expenditures, CMS uses average county FFS expenditures for assignable beneficiaries, including the ACO's assigned beneficiaries, in each county in the ACO's regional service area for the 12-month calendar year corresponding to the relevant benchmark or performance year.[103] [104] CMS weights these county-level FFS expenditure amounts by the proportion of the ACO's assigned beneficiaries residing in each county, with all calculations performed separately by Medicare enrollment type. Refer to § 425.601(c) (calculating county expenditures) and (d) (calculating regional expenditures).
ACOs and other program stakeholders have expressed concerns with the approach to determining regional FFS expenditures using a population of assignable beneficiaries that includes the ACO's assigned beneficiaries, including with respect to the impact on the calculation of the regional adjustment and the blended national-regional growth rate used to trend and update an ACO's historical benchmark, suggesting this policy results in relatively lower benchmarks for ACOs, particularly ACOs with high market penetration in their regional service area, which may tend to be ACOs located in rural areas.[105] For example, the National Association of ACOs' (NAACOS') summary "Fixing the Rural Glitch" explains its belief that by including the costs of all beneficiaries in the regional adjustment—both those assigned to the ACO and those who are not—CMS penalizes an ACO for reducing costs relative to its regional competitors. That is, as an ACO reduces the costs of its own assigned beneficiaries, it also reduces the average regional costs. According to NAACOS, this will ultimately reduce savings for efficient ACOs in all areas, but the effect may be most dramatic for rural ACOs because they will tend to care for a greater portion of their region's total beneficiary population than an urban ACO.[106] As another example, Aledade suggests that incorporating factors based on regional FFS expenditures into the Shared Savings Program's benchmarking methodology systemically penalizes ACOs with a large market share when they reduce costs, leading to disparate payments to ACOs with identical performance.[107] ACOs and other program stakeholders have suggested that CMS remove the effects of the ACO's own performance from factors based on regional FFS expenditures, such as by excluding an ACO's assigned beneficiaries from the population of assignable beneficiaries used to determine regional FFS expenditures.[108] Other alternatives that have been suggested to address these concerns include capping an ACO's penetration in the region at 50 percent by Medicare enrollment type, or expanding the ACO's region.[109] In recent years, legislative changes have been introduced, which if enacted would require the removal of the ACO's assigned beneficiaries from regional expenditure calculations.[110] [111] We appreciate ACOs and other program stakeholders bringing their concerns, and suggested alternatives, to our attention. We have begun to analyze these concerns about the use of factors based on regional FFS expenditures in calculating ACO benchmarks, and to consider possible modifications to the Shared Savings Program's benchmarking methodology to ensure the sustainability of the program's financial models. We note that any such modifications would need to be adopted through notice and comment rulemaking.
In this section of this proposed rule we discuss some of our considerations based on our initial analyses of stakeholders' concerns. We continue to investigate these concerns and perform additional simulations. We seek comment on these considerations and other related issues, as well as suggested approaches to modifying the program's benchmarking methodology, which could inform future rulemaking.
There may be several possible approaches that we could consider for removing an ACO's assigned beneficiaries from the assignable beneficiary population used in regional expenditure calculations, which would vary in the degree of additional program calculations and the level of complexity. We simulated the impact of removing an ACO's assigned beneficiaries from the regional expenditure calculations using an approach that would pose relatively limited operational burden and would leverage data elements already computed under the current benchmarking methodology. This approach relies on the premise that per capita risk-adjusted regional FFS expenditures for all assignable beneficiaries in an ACO's regional service area (a) can be interpreted as a weighted average of per capita risk-adjusted FFS expenditures for the Start Printed Page 39293 ACO's assigned beneficiaries (b) and per capita risk-adjusted FFS expenditures for assignable beneficiaries in the region who are not assigned to the ACO (c), where the weight on (b) is the ACO's regional market share[112] and the weight on (c) is one minus the ACO's regional market share. Shown as an equation this is:
(a) = [(b) × (ACO's regional market share)] + [(c) × (1−ACO's regional market share)].
Thus, to remove the ACO's assigned beneficiaries from the regional expenditure calculation, we would insert the applicable values into the above equation and solve for (c) by rearranging the equation as follows:
(c) = {(a)−[(b) × (ACO's regional market share)]}/(1−ACO's regional market share).
By using such ACO- and regional-level values, this approach, performed separately by Medicare enrollment type, would avoid the need to calculate individualized ACO county-level risk-adjusted expenditures. We seek comment on the approach we have outlined, or alternative approaches to calculating regional FFS expenditures without an ACO's assigned beneficiaries. In particular, we seek comment on specific approaches that would strike the balance of achieving the desired outcome of removing the ACO's assigned beneficiaries from program calculations without introducing an inordinate amount of operational and administrative complexity such that the steps and data included in the calculations can be understood by ACOs and other program stakeholders, and the potential for calculation errors is minimized.
We performed initial simulations, for a subset of Shared Savings Program ACOs, using data for the 6-month performance year starting on July 1, 2019 (sometimes referred to as PY 2019A), for which expenditures were determined based on expenditures for CY 2019, to observe the effects of potential modifications to the benchmarking methodology. In performing these simulations, we used the aforementioned approach for removing expenditures for the ACO's assigned beneficiaries from the calculation of regional FFS expenditures, by removing the impact of an ACO's assigned beneficiaries from the assignable population as weighted by the ACO's regional market share. Specifically, we simulated the effects on the per capita updated benchmark of several alternate policies that would remove an ACO's assigned beneficiaries from regional expenditures used to trend and update the benchmark (either alone or as part of a national-regional blend) or from regional expenditures used to calculate the regional adjustment, or from both. When looking at average impacts by quintile of the ACO's penetration in its regional service area (that is, market share) and rural or non-rural status, the various alternatives resulted in estimated increases in the updated benchmark by amounts ranging from 0.1 percent to 1.4 percent, with ACOs with higher market shares tending to see slightly higher average increases than ACOs with lower market shares and rural ACOs seeing slightly higher average increases than non-rural ACOs. We also observed that some ACOs experienced decreases in their benchmark amounts, ranging from −0.02 percent to −1.5 percent under these simulations of alternate benchmarking policies. We note that additional analysis would be needed to consider the impact of such policies on a broader set of ACOs participating in the Shared Savings Program, to include ACOs that did not participate in a 6-month performance year from July 1, 2019, through December 31, 2019. We seek comment on this estimated range of impacts on ACO benchmark values, and on the potential mixed effects on ACOs that could result from modifications to the benchmarking methodology.
In considering alternative benchmarking methodologies to address ACOs' penetration in their regional service areas, we believe it is important to consider what would constitute heavy penetration by an ACO in its regional service area, and the extent to which market penetration should be considered in benchmark calculations. Based on preliminary analysis of data for CY 2019 using PY 2021 ACO Participant Lists for all ACOs participating in the program as of January 1, 2021, the median ACO regional market share was approximately 16.2 percent, with a minimum of 0.9 percent and a maximum of 59.2 percent. Further, 90 percent of ACOs had a regional market share of less than 37.8 percent, and 80 percent of ACOs had a regional market share of less than 29.3 percent. Accordingly, we seek comment on what would constitute heavy penetration in the ACO's regional service area and how removing the ACO's assigned beneficiaries from regional calculations, dependent on the level of penetration, could either increase or decrease the ACO's benchmark. We also seek comment on approaches that could strike a balance between adjusting program policies to address impacts on potentially few ACOs that are heavily penetrated in their regional service area while maintaining stability for most ACOs that have relatively low penetration in their regional service area.
We seek comment on the following considerations, and other possible unintended consequences that could result from removing an individual ACO's assigned beneficiaries from regional calculations.
- Would this approach create incentives for ACOs to have assigned beneficiaries who are healthier than the remaining comparison population that is the basis for benchmark factors based on regional FFS expenditures (so as to yield a higher benchmark), which could lead ACOs to seek out healthier beneficiaries and avoid at-risk or higher-cost beneficiaries?
++ Would this approach incent the formation of large ACOs within a particular market to obtain the most competitive benchmarks resulting in market consolidation, and discourage participation by relatively smaller ACOs, thus increasing costs for the Medicare Trust Funds if CMS pays larger amounts of shared savings to ACOs that have consolidated to take advantage of the ability to attract more low-cost beneficiaries in their region?
++ Would a change in the regional benchmarking methodology encourage ACOs to avoid at-risk or higher-cost beneficiaries and potentially exacerbate inequities in access to health care?
- We seek comment on the potential for negative impacts on ACOs that serve larger proportions of medically complex beneficiaries such as ACOs whose assigned beneficiary populations include larger proportions of beneficiaries who are medically complex and cared for in ambulatory or home-based settings or who reside in long term care facilities resulting from an approach that removes the ACO's assigned beneficiaries from the assignable beneficiary population used to determine regional FFS expenditures. Would such an approach yield a benchmark so low that such ACOs have little incentive to participate in the Shared Savings Program?
- Would removing an individual ACO's assigned beneficiaries result in regional FFS expenditures based on very small populations, thus introducing significant variability into Start Printed Page 39294 regional FFS expenditure trends used in benchmark calculations?
Additionally, we seek comment on whether removal of an ACO's assigned beneficiaries from regional FFS expenditure calculations would bring about a need to remove ACO assigned beneficiaries from other Shared Savings Program financial calculations based on a broader Medicare population, including factors based on national FFS expenditures, which are used in calculating blended national and regional expenditure trend and update factors, truncation points used in calculating benchmark and performance year expenditures, and the 5 percent cap on the regional adjustment.
We also seek comment on using other approaches to calculating benchmarks under the Shared Savings Program. In particular, we seek comment on alternatives to determining regional FFS expenditures that would reduce the influence of an ACO's assigned beneficiaries on regional expenditure calculations, such as basing these expenditures on a larger geographic area, including using state-level data, Core-Based Statistical Area (CBSA)-level data, or a combination of data for these larger geographic areas and county-level data (such as blended county/state regional expenditures). We also seek comment on alternative benchmarking methodologies that may incorporate data sources other than Medicare FFS expenditure trends, such as by incorporating factors based on Medicare Advantage rates, or other published trends.
We seek comment on considerations related to the potential use of our authority under section 1899(i)(3) of the Act to implement suggested modifications to the benchmarking methodology, in particular alternative approaches to updating the historical benchmark or other alternative benchmarking methodologies that diverge from the requirements of section 1899(d)(1)(B)(ii) of the Act, since to do so we must determine that the alternative payment methodology will improve the quality and efficiency of items and services furnished to Medicare beneficiaries, without resulting in additional program expenditures.
We also note that for each calendar year, CMS releases two public use files (PUFs): (1) County-level Aggregate Expenditure and Risk Score Data on Assignable Beneficiaries PUF, and (2) Number of ACO Assigned Beneficiaries by County PUF. These files are available online at https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/SSPACO/SSP_Benchmark. Stakeholders may find this data helpful to inform their consideration of these issues.
c. Request for Comment on the Shared Savings Program's Risk Adjustment Methodology
CMS takes into account changes in severity and case mix of the ACO's assigned beneficiary population when establishing the benchmark and also in adjusting the benchmark each performance year. In accordance with § 425.601(a)(3), in establishing the benchmark, CMS adjusts expenditures for changes in severity and case mix using prospective HCC risk scores. Pursuant to § 425.601(a)(10), CMS further adjusts the ACO's historical benchmark at the time of reconciliation for a performance year to account for changes in severity and case mix for the ACO's assigned beneficiary population between BY3 and the performance year (refer to § 425.601(a)(10); § 425.605(a)(1), (a)(2); § 425.610(a)(2), (a)(3)). In making this risk adjustment, CMS makes separate adjustments for the population of assigned beneficiaries in each Medicare enrollment type used in the Shared Savings Program (ESRD, disabled, aged/dual eligible, aged/non-dual eligible). CMS uses CMS-HCC prospective risk scores to adjust the historical benchmark for changes in severity and case mix for all assigned beneficiaries, subject to a cap of positive 3 percent for the agreement period. This cap is the maximum increase in risk scores allowed for each agreement period, such that any positive adjustments between BY3 and any performance year in the agreement period cannot be larger than 3 percent. That is, the risk ratios (ratio of performance year risk score to the BY3 risk score) applied to historical benchmark expenditures to capture changes in health status between BY3 and the performance year will never be higher than 1.030 for any performance year over the course of the agreement period. This cap is applied separately for the population of beneficiaries in each Medicare enrollment type.[113]
ACOs and other stakeholders have expressed concerns that the program's methodology for capping any increase in the risk adjustment to the historical benchmark, such that any positive adjustment between benchmark year 3 and any performance year in the agreement period cannot be larger than 3 percent, does not account for risk score growth in the ACO's regional service area, and thereby penalizes ACOs.[114] [115] In earlier rulemaking, commenters expressed that the 3 percent cap on risk score increases was especially problematic for ACOs whose regional service area includes a population of beneficiaries whose risk scores rise more than the cap. One commenter encouraged CMS to adopt a policy of applying a cap on risk score growth after accounting for regional increase in risk scores (85 FR 84784).
We seek comment on—
- Approaches, generally, to improving the risk adjustment methodology for the Shared Savings Program, and specifically for ACOs with medically-complex, high-cost beneficiaries.
- Approaches to risk adjustment that would balance the need for accurate and complete coding, while protecting against incentivizing coding intensity initiatives by ACO participants and ACO providers/suppliers (which may be even more problematic for ACOs with high penetration in their region) that increase risk score growth above the existing 3 percent cap.
- Alternate approaches that would increase the cap on an ACO's risk score growth in relation to risk score growth in the ACO's regional service area, such as:
++ Allowing the ACO risk score growth cap to increase by a percentage of the difference between the current 3 percent cap and risk score growth in the ACO's regional service area. In this alternate approach, the percentage applied would be equal to 1 minus the ACO's regional market share. This approach would raise the existing cap while limiting the ability for ACOs with high penetration in their region to increase their cap by engaging in coding intensity initiatives that raises the regional risk score.
++ Setting the ACO risk score growth cap at some level between the existing 3 percent risk score cap and the regional risk score growth, which would account for a portion of the regional risk score growth that exceeds the current cap.
- The potential interactions between policies to remove assigned Start Printed Page 39295 beneficiaries from the assignable beneficiary population used to calculate regional FFS expenditures and growth rates (described elsewhere in this section of this proposed rule), and policies addressing regional risk score growth.
K. Medicare Ground Ambulance Data Collection System
1. Background on Ambulance Services
Section 1861(s)(7) of the Act establishes an ambulance service as a Medicare Part B service where the use of other methods of transportation is contraindicated by the individual's condition, but only to the extent provided in regulations. Since April 1, 2002, payment for ambulance services has been made under the ambulance fee schedule (AFS), which the Secretary established under section 1834(l) of the Act. Payment for an ambulance service is made at the lesser of the actual billed amount or the AFS amount, which consists of a base rate for the level of service, a separate payment for mileage to the nearest appropriate facility, a geographic adjustment factor (GAF), and other applicable adjustment factors as set forth at section 1834(l) of the Act and § 414.610 of the regulations. In accordance with section 1834(l)(3) of the Act and § 414.610(f), the AFS rates are adjusted annually based on an inflation factor. The AFS also incorporates two permanent add-on payments and three temporary add-on payments to the base rate and/or mileage rate. The two permanent add-on payments at § 414.610(c)(5)(i) are: (1) A 50 percent increase in the standard mileage rate for ground ambulance transports that originate in rural areas where the travel distance is between 1 and 17 miles; and (2) a 50 percent increase to both the base and mileage rate for rural air ambulance transports. The three temporary add-on payments at sections 1834(l)(12)(A) and (13)(A) of the Act and § 414.610 are: (1) A 3 percent increase to the base and mileage rate for ground ambulance transports that originate in rural areas; (2) a 2 percent increase to the base and mileage rate for ground ambulance transports that originate in urban areas; and (3) a 22.6 percent increase in the base rate for ground ambulance transports that originate in "super rural" areas. Section 50203(a)(1) and (2) of the Bipartisan Budget Act (BBA) of 2018 (Pub. L. 115-123, February 9, 2018) includes an extension of the temporary add-on payments through December 31, 2022.
Our regulations relating to coverage of and payment for ambulance services are set forth at 42 CFR part 410, subpart B, and 42 CFR part 414, subpart H.
2. Statutory Requirements for the Ground Ambulance Providers and Suppliers To Submit Cost and Other Information
Section 50203(b) of the BBA of 2018 added paragraph (17) to section 1834(l) of the Act, which requires ground ambulance providers of services and suppliers to submit cost and other information. Specifically, section 1834(l)(17)(A) of the Act requires the Secretary to develop a data collection system (which may include use of a cost survey) to collect cost, revenue, utilization, and other information determined appropriate by the Secretary for providers and suppliers of ground ambulance services. Section 1834(l)(17)(B)(i) of the Act requires the Secretary to specify the data collection system by December 31, 2019, and to identify the ground ambulance providers and suppliers that would be required to submit information under the data collection system. Section 1834(l)(17)(D) of the Act requires that beginning January 1, 2022, the Secretary apply a 10 percent payment reduction to payments made under section 1834(l) of the Act for the applicable period to a ground ambulance provider or supplier that is required to submit information under the data collection system and does not sufficiently submit such information. The term "applicable period" is defined under section 1834(l)(17)(D)(ii) of the Act to mean, for a ground ambulance provider or supplier, a year specified by the Secretary not more than 2 years after the end of the period for which the Secretary has made a determination that the ground ambulance provider or supplier has failed to sufficiently submit information under the data collection system. Section 1834(l)(17)(F) of the Act requires that no later than March 15, 2023 and as determined necessary by MedPAC, MedPAC must submit a report to Congress on the information submitted by the ground ambulance providers and suppliers through the data collection system on the adequacy of payments for ground ambulance services and geographic variations in the cost of furnishing such services.
In the CY 2020 PFS final rule (84 FR 62864 through 62897), we implemented section 1834(l)(17) of the Act and codified regulations governing data reporting by ground ambulance providers and suppliers (referred collectively as "ground ambulance organizations") at §§ 414.601, 414.605, 414.610(c)(9), and 414.626. In the CY 2020 PFS final rule (84 FR 62863 through 629897), we finalized a data collection system that collects detailed information on ground ambulance provider and supplier characteristics including service areas, service volume, costs, and revenue through a data collection instrument, commonly referred to as the Medicare Ground Ambulance Data Collection Instrument, via a web-based system. This instrument includes the specific questions that will be asked of ground ambulance organizations about the total service volume, costs, and revenue associated with a provider or supplier's entire ground ambulance organization in such a way that MedPAC could use to calculate an average cost per ground ambulance transport. We refer the reader to our CY 2020 PFS final rule (84 FR 62863 through 62897) for more specifics on the establishment of the Medicare Ground Ambulance Data Collection System.
3. Proposed Revisions to the Medicare Ground Ambulance Data Collection Instrument
As described in the CY 2020 PFS final rule (84 FR 62867), the Medicare Ground Ambulance Data Collection Instrument uses screening questions and skip patterns so that it is applicable to all ground ambulance organizations regardless of their size, scope of operations and services offered, and structure. We stated that we believe this approach is easier to navigate and less time consuming to complete than a cost report template or instrument and that it minimizes respondent burden by directing ground ambulance organizations to only view and respond to questions that apply to their specific type of organization, all while still collecting the information required in sections 1834(l)(17)(A) of the Act.
The CY 2020 PFS final rule provided a detailed overview of the elements of the data collection instrument, including questions to collect information on costs, revenues, utilization (which CMS defines for the purposes of the data collection instrument as service volume and service mix), as well as the characteristics of ground ambulance organizations. Table 27 includes a high-level summary of the 13 sections of the Medicare Ground Ambulance Data Collection Instrument.
Start Printed Page 39296

We continue to receive ad hoc questions and feedback related to the Medicare Ground Ambulance Data Collection System and the Medicare Ground Ambulance Data Collection Instrument via three primary channels. First, we receive email and other communication from ground ambulance organizations via the CMS Ambulance Data Collection email inbox (AmbulanceDataCollection@cms.hhs.gov) and through other channels (for example, inquiries sent by organizations to Medicare Administrative Contractors (MACs) and then forwarded to CMS). These emails and other communications often include questions seeking clarification of instrument questions and their applicability to specific ground ambulance organization scenarios and context. We continue to update a Medicare Ground Ambulance Data Collection System Frequently Asked Questions (FAQ) document with answers to commonly asked questions. This document is available on the CMS website at https://www.cms.gov/Center/Provider-Type/Ambulances-Services-Center.html. Through review of questions and feedback, we have identified some instances where a clarification to the instrument language itself will likely be more useful and less burdensome to respondents than having to respond with reference to the FAQ document. Second, our contractor also asked a small number of ground ambulance organizations to complete and provide feedback on a paper version of the Medicare Ground Ambulance Data Collection Instrument. This feedback was helpful to identify some additional opportunities for clarification. Third, we continue to identify opportunities to clarify instructions and correct a small number of typos as we work to develop the web-based, programmed version of the Medicare Ground Ambulance Data Collection Instrument.
Based on information that we received via the three sources described above, we are proposing the following changes and clarifications to the Medicare Ground Ambulance Data Collection Instrument. The proposed changes and clarifications aim to reduce burden on respondents, improve data quality, or both.
a. Proposed Change to the Shared Services Questions in Section 2 (Organizational Characteristics)
One component of the data collection instrument is ground ambulance organization characteristics, which is information regarding the identity of the organization and respondent(s) service area, ownership, response time, and other characteristics (84 FR 62871 through 62875). One characteristic on which we sought information is organization type, including whether costs are shared with fire or police response or health care delivery operations (84 FR 62871). The instrument contains a number of questions that are relevant to the issue of shared costs.
Section 2, Question 7 asks "Which category best describes your ground ambulance operation?" and allows respondents to select one of the following options:
(a) Fire department-based; (b) Police or other public safety department-based (including all-hazards public safety organizations); (c) Government stand-alone emergency medical services (EMS) agency; (d) Hospital or other Medicare provider of services (such as skilled nursing facility); (e) Independent/proprietary organization primarily providing EMS services; (f) Independent/proprietary organization primarily providing non-emergency services; or (g) Other (please specify).
Section 2, Question 8 subsequently asks respondents answering a, b, or d to Question 7 to "confirm that your ground ambulance operation shares operational costs, such as building space or personnel, with these other operations." Start Printed Page 39297 Section 2, Question 9 asks "Does your ground ambulance operation share any operational costs, such as building space or personnel, with one of the following," offering respondents the following options: (a) A fire department (not presented if the response to Section 2, Question 7 is "a"); (b) A police or other public safety department (not presented if the response to Section 2, Question 7 is "b"); (c) A hospital or other Medicare provider of services (such as a skilled nursing facility) (not presented if the response to Section 2, Question 7 is "d"); (d) Another healthcare organization (excluding hospitals, skilled nursing facilities, or other Medicare provider of services); (e) Another healthcare organization (excluding hospitals, skilled nursing facilities, or other Medicare provider of services); (f) Other (specify).
Collectively, the purpose of these three questions is to collect information on whether a portion of organizations' costs and revenues may be related to services or operations other than providing ground ambulance services. When this occurs, ground ambulance organizations are presented with additional instructions specifying how they should report costs and revenues associated with providing ground ambulance services rather than these other services or operations.
Based on feedback from ground ambulance organizations, we believe the specific wording of Section 2, Question 9 may be confusing. The question asks respondents whether they share operational costs with "one of the following," implying respondents are limited to a single response, even though in some cases respondents may wish to select multiple responses. Furthermore, ground ambulance organizations may have difficulty interpreting the phrase "share any operational costs." We received questions from some ground ambulance organizations asking whether renting space from a fire department qualified as a "shared operational cost." The intent of the question was to ask about shared ownership and accounting, not renting facility space, sharing a physical space with a separate organization, or similar business and logistical arrangements.
We propose to revise Section 2, Question 9, to read, "Does your organization provide any of the following services or operations (select all that apply)?" retaining the current response options. This change clarifies that the intent of Section 2, Question 9 is to collect information on services or operations provided by the sampled organization. We invite comments on our proposal regarding reporting shared services.
b. Proposed Change to Average Trip Time Question
We stated that the area served by ambulance organizations is an important characteristic and finalized a policy to collect information on the geographic area served by each ambulance organization in Section 3 of the data collection instrument (84 FR 62875). We included questions related to average trip time in primary and secondary service areas (questions 3 and 6 of Section 3) that were important to understand how geographic distance between the ground ambulance organization's facilities and patients affects costs (84 FR 62873).
Section 3 (Service Area), Questions 3 and 6 in the instrument ask ground ambulance organizations to report their "average trip time" using a set of categorical time ranges (for example, 30-60 minutes). These questions define average trip time as "the time the ambulance leaves the station to when that ambulance is available to take another call." Based on feedback from ground ambulance organizations, we believe this definition may be confusing in cases where an ambulance responds to a call from a location other than the station (for example, while en route to another call, from a standby event, or from a hospital). Based on the literal wording of the question, it is not clear whether and if so, how ground ambulance organizations should report trip times for responses not originating at a station when responding to this question, leading to potentially missing or biased data.
We are proposing that this question be revised to ask for "average time on task" defined as "from the time an ambulance begins its response to the time when the ambulance is available to respond to another call (that is, time on task)" to better capture interfacility transfers and situations when an ambulance is already out and responds from a site other than the central station. We believe this change in the wording of the question would be clearer to respondents and would result in higher-quality reported data. We invite comments on our proposal to change the definition of the average trip time.
c. Proposed Change to Secondary Service Area Instructions
In Section 3, Question 4 instructions define the secondary service area for an organization as "outside [its] primary service area, but one where [it] regularly provide[s] services through mutual or auto-aid arrangements. The instruction directs organizations to "not include areas where [they] provide services only under exceptional circumstances." We were notified that some ground ambulance organizations are unsure how to report areas where they (a) did have mutual or auto-aid arrangements in place, which aligns with the definition of secondary service area in the instructions, but where (b) they responded to calls only very rarely, for example once a year, which could be considered an "exceptional circumstance" and ignored for reporting per the instruction.
Although the instructions leave the determination of whether an organization has a secondary service area at the discretion of the sampled ground ambulance organization, we believe that some organizations may benefit from a rule of thumb or example to help assess whether they should or should not report a ZIP code as being part of their secondary service area. We propose to add the following text to the Section 3, Question 4 instructions: "Some, but not all, ground ambulance organizations regularly provide service outside of their primary service area, for example through mutual or auto-aid agreements with nearby municipalities. If this applies to your organization, please report areas that are outside your primary service area but where you regularly provide services as part of your secondary service area. You do not need to report areas where you provide services very rarely or only under exceptional circumstances (for example, when participating in coordinated national or state responses to disasters or mass casualty events). Use your judgment as to whether your organization regularly serves a secondary service area. For example, you may choose to consider ZIP codes outside your primary service area but where you had 5 or more responses during the data collection period as part of your secondary service area if you believe these transports have a significant impact on your organization's costs." Even with this added text, ground ambulance organizations could still determine whether they do or do not have a secondary service area for the purposes of reporting in the Medicare Ground Ambulance Data Collection System. We invite comments on our proposal to revise the secondary service area instructions.
d. Proposed Change to the 90th Percentile Emergency Response Time
Section 4 (Emergency Response Time), Question 3 asks ground ambulance organizations to report the Start Printed Page 39298 90th percentile emergency response time, which the question defines as the time separating the quickest 90 percent of responses from the longest 10 percent of responses. The intent of the question was to collect information to help CMS understand the difference between average response times and atypical "outlier" response time. In the CY 2020 PFS proposed rule (84 FR 40688), we proposed to include a question on average response time. As we noted in the CY 2020 PFS final rule (84 FR 62873), several commenters to the CY 2020 PFS proposed rule recommended asking ground ambulance organizations to provide 90th percentile response time rather than or in addition to the average response time. They believed 90th percentile response time is a more accurate indicator of ambulance services capabilities and quality. They stated that the average time has too wide a range for error, since roughly half of responses are quicker/slower than average. They further stated that using average response time also tends to flatten the data, which means the fastest and slowest organizations did not stand out as much. In response to these comments (84 FR 62874), we finalized an additional question to the instrument asking ground ambulance organizations responding to emergency calls for service to report their 90th percentile response time.
Based on feedback from ground ambulance organizations that we have received on this question since we finalized the instrument, we believe most ground ambulance organizations will find it challenging to interpret this question and report the requested information. Several ground ambulance organizations have indicated that they would misinterpret this question, describing a shorter 90th percentile emergency response time compared to average response time, which, while mathematically possible, is not the intent as we were interested in characterizing outlier emergency responses with unusually long response times.
Thus, we propose to revise the question to ask: "what is your best estimate of the share of responses (enter percentage) that take more than twice as long as the average response time as reported in the prior question?" We believe this would be an easier question for ground ambulance organizations to understand. The goal of this question is to help CMS understand whether the organization has some response times that are much longer than its typical response time. Although the question language would be different, the reported information would still help CMS understand the extent to which a small number of emergency responses may be substantially longer than the average response for each organization. We invite comments on our proposal to revise the question to ask respondents to report the share of responses with more than twice the average response time instead of their 90th percentile emergency response time.
e. Proposed Change to Reporting Paid Ambulance Transports
In the CY 2020 PFS final rule (84 FR 62876 through 62877), we established a series of questions in the data collection instrument to collect data on the volume and the mix of services, including paid ground ambulance transports, that is, ground ambulance transports where the ambulance provider or supplier was paid for a billed amount in part or in full. The general instructions for Section 5 (Ground Ambulance Service Volume) note: "A paid ground ambulance transport refers to a ground ambulance transport for which your organization has been paid in full or in part by a payer and/or patient only. Depending on how your organization collects data, you may report (a) the number of transports furnished during the data collection period that were also paid during the data collection period, or (b) the number of transports paid during the data collection period even if some transports occurred prior to the data collection period." Furthermore, Section 5, Question 7 asks respondents, "what was the total number of paid ground ambulance transports in calendar year 202X [or fill fiscal year as appropriate], across all payer types and regardless of the level of service or geography? (Enter number)."
Based on questions and feedback from ground ambulance organizations that we have received since we finalized the instrument, we believe respondents may have different interpretations of this question, which could lead to inconsistent reported data, including the reported total ground ambulance transports during the data collection period (Section 5, Question 6). The intent of this question was to capture the reported number of ground ambulance transports during the data collection period, provided such transports were paid by the time the information was prepared for reporting to CMS. We did not intend for organizations to report the total number of ground ambulance transports for which they received the payment itself during the data collection period.
We recognize that there is a temporal disconnect between when services are provided and when initial and final payment may be received. In order to standardize the information that is reported by all ground ambulance organizations, and to align the reported information on the number of responses and transports during the data collection period with information reported on the number of paid transports, we propose to clarify Section 5, Question 7 to ask "Of the ground ambulance transports your organization provided in calendar year 202X [or fill fiscal year as appropriate], how many were paid (either in part or in full) across all payer types and regardless of the level of service or geography by the time you are reporting data to CMS?"
We recognize that the "runout period," that is, the time from when services are provided to the time when data is being analyzed, will be short and variable across organizations, particularly for transports towards the end of organizations' data collection periods. Despite this limitation, we believe this approach is preferable to alternatives where (a) respondents have variable interpretations of Section 5, Question 7 and (b) where respondents are asked to report the number of transports for which payment was received during the data collection period, even if the transports for which payment was received happened prior to the data collection period. In the latter case, the number of paid ground ambulance transports could not be directly compared to the number of total ground ambulance transports reported in Section 5, Question 6.
We also are proposing to revise the general instructions in Section 5 to delete the following text as it will no longer be relevant: "Depending on how your organization collects data, you may report (a) the number of transports furnished during the data collection period that were also paid during the data collection period, or (b) the number of transports paid during the data collection period even if some transports occurred prior to the data collection period."
We invite comments on our proposal to revise reporting paid ground ambulance transports.
f. Proposed Change to Questions Related to Labor Hours
Section 7 (Labor Costs) of the data collection instrument asks respondents to report compensation and hours worked for ground ambulance staff. The instrument currently asks respondents to report, separately for each staff category: Total compensation, total hours worked inclusive of all responsibilities, and total hours worked unrelated to either ground ambulance or Start Printed Page 39299 public safety responsibilities. The rationale for asking for total compensation and hours, even if these include compensation and hours for activities other than those related to ground ambulance services, was to preserve the ability to compare compensation between organizations and to external benchmarks such as Bureau of Labor Statistics data. The last item, total hours worked unrelated to either ground ambulance or public safety responsibilities, can be subtracted from overall total hours worked related to ground ambulance and public safety responsibilities combined, and further allocation could separate ground ambulance time and compensation from public safety time and compensation for fire and other public safety-based ground ambulance organizations.
Based on questions received by ground ambulance organizations since we finalized the instrument and feedback through testing on Section 7 questions, we learned that some ground ambulance organizations may misinterpret the Section 7 questions. Specifically, we believe some organizations may assume the question is asking for hours "related" rather than "unrelated" to ground ambulance or public safety responsibilities given the focus of the data collection effort, despite instructions to the contrary. Relatedly, we were notified that some organizations were confused that the Section 7 questions did not provide an opportunity to report total hours worked related to ground ambulance responsibilities, which they assumed was an unintentional omission from the instrument.
We propose to change the instructions in Section 7 to ask respondents to report hours worked on different activities in such a way that the sum of hours worked across different activities equals total hours worked annually. We believe this approach would be easier for respondents to understand and estimate, resulting in less burden for respondents and higher quality reported information.
For stand-alone ground ambulance organizations, we propose to ask respondents to report each of the following per staff category: (a.) Total annual compensation; (b.) Total hours worked annually; (c.) Total hours worked annually related to ground ambulance operations; and (d.) Total hours worked annually related to all other responsibilities. With this change, the instructions in Section 7 would note that "total hours worked annually related to ground ambulance operations" plus "total hours worked annually related to all other responsibilities" should equal "total hours worked annually."
For fire department or other public safety-based ground ambulance organizations, we propose to ask respondents to report each of the following per staff category: (a.) Total annual compensation; (b.) Total hours worked annually; (c.) Total hours worked annually related to ground ambulance operations; (d.) Total hours worked annually related to fire, police, or other public safety operations; and (e.) Total hours worked annually related to all other responsibilities. The Section 7 instructions would note that the sum of total hours worked related to ground ambulance operations; fire, police, or other public safety operations; and all other responsibilities should equal total hours worked annually. We invite comments on our proposal to revise the labor hours.
g. Proposed Change to Instructions Related to Facility, Vehicle, and Equipment Certain Expenses
In the CY 2020 PFS final rule (84 FR 62882 through 62886), we finalized policies to collect cost information related to facilities, vehicles, and other equipment, consumables and supplies. The purpose of Sections 8 (Facilities Costs), 9 (Vehicles Costs), and 10 (Equipment, Consumable, and Supply Costs) in the instrument is to collect total expenses during the data collection period related to facilities, vehicles, and equipment and supplies, respectively. Based on feedback from ground ambulance organizations that we have received since we finalized the instrument, we are concerned that some respondents, particularly those that do not currently depreciate facilities, vehicles, and/or equipment for accounting purposes, may not be sure where to report some components of total expenses in these categories. Although we believe most ground ambulance organizations depreciate facilities, vehicles, and capital medical equipment, we were notified that some ground ambulance organizations do not depreciate these items in their regular accounting practices. Upon a review of the instrument, we found that the instructions and opportunities to report costs for organizations using a cash basis for accounting were inconsistent across Sections 8, 9, and 10 of the instrument. In some instances, ground ambulance organizations are asked to report annual depreciation expenses only, without a clear question related to expenses should the organization not regularly depreciate a certain category of asset. In other cases, there are questions asking respondents to report annual expenses other than annual depreciation expenses, but the instructions provide incomplete guidance on what expenses are in scope.
We considered several factors when developing our proposals to address these inconsistencies. Overall, the purpose of the questions in Sections 8, 9, and 10 is to collect comprehensive information on total expenses related to facilities, vehicles, and equipment and supplies during the organizations' data collection periods. We believe the primary purpose of changes and clarifications to questions in this section should be to ensure all expenses are reported from both organizations that do and do not depreciate facilities, vehicles, and equipment for accounting purposes. We understand that allowing organizations flexibility to report cost information using their current accounting approach will reduce burden. The instructions to the instrument currently state: "In general, you will be able to report information collected under your organization's current accounting practices. We understand that some ground ambulance organizations use accrual-basis accounting while others use cash-basis accounting." We continue to believe this is the correct approach, and that alternatives would impose considerable additional burden on ground ambulance organizations.
We considered several broad alternatives on how to report facility, vehicle, and equipment expenses in Sections 8, 9, and 10. One option is to require all organizations to calculate and report depreciation for facilities, vehicles, and equipment using a standardized approach. Although this would increase burden for respondents, potentially significantly for organizations that do not currently calculate depreciation, it would result in the most standardized information being submitted to CMS and the fewest changes to the layout of the instrument. Another option would be to retain the current structure of the instrument but provide more detailed instructions on how organizations that do and do not depreciate facilities, vehicles, and equipment should report information. A third option is to add new screening questions to the instrument asking individually whether the organization depreciates facilities, vehicles, and equipment. The responses to these screening questions could be used to tailor the instructions, table headings, and question text later in the instrument to avoid confusion.
After considering these options, we propose to add screening questions to the instrument asking individually Start Printed Page 39300 whether the organization depreciates facilities, vehicles, and equipment. We believe this would not substantively affect response burden for organizations and may in some cases reduce burden by clarifying what and how information on expenses must be reported in Sections 8, 9, and 10.
There are two specific places in Sections 8 and 9 in the instrument where we believe the instructions on how to report annual expenses may not be clear. First, Section 8.2, Question 1 asks respondents to report annual expenses for each facility that they report as being related to their ground ambulance operation in Section 8.1, Question 3. Section 8.2, Question 1 is a table with columns for "annual lease or rental costs," "annual depreciation expenses," and "annual mortgage, bond interest, and other costs of ownership." Although the instructions note "do not report depreciation if your organization does not capitalize facilities for accounting purposes," it is not immediately clear where organizations that do not capitalize facilities should report expenses if the facility is owned outright (for example, in cases where a facility is acquired during the data collection period).
Second, Section 9.1, Question 5 and Section 9.2, Question 5 are tables where respondents report costs associated with individual vehicles. Both tables currently ask, "What was the annual depreciation expense for this vehicle?" Although the instructions note "for owned vehicles, do not report depreciation if your organization accounts for vehicles on a cash basis," the instructions do not indicate where expenses for vehicles purchased during the data collection period should be reported by organizations that do not capitalize vehicles for accounting purposes.
We considered several options to clarify the instructions in Sections 8 and 9 specifically. One option is to preserve current table structures and item numbers in both sections while providing additional written instructions. We believe that although this will minimize disruption to the layout of the instrument, it will also do the least to address potential confusion around these questions. Another option is to add new columns in Sections 8 and 9 for facilities and vehicles purchased outright during the data collection period for organizations that do not depreciate these expenses. We propose to add an additional column for clarity, but note that if the screening questions are added as described above not all columns would appear for all respondents, particularly given our proposal to add screening questions related to reporting expenses in Sections 8 and 9.
We also believe there are specific instructions in Section 10 that may not be clear. Section 10.1, Question 1 and Section 10.2, Question 1 asks respondents to report "annual depreciation expenses" for medical and non-medical capital equipment, respectively. The Section 10 instructions note "do not report depreciation if your organization uses a cash basis for accounting" and that "for capital expenditures, medical and non-medical equipment, most organizations will amortize costs over the life of the good" but do not specify that organizations that do not depreciate medical or non-medical equipment should skip these questions and report expenses for equipment acquired during the data collection period in Section 10.1, Question 3, and Section 10.2, Question 3 instead.
We considered several options to clarify the instructions in Section 10 specifically. One option is to clarify in the instructions that organizations that do not depreciate medical or non-medical equipment should skip Section 10.1, Question 1 and Section 10.2, Question 1 and report expenses for equipment acquired during the data collection period in Section 10.1, Question 3, and Section 10.2, Question 3 instead. Although this would involve the least change to the instrument, we would lose the ability to distinguish between expenses for the kinds of equipment that most ground ambulance organizations depreciate for organizations reporting in this way. Another option is to change the instructions for Section 10.1, Question 1 and Section 10.2, Question 1 to refer to broad types of equipment that are typically considered capital medical and non-medical equipment, and then ask respondents to report relevant annual expenses for qualifying equipment in these questions, regardless of whether the expenses are annual depreciation expenses or purchase costs (for organizations not calculating depreciation). We propose to ask organizations that do not depreciate equipment to report expenses associated with purchasing equipment in Section 10.1, Question 1 and Section 10.2, Question 1. This option would preserve our and MedPAC's ability to separately analyze these expenses. We invite comment on these alternatives to address instructions related to facility, vehicle, and equipment expenses.
h. Proposed Changes to Questions Related to National Provider Identifier's (NPIs) Under Broader Parent Organizations
Some ground ambulance NPIs are part of broader parent organization companies that own and/or operate multiple ground ambulance NPIs. Section 2, Question 2 asks, "Did your organization use more than one NPI to bill Medicare for ground ambulance services during the data collection period?" Based on feedback from ground ambulance organizations that we have received since we finalized the instrument, we were notified that the use of "organization" in this question is potentially confusing because it is not clear whether the term applies to the organization sampled to report information to the Medicare Ground Ambulance Data Collection System (which, by definition, is an individual NPI) or to a broader "parent organization." We propose clarifying the question to ask "Is this NPI part of a larger `parent organization' that owns or operates multiple NPIs billing for ground ambulance services?" We are also proposing to clarify the wording of the follow-up instruction for organizations that answer "yes" to this question. The follow-up instruction currently reads, "You are being asked to complete this instrument and enter data only for the following NPI: [pre-populate number]." Because very large parent organizations may have several NPIs sampled and a single or small number of staff collecting and reporting data for multiple NPIs, we are proposing to revise the text to read, "You are being asked to complete this instrument and enter data separately for each sampled NPI. The following questions refer only to the following NPI: [pre-populate number]."
The instrument asks these organizations to report an allocated share of parent organization expenses at the end of most sections of the instrument. For example, Question 3 in Section 7.2 on paid administration, facilities, and medical director staff costs asks, "Please report the allocated portion of administrative labor costs incurred at the level of the parent organization/central office of this NPI based on your organization's approach for allocating costs to specific NPIs. (Enter dollar amount.)"
There are four sections in the instrument that lack similar questions: Section 7.1 (Paid EMT/Response Staff Compensation and Hours Worked), Section 7.3 (Volunteer Labor), Section 9.1 (Ground Ambulance Vehicle Costs), and Section 10.1 (Medical Equipment/ Start Printed Page 39301 Supplies). Without these questions, total reported costs may be biased downward for NPIs that are part of broader parent organizations. We propose to add questions like the one reproduced above to the end of these four sections for completeness. The text would be the same as the above except for replacing "EMT/response staff labor costs," "costs associated with volunteer labor," "ground ambulance vehicle costs," and "medical equipment and supply costs" for "administrative labor costs" in the respective sections.
Relatedly, for completeness, we propose to clarify in the instructions for Section 12 (Total Cost), Question 1, that organizations part of broader parent organizations should include an allocated portion of parent organization (or "central office") costs when reporting their total costs in this question. We invite comments on our proposal to address questions related to NPIs under broader parent organizations.
i. Other Clarifications to the Medicare Ground Ambulance Data Collection Instrument
We propose the following 11 additional clarifications and updates to the instrument:
i. Replacing all first-person language (for example, "we") with third-person language (for example, "CMS") throughout the instrument for editorial consistency.
ii. Section 2, Question 17: There is a typo where this question referred to itself rather than, as is implied by the ordering and framing of the question, the prior item. The question currently asks, "other than what was reported in item 17 . . .," when it should read, "other than what was reported in item 16 . . .".
iii. Section 3, Question 2: This question currently asks, "are you the primary emergency ambulance provider . . .," using "provider" more colloquially than elsewhere in the instrument where the same word is sometimes used to differentiate between Medicare providers of service and Medicare suppliers. We propose to reword this question to read, "are you the primary emergency ambulance organization . . ."
iv. Section 4, Questions 1 and 2 Clarification: The question currently defines response time as "the time from when the call comes in to when the ambulance or another EMS response vehicle arrives on the scene." We propose clarifying this definition to say "the time from when the call comes in to dispatch to when the ambulance or another EMS response vehicle arrives on the scene." Relatedly, for Section 4, Question 2, we propose adding a second answer option for this question that reads, "From the time our organization receives a call from dispatch to the time the ambulance or other EMS vehicle is at the scene." Respondents would still have the option to write-in their own response in Section 4, Question 2, if neither of the pre-programmed options apply to their organization.
v. Section 5, Question 3a. Clarification: This question asks respondents to report the percentage of ground ambulance responses that involve a non-transporting agency and the percentage of ground ambulance transports in which the non-transporting agency continues to provide medical care in the ambulance during a transport. Based on feedback from ground ambulance organizations that we have received since we finalized the instrument, we believe many organizations do not currently track this data and will not easily be able to begin tracking it. We propose clarifying this question to note that estimated percentages are acceptable, as they are in response to certain other questions in the instrument (where noted). We specifically propose to edit Section 5 question 3a. to read: "What is your best estimate of the percentage of total ground ambulance responses that involved a non-transporting agency? (Enter percentage)"
vi. Section 7.1 Instruction Clarification: We propose clarifying "You will report on these staff in a different section" to "You will report on these staff in a later section" to make it clear that the opportunity to report on these staff follows the current instruction.
vii. Sections 7.1 and 7.2 Instruction Clarification: We propose to add "employer payroll taxes" as an additional example of a component contributing to total compensation, without altering any of the definitions or other instructions in these sections.
viii. Section 7.2, Question 3 Clarification: We propose adding a clarification warning for respondents not to consider labor that was reported elsewhere when responding to this question.
ix. Section 7.3, Question 4 Clarification: We propose adding a clarification that medical director volunteer hours do not contribute to this response and a reminder that they are reported separately below (Section 7.3, Question 5).
x. Section 10 Instructions: We propose to correct a typo in the instructions where the instrument describes "operation expenses" rather than "operating expenses" as intended.
xi. Section 13, Question 3 Clarification: Based on the instructions for this question, organizations may report revenue from specific payers that include patient cost-sharing amounts. To ensure patient cost-sharing is not reported twice, we recommend clarifying the item in the chart that currently reads, "Patient self-pay (amount patients pay for deductibles, coinsurance, etc.) to read, "Patient self-pay (cash payment and the amount patients paid for deductibles, coinsurance, and other cost-sharing only if not reported in a row above.)" We invite comments on these proposed clarifications and updates to the instrument.
4. Collection and Reporting of Information Under the Data Collection System
In the CY 2020 PFS final rule (84 FR 62893), we finalized our sampling proposals to implement a 25 percent stratified sample in each of the first four years of data collection and codified the representative sample approach at § 414.626(c). CMS' sampling approach is designed to result in representative samples of ground ambulance organizations in terms of key characteristics including provider versus supplier status, service area population density, volume of transports, and ownership category. The selected ground ambulance organizations for year 1 and year 2 have already been listed on the CMS website at https://www.cms.gov/Center/Provider-Type/Ambulances-Services-Center.html.
In the CY 2020 PFS final rule (84 FR 62894), we finalized the data collection period as a continuous 12-month period of time, which is either the calendar year aligning with the data collection year, or the organization's annual accounting period that begins during the data collection year when an organization has an annual accounting period (such as a fiscal year) that differs from the calendar year and the organization elects to collect and report data over this period rather than the calendar year. We also finalized our proposal to require organizations to report data during a 5-month data reporting period starting immediately following the end of the data collection period. The data collection and reporting requirements for selected ground ambulance organizations were codified at § 414.626(b).
As part of the Medicare Ground Ambulance Data Collection System, sampled ground ambulance Start Printed Page 39302 organizations will report information to CMS using a web-based version of a data collection instrument that is posted on the CMS website at https://www.cms.gov/Center/Provider-Type/Ambulances-Services-Center.html. We are currently developing the Medicare Ground Ambulance Data Collection System and stated in the CY 2020 PFS final rule (84 FR 62867) that the web-based survey would be available before the start of the first data reporting period to allow time for users to register, receive their secure login information, and receive training from CMS on how to use the system.
Due to the COVID-19 public health emergency (PHE), we issued two blanket waivers (May 5, 2020 and November 25, 2020) to delay the data collection and data reporting periods under the Medicare Ground Ambulance Data Collection System. The first waiver delayed the data collection period and data reporting period for selected year 1 ground ambulance organizations and the second waiver delayed the data collection periods and data reporting periods for selected year 1 and year 2 ground ambulance organizations.
This revised modification has been issued on page 32 in the following document: https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf. Specifically, we modified the data collection period and data reporting period, as defined at § 414.626(a), for ground ambulance organizations (as defined at § 414.605) that were selected by CMS under § 414.626(c) to collect data beginning between January 1, 2020 and December 31, 2020 (year 1) and for ground ambulance organizations that were selected to collect data beginning between January 1, 2021 and December 31, 2021 (year 2) for purposes of complying with the data reporting requirements described at § 414.626.
Under this modification, these ground ambulance organizations would select a new continuous 12-month data collection period (organizations may choose a collection period aligning with the calendar year or the organization's fiscal year) that begins between January 1, 2022 and December 31, 2022, to collect data necessary to complete the Medicare Ground Ambulance Data Collection Instrument during their selected data collection period, and submit a completed Medicare Ground Ambulance Data Collection Instrument during the data reporting period that corresponds to their selected data collection period. We modified this data collection and reporting period to increase flexibilities for ground ambulance organizations that would otherwise be required to collect data in 2020-2021 so that they can focus on their operations and patient care during the COVID-19 public health emergency (PHE). We stated, when the blanket waiver was granted, in the COVID-19 Frequently Asked Questions (FAQs) on Medicare Fee-for-Service (FFS) Billing document (page 63 of this document: https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf) that CMS will not allow an option to continue with their current data collection period because the data collected in 2020 and 2021 during the PHE may not be reflective of typical costs and revenues associated with providing ground ambulance services.
As a result of the COVID-19 delay, ground ambulance organizations selected in year 1, 2, and 3 will have the same data collection periods beginning between January 1, 2022 and December 31, 2022 and will have the same data reporting periods beginning between January 1, 2023 and December 31, 2023. In the CY 2020 PFS final rule (84 FR 62893), we finalized our sampling proposals to implement a 25 percent stratified sample in each of the 4 years of data collection. Prior to the delay, we anticipated approximately equal shares of ground ambulance organizations would collect and report data in four consecutive periods. However, as a result of the delays, there will now be approximately 75 percent of the ground ambulance organizations that will have data collection periods that start in the same year and subsequently will have data reporting periods starting in the same year. Later, a final 25 percent sample of ground ambulance organizations in year 4 will collect and report data.
When finalizing our policies in regard to ground ambulance collection and reporting of data, we did not intend to have approximately 75 percent of ground ambulance organizations collect and report data at the same time. To provide MedPAC with the data needed for analysis, acknowledging that due to the COVID-19 delay there will be a delay in CMS providing that data, we believe that we should revise the data collection period and data reporting period for selected ground ambulance organizations in year 3.
Accordingly, we are proposing to revise the data collection period beginning between January 1, 2022 and December 31, 2022 and data reporting period beginning between January 1, 2023 and December 31, 2023 for selected ground ambulance organizations in year 3. Under this proposal, there will be a new data collection period beginning between January 1, 2023 and December 31, 2023 and a new reporting period beginning between January 1, 2024 and December 31, 2024 for selected ground ambulance organizations in year 3. With this proposal, we plan to do the sample in 2022 for selected ground ambulance organizations in year 3 rather than the current plan in 2021. The main advantage of delaying the year 3 sample is that the selected organizations would be more representative of the organizations actually collecting beginning in 2023 and reporting beginning in 2024. The longer the delay between sampling and the data collection and data reporting, the more changes in the industry (for example, NPIs ceasing ground ambulance or all operations). This timeline would align with the data collection period and data reporting period requirements for selected ground ambulance organizations in year 4. As a result, there would be approximately 50 percent of ground ambulance organizations selected in year 1 and 2 with data reporting periods beginning between January 1, 2023 and December 31, 2023 and approximately 50 percent of ground ambulance organizations selected in year 3 and 4 with data reporting periods beginning between January 1, 2024 and December 31, 2024.
Due to the delay caused by the COVID-19 PHE, we examined the possibility of extending the data reporting to encompass 4 years as planned instead of 2 years. We concluded that it would not be feasible to extend the data reporting period over 4 years. Extending the data reporting to encompass four years would further delay MedPAC receiving the data required to analyze for its report to Congress, which is required to be submitted by March 15, 2023. The sampling for year 1 and year 2 selected ground ambulance organizations has already been completed and the lists for the selected ground ambulance organizations in year 1 and year 2 are posted on the CMS website.
With this proposal, more data would be collected in 2023 as there would hopefully be more distance from the peak of the COVID-19 pandemic. Thus, it is our hope that 2023 will be even more reflective of a typical year of costs for ground ambulance organizations than 2022. As the course of the pandemic continues to evolve, we believe that our proposal provides a potential for more even distribution of data over two years for comparison by MedPAC. We invite comments on our proposal to revise the data collection period and data reporting period for Start Printed Page 39303 ground ambulance organizations selected in year 3.
5. Proposed Change to the Notification Process for Selected Ground Ambulance Organizations Required To Report
In the CY 2020 PFS final rule, we codified our notification process at § 414.626(c)(3) and (b)(1). We stated at § 414.626(c)(3) that CMS will notify an eligible ground ambulance organization that it has been selected to report data for a year at least 30 days prior to the beginning of the calendar year in which the ground ambulance organization must begin to collect data by posting a list of selected organizations on the CMS web page and providing written notification to each selected ground ambulance organization via email or U.S. mail.
The Medicare Administrative Contractor (MAC) is responsible for providing written notifications to the selected ground ambulance organizations in their service area. We codified their role at § 414.626(b)(1) which states that within 30 days of the date we notify a ground ambulance organization that it has been selected to report data under this section, the ground ambulance must select a data collection period that corresponds with its annual accounting period and provide the start date of that data collection period to the ground ambulance organization's Medicare Administrative Contractor.
We propose to make a technical revision to § 414.626(b)(1) to state that the selected ground ambulance organization provide the start date of the data collection period to CMS or its contractor instead of the Medicare Administrator Contractor. This change will provide CMS with flexibility to have the MACs or other contracted entities provide written notifications and collect information from the selected ground ambulance organizations. If we find the response rate is low, having the flexibility to contract with other entities that could employ additional outreach resources may be useful. This revision would not preclude CMS from including the MACs in the notification process. We also propose to correct a typographical error at § 414.626(b)(1), which currently states "a ground ambulance must select a data collection period" to read "a ground ambulance organization must select a data collection period." We invite comments on our technical revisions to the citation at § 414.626(b)(1).
6. Payment Reduction for Failure To Report
Section 1834(l)(17)(D)(i) of the Act requires that beginning January 1, 2022, subject to clause (ii), the Secretary reduce the payments made to a ground ambulance organizations under section 1834(l)(17) of the Act for the applicable period by 10 percent if the ground ambulance organization is required to submit data under the data collection system with respect to a data collection period under the data collection period and does not sufficiently submit such data.
We stated in the CY 2020 PFS final rule (84 FR 62895) that we would make a determination that the ground ambulance organization is subject to the 10 percent payment reduction no later than the date that is 3 months following the date that the ground ambulance organization's data reporting period ends. In this final rule, we provided examples of when the determination will be made based on calendar year and fiscal year data collection period beginning in 2020. Due to the delay caused by the COVID-19 PHE, we did not receive data collected in 2020. We will begin to follow this timeline to make a determination that the ground ambulance organization is subject to the 10 percent payment reduction when data collected in 2022 is required to be reported in 2023 for selected ground ambulance organizations in year 1 and year 2.
For example, if a selected ground ambulance organization's data collection period is based on a calendar year, that is, January 1, 2022 through December 31, 2022, we will allow a ground ambulance organization 5 months to report the data collected during the data collection period. For this example, the data reporting period for this organization is January 1, 2023-May 31, 2023. We would make a determination that the ground ambulance organization is subject to the 10 percent payment reduction no later than August 31, 2023. With this timeframe, we would apply the 10 percent reduction in payments, if applicable (no hardship exemption or informal review is granted), for ambulance services provided between January 1, 2024 and December 31, 2024.
As another example, if a selected ground ambulance organization's data collection period is based on a fiscal year, that is, October 1, 2022 through September 30, 2023, we will allow a ground ambulance organization 5 months to report the data collected during the data collection period. For this example, the data reporting period for this organization is October 1, 2023 -February 28, 2024, we would make a determination that the ground ambulance organization is subject to the 10 percent payment reduction no later than June 1, 2024. With this timeframe, we would apply the 10 percent reduction in payments, if applicable (no hardship exemption or informal review is granted), for ambulance services provided between January 1, 2025 and December 31, 2025.
7. Public Availability of Data
We stated in the CY 2020 PFS final rule (84 FR 62897), the data will be made available to the public through posting on our website at least every 2 years and we will post the summary results by the last quarter of 2022. We codified the public availability at § 414.626(f), which states: (f) Public availability of data. Beginning in 2022, and at least once every 2 years thereafter, CMS will post on its website data that it collected under this section, including but not limited to summary statistics and ground ambulance organization characteristics.
Due to the COVID-19 delay, we are proposing to revise § 414.626(f) to state that we will make the data collected under § 414.626 publicly available beginning in 2024. We invite comments on our proposal to revise the timeline when the public availability of data will begin.
L. Medicare Diabetes Prevention Program (MDPP)
The Medicare Diabetes Prevention Program (MDPP) expanded model is a structured intervention that aims to prevent or delay onset of type 2 diabetes among eligible Medicare beneficiaries diagnosed with pre-diabetes. The MDPP expanded model is an expansion of duration and scope of the Diabetes Prevention Program (DPP) model test, which was initially tested through a Round One Health Care Innovation Award. MDPP services are furnished in community and health care settings by organizations that enroll in Medicare as MDPP suppliers, a new supplier type, even if they have an existing Medicare enrollment as another supplier type. MDPP services furnished under the MDPP expanded model are covered as an additional preventive service with no cost-sharing under Medicare. Eligible organizations seeking to furnish MDPP services began enrolling in Medicare as MDPP suppliers on January 1, 2018, and began furnishing MDPP services on April 1, 2018.
We propose to amend our regulation at § 410.79 to preclude the provision of ongoing maintenance sessions unless Start Printed Page 39304 the MDPP beneficiary has started his or her first core session on or before December 31, 2021. In addition, we propose to amend § 414.84(b) and (c) to update the amount of the performance payments for the core sessions and core maintenance sessions and ongoing maintenance sessions (where applicable) to be consistent with the proposal herein. We propose that this change apply to all MDPP beneficiaries starting the MDPP set of services on or after January 1, 2022. Additionally, we propose to amend § 424.205(b) to add a provision to waive the provider enrollment Medicare application fee for all organizations enrolling in Medicare as MDPP suppliers that submit an application on or after January 1, 2022. Finally, we propose to make a conforming amendment to § 424.502 to remove a reference to the CMS-20134 from the definition of "institutional provider." (In accordance with § 424.514, institutional providers generally must pay the enrollment application fee.)
We do not anticipate that the proposed changes would impact our ability to complete an evaluation of the MDPP expanded model, but the evaluation would consider these proposed changes if enacted. The evaluation would continue to use beneficiary-level Diabetes Prevention Recognition Program (DPRP) encounter data and program data furnished by the CDC in combination with Medicare claims data to analyze the long-term utilization of services by beneficiaries who have received the MDPP set of services. We would use these data as planned to assess whether the MDPP expanded model is expected to improve the quality of care without increasing spending, reduce spending without reducing the quality of care, or improve the quality of care and reduce spending. We anticipate that these programmatic adjustments are likely to result in more MDPP suppliers, increased beneficiary access to MDPP services and an ongoing reduction of the incidence of diabetes in eligible Medicare beneficiaries, in both urban and rural communities.
1. Proposed Changes to § 410.79(b), (c), and (e)
We are proposing to amend certain MDPP expanded model policies previously finalized in the CY 2017 PFS final rule (81 FR 80459 through 80475 and 80552 through 80558), the CY 2018 PFS final rule (82 FR 34157 through 34158), and the CY 2021 PFS final rule 85 FR 50074). Previous rules established policies related to the set of MDPP services, beneficiary eligibility criteria, reimbursement structure, and supplier enrollment requirements and compliance standards.
MDPP has experienced challenges recruiting suppliers to participate in the expanded model, which has limited beneficiary access to the preventive services offered under the expanded model. Existing and prospective suppliers have reported that the length of the set of MDPP services and the payment timing and amounts have made implementation and operation of MDPP burdensome and has hindered participation. Currently, MDPP suppliers are required to offer up to 2 years of MDPP services to eligible MDPP beneficiaries. The MDPP set of services, as defined in § 410.79(b), consists of at least 16 sessions offered during the core sessions phase (Months 1-6), monthly maintenance sessions offered during the core maintenance sessions phase (Months 7-12) (collectively the "core sessions phase"), and additional monthly sessions offered during the ongoing maintenance sessions phase (Months 13-24) for eligible beneficiaries. To be eligible for the ongoing maintenance sessions phase, a beneficiary must meet the minimum weight-loss requirement (5 percent weight loss from baseline), as defined in § 410.79(b), and maintain the minimum weight-loss requirement on a quarterly basis to continue to receive MDPP services in subsequent quarters. The ongoing maintenance sessions delivered in year 2 are a unique feature of MDPP. Both the CMS-funded Health Care Innovation Award (HCIA) to the Young Men's Christians Association (YMCA) of the USA (Y-USA), referred to as the DPP model test hereafter, and the CDC's National Diabetes Prevention Program (National DPP) was/are 12 months in length.
CMS included the ongoing maintenance sessions phase in the MDPP set of services to support participants in solidifying the behavioral changes that resulted in weight loss during the first 12 months. In the CY 2017 PFS proposed rule, we proposed adding the ongoing maintenance sessions phase to follow the completion of the 12-month core sessions phase if the beneficiary achieved and maintained the required minimum weight loss of 5 percent from the baseline weight. The proposed rule did not place a limit on the number of ongoing maintenance session phases an eligible beneficiary could attend. In response to stakeholder comments, we modified the proposed policy to limit access to up to 2 years of ongoing maintenance sessions after the 12-month core sessions phase. In the CY 2018 PFS, we again modified the policy to limit access to ongoing maintenance sessions to 1 year after the 12-month core sessions phase as long as MDPP beneficiaries maintained the 5 percent weight loss.
Despite limiting the ongoing maintenance sessions phase to 1 year, we have heard that the MDPP suppliers find the implementation, operation, and costs of the ongoing maintenance sessions phase burdensome. We anticipate that these proposed changes would improve the uptake of organizations enrolling in Medicare to become MDPP suppliers, thus enabling more beneficiaries to access the MDPP set of services. Collectively, this would improve CMS's ability to evaluate the MDPP expanded model as more suppliers and beneficiaries participate in the expanded model test. Currently, more than 1,000 organizations nationally are eligible to become MDPP suppliers based on their preliminary or full CDC DPRP status. However, only 27 percent of eligible organizations are participating in MDPP. Based on an analysis of National Health and Nutrition Examination Survey (NHANES) data, an estimated 16.4 million people are eligible for MDPP;[116] to date, over 3,600 beneficiaries are participating in the MDPP set of services. We anticipate that the removal of the second year of the MDPP set of services on a prospective basis would make MDPP attractive to more MDPP eligible organizations and beneficiaries.
The requirement to offer a second year of the MDPP set of services has also caused confusion among MDPP suppliers because it is inconsistent with the CDC National DPP requirements and curriculum. Because there is no defined curriculum for the ongoing maintenance sessions phase, MDPP suppliers repeat parts of the curriculum they previously used during the core sessions phase per CDC guidance and their updated 2021 DPRP Standards.[117] We have heard anecdotally, through written inquiries and questions asked by MDPP suppliers during MDPP educational events, that MDPP suppliers struggle with discerning the appropriate timing of determining whether a beneficiary has met and/or maintained the 5 percent minimum weight-loss requirement necessary for continued eligibility for and during the ongoing maintenance Start Printed Page 39305 sessions phase. To be eligible to continue to the ongoing maintenance phase of MDPP, beneficiaries must lose and/or maintain a 5 percent weight loss from baseline. MDPP suppliers are responsible for determining if a MDPP beneficiary has met and/or maintained the 5 percent weight loss from baseline during the applicable session and phase. A supplier must submit a claim to the Medicare Administrative Contractor (MAC) for the 5 percent weight loss achievement for each beneficiary, otherwise, all subsequent ongoing maintenance session claims may be rejected by the MAC. Suppliers have 12 months from the date of service to submit claims, if they delay the claim submission for the 5 percent weight loss performance goal, this may impact a supplier's ability to get paid for the ongoing maintenance sessions. For example, if a beneficiary achieves the 5 percent weight loss goal during the first 6 months of MDPP, or during the core services period, and they do not submit the claim for the 5 percent weight loss goal until after the ongoing maintenance interval has started, the supplier risks having their claim for the ongoing maintenance interval rejected. Furthermore, in this scenario, the supplier will need to submit a claim for the second core maintenance session interval with a 5 percent weight loss for the beneficiary to continue with ongoing maintenance sessions. MDPP monitoring data suggest that 82 percent of MDPP beneficiaries for whom we have claims for the 5 percent weight loss goal achievement reach that goal in the first 6 months of the expanded model. However, our monitoring data show claims for MDPP ongoing maintenance sessions for only 10 percent of MDPP beneficiaries and that beneficiary attendance sharply drops after the first quarter of the initial core session. Collectively, these data suggest that suppliers may not be incentivized to retain MDPP beneficiaries after they attend the 9th core session in the set of MDPP services, which MDPP beneficiaries likely reach during the first quarter of the expanded model, or after the MDPP beneficiary has achieved the 5 percent weight loss milestone.
Existing MDPP suppliers report frustration with the requirements associated with the ongoing maintenance phase and we believe that the additional burden and cost of providing the ongoing maintenance sessions is a deterrent to prospective MDPP suppliers. Organizations have communicated to CMS their difficulties in keeping MDPP beneficiaries engaged in the expanded model. For an example, suppliers are reimbursed after they successfully submit claims for beneficiary attendance, after the 1st, 4th, and 9th core sessions during the first 6 months of the MDPP set of services, and then if beneficiaries attend 2 monthly sessions per quarter thereafter. MDPP eligible organizations have cited beneficiary acquisition and retention as a leading barrier to their MDPP supplier enrollment. Stakeholders and suppliers have commented that the payment levels for a second year are inadequate to cover supplier costs given the low volume of beneficiaries who participate in the ongoing maintenance phase and drive up the per-beneficiary costs for the supplier. Stakeholders comment that sessions have the same fixed costs, yet there are a diminishing number of MDPP beneficiaries eligible to participate. As previously noted, our fee-for-service claims-based monitoring data show that only approximately 10 percent of MDPP beneficiaries continue with the ongoing maintenance sessions phase and the majority of MDPP beneficiaries achieve the 5 percent weight loss milestone within the first 6 months of the MDPP set of services. Given our data, stakeholder comments, the lack of the ongoing maintenance year alignment with the CDC's National DPP and the DPP model test, the ongoing maintenance phase is not sufficiently beneficial to continue requiring and may be causing harm to the expanded model's overall goals.
As such, we are proposing to amend our regulations to preclude coverage of ongoing maintenance sessions unless the MDPP beneficiary has started his or her first core session on or before December 31, 2021. Specifically, we propose to amend § 410.79(c)(1)(ii) to provide that an MDPP beneficiary is eligible for the first ongoing maintenance session interval only if the beneficiary started his or her first core session on or before December 31, 2021. If adopted, this change would effectively make the MDPP timeframe consistent with the National DPP for MDPP service periods that begin on or after January 1, 2022. In addition, if finalized, this policy would reduce the administrative burden and costs associated with the ongoing maintenance sessions phase to MDPP suppliers with minimal impact to beneficiaries given their historically low participation rate in the second year of MDPP. This proposed change is consistent with the authority in section 1115A(c) of the Act, and we anticipate this change would improve our ability to evaluate the expanded model test due to an anticipated increase in supplier enrollment, which will increase beneficiary access to the expanded model.
In conjunction with the proposed change to remove the ongoing maintenance sessions phase from the MDPP services period, we are proposing to redistribute a portion of the ongoing maintenance sessions phase performance payments to certain core and core maintenance session performance payments to address stakeholder concerns that the current MDPP payment structure does not cover reasonable costs of MDPP suppliers to deliver the MDPP set of services. For example, the proposed attendance-based performance payments are based on a standardized per-session rate, paid after the 1st, 4th, and 9th sessions attended during the core sessions intervals, and after attending the two (2) sessions during each of the core maintenance intervals. We propose to increase performance payments for MDPP beneficiary achievement of the 5 percent weight loss goal, as well as continued attendance during each core maintenance interval. Although the proposed maximum payment of $661.00 over a 1-year service period is less than the current maximum payment of $704.00 under the original 2-year payment structure, we believe the proposed payment structure would have a net positive effect on the MDPP suppliers. Additionally, the maximum proposed payment is more than the 2021 National Average Facility Medicare Payment of $528.00 for the face-to-face intensive behavioral counseling for obesity (IBT-Obesity) for individuals.118 The IBT-Obesity, a Medicare preventive service benefit whose goal is to promote sustained weight loss among Medicare beneficiaries with a BMI of 30 kg/m2 and higher, pays a similar per session rate as what we are proposing for MDPP, including a maximum of 22 IBT-Obesity sessions in a primary care setting over a 12-month period. Our Office of the Actuary estimated that that the average payment for an MDPP supplier would increase by $100 with the elimination of the second year of MDPP. While the proposed maximum payment available to an MDPP supplier would decrease when compared to the maximum payment under the original 2-year payment structure, the second year of the MDPP set of services have historically been far less utilized than first year set of services. Therefore, it is anticipated that eliminating the second year of payments would have minimal negative impact on the expanded model's costs. Table 28 shows the Start Printed Page 39306 current 2021 non-cumulative performance payments and the proposed performance payments for those MDPP beneficiaries who started their first core service on or after January 1, 2022. We are not proposing to change the payment rates for ongoing maintenance sessions in cases where a beneficiary remains eligible for them (that is, because they started receiving the MDPP set of services on or before December 31, 2021 and achieve the minimum required weight loss); rather, we proposed to maintain those payment rates until such time as ongoing maintenance sessions are phased out.

Our data from the DPP model test showed beneficiaries who finished at least nine (9) sessions of the model were considered "completers" and had better weight loss and lower Medicare spending than non-completers (those who attended fewer than 9 sessions). The DPP model test showed that beneficiaries who attend nine or more sessions will, on average, experience a 6.24 percentage point increase in weight loss compared to beneficiaries attending fewer than nine sessions. Currently, our payment structure does not pay for per session attendance, and stakeholders have commented that the expanded model, in its current state, is creating inequities in access to MDPP among eligible beneficiaries because suppliers cannot invest in the costs to retain beneficiaries who may have access barriers related to transportation or distance of the MDPP location from their home. We anticipate the proposed changes to the payment structure, which would pay a total of $61 more per beneficiary who attends at least 9 session than what is currently paid, would encourage existing suppliers to retain MDPP beneficiaries given the one-year commitment versus two for the MDPP set of services. Continuous beneficiary attendance is critical to reaching key outcomes such as 5 percent weight loss and reduced Medicare spending. Additionally, we expect more eligible organizations will enroll as MDPP suppliers due to our eliminating the ongoing maintenance period, increasing the number of locations beneficiaries may access the MDPP set of services. We expect the proposed changes to the MDPP payment structure would not affect MDPP's qualification for expansion. We would use the CDC DPRP and MDPP claims data as planned to assess whether the MDPP expanded model is expected to improve the quality of care without increasing spending, reduce spending without reducing the quality of care, or improve the quality of care and reduce spending. We anticipate that these programmatic adjustments are likely to result in more MDPP suppliers, increased beneficiary access to MDPP services and an ongoing reduction of the incidence of diabetes in eligible Medicare beneficiaries, in both urban and rural communities.
In our regulatory impact analysis, the CMS Office of the Actuary estimates that these proposed changes would reduce Medicare spending over 10 years, with potential savings starting in 2026. Increasing the first-year payment amounts to suppliers and waiving the Medicare enrollment fee (as discussed below) should increase access to MDPP, resulting in more utilization of the MDPP set of services.
We are proposing a change to our emergency policy at § 410.79(e)(3)(v)(C) to account for the proposed elimination of ongoing maintenance sessions for MDPP beneficiaries who start the set of MDPP services on or after January 1, 2022. Under this proposal, only beneficiaries who start the MDPP set of services between January 1, 2021, and December 31, 2021 and who are in the second year of the set of MDPP services as of the start of an applicable 1135 waiver event may either resume or restart the ongoing maintenance session interval in which they were participating at the start of the applicable 1135 waiver event if they elect not to continue with MDPP services virtually during the applicable 1135 waiver event.
As noted above, we propose to remove the ongoing maintenance sessions phase for all MDPP beneficiaries who start MDPP set of services on or after January 1, 2022. MDPP beneficiaries who start the MDPP Start Printed Page 39307 set of services on or before December 31, 2021 would be able to continue with the ongoing maintenance phase if they meet the eligibility requirements described in § 410.79(c)(3). Table 29 summarizes our proposal for the MDPP services period based on beneficiary start date.

Additionally, we propose to remove the second duplicate paragraph (c)(3)(ii) given that the electronic CFR contains two paragraphs (c)(3)(ii), both containing the exact same language.
We are proposing to amend our regulation at § 410.79(b), (c), and (e). We seek comment on these proposals and ways to simplify the proposed policies.
2. Proposed Changes to § 414.84(b) and (c)
We propose to amend § 414.84(b) and (c) to update the amount of the performance payments for the core sessions, core maintenance sessions and ongoing maintenance sessions (where applicable) to be consistent with the proposal herein. We propose that this change apply to all MDPP beneficiaries starting the MDPP set of services on or after January 1, 2022.
For those MDPP beneficiaries who started the first core session on or before December 31, 2021, we propose that MDPP suppliers continue to submit claims for the ongoing maintenance sessions attended using the existing ongoing maintenance HCPCS G-codes, G9891, G9892, G9893, G9894, and G9895 when submitting claims for those MDPP beneficiaries who attended ongoing maintenance sessions.
We are proposing to amend our regulation at § 414.84(b) and (c). We seek comment on these proposals.
3. Proposed Changes to § 424.205(b)
Medicare requires all organizations that deliver MDPP services to enroll separately in Medicare as a MDPP supplier and pay an enrollment application fee. This places a unique burden on MDPP suppliers. Approximately 39 percent of these entities are non-traditional suppliers that serve their local communities to increase diversity, equity, and inclusion of their services, including but not limited to YMCAs, county health departments, community health centers, and non-profit organizations that focus on health education that otherwise would neither enroll nor be able to enroll as a Medicare supplier. Indeed, they are often very different from most other Medicare providers and suppliers in terms of business model and financial wherewithal, and they frequently furnish non-health care services to the community. In this vein, they cannot be considered in the same light as, for example, hospitals, skilled nursing facilities, ambulance suppliers, or other organizations specifically and exclusively designed for the provision of health care services.
The provider/supplier enrollment fee for CY 2021 is $599. Although MDPP suppliers may submit a written request to CMS for a hardship exception to the application fee in accordance with § 424.514, many would not qualify. We have heard from stakeholders that the enrollment application fee factors into an organization's decision to participate in MDPP. Organizations must submit the provider enrollment fee during the initial start-up phase of their expanded model implementation. This is when costs are likely the highest for organizations and the timing of the first CMS reimbursement is farthest away. MDPP suppliers would need to provide a first core session to at least 24 beneficiaries to simply recoup the Medicare provider enrollment fee. For many potential MDPP suppliers, the provider enrollment application fee, when combined with the additional MDPP requirements, such as the claims processing requirements, result in an organization declining to invest in enrolling as an MDPP supplier.
On April 9, 2020, CMS, through the COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers, waived all provider enrollment application fees. We saw an immediate increase in MDPP supplier enrollment in Q2 2020, the quarter the blanket waivers were announced, but MDPP supplier enrollment slowed thereafter, likely due to the impact of the pandemic and many CDC National DPP organizations pausing their delivery of DPP. We believe that granting a waiver of the fee for MDPP suppliers to extend beyond the COVID-19 Emergency Declaration Blanket Waiver may increase MDPP supplier enrollment, which will ultimately improve beneficiary access to the expanded model and our ability to evaluate the outcome of the MDPP because increasing the number of MDPP suppliers may provide for a more robust evaluation of the expanded model. Given our prior discussion of the unique character of MDPP suppliers in comparison to more traditional provider and supplier types, we believe this policy change is warranted. Start Printed Page 39308
In an effort to minimize the impact of this potential barrier and allow for a more robust expanded model evaluation, we are proposing to utilize CMS' waiver authority under section 1115A(d)(1) of the Act to waive the provider enrollment Medicare application fee (described in sections 1866(j)(2)(C)(i) and (ii) of the Act) for all organizations that submit an application to enroll in Medicare as an MDPP supplier on or after January 1, 2022. We are proposing to amend our regulation at § 424.205 (b) to reflect this waiver.
We seek comment on these proposals.
4. Proposed Changes to § 424.502
We propose to make a conforming amendment to § 424.502 to remove the reference to the CMS-20134 from the definition of "institutional provider." The CY 2018 PFS final rule, which established the application fee for MDPP suppliers, amended the definition of "institutional provider" in section § 424.502 to state that MDPP suppliers that complete the CMS-20134 enrollment application are "institutional provider[s]". Thus, the application fee described in section § 424.514 applies to organizations enrolling in Medicare as MDPP suppliers. We are proposing to reverse this policy by amending § 424.502 to remove the reference to the CMS-20134 thereby removing MDPP suppliers from the list of institutional providers required to pay the Medicare enrollment fee under § 424.514. As proposed, § 424.514 would no longer be applicable to organizations enrolling in Medicare as an MDPP supplier.
We seek comment on this proposal.
M. Clinical Laboratory Fee Schedule: Laboratory Specimen Collection and Travel Allowance for Clinical Diagnostic Laboratory Tests and Use of Electronic Travel Logs
1. Background on the Clinical Laboratory Fee Schedule
Prior to January 1, 2018, Medicare paid for clinical diagnostic laboratory tests (CDLTs) on the Clinical Laboratory Fee Schedule (CLFS), with certain exceptions, under sections 1833(a), (b), and (h) of the Act. Under the previous payment system, CDLTs were paid based on the lesser of: (1) The amount billed; (2) the local fee schedule amount established by the Medicare Administrative Contractor (MAC); or (3) a national limitation amount (NLA), which is a percentage of the median of all the local fee schedule amounts (or 100 percent of the median for new tests furnished on or after January 1, 2001). In practice, most tests were paid at the NLA. Under the previous payment system, the CLFS amounts were updated for inflation based on the percentage change in the Consumer Price Index for All Urban Consumers (CPI-U), and reduced by a productivity adjustment and other statutory adjustments, but were not otherwise updated or changed. Coinsurance and deductibles generally do not apply to CDLTs paid under the CLFS.
Section 1834A of the Act, as established by section 216(a) of the Protecting Access to Medicare Act of 2014 (PAMA) (Pub. L. 113-93, April 1, 2014), required significant changes to how Medicare pays for CDLTs under the CLFS. In the June 23, 2016 Federal Register (81 FR 41036), we published a final rule entitled "Medicare Clinical Diagnostic Laboratory Tests Payment System" (CLFS final rule), that implemented section 1834A of the Act at 42 CFR part 414, subpart G.
2. Payment for Laboratory Specimen Collection and Travel Allowance for COVID-19 Clinical Diagnostic Laboratory Tests
In general, section 1833(h)(3) of the Act requires the Secretary to provide for and establish a nominal fee for specimen collection for laboratory testing and a fee to cover transportation and personnel expenses for trained personnel to collect specimens from homebound patients and inpatients (not in a hospital), in addition to the amounts provided under the Medicare CLFS. Section 1833(h)(3)(A) of the Act provides that the Secretary must establish a nominal fee to cover the appropriate costs in collecting the sample on which a CDLT was performed and for which payment is made under Medicare Part B, except that not more than one such fee may be provided with respect to samples collected in the same encounter. The HCPCS codes for the nominal specimen fees currently listed on the CLFS (HCPCS codes 36415, P9612, and P9615) have a payment rate of $3. Section 216(a) of PAMA added section 1834A(b)(5) to the Act, which increases by $2 the nominal fee that would otherwise apply under section 1833(h)(3)(A) of the Act for a sample collected from an individual in a skilled nursing facility (SNF) or by a laboratory on behalf of a home health agency (HHA). Therefore, effective April 1, 2014, the nominal fee that would otherwise apply for a sample collected from an individual in a SNF or by a laboratory on behalf of a HHA is $5 (see § 414.507(f)), and the relevant HCPCS code is G0471.
In addition, section 1833(h)(3)(B) of the Act requires the Secretary to provide for and establish a fee to cover the transportation and personnel expenses for trained personnel to travel to the location of an individual to collect the sample, except that such a fee may be provided only with respect to an individual who is homebound or an inpatient in an inpatient facility (other than a hospital). In accordance with this provision, Medicare established a travel allowance for a laboratory technician to draw a specimen from homebound patients and non-hospital inpatients. Under current guidance, the travel allowance is intended to cover the estimated travel costs of collecting a specimen from a Medicare beneficiary and to reflect the technician's salary and travel costs. It is paid only when the nominal specimen collection is also payable and is not available if the technician is merely performing a messenger service to pick a specimen drawn by a physician or nursing home personnel. The methodology for determining the travel allowance varies depending on the round trip mileage to patients' homes. For instance, a per mile travel allowance methodology applies when the round trip to patients' homes is greater than 20 miles and a flat rate travel allowance methodology applies when the round trip to patients' homes is less than 20 miles. Medicare Part B MACs calculate the travel allowance for each claim. Stakeholders have reported that, in some cases, the MACs require them to maintain paper logs of miles traveled to receive the travel allowance.
Our general policies for payment of the nominal specimen collection fee and the fee to cover transportation and expenses for trained personnel to collect specimens from homebound patients and non-hospital inpatients are set forth in Pub. 100-04, Medicare Claims Processing Manual, chapter 16, section 60. We also implemented the increased nominal specimen collection fee under section 1834A(b)(5) of the Act in our regulations at § 414.507(f). The manual instructions regarding payment of these fees are available on the CMS website at https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c16.pdf. Neither the annual cash deductible nor the 20 percent coinsurance for Medicare apply to the specimen collection fees or travel allowance for laboratory tests.
In the interim final rule with comment period titled, "Medicare and Medicaid Programs; Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency" (March 2020 COVID-19 IFC), which displayed and became effective on Start Printed Page 39309 March 31, 2020, and appeared in the April 6, 2020 Federal Register (85 FR 19230), we established that Medicare will pay a nominal specimen collection fee and associated travel allowance to independent laboratories for the collection of specimens for COVID-19 clinical diagnostic laboratory testing for homebound and non-hospital inpatients (85 FR 19256 through 19258). This policy provides independent laboratories with additional resources to provide COVID-19 testing and helps with efforts to limit patients' exposure to the general population and alleviate patients' unease with leaving the home specifically for the duration of the public health emergency (PHE) for the COVID-19 pandemic. To identify specimen collection for COVID-19 testing specifically, we established two new level II HCPCS codes: Code G2023 (specimen collection for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), any specimen source); and code G2024 (specimen collection for severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) (Coronavirus disease [COVID19]), from an individual in a SNF or by a laboratory on behalf of a HHA, any specimen source), for independent laboratories to use when billing Medicare for the nominal specimen collection fee for COVID-19 testing for the duration of the PHE for COVID-19.
We stated in the March 2020 COVID-19 IFC (85 FR 19258) that, in establishing a nominal fee for COVID-19 specimen collection, we considered the type of trained laboratory personnel required to collect the specimen and the resources COVID-19 specimen collection could require, and we recognized that the existing specimen collection fees were not intended to address additional resources needed during the PHE for the COVID-19 pandemic. Absent concrete information regarding the costs associated with independent laboratories collecting such specimens for COVID-19 tests in the context of the PHE, we looked to similar services in other settings of care as a potential benchmark. We identified the PFS as the best source for a potential payment amount since physicians and other practitioners often bill for services that involve specimen collection by trained, non-institutional staff.
We described how under the PFS, a Level 1 office visit (CPT® code 99211) typically does not require the presence of a physician or other qualified health care professional and the usual presenting problem(s) are minimal. This code is what is typically reported by physician practices when the patient only sees clinical office staff for services like acquiring a routine specimen sample. CPT® code 99211 describes an: Office visit for E/M of an established patient that may be performed by clinical staff under supervision (may not require a physician's presence). Usually the presenting problem(s) are minimal and typically, 5 minutes are spent supervising or performing the service.
We stated in the March 2020 COVID-19 IFC that, in the case of collecting a specimen for COVID-19 testing, we believe that in the context of and for the duration of the PHE for the COVID-19 pandemic, collecting specimens using nasopharyngeal and oropharyngeal swabs or collection of sputum will require a trained laboratory professional, as well as additional precautions that must be taken to minimize exposure risks in handling specimens that are suspected or confirmed for COVID-19. Thus, we believe that collecting a specimen for COVID-19 testing may incur higher costs than similar specimen collection services, which require a trained laboratory professional, but not additional precautions, to minimize exposure risk.
We indicated in the March 2020 COVID-19 IFC that this specimen collection fee policy was established for the duration of the PHE for COVID-19 (85 FR 19256) and will end once the PHE for the COVID-19 pandemic has ended.
In the CY 2021 PFS proposed rule, we requested comments on whether we should delete HCPCS codes G2023 and G2024 once the PHE for COVID-19 ends (85 FR 50211). Specifically, we sought public input on why these codes, and their corresponding payment amounts, which are higher than the nominal specimen collection fees for other conditions, would be necessary or useful outside of the context of the PHE for COVID-19. We stated that we were particularly interested in why separate, increased payment for specimen collection, specifically for COVID-19 tests, in contrast to other tests, may be needed following the end of the PHE. We noted that comments received may inform a future proposal.
We received public comments on the laboratory specimen collection fees for COVID-19 CDLTs. Commenters expressed support for permanently extending payment for specimen collection for COVID-19 CDLTs after the PHE ends, as commenters expect the COVID-19 virus to be present into CY 2021, thus making it appropriate for CMS to continue to pay laboratories for specimen collection using HCPCS codes G2023 and G2024. Commenters recommended that we expand and permanently authorize the specimen collection fees under HCPCS codes G2023 and G2024 to apply to all CDLTs to compensate for the supplies, equipment, and sterilization protocols required for safe and uncontaminated specimen collection and handling in the presence of COVID-19. Commenters noted that COVID-19 will continue to spread and may become an ongoing and/or seasonal infectious disease event, and because of this possibility, they expect that the heightened safety precautions, the need for personal protective equipment (PPE), and the requirement for special training for specimen collection will persist beyond the immediate PHE. In the CY 2021 PFS final rule (85 FR 84695), we noted that we planned to take this feedback into consideration for possible future rulemaking or guidance regarding the nominal specimen collection fees and associated travel allowance to independent laboratories for the collection of specimens for COVID-19 clinical diagnostic laboratory testing.
After considering these comments, we continue to believe that the laboratory specimen collection fees for COVID-19 CDLTs established in the context of and for the duration of the PHE for the COVID-19 pandemic should conclude at the termination of the PHE, as originally announced in the March 2020 COVID-19 IFC (85 FR 19258). We believe that these increased payments for specimen collection specifically for COVID-19 tests would no longer be warranted beyond the end of the PHE. As described above, the increased fees were intended to address additional resources needed specifically during the PHE for the COVID-19 pandemic, particularly for the collecting of specimens using nasopharyngeal and oropharyngeal swabs or collection of sputum, which required a trained laboratory professional and additional precautions to minimize exposure risks in handling specimens that are suspected or confirmed for COVID-19. Given the advances in the types of COVID-19 CDLTs available to the public and the reduced need for specimen collection by trained laboratory professionals, we believe that the increased laboratory professional resources needed for COVID-19 specimen collection will no longer be necessary after the PHE for the COVID-19 pandemic ends. Additionally, we expect that the termination of the PHE will occur when there is a reduced risk of COVID-19, which will mean the increase of supplies, PPE, and heightened sterilization and safety Start Printed Page 39310 protocols for laboratory specimen collection and handling will be at a more manageable level. Likewise, we expect the potential ongoing spread of COVID-19 likely will diminish after the PHE ends, which will mean that advanced safety precautions, extensive PPE, and specialized training for laboratory specimen collection likely will no longer be required to the same extent as during the PHE. Furthermore, we anticipate that widespread vaccination of both medical professionals as well as the general population will likely reduce the need for intensive PPE.
3. Comment Solicitation on Specimen Collection Fee and Travel Allowance for Clinical Diagnostic Laboratory Tests
Although we expect the increased specimen collection fees for COVID-19 CDLTs will end at the termination of the PHE for the COVID-19 pandemic, we are taking this opportunity to seek broad comment on our policies for specimen collection fees and the travel allowance as we consider updating these policies in the future through notice and comment rulemaking. Therefore, in this proposed rule, we are requesting comments regarding the nominal specimen collection fees for trained personnel to collect specimens from homebound patients and inpatients (not in a hospital). We are also seeking comments related to the calculation of costs for transportation and personnel expenses for trained personnel to collect specimens from such patients.
We are seeking additional public input regarding considerations for the nominal fees for laboratory specimen collection for COVID-19 testing beyond the PHE. We are particularly interested in what additional resources might be needed for specimen collection for COVID-19 CDLTs and other tests after the PHE ends. For example, laboratories may see a benefit to using more PPE for laboratory staff than they did before the onset of the PHE as way to improve safety for personnel and patients. We expect there will be ongoing interest in minimizing outbreaks of diseases such as influenza in various settings, including SNFs, where beneficiaries may not be able to travel to a laboratory for testing. We seek feedback from stakeholders regarding how their specimen collection practices may have changed as a result of, or from insight gained during, the PHE for the COVID-19 pandemic, specifically in terms of changes to supplies and staffing resources as well as related costs. We recognize that the COVID-19 pandemic may have changed the approaches to specimen collection for laboratory testing from homebound patients and inpatients (not in a hospital) in ways that will persist beyond the termination of the PHE for the COVID-19 pandemic, and we want to understand these changes to inform potential modifications to our laboratory specimen collection policies in the future.
We also seek comments on the methodology for calculating the travel allowance, including calculation of mileage specific to per mile or flat rate and proration when there are multiple patients or specimens.
Comments received may inform a future proposal.
4. Medicare Clinical Laboratory Fee Schedule: Electronic Travel Logs
In addition to insights we have gained, and are seeking to gain, from the COVID-19 CDLT laboratory specimen collection fees policies we implemented for the duration of the COVID-19 PHE, we have also had the opportunity to gain insight about other CLFS policies established for the purposes of the PHE for COVID-19.
As described in the March 2020 COVID-19 IFC (85 FR 19256), and noted above, section 1833(h)(3)(B) of the Act requires the Secretary to provide for and establish a fee to cover the transportation and personnel expenses for trained personnel to travel to the location of an individual to collect the sample, except that such a fee may be provided only with respect to an individual who is homebound or an inpatient in an inpatient facility (other than a hospital). In accordance with this provision, Medicare established a travel allowance for a laboratory technician to draw a specimen from homebound patients and non-hospital inpatients. The travel allowance is intended to cover the estimated travel costs of collecting a specimen from a Medicare beneficiary and reflect the technician's salary and travel costs. It is paid only when the nominal specimen collection is also payable and is not available if the technician is merely performing a messenger service to obtain a specimen drawn by a physician or nursing home personnel. The methodology for determining the travel allowance varies depending on the round trip mileage to patients' homes. For instance, a per mile travel allowance methodology applies when the round trip to a patients' home is greater than 20 miles and a flat rate travel allowance methodology applies when the round trip is less than 20 miles.
We stated in the March 2020 COVID-19 IFC (85 FR 19258) that Medicare payment for transportation and expenses for trained personnel to collect specimens from homebound patients and inpatients (not in a hospital) for purposes of COVID-19 testing would be made in accordance with existing instructions found in the Medicare Claims Processing Manual. Our policies for payment of the fee to cover transportation and expenses for trained personnel to collect specimens from homebound patients and non-hospital inpatients are set forth in Pub. 100-04, Medicare Claims Processing Manual, chapter 16, section 60.
Medicare Part B MACs calculate the travel allowance for each claim, and we understand from stakeholders that, in some cases, the MACs have required laboratories to maintain paper logs of miles traveled to receive the travel allowance. In the March 2020 COVID-19 IFC (85 FR 19258), we noted that stakeholders reported to us that maintaining paper logs of miles is burdensome, whereas maintaining electronic logs is less burdensome, especially with the development of GPS systems and various applications for cellular phones in recent years that can track miles traveled. We indicated that, for the duration of the PHE for the COVID-19 pandemic, paper documentation of miles traveled would not be required and that laboratories could maintain electronic logs if they preferred. However, we indicated that laboratories would need to be able to produce these electronic logs in a form and manner that could be shared with MACs, and that the MACs may provide more information on acceptable formats.
We understand that laboratories have benefited from the operational guidance we announced in the March 2020 COVID-19 IFC to permit them to use electronic rather than paper logs of miles traveled. Using electronic logs reduces administrative burden for laboratories and their staffs, as we understand from stakeholders that paper logs are extremely time- and resource-intensive and they have requested that CMS provide flexibilities for the use of electronic logs. Paper logs require storage, which can be a burden to laboratories. Additionally, manipulation of paper logs is time- and labor-intensive, which could increase laboratory costs. Therefore, we are making permanent the option for laboratories to maintain electronic logs of miles traveled for the purposes of covering the transportation and personnel expenses for trained personnel to travel to the location of an individual to collect a specimen sample. To be clear, this option for laboratories to maintain electronic logs is not limited to COVID-19 specimen collection, but Start Printed Page 39311 rather, applies to specimen collection for any CDLT. We are announcing electronic logs as a permanent option in this proposed rule, and will provide guidance in future instructions via forthcoming Change Requests and other materials such as MLN Matters® Articles. Laboratories will need to be able to produce electronic logs in a form and manner that can be shared with MACs, and should continue to consult with their local MACs regarding the format and process for ongoing submission of this information.
N. Medicare Provider and Supplier Enrollment
1. Enrollment Process
a. General Discussion
Section 1866(j)(1)(A) of the Act requires the Secretary to establish a process for the enrollment of providers and suppliers in the Medicare program. The overarching purpose of the enrollment process is to help confirm that providers and suppliers seeking to bill Medicare for services and items furnished to Medicare beneficiaries meet all federal and state requirements to do so. The process is, to an extent, a "gatekeeper" that prevents unqualified and potentially fraudulent individuals and entities from being able to enter and inappropriately bill Medicare. Since 2006, we have taken steps via rulemaking to outline our enrollment procedures. These regulations are generally incorporated in 42 CFR part 424, subpart P (currently §§ 424.500 through 424.570 and hereafter occasionally referenced as subpart P). They address, among other things, requirements that providers and suppliers must meet to obtain and maintain Medicare billing privileges.
As outlined in § 424.510, one such requirement is that the provider or supplier must complete, sign, and submit to its assigned Medicare Administrative Contractor (MAC) the appropriate enrollment form, typically the Form CMS-855 (OMB Control No. 0938 0685). The Form CMS-855, which can be submitted via paper or electronically through the internet-based Provider Enrollment, Chain, and Ownership System (PECOS) process (SORN: 09-70-0532, PECOS), collects important information about the provider or supplier. Such data includes, but is not limited to, general identifying information (for example, legal business name), licensure and/or certification data, and practice locations. After receiving the provider's or supplier's initial enrollment application, CMS or the MAC reviews and confirms the information thereon and determines whether the provider or supplier meets all applicable Medicare requirements.
We believe the Medicare provider enrollment screening process has greatly assisted CMS in executing its responsibility to prevent Medicare fraud, waste, and abuse. As previously mentioned, over the years we have issued various final rules pertaining to provider enrollment. These rules were intended not only to clarify or strengthen certain components of the enrollment process but also to enable us to take further action against providers and suppliers: (1) Engaging (or potentially engaging) in fraudulent or abusive behavior; (2) presenting a risk of harm to Medicare beneficiaries or the Medicare Trust Funds; or (3) that are otherwise unqualified to furnish Medicare services or items. Consistent with this, and as discussed further in this section II.N. of this proposed rule, we propose several changes to our existing provider enrollment regulations.
b. Legal Authorities
There are two principal categories of legal authorities for our proposed provider enrollment provisions. First, section 1866(j) of the Act furnishes specific authority regarding the enrollment process for providers and suppliers. Second, sections 1102 and 1871 of the Act provide general authority for the Secretary to prescribe regulations for the efficient administration of the Medicare program.
2. Proposed Provisions
a. Expansion of Authority To Deny or Revoke Based on Office of Inspector General (OIG) Exclusion
Under §§ 424.530(a)(2) and 424.535(a)(2), respectively, CMS denies or revokes a provider's or supplier's enrollment if the provider or supplier, or any owner, managing employee, authorized or delegated official, medical director, supervising physician, or other health care personnel of the provider or supplier is excluded by the OIG. We propose several changes related to these authorities.
First, we propose to expand the categories of parties within the purview of these denial and revocation provisions to include excluded administrative or management services personnel who furnish services payable by a federal health care program, such as a billing specialist, accountant, or human resources specialist. This change would align with existing OIG guidance stating that providers and suppliers may not employ excluded persons to provide management or administrative services that are payable by a federal health care program.[119] Such individuals can impact a provider's or supplier's operations in a manner harmful to the interests of the Medicare program; for example, an individual in a lower-level administrative position could undertake fraudulent activity to the same extent (and with consequences as severe) as a high-ranking officer. For program integrity purposes, the central issue is not the specific individual who engaged in the abusive conduct, but the conduct itself. Accordingly, we believe this regulatory revision is necessary to protect Medicare and its beneficiaries.
Second, existing § 424.530(a)(2) references "other health care personnel furnishing Medicare reimbursable services who is required to be reported on the enrollment application." (Section 424.535(a)(2) does not contain the entirety of this clause.) To conform to our change described in the previous paragraph, we propose to replace this language with "other health care or administrative or management services personnel furnishing services payable by a federal health care program." This would ensure consistency with the previously referenced OIG guidance, which, we note, is not restricted to services: (1) Only reimbursable by Medicare; or (2) furnished by individuals listed on a Medicare enrollment application. We would also include this language within § 424.535(a)(2) so that the latter aligns with § 424.530(a)(2).
Third, § 424.535(e) states that if the revocation was due to adverse activity (sanction, exclusion, or felony) against an owner, managing employee, authorized or delegated official, medical director, supervising physician, or other personnel of the provider or supplier furnishing Medicare reimbursable services, the revocation may be reversed if the provider or supplier terminates and submits proof that it has terminated its business relationship with that individual within 30 days of the revocation notification. For the reasons already outlined, we propose to replace the language in § 424.535(e) concerning other personnel furnishing Medicare reimbursable services with "other health care or administrative or management services personnel furnishing services payable by a federal health care program." Start Printed Page 39312
b. Deny or Revoke Enrollment for Surrender of Drug Enforcement Administration (DEA) Certificate of Registration in Response To Show Cause Order
We have existing authority under § 424.530(a)(11)(i) to deny a physician's or other eligible professional's enrollment if his or her DEA certificate of registration to dispense a controlled substance is currently suspended or revoked; a concomitant authority to revoke enrollment in this circumstance is outlined in § 424.535(a)(13)(i). We propose to expand these authorities to include situations where the physician or other eligible professional surrenders his or her DEA certificate in response to an order to show cause.
We have encountered situations where a physician or other eligible professional who has engaged in improper prescribing or other DEA-monitored activities relinquishes his or her DEA certificate pending a DEA show cause order so as to avoid a likely suspension or revocation of his or her DEA certificate or other similar circumstance. We believe these scenarios are no less serious from the standpoints of program integrity and beneficiary safety than a DEA certificate suspension or revocation. Hence, we believe this proposed change is warranted.
c. Creation of Specific Rebuttal Rights for Deactivations
As outlined in § 424.540, deactivation means that the provider's or supplier's billing privileges are stopped (but not revoked or terminated). Deactivation is intended to protect the provider or supplier from the misuse of its billing number and to safeguard the Trust Funds from unnecessary overpayments. Under existing regulations, a provider's or supplier's billing privileges may be deactivated if the provider or supplier: (1) Does not submit any Medicare claims for 12 consecutive calendar months; (2) fails to report certain changes in its enrollment information within required timeframes; or (3) fails to fully and accurately comply with a CMS revalidation request within 90 days.[120] To reactivate one's billing privileges, current regulations state that the deactivated provider or supplier must recertify that their enrollment information on file with Medicare is correct and must furnish any missing information as appropriate (or submit a complete Form CMS-855 application if required by CMS).
Since a deactivated provider's or supplier's billing privileges are stopped, § 424.545(b) permits the affected provider or supplier to file a rebuttal in accordance with 42 CFR 405.374 (which allows rebuttals for Medicare payment suspensions). While we have outlined deactivation rebuttal procedures in subregulatory guidance, these procedures are not reflected in regulation. Consequently, we propose to revise 42 CFR part 424, subpart P to describe the deactivation rebuttal process in detail, a process that would generally mirror our existing subregulatory procedures on the topic. This would streamline and clarify the deactivation rebuttal process, promote transparency, and enable the provider community to submit feedback on our proposal.
The specific changes we propose are as follows:
- At § 424.545(b), we propose to change the language that reads "in accordance with § 405.374 of this chapter" to "in accordance with § 424.546." Instead of continuing to reference § 405.374, we are proposing to create a new § 424.546 to address the deactivation rebuttal process. This would enable us to tailor our procedures to the unique facts and circumstances of deactivation rebuttals, which are different from payment suspensions.
- At new § 424.546(a)(1), we propose that if a provider or supplier receives written notice from CMS or its contractor that the provider's or supplier's billing privileges are to be or have been deactivated under § 424.540, the provider or supplier has 15 calendar days from the date of the written notice to submit a rebuttal to CMS. We believe that a 15-day period strikes an ideal balance between the need to expeditiously take measures to safeguard program integrity and the importance of ensuring that the provider or supplier has a reasonable time-period in which to submit a rebuttal.
- At new § 424.546(a)(2), we propose that CMS may, at its discretion, extend the 15-day time-period referenced in § 424.546(a)(1). This would permit CMS to account for special situations, such as the following: (1) A particularly and unusually complex deactivation case that perhaps warrants giving the provider or supplier more time to prepare its rebuttal; or (2) circumstances beyond the provider's or supplier's control prevents or would likely prevent the timely submission of its rebuttal.
- At new § 424.546(b)(1) through (4), we propose that any rebuttal must: (1) Be in writing; (2) specify the facts or issues about which the provider or supplier disagrees with the deactivation's imposition and/or effective date, as well as the reasons for disagreement; (3) submit all documentation the provider or supplier wants CMS to consider in its review of the deactivation; and (4) be submitted in the form of a letter that is signed and dated by the individual supplier (if the latter is enrolled as an individual physician or NPP), the authorized official or delegated official (as those terms are defined in § 424.502), or a legal representative (as defined in 42 CFR 498.10). Concerning proposed paragraph (b)(4), if the legal representative is an attorney, the attorney must include a statement that he or she has the authority to represent the provider or supplier; this statement would be sufficient to constitute notice of such authority. If the legal representative is not an attorney, the provider or supplier must file with CMS written notice of the appointment of a representative; this notice of appointment must be signed and dated by, as applicable, the individual supplier, the authorized official or delegated official, or a legal representative.
We believe that the requirements of proposed § 424.546(b)(1) through (4) are necessary to ensure: (1) A uniform and standard process for submitting deactivation rebuttals; (2) that there is written documentation of the provider's or supplier's contentions; (3) that CMS has sufficient information to perform its review; and (4) that the provider or supplier authorized the rebuttal submission.
- At new § 424.546(c), we propose that the provider's or supplier's failure to submit a rebuttal that is both timely under paragraph (a) and fully compliant with all of the requirements of paragraph (b) constitutes a waiver of all rebuttal rights under this section and § 424.545(b). This provision would not only specify the consequences of an untimely or non-compliant rebuttal but also help encourage the provider or supplier to abide by paragraphs (a) and (b) should it choose to rebut a deactivation.
- At new § 424.546(d), we propose that upon receipt of a timely and compliant deactivation rebuttal, CMS Start Printed Page 39313 reviews the latter to determine whether the imposition of the deactivation and/or the designated effective date are correct. We believe this provision would adequately notify the public of the scope of CMS' review.
- At new § 424.546(e), we propose that nothing in § 424.546 or in § 424.545(b) would require CMS to delay the imposition of a deactivation pending the completion of the CMS review described in paragraph (d). That is, the filing of a rebuttal and the review period associated therewith does not suspend or postpone the deactivation's implementation. This provision, which mirrors our existing subregulatory policy on the matter, is needed so that CMS can expeditiously enforce the program integrity protections that a deactivation affords, all the while recognizing the provider's or supplier's ability to challenge the deactivation via the rebuttal process. If CMS determines under paragraph (d) that the deactivation was erroneous, it would be reversed.
- At new § 424.546(f), and consistent with both current subregulatory policy concerning deactivation rebuttals as well as payment suspension rebuttal regulations at § 405.375(c), a determination made under § 424.546 would not be an initial determination under § 498.3(b) and, therefore, would not be appealable. This would clarify for providers and suppliers that a rebuttal is the only administrative remedy available for a deactivation.
d. Modernizing Enrollment Policies for Emerging Technologies in Independent Diagnostic Testing Facilities
Section 410.33(a) states that CMS pays for diagnostic procedures under the PFS only when performed by the suppliers listed in that section. Among these supplier types are independent diagnostic testing facilities (IDTFs). An IDTF may be a fixed location, a mobile entity, or an individual NPP. It is independent of a physician's office or hospital, although the IDTF regulations outlined in § 410.33(a) also apply when an IDTF furnishes diagnostic procedures in a physician's office.
Section 410.33 as a whole contains provisions with which IDTFs must comply in order to enroll in (and maintain enrollment in) Medicare. This includes requirements for supervising physicians (§ 410.33(b)), nonphysician personnel (§ 410.33(c)), and the ordering of tests (§ 410.33(d)). In addition, § 410.33(g) contains various certification standards that IDTFs must meet. We established these standards to help ensure the quality and safety of IDTF diagnostic testing and to strengthen our ability to verify the IDTF's compliance with enrollment requirements.
IDTFs generally perform diagnostic tests on beneficiaries in, for instance, a health care facility, physician's office, or mobile setting. Indeed, the IDTF standards at § 410.33(g) (as well as other provisions at § 410.33) were designed for traditional IDTF suppliers that engage in direct or in-person beneficiary interaction, treatment, and/or testing. Yet, some health care entities have developed or utilize diagnostic tests that do not require this form of interaction. That is, certain IDTFs perform diagnostic services via computer modeling and analytics, or other forms of testing not involving direct beneficiary interaction; the service is often conducted by a technician who undertakes a computer analysis offsite or at another location at which the patient is not present. The physician then reviews the image to determine the appropriate course of action. In short, these entities generally (though not exclusively) have two overriding characteristics. First, the tests they perform do not involve direct patient interaction, meaning that the test is conducted away from the patient's physical presence and is non-invasive. Second, the test involves off-site computer modeling and analytics.
Despite the comparatively new and innovative forms of testing these entities undertake, they can still qualify as IDTFs (notwithstanding the offsite and indirect nature of the test) so long as they meet the applicable requirements of § 410.33. The dilemma is that these entities often cannot meet certain IDTF requirements (and thus cannot enroll in Medicare) strictly because of the test's indirect nature. In other words, the types of tests at issue do not fall within the category of those to which several of our standards in § 410.33 were intended to apply (specifically, to in-person procedures). To account for such technological advances in diagnostic testing, we believe that revisions to § 410.33 are necessary. To this end, we propose that IDTFs that have no beneficiary interaction, treatment, or testing at their practice location would be either partially or wholly exempt from the following requirements in § 410.33 (hereafter occasionally referenced as "exempted" IDTFs).
Section 410.33(c) requires all nonphysician personnel that the IDTF uses to perform diagnostic tests to demonstrate the basic qualifications to perform these tests as evidenced by state licensure or state certification. In the absence of a state licensing board, the technician must be certified by an appropriate national credentialing body. (The IDTF must also maintain documentation available for review that these requirements have been met.) However, the indirect tests in question often do not require state licensure or state/national credentialing, meaning that § 410.33(c) becomes a difficult requirement for such IDTFs to meet. Indeed, § 410.33(c) has typically been applied to the qualifications needed to perform in-person tests in traditional IDTF settings; that is, the staff at exempted IDTFs often will instead be primarily trained in the test's particular software and computer analytics (or other non-beneficiary based services)). Extending § 410.33(c)'s purview to indirect tests would reduce the number of personnel who can perform them, thus hindering beneficiary access to such services and potentially preventing the enrollment of otherwise qualified IDTFs.
Accordingly, we propose to divide current § 410.33(c) into two paragraphs. New paragraph (c)(1) would contain the existing requirements of § 410.33(c) except as stated in new paragraph (c)(2). We propose in the latter paragraph that, for services that do not require direct or in-person beneficiary interaction, treatment, or testing, any nonphysician personnel performing the test must meet all applicable state licensure requirements for doing so; if there are such state licensure requirements, the IDTF must maintain documentation available for review that these requirements have been met.
While we believe that personnel performing the tests described in proposed paragraph (c)(2) should meet whatever state requirements exist for those services, paragraph (c)(2) would not include any reference to national credentialing bodies. Further, we recognize that, in some instances, states may have no requirements for technicians involved in the particular type of computer analytics involved in the Medicare-covered service.
We also propose that the following IDTF certification standards in § 410.33(g) would not apply to the aforementioned exempted IDTFs:
- The IDTF must have a comprehensive liability insurance policy of at least $300,000 per location that covers both the place of business and all customers and employees of the IDTF (§ 410.33(g)(6)).
- The IDTF must answer, document, and maintain documentation of a beneficiary's written clinical complaint at the physical site of the IDTF (§ 410.33(g)(8)). (For mobile IDTFs, this documentation would be stored at their home office.) Start Printed Page 39314
- The IDTF must openly post the standards outlined in § 410.33(g) for review by patients and the public (§ 410.33(g)(9)).
Concerning § 410.33(g)(8), we note that exempted IDTFs would not be furnishing direct services to beneficiaries that could result in a beneficiary's written clinical complaint. Thus, we believe this standard should be inapplicable to exempted IDTFs, and we would revise paragraph (g)(8) in this vein. We propose a similar approach with § 410.33(g)(9); neither beneficiaries whose tests are sent to the exempted IDTF nor the public in general will visit its physical location, therefore negating the need for a posting of standards.
As previously mentioned, we also propose that § 410.33(g)(6) would not apply to exempted IDTFs. The liability policy addressed therein was designed for IDTFs that provide services to beneficiaries in a facility or mobile unit and thus could have issues concerning medical negligence and/or malpractice. Nevertheless, we recognize that a chain of liability could involve an exempted IDTF if a computer malfunction or other error arose in the IDTF's diagnostic services. To illustrate, a software problem could lead to inaccurate test results, which in turn might result in an incorrect interpretation by a beneficiary's physician and ultimately harm the beneficiary. There could be other instances, too, where the performance of a particular test raises questions of possible liability. Consequently, we are soliciting public comment on the types of situations where this could arise as well as on the following issues: (1) Whether exempted IDTFs should indeed be required to maintain a $300,000 liability policy; (2) if § 410.33(g)(6) remains an exception, whether a liability amount of less than $300,000 is warranted and, if so, what that amount should be (for example, $50,000 or $100,000 or $200,000); and (3) whether no liability policy should be required.
In short, we believe that applying the foregoing exemptions to IDTFs that have developed innovative proprietary software for diagnostic testing where no patient interaction is involved would benefit the Medicare program and its beneficiaries by encouraging new IDTF technologies and services. We welcome comments on our proposed exemptions, and we specifically request comment on whether to retain and modify the IDTF standards in § 410.33(g)(6), (8), and (9) for the aforementioned exempted IDTFs, rather than waive those requirements for them.
e. Proposed Revisions at § 424.535(a)(8)
Under § 424.535(a)(8)(ii), CMS may revoke a provider's or supplier's enrollment if CMS determines that the provider or supplier has a pattern or practice of submitting claims that fail to meet Medicare requirements. The purpose of this provision is to place providers and suppliers on notice that they are legally obligated to always submit correct and accurate claims and that failing to do so could lead to the revocation of their enrollment; indeed, the submission of non-compliant claims places the Trust Funds at risk due to the potential for erroneous payments. In determining whether a revocation is appropriate under § 424.535(a)(8)(ii), CMS considers, as appropriate and applicable, the factors outlined in § 424.535(a)(8)(ii)(A) through (F); respectively, these are:
(A) The percentage of submitted claims that were denied.
(B) The reason(s) for the claim denials.
(C) Whether the provider or supplier has any history of final adverse actions and the nature of any such actions.
(D) The length of time over which the pattern has continued.
(E) How long the provider or supplier has been enrolled in Medicare.
(F) Any other information regarding the provider or supplier's specific circumstances that CMS deems relevant to its determination.
We have recently encountered situations where providers and suppliers have engaged in periods of non-compliant billing that, though comparatively brief, have or could have harmed the Medicare program. While we have attempted revocation action per § 424.535(a)(8)(ii) against such providers and suppliers, the current wording of some of the factors in paragraphs (a)(8)(ii)(A) through (F) have hampered our ability to do so. To increase our flexibility to address periods of abusive billing irrespective of their duration, we believe we must revise § 424.535(a)(8)(ii)(A) through (F) as follows:
- In paragraph (a)(8)(ii)(A), we propose revisions to focus on the percentage of denials within subsets of the provider's or supplier's claim submissions rather than across the entire universe of their claim submissions. Specifically, we would consider the percentage of submitted claims that were denied during the timeframe under consideration. We believe existing paragraph (a)(8)(ii)(A) inhibits our capacity to target brief periods involving a significant percentage of denied claims; this is because this factor has been interpreted to require said percentage to be weighed against claim denials over the entire period of the provider's or supplier's enrollment. As proposed, revised paragraph (a)(8)(ii)(A) would better enable CMS to address these non-compliant periods by restricting the scope of denial percentages to a shorter timeframe. For example, assume Provider X enrolled in Medicare on February 1. Although only a small percentage of its claims were denied through June 30, the denial rate was very high between July 1 and July 31. Under our proposed change, our period of review could be limited to July. This reflects our view that even a comparatively short timeframe of improper billing can threaten the Trust Funds, as evidenced by the aforementioned cases we have seen. We reiterate that the submission of non-compliant claims generates a risk of improper payments, which could lead to thousands or even millions of Medicare dollars being paid pursuant to either a lengthy or brief billing period.
- For reasons similar to our proposed revision of § 424.535(a)(8)(ii)(A), we propose to remove § 424.535(a)(8)(ii)(D) altogether. As already indicated, short but very intense periods of improper billing can endanger the Medicare program no less than a longer pattern of non-compliant yet merely moderate-volume billing. Yet the "length of time" standard in paragraph (a)(8)(ii)(D) often deters us from taking action under paragraph (a)(8)(ii) to address these shorter timeframes. Given this, we believe that eliminating paragraph (a)(8)(ii)(D) would strengthen our program integrity efforts.
- We also propose to remove § 424.535(a)(8)(ii)(E), which addresses the length of the provider's or supplier's enrollment. We consider this factor to be largely immaterial to the issue of whether a pattern of improper billing exists. More importantly, it can hinder our ability to utilize § 424.535(a)(8)(ii) as a whole. We have encountered fraud schemes where providers and suppliers enroll in Medicare, bill inappropriately, and then leave the program after a brief timeframe. We believe the enrollment length in these and other cases of non-compliant billing should have no bearing on whether paragraph (a)(8)(ii) can be applied, for the main issue is the behavior itself and not the period of enrollment.
- We propose to remove § 424.535(a)(8)(ii)(B) as well. Notwithstanding our original inclusion of this factor in paragraph (a)(8)(ii), the overall purpose of paragraph (a)(8)(ii) has always been to deter non-compliant Start Printed Page 39315 billing, regardless of the reason for it. Even if a period of erroneous claim submissions reflected no nefarious intent by the provider, the latter still failed to comply with Medicare billing requirements and this presented a risk to the Medicare program. For this reason, we do not view the claim denial reason as particularly germane to the question of whether paragraph (a)(8)(ii) should apply in a particular case.
- In addition, we propose to add new paragraph (a)(8)(ii)(C) by which we would consider the type of billing non-compliance and the specific facts surrounding said non-compliance (to the extent this can be determined). We believe this paragraph would provide greater specificity than the broader, catch-all factor at § 424.535(a)(8)(ii)(F) (which we would nonetheless retain). It would also allow us to more narrowly tailor our review to the unique facts of the case, thus also strengthening our ability to consider any aggravating or mitigating circumstances.
Given the foregoing, paragraph (a)(8)(ii) would include the following factors, respectively designated as paragraphs (A) through (D):
- The percentage of submitted claims that were denied during the period under consideration.
- Whether the provider or supplier has any history of final adverse actions and the nature of any such actions.
- The type of billing non-compliance and the specific facts surrounding said non-compliance (to the extent this can be determined).
- Any other information regarding the provider or supplier's specific circumstances that CMS deems relevant to its determination.
We recognize that these revisions would represent a reduction in the number of factors we would consider. However, we believe the remaining criteria would still give the provider or supplier fair consideration in our determinations while permitting us to address a wider range of non-compliant billing periods in order to protect the Medicare program.
2. Provider/Supplier Medical Review Requirements
a. Background
CMS identifies improper payments in the Medicare Fee-for-Service (FFS) program through a variety of program integrity-related activities, and we use a network of contractors to carry out program integrity initiatives, including Recovery Audit contractors (RACs), the Supplemental Medical Review Contractor (SMRC), Unified Program Integrity Contractors (UPICs), Medicare Administrative Contractors (MACs), and the Comprehensive Error Rate Testing (CERT) contractor. (We are purposely excluding Quality Improvement Organizations (QIOs) from this discussion and the following proposals since QIOs are governed by separate and distinct statutory and regulatory requirements. For information about the QIOs, see sections 1151-1163 of the Act and 42 CFR parts 475-480.)
Both UPICs and MACs perform prepayment medical review, while the RACs, SMRC, UPICs, MACs, and CERT all perform post-payment medical reviews. Both prepayment medical reviews and post-payment medical reviews are used by our contractors to determine, among other things, whether items or services are reasonable and necessary under section 1862(a)(1) of the Act. In carrying out these reviews, each contractor requests additional documentation from providers and suppliers, which the contractors then assess to either support the payment of claims or conversely, deny (in full or in part) claims thereby protecting the Medicare Trust Funds against improper payments. Our contractors may also carry out follow-up prepayment or post-payment reviews on the same providers or suppliers to ensure improper payments are not continuing.
Our contractors are authorized to request additional documentation through multiple statutory authorities, including sections 1815(a), 1833(e) and 1862(a)(1)(A) of the Act. Sections 1815(a) and 1833(e) of the Act provide that no payments shall be made to any provider or supplier unless it has furnished such information as the Secretary may request in order to determine the amounts due such provider for the period with respect to which the amounts are being paid or any prior period. Under section 1862(a)(1)(A) of the Act, payment must generally be limited to those items and services that are reasonable and necessary.
b. Proposal for Regulations Governing Prepayment and Post-Payment Medical Review
Despite the statutory authority authorizing our contractors' activities, we do not have regulatory provisions governing certain medical review activities, specifically prepayment and post-payment medical reviews. In this proposed rule, we are proposing key terms and definitions associated with these two review types; language codifying a contractors' authority to request additional documentation within established timeframes; and provisions detailing a provider's or supplier's responsibility to comply with requests for additional documentation, including the impact should a provider or supplier fail to comply with a request. These provisions are based on existing operational practices used by our contractors. We believe that adding these provisions in regulation will enhance provider and supplier understanding of our review processes, as well as, improve consistency among our contractors.
c. Proposed Key Terms and Definitions
To ensure consistency across prepayment and post-payment reviews and establish clear requirements, we propose adding the following key terms and their definitions to § 405.902: "Additional documentation" means the information requested by a contractor when conducting a prepayment review or post-payment review; "Additional Documentation Request (ADR)" means a contractor's initial documentation request in reviewing claims selected for prepayment review or post-payment review; "Post-payment medical review (or post-payment review)" means a review that occurs after payment is made on the selected claim to determine whether the initial determination for payment was appropriate; and "Prepayment medical review (or prepayment review)" means a review that occurs before an initial determination for payment is made on the selected claim to determine whether payment should be made. These definitions would be consistent with longstanding manual language and common use of these terms by our contractors.
d. Prepayment and Post-Payment Medical Review
We are proposing to add new § 405.903 to outline the prepayment medical review provisions.
- At paragraph (a), we are proposing to codify our contractors' authority to conduct prepayment medical review on selected claims in order to determine whether and how much payment should be made.
- At paragraph (b), we are proposing language detailing our contractors' authority to request additional documentation while conducting a prepayment review.
- At paragraph (b)(1), we are proposing that a provider or supplier will be provided 45 calendar days to submit additional documentation in response to a contractor's request except as stated in paragraphs (b)(2) and (c).
- At paragraph (b)(2), we are proposing that a contractor may accept documentation received after 45 Start Printed Page 39316 calendar days for good cause. Good cause means situations such as natural disasters, interruptions in business practices, or other extenuating circumstances that the contractor deems good cause in accepting the documentation.
- At paragraph (c), we are proposing language detailing a UPIC's authority to provide 30 calendar days to a provider or supplier submitting additional documentation and that a UPIC may accept documentation received after 30 calendar days for good cause. Good cause means situations such as natural disasters, interruptions in business practices, or other extenuating circumstances that the UPIC deems good cause in accepting the documentation.
These provisions reflect longstanding requirements MACs and UPICs have used in conducting prepayment reviews. The different time-periods within which additional documentation must be received is based on unique processing requirements for each contractor. Although both conduct prepayment reviews, the UPICs work directly with law enforcement and focus on potentially fraudulent providers or suppliers. Thus, the different timeframes for receiving additional documentation is necessary to account for the distinction and enables each type of contractor to appropriately balance their need for documentation in completing reviews with the potential burden on providers and suppliers should reviews take longer than is warranted. Efforts to limit the burden placed on providers and suppliers as much as possible is also warranted so that patient care is not unnecessarily impacted.
Additionally, both MACs and UPICs historically have had the authority to accept documentation received after the initial timeframe has expired based on good cause, such as natural disasters, interruptions in business practices, or other extenuating circumstances. These circumstances are best determined on a case-by-case basis by the MAC or UPIC, and the proposed language at paragraphs (b)(2) and (c), respectively, convey the MAC and UPIC authority to determine that good cause exists to warrant accepting documentation received after the initial timeframe given.
- At paragraph (d), we propose to specify that a contractor's prepayment review will result in an initial determination under § 405.920. Again, this has been the longstanding approach to the results of prepayment reviews.
We are also proposing similar provisions at new § 405.929 regarding post-payment medical reviews.
- At paragraph (a), we are proposing language outlining our contractors' authority to select claims and conduct post-payment medical reviews.
- At paragraph (b), we are proposing language that specifies our contractors' authority to request additional documentation.
- At paragraph (b)(1), we are proposing that a contractor will give a provider or supplier 45 calendar days to submit additional documentation in response to a request except as stated in paragraphs (b)(2) and (c).
- At paragraphs (b)(2) and (c), we propose that a contractor may accept documentation received after 30 days for good cause. Good cause means situations such as natural disasters, interruptions in business practices, or other extenuating circumstances that the contractor deems good cause in accepting the documentation.
- At paragraph (c), we are proposing language that specifies the UPIC's authority to provide 30 calendar days when requesting additional documentation and that a UPIC may accept documentation received after 30 calendar days for good cause. Good cause means situations such as natural disasters, interruptions in business practices, or other extenuating circumstances that the UPIC deems good cause in accepting the documentation.
- At paragraph (d), we propose that when conducting a post-payment review, a contractor's review will result in either no change or a revised determination under § 405.984.
As with prepayment reviews, these provisions reflect longstanding requirements UPICs and MACs, RACs, the CERT contractor, and SMRC have used in conducting post-payment reviews. While the MACs, RACs, CERT contractor, and SMRC have relatively comparable medical review processes, the UPICs are somewhat different given their close working relationship with law enforcement and focus on potentially fraudulent providers or suppliers. Thus, the different timeframes for receiving additional documentation is necessary to account for the distinction and enables each contractor to appropriately balance their need for documentation in completing reviews with the potential burden on providers and suppliers should reviews take longer than may be expected. Efforts to limit the burden placed on providers and suppliers as much as possible is also warranted so that patient care is not unnecessarily impacted.
Given that for post-payment reviews the claims have already been paid, all the contractors have historically had the authority to accept documentation received after the initial timeframe has expired based on good cause. As with prepayment reviews, this may include situations such as natural disasters, interruptions in business practices, or other extenuating circumstances that the specific contractor deems good cause in accepting the document after 30 or 45 calendar days. These circumstances are best determined on a case-by-case basis, and the proposed language at paragraphs (b)(2) and (c) convey the authority to determine that good cause exists to warrant accepting documentation received after 30 or 45 calendar days.
We are also proposing to add new § 405.930 to clearly outline our contractors' authority to deny a claim should a provider or supplier fail to convey the additional documentation in response to a request. The proposed language clarifies that the contractor must give the provider or supplier notice and time to respond to the additional documentation request. Contractors have authority to require additional documentation through multiple statutory provisions, including sections 1815(a), 1833(e) and 1862(a)(1)(A) of the Act. While our contractors maintain discretion to provide additional time to a provider or supplier in responding to an additional documentation request, our contractors also have the authority to deny additional time and the associated claim(s) when the additional documentation is not received within the requested timeframe.
We are also proposing to revise the section heading of § 405.986(a) to read, "Establishing good cause for reopening." This revision clarifies the distinction made between the process for establishing good cause to reopen an initial determination made on a claim, and the good cause factors that may be applied in accepting documentation submitted after the applicable timeframes in §§ 405.903 and 405.929. In establishing criteria to determine whether to accept late documentation in response to an ADR, we are adopting the criteria set forth in §§ 405.903 and 405.929, and we are not utilizing the good cause criteria for reopening an initial determination on a claim in § 405.986. We believe this change will add further clarification to the substantive text to reflect that the section only applies to reopenings of initial determinations on a claim. Start Printed Page 39317
O. Modifications Related to Medicare Coverage for Opioid Use Disorder (OUD) Treatment Services Furnished by Opioid Treatment Programs (OTPs)
1. Background
Section 2005 of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities (SUPPORT) Act established a new Medicare Part B benefit category for OUD treatment services furnished by OTPs during an episode of care beginning on or after January 1, 2020. In the CY 2020 PFS final rule (84 FR 62630 through 62677 and 84 FR 62919 through 62926), we implemented Medicare coverage and provider enrollment requirements and established a methodology for determining the bundled payments for episodes of care for the treatment of OUD furnished by OTPs. We established new codes for and finalized bundled payments for weekly episodes of care that include methadone, oral buprenorphine, implantable buprenorphine, injectable buprenorphine or naltrexone, and non-drug episodes of care, as well as add-on codes for intake and periodic assessments, take-home dosages for methadone and oral buprenorphine, and additional counseling. In the CY 2021 PFS final rule (85 FR 84683 through 84688), we adopted new add-on codes for take home supplies of nasal naloxone and injectable naloxone. We are continuing to monitor Medicare enrollment by OTPs and utilization of the new benefit to ensure that Medicare beneficiaries have appropriate access to care as well as monitoring for fraud, waste, and abuse. For CY 2022, we are proposing several refinements to the regulations governing Medicare coverage and payment for OUD treatment services furnished by OTPs.
2. Annual Updates
In the CY 2020 PFS final rule (84 FR 62667), we finalized a policy under which the payment for the drug component of episodes of care will be updated annually using the most recent data available from the applicable pricing mechanism at the time of ratesetting for the applicable calendar year. The payment for the non-drug component of the bundled payment for OUD treatment services will be updated annually based upon the Medicare Economic Index (MEI) (84 FR 62668 and 62669). The current payment rates, as finalized in the CY 2021 PFS final rule, both with and without locality adjustments, can be found on the CMS OTP website under Billing and Payment at https://www.cms.gov/files/document/otp-billing-and-payment-fact-sheet.pdf. The list of the payment rates for OUD treatment services furnished by OTPs, with the annual update applied for CY 2022, will be made available at the time of publication of the CY 2022 PFS final rule.
3. Proposed Refinements to Regulations Governing Medicare Payment to OTPs
In the CY 2021 PFS final rule (85 FR 84684 through 84685), we extended the definition of OUD treatment services to include short acting opioid antagonist medications for the emergency treatment of known or suspected opioid overdose, such as naloxone, and overdose education furnished in conjunction with opioid antagonist medication. We also established an adjustment to the weekly bundled payments when the OTP furnishes take-home supplies of these medications at § 410.67(d)(4)(i)(E). This adjustment includes both a drug component and a non-drug component for overdose education. The payment for the drug component of the adjustment will be determined using the methodology in § 410.67(d)(2)(i), and will be updated annually using the most recent data available at the time of ratesetting. The amount of the non-drug component of the adjustment, which includes overdose education, will be determined based on the CY 2020 Medicare payment rate for CPT code 96161; however, we did not explicitly address either geographic adjustments or annual updates to this payment rate.
In the CY 2020 PFS final rule (84 FR 62666 through 62667), we finalized the application of a geographic adjustment to the non-drug component of the OTP bundled payments, as well as the add-on payment adjustments for non-drug services, using the Geographic Adjustment Factor (GAF). We explained that unlike the national pricing of drugs, the costs for the services included in the non-drug component of the OTP bundled payment for OUD treatment services are not constant across all geographic localities. For example, OTPs' costs for rent or employee wages could vary significantly across different localities and could potentially result in disparate costs for the services included in the non-drug component of OUD treatment services, therefore, we stated we believed it would be appropriate to apply a geographic locality adjustment to the non-drug component of the bundled payments. We also specifically stated our belief that the same logic regarding the differential costs for those services included in the bundled payments would apply and should be recognized for add-on payment adjustments for non-drug services. This geographic adjustment is codified in the regulations at § 410.67(d)(4)(ii).
Additionally, in the CY 2020 PFS final rule we finalized an annual update to the non-drug component of the bundled payment for an episode of care based upon the MEI (84 FR 62668 through 62669). We noted that we believed the same logic regarding the potential for changes in the costs of the services included in the non-drug component of the bundled payment rates also applied to the add-on payment adjustments for non-drug services. This annual update is codified in the regulations at § 410.67(d)(4)(iii).
As noted previously, when we adopted the adjustment to the weekly bundled payments for take-home supplies of opioid antagonist medications in the CY 2021 PFS final rule, we did not specifically address either geographic adjustments or annual updates to the non-drug component of this adjustment and did not update the provisions governing the geographic adjustment and annual update in order to reference the new adjustment. Because the adjustment for take-home supplies of opioid antagonist medications includes a non-drug component, we believe the same considerations regarding varying costs based on geographic locality and the need for annual updates apply. Accordingly, we are proposing to revise the regulation at § 410.67(d)(4)(ii) to include the adjustment for take-home supplies of opioid antagonist medications in the list of items for which the non-drug component will be geographically adjusted using the GAF. We are also proposing to revise the regulation at § 410.67(d)(4)(iii) to include the adjustment for take-home supplies of opioid antagonist medications in the list of items that will be updated annually using the MEI.
Additionally, in the CY 2021 PFS final rule (85 FR 84688), we explained that consistent with § 410.67(d)(5), any payment to an OTP for naloxone would be duplicative if a claim for the same medication is separately paid under Medicare Part B or Part D for the same beneficiary on the same date of service, and that we would recoup any duplicative payment made to an OTP for naloxone. However, the regulation on duplicative payments at § 410.67(d)(5) does not specifically reference payments for medications that are furnished as part of an adjustment to the bundled payment. Accordingly, we are also proposing to revise § 410.67(d)(5) to state explicitly that payments for medications that are delivered, administered or dispensed to Start Printed Page 39318 a beneficiary as part of an adjustment to the bundled payment are considered a duplicative payment if a claim for delivery, administration or dispensing of the same medication(s) for the same beneficiary on the same date of service was also separately paid under Medicare Part B or Part D. Consistent with the policies finalized in the CY 2020 PFS final rule (84 FR 62663 through 62664) regarding duplicative payments for medications dispensed as part of the weekly bundle, we believe that it is appropriate to also ensure that Medicare payments for drugs provided as an add-on to the bundled payment rate are not duplicative. We note that this proposed revision would apply not only to duplicative payments for take-home supplies of naloxone, but also to duplicative payments for additional take-home supplies of other medications that are made under § 410.67(d)(4)(i)(D).
We seek comment on these proposed changes.
4. Proposed OTP Coding and Payment for New Nasal Naloxone Product
We are aware that the FDA recently announced the approval of a new, higher dose naloxone hydrochloride nasal spray product used to treat opioid overdose and that the newly approved product delivers 8mg of naloxone.[121] In the CY 2021 PFS final rule (85 FR 84683 through 84685), we finalized payment for HCPCS code G2215 (Take-home supply of nasal naloxone (provision of the services by a Medicare-enrolled Opioid Treatment Program); List separately in addition to code for primary procedure). HCPCS code G2215 was priced based on an assumption of a typical case in which the beneficiary would be provided with a box of two 4mg nasal spray products. At the time of drafting this proposed rule, we do not yet have any available pricing information for this newly approved product. However, in order to be able to make payment to OTPs under Medicare for this product, we are proposing to create a new G-code describing a take-home supply of this higher dose naloxone hydrochloride nasal spray product.
Under this proposal, we would price this new add-on code based on the established methodology under the OTP benefit for determining the adjustment for take-home supplies of opioid antagonist medications at § 410.67(d)(4)(i)(E). This adjustment includes both a drug component and a non-drug component. The amount of the drug component of the adjustment would be determined using the methodology for pricing the drug component of an episode of care at § 410.67(d)(2)(i). Accordingly, consistent with the approach used to price the drug component of HCPCS code G2215, we would apply the payment methodology set forth in section 1847A of the Act to determine the payment for the new naloxone hydrochloride nasal spray product, except that payment amounts that are determined based on ASP or wholesale acquisition cost (WAC) would not include any add-on percentages (85 FR 84685). As stated in the CY 2021 PFS final rule (85 FR 84685), we believe using ASP provides a transparent and public benchmark for manufacturers' actual pricing as it reflects the manufacturers' actual sales prices to all purchasers (with limited exceptions as noted in section 1847A(c)(2) of the Act) and is the only pricing methodology that includes off-invoice rebates and discounts as described in section 1847A(c)(3) of the Act. Therefore, we believe ASP to be the most market-based approach to set drug prices. Additionally, we would price the drug component of the code based on an assumption of a typical dosage for a take-home supply of this new product to be a box of two 8mg nasal sprays. Consistent with the methodology established in § 410.67(d)(4)(i)(E), the amount of the non-drug component of the code would be determined based on the CY 2020 Medicare payment rate for CPT code 96161. In addition, payment for the add-on code would be limited to once every 30 days except when a further take home supply of the medication is medically reasonable and necessary. We welcome comment on this proposal.
4. Counseling and Therapy Furnished via Audio-Only Telephone
In the CY 2020 PFS final rule (84 FR 62645 and 62646), we finalized allowing the use of two-way interactive audio/video communication technology, as clinically appropriate, to furnish the counseling and therapy portions of the weekly bundle of services and additional counseling or therapy services furnished by OTPs. Due to the Public Health Emergency (PHE) for COVID-19, in the interim final rule with comment period (IFC) entitled "Medicare and Medicaid Programs; Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency," which appeared in the April 6, 2020 Federal Register (85 FR 19230) (hereinafter referred to as the "March 31, 2020 COVID-19 IFC"), we revised § 410.67(b)(3) and (4) to allow the therapy and counseling portions of the weekly bundles, and any additional counseling or therapy, to be furnished using audio-only telephone calls rather than via two-way interactive audio/video communication technology for the duration of the PHE for COVID-19. Under the policy adopted in the March 31, 2020 COVID-19 IFC, counseling and therapy could be furnished using audio-only telephone calls only where two-way audio/video communications technology is not available to the beneficiary, and provided all other applicable requirements were met. In the March 31, 2020 COVID-19 IFC, we stated that we believed this change was necessary to ensure that beneficiaries with opioid use disorders would be able to continue to receive these important services during the PHE during which the public has been instructed to practice self-isolation or social distancing, and because interactive audio/video communication technology may not be available to all beneficiaries.
We have continued to evaluate whether this flexibility would be needed after the end of the PHE. According to MedPAC's March 2021 Report to the Congress, allowing audio-only interaction for certain telehealth services can improve beneficiary choice and equity in access to care for beneficiaries who do not have access to the technology for a video telehealth visit.[122] Additionally, public comments received in response to the CY 2021 PFS proposed rule (85 FR 84691) encouraged us to reconsider our position on coverage of audio-only services following the conclusion of PHE for COVID-19 and commenters suggested that CMS consider permanently allowing OTPs to furnish certain OUD treatment services using audio-only telephone calls. Commenters stated that allowing OTPs to furnish services via audio-only interactions facilitates broader access to services, particularly for vulnerable populations, and ensures providers have flexibility to deliver care to beneficiaries as efficiently and seamlessly as possible. Given the sensitivity of OUD treatment services, commenters noted that this is an area in which more flexibility will promote not only access but also effective and sustained treatment for beneficiaries in need of care. Other commenters stated that the use of communication technology has reduced stress and stigma for those who require OUD treatment services and the allowance of Start Printed Page 39319 audio-only services has greatly expanded access for beneficiaries who may not be able to use interactive video. Another commenter stated that allowing use of audio-only communication to continue after the PHE for COVID-19 would be essential in addressing disparities in healthcare, especially for dually eligible beneficiaries who do not have access to audio-visual communication technology.
After further consideration, we are persuaded by the public comments and other stakeholder feedback that using audio-only telephone calls to furnish therapy and counseling in cases where two-way audio/video communication technology is not available to the beneficiary after the end of the PHE for the COVID-19 pandemic would facilitate broader access to services. Therefore, we are proposing to allow OTPs to continue to furnish the therapy and counseling portions of the weekly bundles, as well as any additional counseling or therapy that is billed using the add-on code, using audio-only telephone calls rather than via two-way interactive audio/video communication technology following the end of the PHE for COVID-19 in cases where audio/video communication technology is not available to the beneficiary, provided all other applicable requirements are met. Accordingly, we are proposing to revise the regulations at § 410.67(b)(3) and (4) to allow OTPs to furnish therapy and counseling using audio-only telephone calls rather than via two-way interactive audio/video communication technology after the conclusion of the PHE for COVID-19 in cases where audio/video communication is not available to the beneficiary, provided all other applicable requirements are met. We note that we interpret the requirement that audio/video technology is "not available to the beneficiary" to include circumstances in which the beneficiary is not capable of or has not consented to the use of devices that permit a two-way, audio/video interaction because in each of these instances audio/video communication technology is not able to be used in furnishing services to the beneficiary.
Additionally, we are proposing that after the conclusion of the PHE for COVID-19, when two-way interactive audio/video communication technology is used to furnish additional counseling and therapy services billed under the add-on code, OTPs would be required to append modifier 95 (Synchronous Telemedicine Service Rendered via Real-Time Interactive Audio and Video Telecommunications System) to the claim. We are not proposing to require the use of this modifier when counseling and therapy services included in the weekly bundle are furnished using two-way interactive audio/video communication technology. We recognize that it may be difficult to determine which modifier to use in cases where multiple services within the bundle are furnished using different modalities, therefore, we are limiting our proposal regarding the use of modifier 95 to claim lines for the counseling and therapy add-on code (HCPCS code G2080). We are also proposing that, following the conclusion of the PHE for COVID-19, when counseling or therapy services are furnished using audio-only telephone calls, either as part of a weekly bundle or billed using the counseling and therapy add-on code (HCPCS code G2080), OTPs would be required to document in the beneficiary's medical record that the counseling or therapy was furnished via audio-only telephone call and the rationale for doing so. In addition, we are proposing the use of a new service-level modifier to be appended to claims submitted for the counseling and therapy add-on code (HCPCS code G2080) when furnished via an audio-only interaction, which would serve to certify that the practitioner had the capacity to furnish the services using two-way, audio/video communication technology, but instead, used audio-only technology because audio/video communication technology was not available to the beneficiary. The use of this modifier would allow CMS to track utilization of this flexibility in the claims data and evaluate that data as we consider ongoing refinements to the OTP benefit in the future. To avoid placing additional burden on OTPs during the PHE for COVID-19, these proposed new requirements would take effect on January 1, 2022, but would apply only for services furnished after the conclusion of the PHE for COVID-19. Accordingly, if the PHE for COVID-19 extends into 2022, OTPs that furnish counselling and therapy services using either two-way audio/video technology or audio-only telephone calls would not be required to use the applicable modifier or to comply with the new documentation requirements until after the end of the PHE.
Accordingly, we are proposing to revise § 410.67(d) to add a new paragraph (6) to state that when substance use counseling under paragraph (b)(3) of this section or therapy services under paragraph (b)(4) of this section are furnished using audio-only telephone calls after the end of the PHE, as defined in § 400.200 of this chapter, the practitioner must document in the beneficiary's medical record that the services were furnished using audio-only technology and the rationale for doing so. For purposes of the add-on code for additional counselling and therapy services, the practitioner must also certify, in a form and manner specified by CMS, that they had the capacity to furnish the services using two-way, audio/video communication technology, but used audio-only technology because the beneficiary did not have access to two-way audio/video communications technology. Under these proposals, we would defer to clinician judgment in determining whether in-person counseling or therapy, rather than the use of audio-only telephone calls, would be most appropriate in certain circumstances, such as for patients who are considered to be high risk. Additionally, we are seeking comment on whether we should put any additional or alternative conditions in place to promote program integrity, minimize patient safety concerns, and ensure that beneficiaries have access to the most appropriate form of care.
P. Physician Self-Referral Updates
1. The Physician Self-Referral Statute and Regulations
Section 1877 of the Act, also known as the physician self-referral law: (1) Prohibits a physician from making referrals for certain designated health services payable by Medicare to an entity with which he or she (or an immediate family member) has a financial relationship, unless an exception applies; and (2) prohibits the entity from filing claims with Medicare (or billing another individual, entity, or third party payer) for those referred services. A financial relationship is an ownership or investment interest in the entity or a compensation arrangement with the entity. The statute establishes a number of specific exceptions and grants the Secretary of the Department of Health and Human Services (the Secretary) the authority to create regulatory exceptions for financial relationships that do not pose a risk of program or patient abuse. Section 1903(s) of the Act extends aspects of the physician self-referral prohibitions to Medicaid. For additional information about section 1903(s) of the Act; see 66 FR 857 through 858.
The following discussion provides a chronology of our more significant and comprehensive rulemakings; it is not an exhaustive list of all rulemakings related to the physician self-referral law. After the passage of section 1877 of the Act, we proposed rulemakings in 1992 Start Printed Page 39320 (related only to referrals for clinical laboratory services) (57 FR 8588) (the 1992 proposed rule) and 1998 (addressing referrals for all designated health services) (63 FR 1659) (the 1998 proposed rule). We finalized the proposals from the 1992 proposed rule in 1995 (60 FR 41914) (the 1995 final rule), and issued final rules following the 1998 proposed rule in three stages. The first final rulemaking (Phase I) was a final rule with comment period published in the January 4, 2001 Federal Register (66 FR 856). The second final rulemaking (Phase II) was an interim final rule with comment period (69 FR 16054) published in the March 26, 2004 Federal Register. Due to a printing error, a portion of the Phase II preamble was omitted from the March 26, 2004 Federal Register publication. That portion of the preamble, which addressed reporting requirements and sanctions, was published in the April 6, 2004 Federal Register (69 FR 17933). The third final rulemaking (Phase III) was a final rule published in the September 5, 2007 Federal Register (72 FR 51012).
In addition to Phase I, Phase II, and Phase III, we issued final regulations on August 19, 2008 in the Fiscal Year (FY) 2009 Inpatient Prospective Payment System final rule with comment period (73 FR 48434) (the FY 2009 IPPS final rule). That rulemaking made various revisions to the physician self-referral regulations, including: (1) Revisions to the "stand in the shoes" provisions; (2) establishment of provisions regarding the period of disallowance and temporary noncompliance with signature requirements; (3) prohibitions on per unit of service (often referred to as "per-click") and percentage-based compensation formulas for determining the rental charges for office space and equipment lease arrangements; and (4) expansion of the definition of "entity."
After passage of the Patient Protection and Affordable Care Act of 2010 (Pub. L. 111-148) (Affordable Care Act), we issued final regulations on November 29, 2010 in the CY 2011 PFS final rule with comment period that codified a disclosure requirement established by the Affordable Care Act for the in-office ancillary services exception (75 FR 73443). We also issued final regulations on November 24, 2010 in the CY 2011 OPPS final rule with comment period (75 FR 71800), on November 30, 2011 in the CY 2012 OPPS final rule with comment period (76 FR 74122), and on November 10, 2014 in the CY 2015 OPPS final rule with comment period (79 FR 66987) that established or revised certain regulatory provisions concerning physician-owned hospitals to codify and interpret the Affordable Care Act's revisions to section 1877 of the Act.
On November 16, 2015, in the CY 2016 PFS final rule, we issued regulations to reduce burden and facilitate compliance (80 FR 71300 through 71341). In that rulemaking, we established two new exceptions, clarified certain provisions of the physician self-referral regulations, updated regulations to reflect changes in terminology, and revised definitions related to physician-owned hospitals. The new exception at § 411.357(y) for timeshare arrangements included a limitation on certain per unit of service and percentage-based compensation formulas. On November 15, 2016, in the CY 2017 PFS final rule, we again finalized requirements that the rental charges for the lease of office space or equipment are not determined using a formula based on per unit of service rental charges, to the extent that such charges reflect services provided to patients referred by the lessor to the lessee (81 FR 80534). The requirements are identical to those in effect since October 1, 2009, and are included in the exceptions for the rental of office space at § 411.357(a)(5)(ii)(B), the rental of equipment at § 411.357(b)(4)(ii)(B), fair market value compensation at § 411.357(l)(3)(ii), and indirect compensation arrangements at § 411.357(p)(1)(ii)(B).
In the December 2, 2020 Federal Register, we published a final rule entitled "Modernizing and Clarifying the Physician Self-Referral Regulations" (the "MCR final rule") (85 FR 77492) that established three new exceptions to the physician self-referral law applicable to compensation arrangements that qualify as "value-based arrangements," established exceptions for limited remuneration to a physician and the donation of cybersecurity technology and services, and revised or clarified several existing exceptions. The MCR final rule also provided guidance and updated or established regulations related to the fundamental terminology used in many provisions of the physician self-referral law. Most notably, we defined the term "commercially reasonable" in regulation, established an objective test for evaluating whether compensation varies with the volume or value of referrals or other business generated between the parties, and revised the definitions of "fair market value" and "general market value." The MCR final rule also revised the definition of "indirect compensation arrangement."
2. Indirect Compensation Arrangements (§ 411.354(c)(2))
a. Summary of Proposals
We are proposing to revise the regulation at § 411.354(c)(2) that sets forth the conditions for the existence of an indirect compensation arrangement. First, we are proposing to revise § 411.354(c)(2)(ii), which identifies when aggregate compensation to a physician results in an indirect compensation arrangement (if the other conditions of § 411.354(c)(2) are met), to more precisely address the concerns and effectuate the policies that we articulated in the MCR final rule. Specifically, we are proposing to revise the regulation to include as a potential indirect compensation arrangement any unbroken chain of financial relationships in which the compensation arrangement closest to the physician (or immediate family member of the physician) involves compensation for anything other than services that he or she personally performs. This would include arrangements for the rental of office space or equipment that meet the other conditions of the regulation at § 411.354(c)(2), which would be subject to, among other requirements, the prohibition on percentage-based and unit-based (often referred to as "per-click") compensation formulas at § 411.357(p)(1)(ii) in the exception for indirect compensation arrangements (or the requirements of another applicable exception). Second, following the publication of the MCR final rule, we received inquiries from stakeholders requesting clarification on the term "unit" in § 411.354(c)(2)(ii)(A). We are proposing to define the term "unit" for purposes of applying the regulation. We are also proposing to define "services that are personally performed" for purposes of applying proposed § 411.354(c)(2)(ii)(A)(4).
b. Definition of "Indirect Compensation Arrangement"
Although section 1877(h)(1) of the Act defines the term "compensation arrangement" as including both direct and indirect compensation, the statute does not define the term "indirect compensation arrangement." In Phase I, relying on the Secretary's authority under section 1877(b)(4) of the Act, we set forth in regulation the conditions under which an indirect compensation arrangement exists and a corresponding exception for such arrangements (66 FR 684 through 687). In Phase II, we revised the regulation at § 411.354(c)(2)(ii) to distinguish the language identifying when an indirect Start Printed Page 39321 compensation arrangement exists from the language of the exception for indirect compensation arrangements at § 411.357(p) (69 FR 16069). Most recently, in the MCR final rule, we further revised the regulation at § 411.354(c)(2) that identifies when an indirect compensation arrangement exists (85 FR 77544 through 77546).
Prior to the MCR final rule, an unbroken chain of financial relationships between a referring physician (or a member of his or her immediate family) and the entity furnishing designated health services established an "indirect compensation arrangement" if all the elements of § 411.354(c)(2), as then in effect, existed. The indirect compensation arrangement must satisfy the requirements of an applicable exception in order to avoid the referral and billing prohibitions of the physician self-referral law. (In the alternative, the parties could use an exception at § 411.355 to except the physician's referrals on a service-by-service basis.) This two-step process, which first identified the universe of unbroken chains of financial relationships that might be of concern and then excepted from the physician self-referral law's prohibitions those unbroken chains of financial relationships that did not pose a risk of program or patient abuse, was developed to closely correspond to the statutory treatment of compensation arrangements directly between an entity and a referring physician (or an immediate family member of the referring physician) (69 FR 16059). When analyzing compliance with the requirement that compensation does not take into account the volume or value of a physician's referrals or the other business generated by the physician for the entity, which is included in the exception for indirect compensation arrangements at § 411.357(p) and certain exceptions for direct compensation arrangements, special rules on unit-based compensation at § 411.354(d)(2) and (3) that deemed certain compensation not to take into account the volume or value of the physician's referrals or the other business generated by the physician could be applied.
As noted above, in the MCR final rule, we established an objective test for evaluating whether compensation varies with the volume or value of referrals or other business generated between the parties and responded to commenters that questioned whether compensation to a physician would run afoul of the objective tests under specified circumstances (85 FR 77539 through 77547). Inquiring about proposed modifications to § 411.354(c)(2)(ii) that we did not ultimately finalize, one commenter presented the example of a physician who performs surgeries at a hospital and receives a fixed amount per personally-performed relative value unit that is consistent with the fair market value of the physician's services (85 FR 77544). In developing our response to the commenter (and other commenters), we revisited the regulatory construct for determining which unbroken chains of financial relationships between entities and physicians (or immediate family members of physicians) establish indirect compensation arrangements and how to determine if they pose a risk of program or patient abuse (85 FR 77545).
With the underlying goal of reducing unnecessary burden on providers and suppliers, we stated that we do not see a need to treat compensation arrangements that may qualify as "indirect compensation arrangements" in the exact same way that the statute treats direct compensation arrangements when that construct creates unnecessary burden on the regulated industry (85 FR 77545 through 77546). We stated that it is possible to simplify the analysis of whether an unbroken chain of financial relationships presents a risk of patient or program abuse or poses program integrity concerns (85 FR 77546), and finalized revisions to § 411.354(c)(2) intended to achieve the same result as the two-step Phase I regulatory construct in protecting against program or patient abuse while reducing unnecessary burden on the regulated industry (88 FR 77546). The revised (now current) regulation at § 411.354(c)(2)(ii) effectively incorporates and applies the conditions of the special rules on unit-based compensation at § 411.354(d)(2) and (3) at the definitional level when determining whether there exists an indirect compensation arrangement that must satisfy the requirements of an applicable exception in order to avoid the prohibitions of the physician self-referral law.
Under the regulation finalized in the MCR final rule, an unbroken chain of financial relationships between an entity and a physician is considered an indirect compensation arrangement if the physician (or immediate family member of the physician) receives aggregate compensation from the person or entity in the chain with which the physician (or immediate family member) has a direct financial relationship that varies with the volume or value of referrals or other business generated by the physician for the entity furnishing the designated health services, and any of the following are true: (1) The individual unit of compensation received by the physician (or immediate family member) is not fair market value for items or services actually provided; (2) the individual unit of compensation received by the physician (or immediate family member) is calculated using a formula that includes the physician's referrals to the entity furnishing designated health services as a variable, resulting in an increase or decrease in the physician's (or immediate family member's) compensation that positively correlates with the number or value of the physician's referrals to the entity; or (3) the individual unit of compensation received by the physician (or immediate family member) is calculated using a formula that includes other business generated by the physician for the entity furnishing designated health services as a variable, resulting in an increase or decrease in the physician's (or immediate family member's) compensation that positively correlates with the physician's generation of other business for the entity. In addition, the entity must have actual knowledge of, or act in reckless disregard or deliberate ignorance of, the fact that the referring physician (or immediate family member) receives aggregate compensation that varies with the volume or value of referrals or other business generated by the referring physician for the entity. Under the regulation, unless all the elements of § 411.354(c)(2)(i), (ii), and (iii) exist, an unbroken chain of financial relationships between an entity furnishing designated health services and a physician (or immediate family member of a physician) is not considered an indirect compensation arrangement.
As explained previously, the changes to the regulations that identify indirect compensation arrangements of concern under the physician self-referral law occurred in response to comments and inquiries primarily in the context of compensation paid to physicians for their personally performed services (85 FR 55739 through 55747). The revisions to § 411.354(c)(2)(i) through (iii) were intended to more precisely identify arrangements that pose a risk of overutilization, patient steering, and other abusive conduct at an earlier stage of the analysis (85 FR 77546). However, in streamlining the former two-step process for analyzing unbroken chains of financial relationships, we inadvertently omitted an important program integrity requirement that previously applied when determining satisfaction of the requirements of the Start Printed Page 39322 exception at § 411.357(p) for indirect compensation arrangements. As a result, we inadvertently excluded from the definition of "indirect compensation arrangement" a subset of unbroken chains including compensation arrangements that we have long identified as presenting significant program integrity concerns: Certain arrangements involving unit of service-based payment for the rental of office space or equipment.
We have repeatedly stated our view that unit of service-based compensation formulas in arrangements for the lease of space and equipment are inherently susceptible to abuse because the physician lessor has an incentive to profit from referring a higher volume of patients to the lessee. Beginning with the 1998 proposed rule, we stated that unit of service-based payments for patients who are referred for the service by the lessor physician were not consistent with the requirement that compensation not reflect the volume or value of a physician's referrals or other business generated (63 FR 1714). In Phase I, we revisited the issue, reviewed the legislative history, and concluded that, as long as the per-unit payment reflected fair market value in arms' length bargaining and did not vary over the course of the arrangement, unit of service-based payments could qualify for the protection of an exception, provided that the other requirements of the applicable exception are met. (66 FR 876). We noted that such arrangements might run afoul of the anti-kickback statute and stated our intent to continue to monitor such arrangements for potential abuse (66 FR 878).
Subsequently, in the 2009 IPPS final rule, based on our observations of program integrity concerns and comments in support of prohibiting unit of service-based compensation formulas in office space and equipment leases, we finalized revisions to the exceptions for the rental of office space at § 411.357(a), the rental of equipment at § 411.357(b), fair market value compensation at § 411.357(l), and indirect compensation arrangements at § 411.357(p). The revised exceptions required that, to the extent that such arrangements related to the lease of office space or equipment, the rental charges may not be determined using a formula based on: (1) A percentage of the revenue raised, earned, billed, collected, or otherwise attributable to the service performed or business generated in the office space; or (2) unit of service-based rental charges, to the extent that such charges reflect services provided to patients referred by the lessor to the lessee (73 FR 48713 through 48714). Commenters largely supported the change. A significant number of commenters reported their own experiences of situations in which unit of service-based compensation arrangements resulted in patients being referred for medically unnecessary treatment. Some hospitals reported being effectively compelled to lease equipment from physician groups (73 FR 48715). In the 2016 PFS final rule, we included similar restrictions on percentage-based and unit of service-based compensation formulas in the new exception at § 411.357(y) for timeshare arrangements. In support of that limitation, we again cited concerns that unit of service-based compensation formulas in arrangements involving the use of office space or equipment could lead to overutilization and patient steering (80 FR 71331 through 71332).
We most recently addressed the issue of unit of service-based compensation formulas in depth in the 2017 PFS proposed and final rules. In those rules, at the direction of the D.C. Circuit Court in Council for Urological Interests v. Burwell, 790 F.3d 212 (D.C. Cir. 2015), we explained our rationale for the restrictions as they apply to arrangements for the lease or use of office space or equipment, again identifying overutilization and patient steering as the primary program integrity concerns supporting our conclusion that such compensation provisions present a significant program risk (81 FR 46452 through 46453 and 80528 through 80534). We reiterated that unit of service-based compensation formulas, in particular in arrangements for the lease of equipment:
- Create an incentive for overutilization of imaging services (as described by MedPAC in its comments to our proposal in the CY 2008 PFS proposed rule), as well as other services, including therapeutic services;
- Create an incentive for physicians to narrow their choice of treatment options to those for which they will realize a profit, even where the best course of action may be no treatment;
- Influence physicians to refer to the lessee instead of referring to another entity that utilizes the same or different (and perhaps more efficacious) technology to treat the patient's condition;
- Result in physicians steering patients to equipment they own, even if it means having the patient travel to a non-convenient site for services using the leased equipment; and
- Increase costs to the Medicare program when referring physicians pressure hospitals to use their leasing company despite not being the low cost provider.
We also identified two advisory opinions issued by OIG in which OIG voiced concerns about unit of service-based compensation arrangements and indicated that such arrangements are disfavored under the anti-kickback statute (81 FR 80528). Commenters again were largely supportive of the proposal, which merely re-proposed the then-existing prohibitions on such compensation formulas in arrangements for the lease or use of office space or equipment (81 FR 80528 through 80529).
Our position on the inherent risks presented by unit of service-based compensation formulas in the context of the rental of space or equipment has not changed since the 2017 PFS final rule. This fact is evident elsewhere in the MCR final rule. For example, the rule finalized changes to the exception for fair market value items and services, making it applicable to the rental of office space (85 FR 77606). With this change, we also revised the exception to state that the previously-established restrictions at § 411.357(l)(3)(i) and (ii) applicable to fair market value equipment leases also apply to leases of office space. We reiterated our longstanding concerns with unit of service-based compensation formulas for leases of office space and equipment, described the history of such concerns, and stated, in response to a comment supporting the inclusion of the restriction, that it was "a necessary safeguard" for the reasons articulated in our prior rulemakings (85 FR 77607). We included a similar restriction in the newly-finalized exception for limited remuneration to a physician at § 411.357(z), citing the same concerns (85 FR 77624).
We continue to believe that arrangements involving unit of service-based compensation for the rental of office space or equipment, whether direct or indirect, may pose a significant risk of program abuse, and are proposing revisions to § 411.354(c)(2)(ii) that would ensure that the prohibition on certain unit of service-based compensation formulas for the rental of office space or equipment applies to all compensation arrangements that include them. Under proposed § 411.354(c)(2)(ii), an unbroken chain of financial relationships in which the compensation arrangement closest to the physician (or immediate family member) is an arrangement for the rental of office space or equipment would be an indirect compensation arrangement if all other conditions of § 411.354(c)(2)(i) through (iii) are met. If Start Printed Page 39323 the parties to the compensation arrangement elect to use the exception at § 411.357(p) instead of another applicable exception, if any, the compensation for the rental or office space or equipment may not be determined using a formula based on per-unit of service rental charges to the extent that such charges reflect services provided to patients referred by the lessee to the lessor.
Arrangements involving compensation to a physician for items or the services of others where the physician's referral of designated health services to an entity or other business generated by the physician for an entity may contribute to the compensation received by the physician are distinguishable from arrangements that solely involve compensation for a physician's personally performed services. Program integrity concerns arise when payment for items or services provided as the result of a physician's referrals or the other business the physician generates, rather than the physician's own labor, is included in the calculation of compensation. As discussed previously, the MCR final rule policy that identifies indirect compensation arrangements of concern under the physician self-referral law in a single-step process was focused on reducing unnecessary burden related to the analysis of unbroken chains of financial relationships that do not pose a risk of program or patient abuse, and was developed in the context of compensation paid to physicians for their personally performed services. However, the current regulations, as finalized in the MCR final rule, are not limited to indirect compensation arrangements under which a physician (or immediate family member) is paid solely for services that he or she personally performs, which, as a general matter, do not raise significant program integrity concerns, provided that the compensation is consistent with fair market value for the personally performed services.
To better align with our view regarding the reduced risk of program or patient abuse where compensation to a physician (or an immediate family member of a physician) is solely for services that he or she personally performs, the proposed revisions to § 411.354(c)(2)(ii) would require a two-step analysis of any unbroken chain of financial relationships in which the compensation paid under the arrangement closest to the physician (or immediate family member) is for anything other than services personally performed by the physician (or immediate family member), including, as noted above, arrangements for the rental of office space or equipment. Specifically, we are proposing to revise the condition at § 411.354(c)(2)(ii)(A) to consider an unbroken chain of financial relationships between a physician and an entity that meets the other conditions of § 411.354(c)(2)(i) through (iii) to be an indirect compensation arrangement for purposes of the physician self-referral law if the unit of compensation received by the physician (or immediate family member) is payment for anything other than services personally performed by the physician (or immediate family member). We are also proposing slight revisions to the language of § 411.354(c)(2)(ii)(A)(2) and (3) to clarify that these conditions relate to the formula for calculating of the amount of compensation per unit. As proposed, the condition at § 411.354(c)(ii)(A) would state that the referring physician (or immediate family member) receives aggregate compensation from the person or entity in the chain with which the physician (or immediate family member) has a direct financial relationship that varies with the volume or value of referrals or other business generated by the referring physician for the entity furnishing the designated health services and the individual unit of compensation received by the physician (or immediate family member): (1) Is not fair market value for items or services actually provided; (2) Is calculated using a formula that includes the physician's referrals to the entity furnishing designated health services as a variable, resulting in an increase or decrease in the amount of compensation that positively correlates with the number or value of the physician's referrals to the entity; (3) Is calculated using a formula that includes other business generated by the physician for the entity furnishing designated health services as a variable, resulting in an increase or decrease in the amount of compensation per unit that positively correlates with the physician's generation of other business for the entity; or (4) Is payment for anything other than services personally performed by the physician (or immediate family member). For purposes of proposed § 411.354(c)(2)(ii)(A)(4), we would consider services that are performed by any person other than the physician (or immediate family member), including, but not limited to, the referring physician's (or immediate family member's) employees, independent contractors, group practice members, or persons supervised by the physician (or the immediate family member) not to be personally performed by the physician. We are proposing to codify this policy at § 411.354(c)(2)(ii)(B)(3).
c. Definition of "Unit" for Purposes of Applying § 411.354(c)(ii)(A)
As explained above, under current § 411.354(c)(2)—which was finalized in the MCR final rule—the determination of whether an indirect compensation arrangement exists requires the evaluation of the individual unit of compensation that the physician (or immediate family member) receives. If the individual unit of compensation does not meet any of the conditions at § 411.354(c)(2)(ii)(A)(1) through (3), the unbroken chain of financial relationships does not constitute an indirect compensation arrangement. Since the publication of the MCR final rule, we have received inquiries from stakeholders regarding how the provisions of § 411.354(c)(2)(ii)(A) should be applied in situations where compensation does not appear to be unit-based or is calculated using two or more different units or types of units. We are proposing revisions to § 411.354(c)(2)(ii)(B) to clarify how to identify the unit to analyze against the conditions of current § 411.354(c)(2)(ii)(A)(1) through (3) and proposed § 411.354(c)(2)(ii)(A)(4).
As a preliminary matter, it is our position that all compensation essentially is unit-based compensation. The underlying unit may be a discrete item, a unit of service, a unit of time, or a unit that results from combining different types of units into a single unit used to calculate the compensation. The identification of purely time-based or service-based units is straightforward. With respect to compensation that is entirely paid per hour, per day, per month, per year, or per similar period of time, the individual unit of compensation is the smallest unit of time for which the compensation is paid. For example, where a physician is paid $150 per hour for his or her medical director services, the unit is an hour. Similarly, where a physician is paid $350,000 per year for his or her full-time professional services, the unit is a year. With respect to compensation that is entirely paid per service, such as a work relative value unit (wRVU) or the provision of a training seminar, the unit is the individual service. For example, where a physician is paid $30 per wRVU that he or she personally performs, the unit is a wRVU. Similarly, where a physician is paid $1000 to provide a training session on infection control measures for an organization's employees, the unit is a training session. Start Printed Page 39324 Compensation formulas that incorporate a percentage of a variable are also unit-based. For example, if a physician is paid 50 percent of the amount collected for the professional services that he or she performs in a calendar year, the unit is a calendar year. If a physician is paid 95 percent of the Medicare PFS amount for a particular service that he or she personally performs, the unit is the service.
We are aware that compensation arrangements may include different units of compensation paid to a physician. According to stakeholders inquiring about the application of § 411.354(c)(2)(ii)(A), a physician employed by a physician organization may receive an annual salary for his or her full-time professional services furnished to patients of the physician organization plus a productivity bonus for each wRVU that he or she personally performs. The stakeholders inquired how to identify the unit that results from combining different types of units into a single unit used to calculate the physician's compensation. In such instances, we consider the unit of compensation to be time-based and reflect the aggregate compensation paid to the physician during the period of time applicable to the payment; that is, the time-period during which compensation is paid (for example, per month or per year) or over the entire term of the arrangement. It is our understanding that fair market valuations generally follow this construct, determining the fair market value of various types of compensation for a physician's personally performed services, such as fixed salary payments and productivity or bonus compensation, by assessing the physician's compensation in the aggregate over a period of time. Further, a service-based unit of compensation is easily converted to a time-based unit by incorporating the period of time applicable to the payment for the services (for example, $30 per wRVU per month), while the reverse is not true. It is for these reasons that we believe that "hybrid" compensation—that is, compensation that has both a time-based unit component and a service-based unit component—is appropriately analyzed by converting it to compensation for a unit of time for purposes of applying § 411.354(c)(2)(ii).
To illustrate, assume that an employment arrangement between a physician and a physician organization specifies compensation of $200,000 per calendar year for the physician's full-time professional services plus a productivity bonus of $10 for each wRVU that he or she personally performs, and that the physician is paid on a monthly basis. The unit of compensation would be a month, and the formula for determining the compensation per month would be ($200,000 ÷ 12 months) + ($10 × the number of wRVUs personally performed during the month). (In the alternative, the parties could analyze the arrangement under § 411.354(c)(2)(ii)(A) using a calendar year as the unit of compensation.) However, if the employment arrangement specified productivity bonus compensation of $10 per wRVU only for those personally performed wRVUs above a predetermined target, the unit would be the period of time for which the target is applicable. To illustrate, instead of $10 for each wRVU that the physician personally performs, assume that the physician receives $10 for the wRVUs that he or she personally performs in excess of 4,000 wRVUs per calendar year. The unit of compensation would be a calendar year, and the formula for determining the compensation per year would be $200,000 + $10 × (actual number of wRVUs personally performed during the calendar year−4,000).
We note that a compensation arrangement may also involve multiple units of the same type. For example, a physician employed by a physician organization may receive a salary of $200,000 per year for his or her full-time professional services plus $150 per hour for his or her personally performed medical director services or $500 per month for each of the physician organization's NPPs that he or she supervises. Or, a physician may receive compensation for services based on a fee schedule; for example, $50 for service A, $75 for service B, and $100 for service C. In circumstances where more than one unit of the same type is used to calculate the physician's compensation, each unit must be analyzed under § 411.354(c)(2)(ii)(A)(1) through (4) to determine whether the conditions for an indirect compensation arrangement exist.
To facilitate compliance with the physician self-referral law as it applies to indirect compensation arrangement, we are proposing a new regulation at § 411.354(c)(2)(ii)(B)(2) that expressly identifies the unit to consider for purposes of applying the regulation at § 411.354(c)(2)(ii)(A) and determining the existence of an indirect compensation arrangement that must satisfy the requirements of an applicable exception. Under proposed § 411.354(c)(2)(ii)(B)(2), for purposes of applying § 411.354(c)(2)(ii)(A), the individual unit is: (1) Time, where the compensation paid to the physician (or immediate family member) is based solely on the period of time during which the services are provided; (2) service, where the compensation paid to the physician (or immediate family member) is based solely on the service provided; and (3) time, where the compensation paid to the physician (or immediate family member) is not based solely on the period of time during which a service is provided or based solely on the service provided.
We seek comment on the proposals discussed above and whether additional guidance is needed with respect to the determination of whether an indirect compensation arrangement exists.
3. Exception for Preventive Screening Tests, Immunizations, and Vaccines (§ 411.355(h))
As a general matter, vaccines fall within the definition of "outpatient prescription drugs" at § 411.351, and therefore, are considered designated health services for purposes of the physician self-referral law. Because the federal government purchased the initial supply of COVID-19 vaccines, Medicare does not make payment for COVID-19 vaccines at this time,[123] and COVID-19 vaccines do not fall within the definition of "designated health service" at § 411.351. However, should COVID-19 vaccines become payable by Medicare, unless the requirements of an applicable exception to the physician self-referral law are satisfied, the physician self-referral law's prohibitions under section 1877(a)(1) of the Act and § 411.353(a) and (b) would apply to the referral and billing of COVID-19 vaccines.
In Phase I, using the Secretary's authority at section 1877(b)(4) of the Act to create additional exceptions that do not pose a risk of program or patient abuse, we finalized an exception at § 411.355(h) that excludes from the physician self-referral law's referral and billing prohibitions certain preventive screening tests, immunizations, and vaccines covered under Medicare (66 FR 939). As finalized in Phase I, in addition to requirements related to compliance with the federal anti-kickback statute and federal and state laws and regulations related to billing and claims submission, the exception at § 411.355(h) required that the preventive screening test, immunization, or vaccine is subject to CMS-mandated frequency limits, reimbursed by Medicare based on a fee schedule, and listed on the CMS website Start Printed Page 39325 and in annual PFS Updates. In Phase II, we removed the requirement that the preventive screening test, immunization, or vaccine is reimbursed based on a fee schedule, in recognition that some of the vaccines eligible for the exception may be paid by Medicare using different reimbursement methods (69 FR 16116). In the MCR final rule, as part of a broader effort to decouple the physician self-referral law from the federal anti-kickback statute and federal and state laws or regulations governing billing or claims submission, we removed the requirement at former § 411.355(h)(2) that the arrangement does not violate the federal anti-kickback statute as well as the requirement at former § 411.355(h)(3) that the arrangement does not violate any federal or state law or regulation governing billing or claims submission (85 FR 77567).
Services to which the exception at § 411.355(h) is applicable remain designated health services for purposes of the physician self-referral law; however, referrals may be made and claims submitted for such services if all requirements of the exception are satisfied (69 FR 16100). In the CY 2021 PFS final rule, we added COVID-19 vaccines to the list of immunization and vaccine codes to which the exception at § 411.355(h) is applicable (85 FR 84954 through 85955). We did so to ensure that the physician self-referral law would not impede the availability of COVID-19 vaccines for Medicare and other patients if they become payable by Medicare (85 FR 84955).
Under current § 411.355(h)(1), a preventive screening test, immunization, or vaccine must be subject to CMS-mandated frequency limits, among other requirements. Frequency limits determine the maximum number of times that Medicare will pay for a service for a particular beneficiary during an established period, often a calendar year or 12-month period. CMS-mandated frequency limits also serve to minimize the risk of program or patient abuse due to a physician's financial self-interest because Medicare would not pay for additional services referred and furnished in excess of the frequency limitation. In Phase I, we stated our belief that, under the terms of the exception at § 411.355(h) as finalized in Phase I—which included the requirement that the service is subject to CMS-mandated frequency limits—the risk of abuse is extremely low. We also stated that the exclusion of certain preventive screening tests, immunizations, and vaccines from the reach of the physician self-referral law is consistent with the statutory language and structure and the expressed Congressional intent to provide preventive care to Medicare beneficiaries (66 FR 939).
The United States continues to respond to the outbreak of COVID-19 caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). At this time, we have not mandated frequency limits for the COVID-19 vaccines identified on the List of CPT/HCPCS Codes (Code List) to which the exception at § 411.355(h) is applicable and we are uncertain whether or, if so, when CMS may mandate frequency limits for COVID-19 vaccines. Thus, although COVID-19 vaccines are identified on the List of CPT/HCPCS Codes as codes to which the exception at § 411.355(h) is applicable, they would not satisfy the requirement at current § 411.355(h)(1) that the preventive screening test, immunization, or vaccine is subject to CMS-mandated frequency limits. We are concerned that the current absence of CMS-mandated frequency limits on the available COVID-19 vaccines could impede the availability of critically important COVID-19 vaccines for Medicare and other patients, as physician referrals for COVID-19 vaccines would be prohibited unless another exception to the physician self-referral law is applicable and all its requirements are satisfied. Therefore, we are proposing to permit the use of the exception at § 411.355(h) for COVID-19 vaccines even when they are not subject to CMS-mandated frequency limits, provided that all other requirements of the exception are satisfied. Specifically, we are proposing to revise and renumber the regulation at § 411.355(h). Revised § 411.355(h)(1) would include the conditions that must be met to avoid the physician self-referral law's referral and billing prohibitions, and revised § 411.355(h)(2) would state that the requirement at § 411.355(h)(1)(iii) does not apply to a COVID-19 vaccine code during such period that the vaccine is not subject to a CMS-mandated frequency limit. In light of the impact of the COVID-19 pandemic on the United States and the vital need to protect beneficiaries (and others) from the SARS-CoV-2 virus, we do not believe that making the exception at § 411.355(h) available for COVID-19 vaccines to which no CMS-mandated frequency limits apply would pose a risk of program or patient abuse. We seek comment on our approach and whether we should limit relief from the requirement at proposed § 411.355(h)(1)(iii) to the period during which the current public health emergency is in effect, until such time as CMS-mandated frequency limits apply for COVID-19 vaccines, or some other period of time.
In the alternative, we are proposing to remove the CMS-mandated frequency limit requirement for all vaccines. We seek comment on whether it would then be necessary to include alternative program integrity requirements in the exception at § 411.355(h). We are interested in comments regarding whether physicians are likely to order vaccines more frequently than recommended by the Department and any other organization the Department identifies as an authority on this matter.
We are not proposing to remove the CMS-mandated frequency limit requirement with respect to preventive screening tests. We remain concerned that a physician's ability to refer frequently for preventive screening tests could lead to program or patient abuse, and do not believe that the current COVID-19 pandemic or any other circumstances necessitate the removal of this important program integrity protection with respect to preventive screening tests.
We are also proposing to revise the terminology used in the exception at § 411.355(h) for clarity and consistency. Specifically, we are proposing to remove the terms "immunization" and "immunizations" throughout § 411.355(h) and the headers used in the Code List. The Centers for Disease Control and Prevention (CDC) defines immunization as a process by which a person becomes protected against a disease through vaccination. This term is often used interchangeably with vaccination or inoculation. Vaccine is defined as a product that stimulates a person's immune system to produce immunity to a specific disease, protecting the person from that disease.[124] All of the codes currently on the Code List to which the exception at § 411.355(h) is applicable have a descriptor containing "vaccine" or a derivative of "vaccine."
Vaccines fall within the definition of "outpatient prescription drugs" at § 411.351, and therefore, are considered designated health services for purposes of the physician self-referral law. As defined by the CDC, an immunization is not an item or service that is a "designated health service" (as defined in § 411.351) and to which the physician self-referral law applies. We believe that "vaccine" is the appropriate term to use in § 411.355(h) and in the Start Printed Page 39326 headers in the Code List. Although we are not aware that including both terms in § 411.355(h) and the Code List has caused stakeholder confusion to date, we are proposing to improve the accuracy of the terminology at this time to prevent any possible confusion in the future. The proposed revisions to § 411.355(h), if finalized, would not affect whether we consider a code to be a designated health service.
4. List of CPT/HCPCS Codes (§ 411.351)
As described in section III.P.1. of this proposed rule, unless an exception applies and its requirements are satisfied, the physician self-referral law prohibits a physician from making a referral for the furnishing of certain designated health services if the physician has a financial relationship with the entity to which the referral is made. Recognizing that providing precise definitions of which designated health services implicate the physician self-referral law would facilitate compliance with the law, in the Phase I final rule, we determined to define certain designated health services by publishing specific lists of CPT and HCPCS codes that physicians and providers most commonly associate with a given designated health service (66 FR 922). This list of codes defines the entire scope of the designated health services category for purposes of the physician self-referral law and is controlling vis-à-vis the definition of the category at § 411.351, which contains a general explanation of the principles used to select the codes.
In Phase I, we stated that, because HCPCS Level I and II codes change and can quickly become out-of-date, we would not include the list of codes that are designated health services in the text of our regulations (66 FR 923). We also stated that, the definitions of specific services in our regulations would cross refer to a comprehensive table that would appear initially in the Federal Register along with Phase I and thereafter in an addendum to the annual final rule concerning payment policies under the PFS rule. We defined at § 411.351 the term "List of CPT/HCPCS Codes Used to Describe Certain Designated Health Services Under the Physician Referral Provisions (Section 1877 of the Social Security Act)" to mean the list of certain designated health services under section 1877 of the Act initially posted on the CMS website and updated annually thereafter in an addendum to the PFS final rule and on the CMS website. In the Phase II interim final rule, we revised the term to "List of CPT/HCPCS Codes" and its definition to "the list of CPT and HCPCS codes that identifies those items and services that are designated health services under section 1877 of the Act or that may qualify for certain exceptions under section 1877 of the Act." The Phase II definition also stated that the list is updated annually, as published in the Federal Register, and is posted on the CMS website at http://www.cms.gov/medlearn/refphys.asp. Other than including an updated URL for the location of the list on the CMS website, the current definition of List of CPT/HCPCS Codes is identical to the Phase II definition. The CMS website currently identifies this list as the Code List for Certain Designated Health Services (the Code List).[125]
Coding changes have become more frequent since we initially began publishing the Code List. Currently, CPT codes are updated annually and effective for use on January 1 of each year, with some exceptions for Category I, II, and III codes, which are published more frequently. CMS has updated its HCPCS Level II coding procedures to enable shorter and more frequent HCPCS coding cycles. For example, we have implemented quarterly HCPCS code application opportunities for drugs and biologicals; and bi-annual application opportunities for Durable Medical Equipment (DME) and Orthotics, Prosthetics (O&P), and Supplies, as part of our comprehensive initiative to foster innovation and expedite adoption of and patient access to new medical technologies. (See https://www.cms.gov/Medicare/Coding/MedHCPCSGenInfo.)
In order to make available the most recent updates in a timelier manner for purposes of the physician self-referral law, we are proposing to update the Code List on a more frequent basis. Specifically, we are proposing to update the Code List each calendar quarter, and provide public notification in advance of Code List updates. Advance notification would be posted on the CMS website on March 1, June 1, September 1, and December 1 of each year, with corresponding Code List updates effective on April 1, July 1, October 1, and January 1, respectively. We are also proposing a 30-day public comment period following the posting of each advance notification of the upcoming quarterly Code List update. Under this proposal, we would provide information on our website regarding the process for submitting public comments through www.regulations.gov and address all public comments on the Code List on the CMS website. We anticipate that most comments would be addressed within 90 calendar days of the effective date of the Code List update to which they pertain; however, a longer timeframe may be necessary to address complex comments or those that require coordination with external parties. This new process and schedule would begin with the update effective April 1, 2022. The Code List that is effective January 1, 2022 would be included in the CY 2022 PFS final rule. We believe that predetermined dates for the updates will provide clarity and transparency for stakeholders regarding any revisions to the Code List.
In addition, we are proposing to publish the Code List solely on the CMS website (commencing after the publication of the January 1, 2022 Code List in the CY 2022 PFS final rule, as proposed above). We believe that publication via the CMS website would facilitate compliance with the physician self-referral law and allow access to the most up-to-date Code List. Further, this approach would provide a more comprehensive list of codes identifying designated health services for purposes of the physician self-referral law that better aligns with the current coding cycles for CPT and HCPCS codes.
Finally, we are proposing corresponding revisions to the definition of List of CPT/HCPCS Codes at § 411.351 to update the URL that indicates where the Code List is published on the CMS website. Specifically, we are proposing to revise the definition of List of CPT/HCPCS Codes at § 411.351 to state "List of CPT/HCPCS Codes means the list of CPT and HCPCS codes that identifies those items and services that are designated health services under section 1877 of the Act or that may qualify for certain exceptions under section 1877 of the Act. It is updated each calendar quarter and posted on the CMS website at https://www.cms.gov/Medicare/Fraud-and-Abuse/PhysicianSelfReferral/List_of_Codes."
We seek comment on our proposals and whether more or less frequent Code List updates would be appropriate.
Q. Requirement for Electronic Prescribing for Controlled Substances for a Covered Part D Drug Under a Prescription Drug Plan or an MA-PD Plan
1. SUPPORT Act Requirements
Section 2003 of the SUPPORT Act generally mandates that the prescribing of a Schedule II, III, IV, or V controlled substance under Medicare Part D be done electronically in accordance with an electronic prescription drug program Start Printed Page 39327 beginning January 1, 2021, subject to exceptions, which the Secretary may specify. Section 2003 of the SUPPORT Act requires that the Secretary use rulemaking to specify circumstances and processes by which the Secretary may waive the Electronic Prescribing for Controlled Substances (EPCS) requirement, and provides the Secretary with authority to enforce and specify appropriate penalties for non-compliance with EPCS. The SUPPORT Act specifies some circumstances under which the Secretary may waive the electronic prescribing requirement with respect to controlled substances that are covered Part D drugs and permits HHS to develop other appropriate exceptions. Since the statute states that the Secretary shall through rulemaking specify circumstances and processes by which the Secretary "may waive" the EPCS requirement, we consider the list to be illustrative. The circumstances that are listed in the statute under which the Secretary may waive the EPCS requirement are at section 1860D-4(e)(7) of the Act, as added by section 2003 of the SUPPORT Act, and include:
- A prescription issued when the practitioner and dispensing pharmacy are the same entity;
- A prescription issued that cannot be transmitted electronically under the most recently implemented version of the National Council for Prescription Drug Programs SCRIPT standard, which is the SCRIPT 2017071 standard;
- A prescription issued by a practitioner who received a waiver or a renewal thereof for a period of time as determined by the Secretary, not to exceed 1 year, from the requirement to use electronic prescribing due to demonstrated economic hardship, technological limitations that are not reasonably within the control of the practitioner, or other exceptional circumstance demonstrated by the practitioner;
- A prescription issued by a practitioner under circumstances in which, notwithstanding the practitioner's ability to submit a prescription electronically as required by this subsection, such practitioner reasonably determines that it would be impractical for the individual involved to obtain substances prescribed by electronic prescription in a timely manner, and such delay would adversely impact the individual's medical condition involved;
- A prescription issued by a practitioner prescribing a drug under a research protocol;
- A prescription issued by a practitioner for a drug for which the FDA requires a prescription to contain elements that are not able to be included in electronic prescribing, such as a drug with risk evaluation and mitigation strategies that include elements to assure safe use;
- A prescription issued by a practitioner—
++ For an individual who receives hospice care under title XVIII of the Act; and
++ That is not covered under the hospice benefit under title XVIII of the Act; and
- A prescription issued by a practitioner for an individual who is—
++ A resident of a nursing facility (as defined in section 1919(a) of the Act); and
++ Dually eligible for benefits under title XVIII and title XIX of the Act.
2. Previous Regulatory Action
To begin the process of implementing section 2003 of the SUPPORT Act, in August 2020, we released a Request for Information entitled "Medicare Program: Electronic Prescribing of Controlled Substances; Request for Information (RFI)" (85 FR 47151) (hereinafter referred to as the August 2020 RFI). In August 2020, we released the CY 2021 PFS proposed rule (85 FR 50074) (hereinafter referred to as the CY 2021 PFS proposed rule), which proposed that Part D prescribers be required to use the NCPDP SCRIPT 2017071 standard for EPCS prescription transmissions. We proposed that this mandate would not become effective until January 1, 2022.
We received a combined total of 155 timely comments in response to the August 2020 RFI and the CY 2021 PFS proposed rule. Most commenters supported implementing EPCS and use of the NCPDP SCRIPT 2017071 standard. Comments were mixed as to when compliance actions for EPCS should begin. Some commenters requested that CMS adhere to the January 1, 2021 date specified in the SUPPORT Act because of the many safety benefits associated with EPCS articulated in the rule. Some prescriber groups supported the proposed January 1, 2022 date, while others requested even more time for implementation. To balance the needs of prescribers who wanted more time to implement EPCS and commenters who wanted adherence to the January 1, 2021 date, we finalized this provision with an effective date of January 1, 2021 and a compliance date of January 1, 2022 in the CY 2021 Physician Fee Schedule final rule (85 FR 84472) (hereinafter referred to as the CY 2021 PFS final rule). Due to the consensus among commenters that the NCPDP SCRIPT 2017071 standard was the best choice for EPCS, we required in the CY 2021 PFS final rule that Part D prescribers use this standard.
3. Current EPCS Environment
A variety of Part D medications are classified as controlled substances by the Drug Enforcement Administration (DEA). Among these are medications used for the treatment of acute and chronic pain, (for example, hydrocodone, fentanyl, codeine, methadone), and stimulant medications (for example, Adderall®, Ritalin®). Buprenorphine (for example, Suboxone®) is one of only three of the most effective drugs approved by the FDA to treat opioid use disorders (OUD) including in outpatient settings, and is a Schedule III drug. Benzodiazepines and sedative-hypnotics (including Xanax®, Valium®, Ativan®, Restoril®, Midazolim®, and Halcion®) are used for sleep, agitation, and seizure disorders. Anabolic steroids (for example, Depo-testosterone®) are used to treat impotence, delayed puberty, hormonal imbalance, and inoperable breast cancers.
As discussed in the CY 2021 PFS proposed and final rules, we noted that electronic prescribing of controlled substances provides multiple advantages over the traditional processing of paper prescriptions. These advantages include, but are not limited to, improved workflow efficiencies; deterring and detecting prescription fraud and irregularities by requiring an extra layer of identity proofing, two-factor authentication and digital signature processes; enhanced patient safety through patient identity checks, safety alerts, medication menus, electronic history files, and medication recommendations that lower the risk of errors and potentially harmful interactions; and providing more timely and accurate data than paper prescriptions by avoiding data entry errors and pharmacy calls to a prescriber to clarify written instructions. By allowing for the direct transmission of prescriptions for controlled substances between prescribers and pharmacies or facilities, EPCS may also reduce the burden on prescribers who need to coordinate and manage paper prescriptions between staff, patients, facilities, other care sites, and pharmacies. EPCS can also assure prescribers' identity more easily and may permit a single workflow for prescribing both controlled and non-controlled drugs, improving the overall prescribing process.
From the patient standpoint, EPCS may reduce the logistical burden on patients and caregivers who may Start Printed Page 39328 otherwise be required to make multiple trips between prescribers and pharmacies to transport paper prescriptions when filling time-sensitive prescriptions, while in pain, or otherwise in need of medical treatment with controlled substances. EPCS can lessen the time needed to obtain prescriptions by minimizing trips to the prescriber to pick up paper prescriptions for refills and minimize transportation costs to and from the prescriber's office. EPCS's identity and security requirements assure prescribers, patients, and pharmacies that prescriptions are processed as intended. In addition to helping with the reduction in fraud previously described, EPCS minimizes the likelihood that prescriptions have been tampered with, since electronic prescriptions are securely transmitted directly to the pharmacy from health information technology, which minimizes the likelihood of exposure to patients or other third parties. During the PHE for COVID-19, EPCS also helps parties observe social distancing.
It is due to these advantages, coupled with the SUPPORT Act's EPCS mandate, that we encourage all prescribers to conduct EPCS as soon as is feasible for them. We believe that although EPCS is ultimately more efficient, implementing EPCS does take additional time and resources. Prescribers must follow DEA guidance for EPCS, which is summarized at https://deadiversion.usdoj.gov/ecomm/e_rx/. Prescribers must first ensure that their current ePrescribing software can support EPCS and meets DEA requirements pursuant to 21 CFR part 1311. Further, DEA also requires prescribers to have their identities verified prior to being issued the authentication credentials needed to sign and issue electronic controlled substance prescriptions. For individual prescribers, identity proofing (that is, verification that the prescriber is who he or she claims to be) is conducted by a credential service provider (CSP) or certification authority (CA). Institutional practitioners, as defined under 21 CFR 1300.01, have the option of conducting in-house identity proofing of the practitioners authorized to use the institution's e-prescribing software. Alternatively, institutional practitioners may require their practitioners to undergo identity proofing by a CSP or CA. Once their identities have been confirmed, prescribers may be issued their authentication credentials. The authentication credentials must be two-factor, meaning that prescribers must be required to supply two factors to confirm both their identity and their authorization to access the e-prescribing software. The factors may be something the prescriber knows (such as a password or PIN), something the prescriber has (such as a smartcard or token), or a biometric (such as a fingerprint). For institutional practitioners, the authentication credentials may be issued by an entity within the institution that is separate from the entity that conducted identity proofing, if identity proofing was conducted in-house. Otherwise, authentication credentials are issued by a CSP or CA. Once a prescriber has received his or her two-factor authentication credentials, the prescriber must be granted access to sign and issue electronic controlled substance prescriptions using the e-prescribing software. This step is completed by certain individuals specifically designated to manage the e-prescribing software's logical access controls. Prior to granting a prescriber access, the individuals managing logical access controls must verify that the prescriber's state authorization to practice and, where applicable, state authorization to prescribe controlled substances, are valid. Additionally, for individual prescribers (those prescribers not prescribing under an institutional practitioner's DEA registration), the individuals managing logical access controls must verify that their DEA registration is valid. This step is required even if they are already prescribing controlled substances on paper. After being granted access, practitioners may sign and issue electronic prescriptions for controlled substances using their two-factor authentication credentials. The EPCS application must require two-factor authentication for each transaction. Software and workflow training is available for each step of the process. When writing prescriptions, the prescriber may wish to talk with the patients and/or caregivers about electronic prescribing, so there is awareness of the general mechanics of how the prescription(s) will be conveyed to the pharmacy.
We recognize that section 2003(c) of the SUPPORT Act tasked the Department of Justice (DOJ) with updating the requirements for the biometric component of multifactor authentication. As shown on the Spring 2021 Unified Agenda,[126] rulemaking to address this mandate is currently in progress. After reviewing comments on the August 2020 RFI and CY 2021 PFS proposed rule and talking with industry stakeholders, we recognize that commenters believe that an update in the DOJ requirements should allow prescribers to start conducting EPCS with greater ease.
The comments also stated that prescribers have felt strained by the COVID-19 pandemic. Prescribers reported feeling financially strained, worried about their own health and the health of their employees, and concerned about having to make rapid changes during a time when they are continuing to cope with the effects of the COVID-19 pandemic on their practices, and their patients. Despite the strain that has been experienced by prescribers, we have noted an increase in EPCS during this PHE. In CY 2021, EPCS increased to 70 percent of all prescription drug events (PDEs) for controlled substances as compared to 38 percent in CY 2019.[127] We believe that social distancing is likely to be at least partly responsible for the increase in EPCS during this PHE for COVID-19. With the use of electronic prescribing, one potential prescriber-patient interaction in which COVID-19 could be transmitted is eliminated, and any necessary prescriptions can be electronically transmitted to the pharmacy without the prescriber and patient having to see each other in-person and risk transmitting COVID-19. Some insurers, including Part D plans, have been permitting medication refills, including for controlled substances, earlier than usual or for a more extended period of time than is allowed. Pharmacies that were not doing so before the pandemic have been delivering medications, or delivering them at no charge, and communities and individuals have worked together to design ways for vulnerable persons to continue to receive access to prescribed medications in tandem with government and private sector flexibilities during the PHE. We believe that these additional flexibilities may have encouraged prescribers to more broadly use EPCS, since it prevented them from having their prescription transmissions automatically denied. The reason for this is that EPCS transaction sets can pull certain pieces of required information for use in their transactions, which prevent the transactions from hitting system edits that would have previously prevented these practices.
Start Printed Page 39329
4. Proposed Timeframe for EPCS Adoption
Section 2003 of the SUPPORT Act mandates that EPCS for Part D controlled substances begin on January 1, 2021. Due to this statutory mandate coupled with the aforementioned advantages provided by EPCS, we encourage all prescribers to adopt EPCS as soon as is feasible for them. However, as stated in our CY 2021 PFS final rule, we recognize that although EPCS is ultimately more efficient, implementing EPCS takes additional time and resources. It is for this reason that, in our CY 2021 PFS final rule, we finalized a policy stating that CMS would not take compliance actions before January 1, 2022.
In crafting this policy, we also examined responses from commenters encouraging earlier adoption of EPCS, due to its benefits for social distancing, improved patient safety and workflow efficiencies, fraud deterrence, adherence management, and reduced burdens. We agreed with commenters that EPCS has many benefits, which is why we specified an effective date of January 1, 2021 in our regulations, even though we declined to take compliance actions until January 1, 2022.
Since finalizing the CY 2021 PFS final rule, we have received additional prescriber feedback indicating concern with having to implement EPCS rapidly. We have also learned more about the degree to which prescribers have been adversely affected by the COVID-19 pandemic, and that the PHE and the widespread effects of the pandemic may last longer than we had anticipated last year. We want to ensure that our actions do not have unintended consequences, such as the abrupt discontinuation of prescribers' ability to prescribe controlled substances to vulnerable populations, including Part D beneficiaries who need pain treatment or who have SUDs. In addition, once DOJ has had the opportunity to implement updates to EPCS requirements, such updates will allow prescribers to start conducting EPCS more rapidly and easily. It is for these reasons that we are proposing to revise § 423.160(a)(5) to change the EPCS compliance date from January 1, 2022 to January 1, 2023. We welcome comments on this proposal, including whether commenters believe that we should maintain the January 1, 2022 compliance date, given the benefits of EPCS, and the feasibility for prescribers to adopt EPCS for Part D prescriptions by January 1, 2023.
We propose to extend the compliance deadline for Part D controlled substance prescriptions written for beneficiaries in long-term care (LTC) facilities, excluding beneficiaries who are residents of nursing facilities and whose care is provided under Part A of the benefit, from January 1, 2022 to January 1, 2025. The intent of this extension is to strike a balance between being responsive to stakeholder concerns surrounding the increased implementation barriers faced by LTC facilities, while at the same time helping ensure that these facilities eventually implement EPCS, due to its aforementioned benefits.
After considering the comments in response to our August 2020 RFI and CY 2021 PFS proposed rule, in addition to our conversations with stakeholders, we believe that LTC facilities face additional barriers to EPCS adoption that most prescribers do not face. In addition to the current challenge of having to manage care for vulnerable residents during the current COVID-19 pandemic, prescribers who work in LTC facilities or who provide care to residents in LTC facilities face technological barriers that other prescribers do not face. One such barrier is that the NCPDP SCRIPT 2017071 standard lacks appropriate guidance for LTC facilities. We understand that this is because early versions of the NCPDP SCRIPT Standard, such as NCPDP SCRIPT Standard versions 5.0 and 8.1, did not support the workflows in the LTC setting that require prescribers to issue a prescription for a patient to a non-prescriber (such as a nursing facility) that in turn forwards the prescription to a dispenser (LTC pharmacy). We nevertheless adopted the NCPDP SCRIPT 2017071 standard in the CY 2021 PFS final rule [85 FR 84807] because it is the most commonly used standard for Part D e-prescribing, and we sought to minimize disruption and provider burden when implementing this statutory mandate. However, we understand that NCPDP is in the process of creating specific guidance for LTC facilities within the SCRIPT 2017071 standard, which would allow willing partners to enable three-way communication between the prescriber, LTC facility and pharmacy to bridge any outstanding gaps that impede adoption of the NCPDP SCRIPT 2017071 standard in the LTC setting. We understand that NCPDP may be able to adopt these changes and integrate them into the LTC workflow by January 1, 2023.
We also understand that some LTC settings/services in rural communities do not have sufficient capabilities to support the NCPDP SCRIPT 2017071 standard. This concern is exacerbated by the fact that based on stakeholder feedback and information in several reports,[128] we believe LTC settings often include practitioners and staff serving large numbers of residents across multiple nursing homes. This unique set of circumstances means that some practitioners who primarily practice in suburban or urban areas may have to travel to see residents in rural facilities where there is limited broadband, making EPCS transmission set-ups difficult across LTC facilities. However, we believe that as broadband access increases and the impact of the pandemic decreases, LTCs should be able to more easily conduct EPCS.
As a result, we propose to revise § 423.160(a)(5) to clarify that compliance actions for prescriptions written for beneficiaries in an LTC facility will not begin until January 1, 2025. We do not propose a specific LTC waiver or exception to the EPCS requirement, and we do not anticipate extending the compliance deadline beyond January 1, 2025. We solicit comments on the benefits, burdens, and challenges of this approach.
5. Proposed Compliance Threshold
The EPCS requirement applies to all controlled substance prescriptions for Part D drugs under a Part D plan, unless an exception to the requirement applies. In order to implement this mandate effectively, however, we seek to implement it in a manner that balances the mandate with helping ensure that prescribers are not overly burdened, and are able to issue prescriptions for their patients during the rare occurrences when EPCS is not feasible, such as:
- When it would be impractical for the patient to obtain medication(s) prescribed by electronic prescription in a timely manner and such delay would adversely impact the patient's medical condition,
- When the NCPDP standard does not support transmitting the prescription,
- When the prescriber is unable to meet DEA requirements for identity proofing for reasons beyond their control;
- Where EPCS is not available due to temporary technological failure.
Based on our review of PDE data, the NCPDP standard, and our conversations with Part D stakeholders, we believe that there are very few scenarios under which a prescription could not be transmitted using the NCPDP standard. Start Printed Page 39330
We note that the section 1860D-4(e)(7)(B)(vi) of the Act provides that the Secretary may grant an exception for a prescription issued for a drug for which the FDA requires a prescription to contain elements that cannot be included in electronic prescribing. However, after reviewing the NCPDP standard implementation guide, we do not believe that there are any such prescriptions under the current standard. The statute gives as an example a drug with risk evaluation and mitigation strategies that include elements to assure safe use. Based on our review of the current NCPDP standard, all opioids have risk evaluation and mitigation strategies and as a result, would fall into the exception if there were one, which would frustrate the purpose of this statute.[129] As a result, we decline to propose to adopt this suggested exception. However, we seek comment on this decision.
We do believe that other reasons could make EPCS not feasible for prescribers who currently conduct EPCS, such as the aforementioned cases of temporary technological failures or cases where it would be impractical for the patient to obtain medication(s) prescribed by electronic prescription in a timely manner and such delay would adversely impact the patient's medical condition. However, we are not proposing a specific exception for these cases, since based on our stakeholder feedback and review of PDE data, we believe that EPCS is not feasible in no more than an estimated 30 percent of instances due to circumstances such as the ones described previously. We believe that Part D prescribers should be able to conduct EPCS on 70 percent of their Part D controlled-substance prescriptions without being overly burdened or burdening patients. Under section 1860D-4(e)(7)(D) of the Act, we have authority to specify appropriate penalties for non-compliance with the EPCS requirement. It follows, then, that we similarly have the authority to specify a threshold for when we would penalize non-compliance. For this reason, we propose that in order for prescribers to be considered compliant with the EPCS mandate, they must prescribe at least 70 percent of their Part D controlled substance prescriptions electronically.
Specifically, we are proposing to revise § 423.160(a)(5) to specify that 70 percent of all prescribing under Part D for Schedule II, III, IV, and V controlled substances be done electronically per calendar year, excluding from that calculation any prescriptions issued while a prescriber falls within an exception or a waiver. We would conduct this calculation by examining PDE data at the end of the calendar year and dividing the number of Part D controlled substances that the prescriber e-prescribed by the total number of Part D controlled substance prescriptions that the prescriber prescribed. We seek comment on this method and the proposal to make 70 percent the compliance threshold for adherence to the EPCS mandate, and what circumstances would make EPCS not feasible.
6. Proposed Classes of Exceptions
a. Prescriptions Issued When the Prescriber and Dispensing Pharmacy Are Same Entity
Section 2003 of the SUPPORT Act requires that we specify circumstances by which we may waive the EPCS requirement, and the statute lists several possible circumstances to consider. We listed and sought comment on these circumstances in the August 2020 RFI. The first of these circumstances, which is listed at section 1860D-4(e)(7)(B)(i) of the Act, is when the practitioner issuing the prescription and dispensing pharmacy are the same entity.
All August 2020 RFI commenters who commented on this exception supported it, stating that such an exception would promote patient safety, workflow efficiency, and health IT performance. Several commenters noted that requiring EPCS in this circumstance may create an unwarranted artificial workflow structure. We believe that this may be because the EPCS transactions conducted within an organization are commonly handled by a single database that exists within the organization, and should we not grant this exemption, these entities would be required to reconfigure their own processes, rather than leverage their own integrated databases. Were we to implement a requirement to use the NCPDP SCRIPT 2017071 standard within this closed system, this requirement could increase costs and the rate of performance errors, such as data corruption and patient matching errors, which we understand often happens when an entity is forced to split a unified database into a transaction system that relays information to and from the same entity. We seek comment on this assumption.
As stated in our current regulation at § 423.160(a)(3)(iii), we currently allow Part D plans to use either HL7 messages or the NCPDP SCRIPT standard to transmit prescriptions or prescription-related information internally when the sender and the beneficiary are part of the same legal entity. This allowance stands in contrast to our overarching requirement at § 423.160(a)(1) and (3), for prescribers to use the NCPDP SCRIPT standard for most external transactions. We believe that allowing Part D plans to continue to have more discretion over their internal transactions is consistent with our current policy. Therefore, we propose to adopt at § 423.160(a)(5)(i) the EPCS exception listed in section 1860D-4(e)(7)(B)(i) of the Act, for prescriptions issued where the prescriber and dispensing pharmacy are the same entity. We seek comment on this proposal.
b. Cases Where Prescribers Issue Only a Small Number of Part D Prescriptions
As we develop regulations to implement section 2003 of the SUPPORT Act, we seek to help ensure that Part D prescribers, including small prescribers (which CMS will define in subsequent rulemaking), are not overly burdened by our regulation. Based on the comments received from the August 2020 RFI and the stakeholder feedback that we received about EPCS in general, we believe it is appropriate to specify circumstances for a waiver of the EPCS requirement in cases where a prescriber issues a very low volume of controlled substance prescriptions for Part D drugs. For prescribers of very few Part D controlled substance prescriptions, the cost of installing EPCS equipment and software may be unduly burdensome relative to its benefit in terms of improving the security of prescriptions for controlled substances. As noted above, we do not want to disincentivize prescribers from prescribing controlled substances to Part D beneficiaries altogether, especially those who have few beneficiaries who need them.
After reviewing the current PDE data and the costs associated with implementing EPCS, we propose to exempt prescribers who prescribe 100 or fewer Part D controlled substance prescriptions per year. Based on our stakeholder feedback, we understand that EHR companies provide the initial electronic prescribing set-up free of charge, provided the prescribers transmit a minimum number of transactions per year. We estimate that this amount is, on average, 100 Part D controlled substance transactions. In order to do EPCS, prescribers would have to have the capability to e-prescribe more broadly. It is for this reason that we weighed the cost of e-prescribing set-up in general, even though we do not intend to include non-part D prescriptions of controlled or Start Printed Page 39331 non-controlled substances in our calculation of whether or not prescribers meet the threshold of 100 Part D controlled substance prescriptions per year. Since, based on our conversations with stakeholders, the cost of EPCS transactions is less than the cost of transmitting certain transactions manually, we believe that the initial investment to install EPCS equipment and software is likely justified once prescribers transmit more than 100 Part D controlled substance prescriptions per year. We seek comment on this assumption. Although we understand that prescribers will be required to purchase third party applications with additional identity and security measures so that EHRs meet DEA requirements, we have not included this cost in our calculation, due to the wide variability of these costs for which there is a dearth of information. We seek stakeholder feedback on the costs of these third-party applications.
We believe that requiring prescribers who prescribe 100 or fewer Part D controlled substance prescriptions per year to purchase and construct EPCS hardware and software may be financially burdensome for these prescribers compared to the benefits of EPCS, since any reduced costs from EPCS transactions may not be enough to justify the initial start-up costs for purchasing and installation of EPCS hardware and software. We also believe that the cost of any future CMS compliance actions may be too great to justify when 100 or fewer Part D controlled substance prescriptions per year are at issue. Although we considered using a lower threshold (such as 50) or a higher threshold (such as 200), we believe that 100 Part D controlled substance prescriptions per year strikes the right balance between helping ensure that we implement section 2003 of the SUPPORT Act's EPCS mandate and that prescribers can use resources appropriately.
In order to implement this exception using the data that we have available, we are proposing that this exception be given to individual prescribers, regardless of the size of the group practice that they belong to. We also believe that this exception would protect these small prescribers, should they change their place of employment or if their place of employment does not offer support for implementing EPCS. We seek comment on this proposal.
Based on our examination of PDE data and conversations with stakeholders, we believe that prescribers working under most research protocols would fall under the proposed exception for small prescribers. However, we seek comment on this assumption. Although we have not proposed to adopt the suggested exception listed in section 1860D-4(e)(7)(B)(v) of the Act, which describes an exemption for prescribers working under a research protocol, we believe that in most cases prescribers who would fall within this category would be included in the exception for small prescribers or in the exception for cases where the prescriber and dispenser are the same entity. We decline to propose to specifically create an exception for prescribers working under a research protocol in the regulations, since we believe that so few prescribers would fall outside of these other exceptions. We believe an exception for prescribers working under a research protocol who do not otherwise meet these exceptions is unnecessary because we believe that EHR companies will set up the appropriate EHR equipment, provided around 100 Part D controlled substance prescriptions are transmitted per year. We propose to implement this proposal by examining PDE claims as of December 31 of the prior year to determine which prescribers fall within this exception. Prescribers can ascertain whether they meet this exception by looking at how many prescriptions for Part D controlled substances they conducted the prior year or by contacting the CMS contractor responsible for administering the compliance portion of this mandate. CMS and its contractor will be using PDE data from the prior year to determine whether the prescriber qualifies for the exception based on the number of Part D controlled substance claims the prescriber had issued the previous year. CMS will use the previous year's data to determine whether or not the prescriber falls under this exception for the year-in-question. We do not see a compelling reason to exempt prescribers conducting a research protocol on that basis alone.
Based on our conversations with Prescription Drug Plans (PDPs), MA-PD plans, and other organizations with which prescribers are affiliated, we are aware that some are willing to donate the technology and services necessary for prescribers to adopt EPCS. Based on those conversations, we believe that they are more willing to donate these technology and services to prescribers who are working under a research protocol, than to prescribers not working under such a protocol. However, we seek comment on such an assumption. We believe that, to the extent this is an accurate assumption, such donations further decrease the burden for prescribers working under a research protocol. It is for these additional reasons that we have declined to propose an exception for those working under a research protocol. We seek comment on this decision.
We propose to amend § 423.160(a)(5) by adding § 423.160(a)(5)(ii), which creates an exception for prescribers who issue 100 or fewer controlled substance prescriptions for Part D drugs per calendar year as determined using CMS claims data as of December 31st of the preceding year, so that these prescribers will not be required to meet the EPCS requirement. We seek comment on this proposal, including regarding the maximum number of Part D controlled substance prescriptions a prescriber can issue to be still considered a small prescriber and, so, to fall within this exception.
c. Cases of Recognized Emergencies and Extraordinary Circumstances
Section 1860D-4(e)(7)(B)(iii) of the Act, as added by section 2003 of the SUPPORT Act, lists an exception for consideration by the Secretary for cases of exceptional circumstance demonstrated by the prescriber. As stated in our proposal regarding the EPCS compliance threshold, we seek to help ensure that prescribers are able to issue prescriptions for their patients during the rare occurrences when EPCS is not feasible. We believe that the exception listed in the statute, which includes economic hardship, technological limitations that are not reasonably within the control of the prescriber, and other exceptional circumstances, includes prescribers who are overwhelmed due to having to treat patients during a pandemic or a natural disaster such as a hurricane, flood, or earthquake. It is our goal not to penalize prescribers for such circumstances, and we do not want to unduly increase their burden during difficult situations that impact them, and their patients. We seek comment on what other extraordinary circumstances may prevent prescribers from being able to conduct EPCS.
In order to help ensure that these extraordinary circumstances are accounted for, we are proposing two exceptions to the EPCS requirement. The first exception, at proposed § 423.160(a)(5)(iii), is for prescribers who are prescribing during a recognized emergency, such as a natural disaster, a pandemic, or a similar situation where there is an environmental hazard. We want to help ensure that the EPCS mandate does not interfere with necessary care for patients, especially during natural disasters or pandemics. Start Printed Page 39332 As a result, we are proposing to exempt prescribers who are issuing prescriptions in areas that are affected by such circumstances. To qualify for this exception, this circumstance would have to arise from an emergency or disaster declared by a federal, state, or local government entity. We would determine whether a prescriber qualifies for this exception based on whether the prescriber's NCPDP database address is located in the geographic area of an emergency or disaster declared by a federal, state or local government entity.
The second exception, at proposed § 423.160(a)(5)(iv), is for prescribers who request and receive from CMS a waiver, which CMS would grant to prescribers who are facing extraordinary circumstances that prevent them from electronically prescribing a controlled substance to a Part D beneficiary, but who are not in an emergency or disaster area. We define "extraordinary circumstance" to mean a situation, other than an emergency or disaster, outside of the control of a prescriber that prevents the prescriber from electronically prescribing a controlled substance to a Part D beneficiary. An example of such a circumstance would be if a prescriber was in a service area that lacks broadband access or EPCS providers refuse to install systems for the prescriber. The prescriber would have to be able to submit evidence of such an extraordinary circumstance to CMS.
For purposes of the exception at proposed § 423.160(a)(5)(iii), prior to imposing any compliance actions on a prescriber, we would ascertain whether there is an emergency or disaster declared by a federal, state, or local government entity for the geographic area associated with the prescriber's address in the NCPDP database.
For purposes of the proposed exception at proposed § 423.160(a)(5)(iv), we are proposing that prescribers will be excepted from the EPCS requirements if they request and receive a waiver from CMS. We intend that prescribers will be able to submit a request for a waiver to inform CMS of any extraordinary circumstances that they may be facing and that would prevent the prescriber from conducting EPCS. This waiver could be for any circumstance outside of the prescriber's control and does not require an official declaration by a state or local government. To meet the standard for a waiver, prescribers must provide documentation showing the existence of a circumstance beyond their control and that such a circumstance prevents them from conducting EPCS. Section 1860D-4(e)(7)(B)(iii) of the Act, as added by section 2003 of the SUPPORT Act, refers to a waiver or a renewal thereof for a period of time as determined by the Secretary, not to exceed one year, which suggests a timeframe not to exceed one year, but to be determined by the Secretary.
We have proposed the first part of the waiver process below and will include more information about it in subsequent rulemaking. We welcome stakeholder comments on a potential waiver process.
To implement this proposal, we propose to amend § 423.160(a)(5) by adding paragraphs (a)(5)(iii) and (iv). Section 423.160(a)(5)(iii) would specify an exception for prescribers in the geographic service area of an emergency or disaster declared by a federal, state or local government entity. Section 423.160(a)(5)(iv) would clarify that prescribers would be exempt from the EPCS requirements if they have received a CMS-approved waiver certifying that the prescriber is unable to conduct EPCS due to circumstances beyond the prescriber's control. In order to receive a CMS-approved waiver, the prescriber would have to submit an attestation using a form, which would be made available on a CMS-supported website, so that prescribers will be able to request a waiver via an online portal.
The following minimum set of information would be required on the attestation:
- Prescriber's first and last name;
- Prescriber's NPI;
- Prescriber's taxpayer identification number (TIN) or TIN associated with his or medical practice, when applicable;
- Prescriber's contact information, including name, email address, telephone number, and mailing address; and
- A description of the extraordinary circumstance necessitating a waiver and how it affects the prescriber.
Following receipt of the attestation, we will: (1) Provide a written acknowledgement of receipt of the request using the contact information submitted via the portal and (2) provide a decision formally granting the attestation using the contact information submitted via the portal. Under the proposed policy, the prescriber would submit their attestation about the circumstance and receive a waiver based on such an attestation. We welcome comments on the different aspects of this proposal.
d. Individuals in Hospice and Nursing Facilities
Section 2003 of the SUPPORT Act, in adding section 1860D-4(e)(7)(B)(vii) to the Act, tasked the Secretary to consider whether prescriptions for individuals under the Part D benefit for an individual enrolled in the Medicare Part A Hospice benefit should be exempt from the EPCS requirement. After considering this issue, we believe that an exception for a prescription made for an individual enrolled in hospice would be inappropriate for several reasons. First, when electing hospice, patients have chosen to move from a curative model of care to a holistic palliative model of care. Regulations at 42 CFR 418.202(f) stipulate that the Medicare hospice benefit covers only drugs and biologicals used primarily for the relief of pain and symptom control for the terminal illness and related conditions. Under section 1860D-2(e)(2)(B) of the Act, a drug is excluded from Part D coverage if payment for such drug, as prescribed and dispensed for the beneficiary, is available under Medicare Part A or Part B. Thus, in cases where, with respect to a beneficiary, the hospice benefit covers a drug or biological used primarily for the relief of pain or symptom control for the terminal illness or related conditions, such drug is excluded from Part D under section 1860D-2(e)(2)(B) of the Act. The HHS OIG worked with CMS and the National Hospice and Palliative Care Organization (NHPCO) to identify four common categories of prescription drugs that are typically used to treat symptoms often experienced during the end of life, regardless of an individual's terminal diagnosis.[130] The OIG has found that these categories of drugs should generally be paid under the hospice benefit.[131] Thus, there may be very few instances in which a controlled substance prescribed for a Part D enrollee who has elected hospice could be covered under Part D. We believe an exception that would apply only in these rare instances could be confusing and burdensome for prescribers who furnish care to some Part D beneficiaries who are enrolled in hospice and for some who are not because to qualify for the exception they would have to determine when a particular enrollee has elected hospice. Further, a beneficiary is free to elect the hospice benefit and cancel that election as they choose, which would make it difficult for a prescriber to be sure at any point in time whether a beneficiary is, or is not, currently enrolled in hospice and therefore whether a paper prescription is permitted. We note that the EPCS Start Printed Page 39333 requirement would not apply to any prescriptions for Part A or Part B controlled substances in any event.
Further, were CMS to provide an exception for prescriptions for Part D-covered controlled substances for hospice enrollees, it would pose an operational challenge to accurately match prescription data records with hospice enrollment data where the patient's hospice status can be fluid. It would be operationally challenging to ensure that paper prescriptions were only issued for beneficiaries enrolled in hospice (which would be permitted), and not for patients not enrolled in hospice (where EPCS would be required). We believe the cost of this potentially confusing and laborious analysis for the small number of prescriptions dispensed for beneficiaries enrolled in hospice but covered under Part D exceeds the benefit creating the exception would provide to prescribers.
Therefore, we decline to propose an exemption for prescribers issuing prescriptions for individuals enrolled in hospice. However, we seek comment on this decision.
Section 1860D-4(e)(7)(B)(viii) of the Act suggests an exemption for prescribers issuing prescriptions for individuals who are residents of a nursing facility and eligible for Medicare and Medicaid benefits. We sought stakeholder feedback on this exemption in our August 2020 RFI and discussed it with our federal partners at the DEA, and have been informed that there are situations where nursing facilities experience or are at risk of drug diversion. This stakeholder feedback did not inform us of any compelling reasons to include an exemption for prescribers issuing prescriptions for individuals who are residents of a nursing facility and eligible for Medicare and Medicaid benefits. We have also seen the severe impact that the COVID-19 pandemic has had on nursing facility residents, who are at high risk for infection, serious illness, and death from COVID-19, as well as other infectious diseases including clostridium difficile and the seasonal flu. It is for these reasons that we decline to propose an exemption for prescribers issuing prescriptions for individuals who are residents of a nursing facility and eligible for Medicare and Medicaid benefits. However, we seek comment on this issue.
7. Fraud and Abuse Laws
We are aware that Prescription Drug Plans (PDPs), MA-PD plans, or other organizations with which prescribers are affiliated may wish to assist prescribers with satisfying the mandate for electronic prescribing of controlled substances for a covered Part D drug by providing technology and services necessary to effectuate the electronic prescribing of such drugs. Such assistance may implicate the payment and fraud and abuse laws that govern the financial relationships in the health care industry. Specifically, the donation of free or below-fair market value electronic prescribing technology or services to a practitioner (or any other person) may implicate the physician self-referral law and the federal anti-kickback statute. However, there is an exception to the physician self-referral law's prohibition and a corresponding safe harbor under the federal anti-kickback statute that would permit certain donations in the form of items or services (not including cash or cash equivalents) necessary and used solely to receive and transmit electronic prescription information if all requirements of the applicable exception or safe harbor are satisfied. In addition, other exceptions to the physician self-referral law and safe harbors under the federal anti-kickback statute may apply.
Section III.P.1. of this proposed rule provides a general discussion of the application of, prohibitions of, and exceptions to the physician self-referral law. For information specific to the exception for donations of electronic prescribing items and services, we refer readers to our August 8, 2006 final rule entitled "Physicians' Referrals to Health Care Entities With Which They Have Financial Relationships; Exceptions for Certain Electronic Prescribing and Electronic Health Records Arrangements" (71 FR 45140) and found at https://www.govinfo.gov/content/pkg/FR-2006-08-08/pdf/06-6667.pdf, and the regulations interpreting the physician self-referral law, including additional exceptions to its prohibitions, which are found in 42 CFR part 411, subpart J. Information regarding the federal anti-kickback statute and its applicable safe harbors can be found at www.oig.hhs.gov.
8. Penalties
Section 1860D-4(e)(7)(D) of the Act gives the Secretary the authority to enforce and specify appropriate penalties for non-compliance with the EPCS requirement. We sought stakeholder feedback on whether CMS should impose penalties and if so, what those penalties should be. We have also examined state EPCS requirements and their accompanying penalties. However, because these requirements have only been recently implemented and most states do not have penalties for failing to adopt EPCS, we have not been able to evaluate what type of penalties have been effective for state mandates.
In implementing the EPCS requirement, we seek to help ensure that we do not place too much of a burden on prescribers, as we do not want this requirement to have an unintended consequence of incentivizing prescribers to stop prescribing controlled substances to Part D beneficiaries, as appropriate, should they not have EPCS set-up. We also need sufficient time to gather more stakeholder feedback on the most effective and most appropriate type of penalties.
Therefore, we propose that with respect to compliance from January 1, 2023 through December 31, 2023, CMS compliance actions will consist of sending letters to prescribers that we believe are violating the EPCS requirement during that period of time. These letters will consist of a notification to prescribers that they are violating the EPCS requirement, information about how they can come into compliance, the benefits of EPCS, an information solicitation as to why they are not conducting EPCS, and a link to the CMS portal to request a waiver. We will re-evaluate whether further compliance actions will be necessary and what those compliance actions will be in future rulemaking. We seek comment on this proposal, including what type of compliance action may be appropriate after the initial period described above, including whether any penalties should be phased in over time.
R. Open Payments
1. Background
a. Open Payments Policies
The Open Payments program is a statutorily-mandated program that promotes transparency by providing information to the public about the financial relationships between the pharmaceutical and medical device industry, and certain types of health care providers. Section 1128G of the Act requires manufacturers of covered drugs, devices, biologicals, or medical supplies (referred to as "applicable manufacturers"), as well as applicable group purchasing organizations (GPOs), to annually submit information for the preceding calendar year about certain payments or other transfers of value made to "covered recipients," currently defined as physicians, teaching hospitals, physician assistants (PAs), nurse practitioners (NPs), clinical nurse specialists (CNSs), certified registered Start Printed Page 39334 nurse anesthetists (CRNAs) & anesthesiologist assistants (AAs), and certified nurse-midwives (CNMs).
Payments or other transfers of value that must be reported include such things as research-related payments, honoraria, gifts, travel expenses, meals, grants, and other compensation. The type of information required to be reported includes, but is not limited to, the date and amount of the payment or other transfer of value, identifying information about the covered recipient, and details about products associated with the transaction. When a payment or other transfer of value is related to marketing, education, or research specific to a covered drug, device, biological or medical supply, the name of that covered drug, device, biological or medical supply also must be reported. The estimated burden of these reporting requirements, as outlined under OMB control number 0938-1237, is approximately 1.9 million hours over the course of 1 year.
Section 1128G of the Act establishes certain minimum dollar thresholds for required reporting, with two bases for reporting: Individual and aggregate payments or transfers of value. To determine if multiple small individual payments or other transfers of value made to a covered recipient exceed the aggregate threshold and therefore must be reported, applicable manufacturers and applicable GPOs must aggregate all individual payments made across all payment categories within a given reporting year. The statutory threshold established in 2013 was $10 for individual payments and $100 for aggregated payments, and this amount has increased with the consumer price index each year. For CY 2021, the annual reporting thresholds for individual payments or other transfers of value is $11.04 and the aggregate amount is $110.40.
The Open Payments program yields information to the general public about providers, as well as information that researchers may use to look into potential correlations between financial relationships and provider behaviors. Between August 2013 and the June 2020 publication, more than 76 million records have been disclosed under the Open Payments program, enabling significant transparency into applicable exchanges of value. We have been committed to stakeholder engagement in an effort to limit the burden in the Open Payments program reporting processes and improve clarity for the public. Additional background about the program and guidance, including frequently asked questions, regarding how the program works and what type of information is required to be reported is available at www.cms.gov/OpenPayments.
In the February 8, 2013 Federal Register (78 FR 9458), we published regulations implementing section 1128G of the Act and establishing the Open Payments program. Section 1128G of the Act requires applicable manufacturers and applicable GPOs to submit information annually about certain payments or other transfers of value made to covered recipients during the course of the preceding calendar year. Additionally, section 1128G of the Act defines covered drugs, devices, biologicals, or medical supplies as those covered under Medicare, a state plan under Medicaid, or the Children's Health Insurance Program (CHIP) (or a waiver of either such state plan), and requires applicable manufacturers and applicable GPOs to disclose any ownership or investment interests in such entities held by physicians or physicians' immediate family members, as well as information on any payments or other transfers of value provided to such physician owners or investors. Under section 1128G(e)(10)(A) of the Act, the term "payment or other transfer of value" refers to a transfer of anything of value, though some exclusions apply.
In the CY 2015 PFS final rule with comment period (79 FR 67548), we amended the regulations by standardizing reporting in the Open Payments program. Specifically, we: (1) Deleted the definition of "covered device"; (2) removed the special rules for payments or other transfers of value related to continuing education programs; (3) clarified the marketed name reporting requirements for devices and medical supplies; and (4) required stock, stock options, and any other ownership interests to be reported as distinct forms of payment.
In the CY 2017 PFS proposed rule (81 FR 46395), we solicited information from the public on a wide variety of topics regarding the Open Payments program. Since the implementation of the program and changes made in the CY 2015 PFS final rule with comment period, various commenters have provided us feedback. Consequently, we identified areas in the rule that might benefit from revision and solicited public comments to inform future rulemaking. We sought comment on whether the payment categories listed at 42 CFR 403.904(e)(2) are adequately inclusive to facilitate reporting of all payments or transfers of value, as well as ways to streamline or make the reporting process more efficient while facilitating our role in oversight, compliance, and enforcement, along with posing other program-specific questions. A summary of the comments we received was published in the CY 2017 PFS final rule (81 FR 80428 through 80429).
On October 24, 2018, the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (SUPPORT Act) (Pub. L. 115-271) was signed into law. Section 6111 of the SUPPORT Act amended the definition of "covered recipient" under section 1128G(e)(6) of the Act with respect to information required to be submitted on or after January 1, 2022, to include PAs, NPs, CNSs, CRNAs, and CNMs, in addition to the previously listed covered recipients of physicians and teaching hospitals. In the CY 2020 PFS final rule, we codified the Open Payments provisions of the SUPPORT Act and addressed public comments received from the CY 2017 PFS proposed rule by simplifying the process for reporting data by adjusting the Nature of Payment categories, and standardized data on reported covered drugs, devices, biologicals, or medical supplies.
In this rule, we propose to clarify existing Open Payments requirements, as well as add provisions that program stakeholders have requested and we agree would improve the quality of the data. We propose the following revisions effective for data collection beginning in CY 2023 and reporting in CY 2024: (1) Adding a mandatory payment context field for records to teaching hospitals; (2) adding the option to recertify annually even when no records are being reported; (3) disallowing record deletions without a substantiated reason; (4) updating the definition of ownership and investment interest; (5) adding a definition for a physician-owned distributorship as a subset of applicable manufacturers and group purchasing organizations, for the purposes of Open Payments program reporting only, which definition would not apply for purposes of any other laws or regulations, including, but not limited to, section 1128B of the Act (the federal Anti-Kickback statute), the regulations at 42 CFR 1001.952, and materials interpreting the anti-kickback statute, such as Special Fraud Alerts; and section 1877 of the Act and the regulations at 42 CFR part 411, subpart J (collectively, the physician self-referral law); (6) requiring reporting entities to disclose relationships they have with other companies for the purposes of transparent reporting; (7) disallowing publications delays for general payment records; (8) clarifying the exception for short-term loans applies for 90 total days in a calendar year, regardless of Start Printed Page 39335 whether the 90 days were consecutive; and (9) removing the option to submit and attest to general payment records with an "Ownership" Nature of Payment category. We believe these changes will increase the usability of the data, address concerns we have heard from stakeholders, and give reporting entities sufficient time to prepare for changes to their data collection and reporting procedures.
b. Legal Authority
Four legal authorities from the statute ground our provisions:
- Sections 1102 and 1871 of the Act, which provide general authority for the Secretary to prescribe regulations for the efficient administration of the Medicare program.
- Section 1861 of the Act, which defines providers and suppliers.
- Section 1128G of the Act, as amended by section 6111 of the SUPPORT Act, which requires applicable manufacturers of drugs, devices, biologicals, or medical supplies covered under Medicare or a state plan under Medicaid or CHIP to report annually to the Secretary certain payments or other transfers of value to physicians and teaching hospitals, and to PAs, NPs, CNSs, CRNAs, and CNMs for information required to be submitted under section 1128G of the Act on or after January 1, 2022.
c. Provisions of the Proposed Regulations
(1) Payment Context Field for Teaching Hospitals
We have received feedback from teaching hospitals during informal interviews that Open Payments submissions do not contain sufficient information to identify reported payments or transfers of value in their own records. This means that teaching hospitals are unable to verify records during the review and dispute process and must dispute the record in order to obtain additional information, which yields additional and unnecessary work for both teaching hospitals and reporting entities.
To reduce the burden created by disputes for both reporting entities and teaching hospitals, we are proposing a mandatory context field for payments or transfers of value attributed to teaching hospitals, which would contain information to better identify the payment as deemed appropriate by the applicable manufacturer or GPO. Examples of data that the reporting entity may choose to include are: The check number or electronic wire number for the payment; related department of the hospital; or other pieces of relevant information.
(2) Optional Annual Recertification
Over the course of the program, we have received feedback from several companies that they would like the ability to attest that they do not have any reportable records for that year. At this time, any entity that does not have any reportable payments or transfers of value does not need to recertify in Open Payments, but also does not have a way to communicate to CMS that it believes it is still compliant even though it has not reported.
We propose to make it optional for a company that does not have reportable payments or transfers of value for the program year to recertify their registration in Open Payments and attest that it does not have any records to submit, which would give peace of mind to reporting entities which are correctly not reporting records. We believe this optional recertification for entities without reporting requirements would be low burden to reporting entities, but would be invaluable to ensuring the integrity of the data. We propose adding the following language to an option for entities that are recertifying without submitting records:
"1. I attest that I am a Chief Executive Officer, Chief Financial Officer, Chief Compliance Officer, or other Officer equivalent authorized representative for the reporting applicable manufacturer or applicable group purchasing organization with the authority to attest to the information submitted in the Open Payments system.
2. I attest that, to the best of my knowledge, belief, and ability, my organization does not have any reportable payments or transfers of value or ownership and investment interest to report for the current program year.
3. If I become aware of any information that my entity is required to report, I will submit this information to CMS as required per 42 CFR 403.908(h)(1), which states that if an applicable manufacturer or applicable group purchasing organization discovers an error or omission in its annual report, it must submit corrected information to CMS immediately upon confirmation of the error or omission."
(3) Defining a Physician-Owned Distributorship
The preamble of the 2013 Open Payments final rule (78 FR 9458) discusses physician-owned distributorships (PODs), as a subset of group purchasing organizations (GPOs), but does not provide a specific definition for this type of entity. Reporting entities currently have the ability to self-identify as a POD when registering with Open Payments, but due to the lack of a definition of the term "physician-owned distributorship" or "POD," this designation is not required. We believe that the disclosure of an entity's status as a POD is essential to the transparency that is central to the program, and will also help clear up confusion about whether PODs are required to report. Accordingly, we propose to include the definition of a POD as set out at § 403.902 as a subset of either an applicable manufacturer or applicable GPO.
We are also proposing to include language at § 403.908(c)(4) to require PODs to self-identify when registering or recertifying.
Furthermore, to better align the Open Payments program with the updated definition of ownership and investment interest at § 411.354(b)(3) (see 85 FR 77587), we are including the exceptions for titular ownership and employee stock ownership programs (ESOPs) that are qualified under IRS regulations for consistency in application.
In addition, we emphasize that:
- The proposed definition of a physician-owned distributorship does not apply for purposes of any other laws or regulations, including, but not limited to, section 1877 of the Act, the regulations at 42 CFR part 411, subpart J, section 1128B of the Act, or the regulations at 42 CFR 1001.952.
- "Ownership or investment interest" is defined at § 403.902 of the Open Payments regulations and would not include publicly traded securities or mutual funds.
- To be considered a physician owner(s), the owner would have to hold at least one active professional license to practice as a physician issued by a U.S. state or territory.
- If a company with common ownership reports in a consolidated report with the POD, the reporting company would only be required to register as a POD if it meets the 5 percent ownership requirement when ownership of all entities in the report is calculated.
- The POD would be required to report ownership and investment interest as required by existing Open Payments requirements. Ownership or investment interest is defined at § 403.902 to include, but is not limited to: Stocks, stock option(s) (other than those received as compensation, until they are exercised); partnership shares, both limited and non-limited; limited liability company memberships; loans, bonds, or other financial instruments Start Printed Page 39336 that are secured with an entity's property or revenue or a portion of that property or revenue. This definition explicitly excepts titular ownership and ESOPs that are qualified under IRS regulations.
- The POD would be required to identify as a POD whether or not the physician has a controlling interest in the reporting entity (for example, a silent partner whose only role is to provide capital and is not involved in the company's operations would still meet requirements for reporting).
- Five percent interest would be calculated as 5 percent of the total dollar value in USD of all ownership in the POD as of December 31, or the latest date that the ownership was held, as of the calendar year proceeding the Program Year. For example, if reporting ownership in a POD for Program Year 2022, the ownership would be calculated as of December 31, 2022, or the latest date in the calendar year that the physician held the ownership or investment interest.
- Indirect ownership interest would also have to be reported as required by § 403.902. Indirect ownership is often the result of the use of holding companies and parent/subsidiary relationships.
- Any entity meeting this definition would be required to identify itself as a POD when submitting and attesting to its records. For example, if an applicable manufacturer meets the definition of a POD, it may not choose to identify itself simply as an "Applicable Manufacturer" but would have to choose its business type as "Applicable Manufacturer—Physician Owned Distributorship."
- We believe that this proposed definition should not increase industry burden because it is a subset of existing definitions, but should clarify confusion about PODs being outside of reporting requirements.
(4) Disallowing Record Deletion Without Reason
While we have not seen evidence of the following behavior, we believe that our existing regulations might allow entities to be compliant by reporting and attesting to records, then deleting those records so that they are never publicly available. We propose to prevent reporting followed by deletion by adding language at § 403.904(a)(3) that would state that an entity that has reported payments or transfers of value under the scope of this rule may not remove, delete, or alter the records in the Open Payments system unless it discovers an error in the information furnished, or the record is otherwise believed to meet existing exceptions for reporting that were previously unknown.
An example of a properly deleted record would be the deletion of ownership records that were reported for a publicly traded company, since publicly traded companies are not required to report ownership and investment interest. We would add a dialogue box in the system for reporting entities to provide a reason for record deletion. We note that deletions will continue to undergo additional scrutiny to ensure the integrity of the data.
(5) Disallow Publication Delays of General Payments
Delayed publication is permitted for Open Payments records based on concerns that the information provided in the record details may reveal proprietary information about a company's research activities. According to § 403.910, only payments that are made in connection with (1) Research or on development of a new drug, device, biological, or medical supply, or a new application of an existing drug, device, biological, or medical supply or (2) Clinical investigations regarding a new drug, device, biological, or medical supply" are allowed to be delayed from publication. As of December 26, 2020, there were 20,930 general records with a value of $26.4M that were delayed from publication for at least one Program Year, and based on the information provided in the current format required for the submission of general records, we are unable to verify these records' connection with research or clinical investigations. Therefore, we propose to eliminate the ability to delay general payments from publication and only permit publication delay of research payments, whose formatting does require the appropriate information to be provided, the details of which are specified at § 403.904(f).
Reporting entities may hesitate to include records that are currently being delayed as general payments because they are associated with a research study, but not directly outlined in that research agreement. For example, a company may pay for an airline ticket for a physician to conduct research that is associated with a research agreement, but that travel was not explicitly outlined in that agreement. However, we do not believe that the current requirements for a research payment would exclude these types of payments from being reported as research payments, as long as they are made in connection with, and subject to, a research agreement.
(6) Short Term Loans
The Open Payments final rule makes a reporting exception for short term equipment loans. A short term medical supply or device loan means the loan of a covered device or a device under development, or the provision of a limited quantity of medical supplies for a short-term trial period, not to exceed a loan period of 90 days or a quantity of 90 days of average daily use, to permit evaluation of the device or medical supply by the covered recipient.
The Open Payments regulations also clarify that for a single product the total number of days for the loan should not exceed 90 days for the entire year, regardless of whether the 90 days were consecutive. We believe that this aligns with the intention to limit the loan period to 90 days and not allow a new loan to start at the end of the previous loan period, thus avoiding the reporting requirements. We propose to clarify this by stating that short term medical supply or device loan means the loan of a covered device or a device under development, or the provision of a limited quantity of medical supplies for a short-term trial period, not to exceed a loan period of 90 cumulative days per calendar year or a quantity of 90 cumulative days of average daily use per calendar year, to permit evaluation of the device or medical supply by the covered recipient.
g. Remove General Ownership Records
We currently have two ways for an entity to report ownership: Entities may submit an ownership record or a general record with a Nature of Payment category of "Ownership." We propose to remove the "Ownership" Nature of Payment category. The statute requires special rules for the reporting of ownership interest, including dollar amount invested and value of interest, which is not captured by the general payment with the Nature of Payment category of "Ownership." Furthermore, this proposal would create a cleaner and more consistent data set.
(7) Updated Contact Information
When sending communications to entities, the Open Payments program often finds that their contact information is outdated, especially if the entity has not recertified recently. It is important for the integrity of the data that the program is able to contact the reporting entities in case of perceived irregularities or potential noncompliance. We propose to make it mandatory for a company that has had reportable payments or transfers of Start Printed Page 39337 value within the past 2 calendar years to keep its contact information current within the Open Payments system. For example, if an applicable manufacturer or group purchasing organization had reported records in Program Years 2018 and 2022, but did not have records for Program Years 2019, 2020, or 2021, it would be required to keep updated contact information in the system during Program Years 2019 and 2020. The applicable manufacturer or group purchasing organization would not have to update its contact information for Program Year 2021. In Program Year 2022, since it once again had reportable records, it would be required to recertify and update its contact information as usual. We propose to include this requirement at § 403.908(c)(3).
IV. Summary of the Quality Payment Program Proposed Provisions
A. CY 2022 Updates to the Quality Payment Program
1. Executive Summary
a. Overview
This section of the final rule sets forth changes to the Quality Payment Program starting January 1, 2022, except as otherwise noted for specific provisions. The 2022 MIPS performance period/2024 MIPS payment year of the Quality Payment Program continues to build on the first few years of implementation of the Quality Payment Program to focus more on our measurement efforts, refine how clinicians will be able to participate in a more meaningful way and encourage participation in Advanced Alternative Payment Models (APMs).
Authorized by the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) (Pub. L. 114-10, April 16, 2015), the Quality Payment Program is an incentive program that includes two participation tracks, the Merit-based Incentive Payment System (MIPS) and Advanced APMs. MIPS eligible clinicians are subject to a MIPS payment adjustment based on their performance in four performance categories: Cost, quality, improvement activities, and Promoting Interoperability. The weights of those four performance categories are specified in statute. For CY 2022, those weights are as follows: 30 percent for the quality performance category, 30 percent for the cost performance category, 15 percent for the improvement activities performance category, and 25 percent for the Promoting Interoperability performance category. If an eligible clinician participates in an Advanced APM and achieves Qualifying APM Participant (QP) status, they are excluded from the MIPS reporting requirements and payment adjustment. Those that are qualifying APM participants (QPs) for the year receive a 5 percent lump sum incentive payment during the corresponding payment year through CY 2024, or a differential payment update under the PFS for payment years beginning in 2026.
Participation in the Quality Payment Program rose in the third year. We saw 99.99 percent of eligible clinicians participate in MIPS in 2019 with 954,614 MIPS eligible clinicians receiving a payment adjustment, which exceeded our 2018 participation rates. In addition, 97.6 percent of eligible clinicians participating in MIPS received a positive payment adjustment for 2021 based on 2019 performance year results. Regarding performance in Advanced APMs, for the 2019 QP Performance Period, 195,564 eligible clinicians earned Qualifying APM Participant (QP) status while another 27,995 eligible clinicians earned partial QP status.[132] We note that due to the Public Health Emergency (PHE) for COVID-19, 65,237 (or about 6.83 percent of 954,614) MIPS eligible clinicians received reweighting for performance year 2019 of one or more MIPS performance categories due to our MIPS extreme and uncontrollable circumstances policy.
We plan to continue developing Quality Payment Program policies that more effectively reward high-quality of care for patients and increase opportunities for Advanced APM participation. We are moving forward with MIPS Value Pathways (MVPs) as MVPs allow for a more cohesive participation experience by connecting activities and measures from the 4 MIPS performance categories that are relevant to a specialty, medical condition, or a particular population. The MVPs would include the Promoting Interoperability performance category as a foundational element and incorporate population health claims-based measures, as feasible, along with relevant measures and activities for the quality, cost, and improvement activities performance categories. To provide clinicians and third party intermediaries with sufficient time to prepare for a shift to this new participation framework, in this rule, we are proposing to begin transitioning to MVPs in the 2023 MIPS performance year.
As we make long-term improvements, evolve MIPS policies, and plan to implement MVPs in the future, we remain committed to our program goals. We are aligning with broader CMS initiatives, such as the CMS Quality Measure Action Plan (https://www.cms.gov/files/document/2021-cms-quality-conference-cms-quality-measurement-action-plan-march-2021.pdf), to unify strategic efforts to adopt measures most critical to providing high quality care and accelerate strategic improvements for quality programs and measures. The vision for the CMS Quality Measure Action Plan is to use impactful quality measures to improve health outcomes and deliver value by empowering patients to make informed care decisions while reducing burden to clinicians. This plan supports CMS's work to identify activities for transformation of quality measurement and value-based programs and recognizes the need to modernize the current quality ecosystem of measurement and programs. Additionally, it will encourage further reductions to the burden of quality measure reporting and address the current lack of alignment. These efforts will also support identifying activities that are driving better patient outcomes at lower costs. The planned implementation of MVPs aligns with many of the objectives and goals the CMS Quality Measure Action Plan will strive to achieve.
Through the proposals we describe below, we intend to transform and simplify the MIPS program through MVPs, promote the use of connected measures and activities, reward clinicians for providing high value care, and help all clinicians improve care and engage patients. We also intend to gather information from stakeholders to help guide efforts to advance health equity throughout CMS quality programs.
b. Summary of Major Provisions
(1) Major MIPS Provisions
The MIPS program aims to drive value through the collection, assessment, and public reporting of data that informs and rewards the delivery of high-value care. Within MIPS we intend to pay for health care services in a way that drives value by linking performance on cost, quality, and the patient's experience of care.
We have heard from clinicians that MIPS requirements are confusing, burdensome, and that it is difficult to choose measures from the several hundred MIPS and QCDR quality Start Printed Page 39338 measures that are meaningful to their practices and have a direct benefit to patients. We have also heard concerns from stakeholders that MIPS does not allow for sufficient differentiation of performance across practices due in part to clinician quality measure selection bias. These aspects detract from the program's ability to effectively measure and compare performance, provide meaningful feedback, and incentivize quality. MVPs are intended to lead to a simplified MIPS clinician experience, improve value, reduce burden, and better inform patient choice in selecting clinicians. We noted that the MVP framework would connect measures and activities across the 4 MIPS performance categories, incorporate a set of administrative claims-based quality measures that focus on population health, provide data and feedback to clinicians, and enhance information provided to patients. We intend to focus the future of MIPS on MVP development and implementation.
Additionally, we have heard from patients, clinicians, and other stakeholders that they would like more comprehensive and granular reporting from the MIPS program. To that end, we are proposing to establish voluntary subgroup reporting to help provide patients and clinicians information that is clinically meaningful at a more granular level.
We are additionally issuing a request for information (RFI) to address the Advancing to Digital Quality Measurement and the Use of Fast Healthcare Interoperability Resources (FHIR) in Physician Quality Programs please refer to section IV.A.1.c. of this rule for more information. We are also issuing an RFI to address Closing the Health Equity Gap in CMS Clinician Quality Programs in section IV.A.1.d. of this rule.
(a) Basis and Scope
At § 414.1300, we previously codified the basis and scope of the MIPS and APMs. In order to support the continued application of voluntary reporters, we propose to revise the basis and scope at § 414.1300(a)(2) to remove reference to section 1848(a) of the Act—Payment for Physicians' Services Based on Fee Schedule and instead redesignate the text at § 414.1300(a)(3) to § 414.1300(a)(2) to state section 1848(k)—Quality Reporting System. At § 414.1400(a)(3), we also propose to add new language to state section 1848(m)—Incentive Payments for Quality Reporting.
(b) MIPS Value Pathways and APM Performance Pathway
We recognize that the transition to MVPs will take time and we will continue to evaluate the readiness of clinicians in making this transition, while balancing our strong interest in improving measurement and making MIPS more focused on value.
As discussed in section IV.A.3.b. of this proposed rule, for MIPS Value Pathways (MVPs) we propose:
- To define who can report MVPs, through the term MVP Participant.
- A delay to the CY 2023 MIPS performance period/CY 2025 MIPS payment year: MVP implementation and subgroup reporting timelines. Beginning in the CY 2025 MIPS performance period/CY 2027 MIPS payment year, multispecialty groups would be required to form subgroups in order to report MVPs.
- An introductory set of 7 MVPs to be available beginning with the 2023 performance period.
- MVP reporting requirements that account for the four MIPS performance categories.
- During the CY 2023 and CY 2024 performance periods, voluntary subgroup reporting within MIPS limited to reporting through MVPs or the APP. For the MIPS program, eligibility, special status determination, and QP determination would continue to be determined at the group level for subgroup participants. Subgroup performance will be assessed at the subgroup level for three performance categories (the quality, cost, and improvement activities performance categories) and will be assessed at the group level for one performance category (the Promoting Interoperability performance category). Additionally, subgroups will continue to be included in group level reporting but will receive scores separate from their affiliated group.
- MVP scoring policies closely align with those used in traditional MIPS, with few exceptions.
- MVP scoring policies including policies for scoring administrative claims measures, including population health measures, scoring only the cost measures specified in the MVP, assigning 20 points for each medium-weighted and 40 points for each high-weighted improvement activity specified in the MVP, scoring subgroups on their affiliated group's data for the Promoting Interoperability performance category, reweighting performance categories for subgroups in certain circumstances, and requirements that the quality performance category be scored with few exceptions for reweighting.
- To provide comparative feedback within performance feedback, comparing the performance of like clinicians who report on the same MVP.
We also discuss in section IV.A.3.b. of this proposed rule, future considerations and goals of the MIPS program:
- We request comment on the timeline to sunset traditional MIPS in the future, and to eventually make MVP reporting mandatory. Note: we are referring to the established MIPS participation options collectively as traditional MIPS (85 FR 84844).
- Through the MVP development work, gradually implement MVPs for all specialties and subspecialties that participate in the program.
As discussed in section IV.A.3.c. of this proposed rule, for the APM Performance Pathway, to create stability within the APP, we are not proposing any major changes to the APP.
(c) Other MIPS and APM Policies
We are proposing the following provisions for MIPS beginning with the 2022 performance period:
- As discussed in section IV.A.3.d. of this proposed rule, for the MIPS Performance Measures and Activities, we propose:
++ In section IV.A.3.d.(1) of this proposed rule, for the quality performance category, to maintain the data completeness criteria threshold at 70 percent for the 2021 and 2022 MIPS performance periods (2023 and 2024 MIPS payment years); establish the data completeness criteria threshold at 80 percent for the 2023 MIPS performance period (2025 MIPS payment year); extend the availability of the CMS Web Interface as a collection and submission type for the 2022 MIPS performance period; establish a set of 195 MIPS quality measures; and seek public comment through a request for information (RFI) regarding the draft COVID-19 Vaccination by Clinicians measure specifications.
++ In section IV.A.3.d.(2) of this proposed rule, for the cost performance category, to establish 5 new cost measures for implementation into MIPS, which adds to the 2 global or population-based measures and 18 episode-based measures.
In section IV.A.3.e.(2) of this proposed rule, in regard to calculating the final score, we propose formulas for the complex patient bonus with two separate components (one for medical complexity and one for social complexity) and an overall cap of 10 bonus points. Lastly, we propose Start Printed Page 39339 updating the formulas for the bonuses to base them on standardized scores and to reward those who fall in higher quintiles and not reward those who fall below a cut-off point.
- As discussed in section IV.A.3.f. of this proposed rule, beginning with year 6 of MIPS (2024 MIPS payment year), the performance threshold must be either the mean or median of the final scores for all MIPS eligible clinicians for a prior period. We are proposing to establish the performance threshold using the mean and the 2017 performance period/2019 MIPS payment year data, which would result in a performance threshold of 75 points. In addition, for the 2024 MIPS payment year, the additional performance threshold must be set at either (1) the 25th percentile of the range of possible final scores above the performance threshold, or (2) the 25th percentile of the actual final scores for MIPS eligible clinicians with final scores at or above the performance threshold with respect to a prior period. We note that under section 1848(q)(6)(C) of the Act, the additional MIPS payment adjustment factors for exceptional performance are available through the 2024 MIPS payment year, making this the last year of the additional performance threshold and the associated additional MIPS payment adjustment factors for exceptional performance. We are proposing to establish an additional performance threshold of 89 points. This is the 25th percentile of actual final scores from the 2017 performance period/2019 MIPS payment year at or above 75 points.
- As discussed in section IV.A.3.h. of this proposed rule, for Third Party Intermediaries, we are proposing to modify third party intermediary requirements, remedial actions and termination policies. Specifically, beginning with the 2023 MIPS performance period/2025 MIPS payment year, QCDRs, qualified registries, and health IT vendors must support MVPs that are applicable to the MVP participants on whose behalf they submit MIPS data. QCDRs, qualified registries, and, health IT vendors may also support the APP. We also propose to require QCDRs, qualified registries, health IT vendors, and CMS-approved survey vendors to support subgroup reporting beginning with the 2023 MIPS performance period/2025 MIPS payment year.
- As discussed in section IV.A.3.i. of this proposed rule, for Public Reporting on Compare Tools hosted by the U.S. Department of Health and Human Services (Compare Tools), we are proposing to publicly report clinician affiliations to certain types of facilities (for example, LTCHs, IRFs, etc.). We also seek comment through a RFI to inform the ways in which utilization data may be useful to patients and caregivers for their health care decisions. In order to give MIPS eligible clinicians time to familiarize themselves with MVPs and subgroup reporting, we are proposing to delay public reporting of new improvement activities and Promoting Interoperability measures and attestations reported via MVPs by 1 year, and begin publicly reporting subgroup-level performance information in PY 2024, on the compare tool hosted by the U.S. Department of Health and Human Services. We are also proposing to create a separate subgroup workflow that would allow subgroup performance information to be publicly reported in an online location that can be navigated to and from an individual clinician or group profile page. This also aligns with the historical approach to report performance information at the level that it is submitted.
- As discussed in section IV.A.4.b. and IV.A.4.c. of this rule, we are proposing a change to the APM Incentive Payment payee hierarchy to include payment to TINs associated with QPs during the payment year.
c. Advancing to Digital Quality Measurement and the Use of Fast Healthcare Interoperability Resources (FHIR) in Physician Quality Programs—Request for Information
We aim to move fully to digital quality measurement in CMS quality reporting and value-based purchasing programs by 2025. As also detailed in the FY 2022 Hospital Inpatient PPS proposed rule (86 FR 25549 through 25554), as part of this modernization of our quality measurement enterprise, we are issuing this request for information (RFI). The purpose of this RFI is to gather broad public input solely for planning purposes for our transition to digital quality measurement. Any updates to specific program requirements related to providing data for quality measurement and reporting provisions would be addressed through future rulemaking, as necessary. This RFI contains five parts:
- Background. This part provides information on our quality measurement programs and our goal to move fully to digital quality measurement by 2025. This part also provides a summary of recent HHS policy developments that are advancing interoperability and could support our move towards full digital quality measurement.
- Definition of Digital Quality Measures (dQMs). This part provides a potential definition for dQMs. Specific requests for input are included in the section.
- Use of Fast Healthcare Interoperability Resources (FHIR®) for current electronic clinical quality measures (eCQMs). This part provides information on current activities underway to align CMS eCQMs with the FHIR standard and support quality measurement via application programming interfaces (APIs), and contrasts this approach to current eCQM standards and practice.
- Changes Under Consideration to Advance Digital Quality Measurement: Actions in Four Areas to Transition to Digital Quality Measures by 2025. This part introduces four possible steps that would enable transformation of CMS' quality measurement enterprise to be fully digital by 2025. Specific requests for input are included in the section.
- Solicitation of Comments. This part lists all requests for input included in the sections of this RFI.
(1) Background
As required by law, we implement quality measurement and value-based purchasing programs across a broad range of inpatient acute care, outpatient, and post-acute care (PAC) settings consistent with our mission to improve the quality of health care for Americans through measurement, transparency, and increasingly, value-based purchasing. These quality programs are foundational for incentivizing value-based care, contributing to improvements in health care, enhancing patient outcomes, and informing consumer choice. In October 2020, we launched the CMS Quality Measure Action Plan.[133] One key goal of the plan is to improve the efficiency of quality measures by a transition to digital measures and use of advanced data analytics. Our objective is to use data and information as essential aspects of a healthy, robust healthcare infrastructure to allow for payment and management of accountable, value-based care and development of learning health organizations.[134] Consistent with the CMS Quality Measure Action Plan, we aim to move fully to digital quality measurement by 2025. We acknowledge providers within the various care and practice settings covered by our quality programs may be at different stages of readiness, and therefore, the timeline for Start Printed Page 39340 achieving full digital quality measurement across our quality reporting programs may vary.
We also continue to evolve the Medicare Promoting Interoperability Program's focus on the use of certified electronic health record (EHR) technology, from an initial focus on electronic data capture to enhancing information exchange and expanding quality measurement (83 FR 41634). However, reporting data for quality measurement via EHRs remains burdensome, and our current approach to quality measurement does not readily incorporate emerging data sources such as patient-reported outcomes (PRO) and patient-generated health data (PGHD).[135] There is a need to streamline our approach to data collection, calculation, and reporting to fully leverage clinical and patient-centered information for measurement, improvement, and learning.
Additionally, advancements in technical standards and associated regulatory initiatives to improve interoperability of healthcare data are creating an opportunity to significantly improve our quality measurement systems. In May 2020, we finalized interoperability requirements in the CMS Interoperability and Patient Access final rule (85 FR 25510) to support beneficiary access to data held by certain payers. At the same time, the Office of the National Coordinator for Health Information Technology (ONC) finalized policies in the ONC 21st Century Cures Act final rule (85 FR 25642) to advance the interoperability of health information technology (IT) as defined in section 4003 of the Cures Act, including the "complete access, exchange, and use of all electronically accessible health information." Closely working with ONC, we collaboratively identified Health Level 7 (HL7®) FHIR Release 4.0.1 as the standard to support Application Programming Interface (API) policies in both rules. ONC, on behalf of HHS, adopted the HL7 FHIR Release 4.0.1 for APIs and related implementation specifications at 45 CFR 170.215. We believe the FHIR standard has the potential to be a more efficient and modular standard to enable APIs. We also believe this standard enables collaboration and information sharing, which is essential for delivering high-quality care and better outcomes at a lower cost. By aligning technology requirements for payers, health care providers, and health IT developers HHS can advance an interoperable health IT infrastructure that ensures providers and patients have access to health data when and where it is needed.
In the ONC 21st Century Cures Act final rule, ONC adopted a "Standardized API for Patient and Population Services" certification criterion for health IT that requires the use of FHIR Release 4 and several implementation specifications. Health IT certified to this criterion will offer single patient and multiple patient services that can be accessed by third party applications (85 FR 25742).[136] The ONC 21st Century Cures Act final rule also requires health IT developers to update their certified health IT to support the United States Core Data for Interoperability (USCDI) standard.[137] The scope of patient data identified in the USCDI and the data standards that support this data set are expected to evolve over time, starting with data specified in Version 1 of the USCDI. In November 2020, ONC issued an interim final rule with comment period extending the date when health IT developers must make technology meeting updated certification criteria available under the ONC Health IT Certification Program until December 31, 2022 (85 FR 70064).[138]
The CMS Interoperability and Patient Access final rule (85 FR 25510) and program policies build on the ONC 21st Century Cures Act final rule (85 FR 25642). The CMS Interoperability and Patient Access final rule and policies require certain payers (for example, Medicare Advantage organizations, Medicaid and CHIP Fee-for-Service programs, Medicaid managed care plans, CHIP managed care entities, and issuers of certain Qualified Health Plan [QHP] on the Federally-facilitated Exchanges [FFEs]) to implement and maintain a standards-based Patient Access API using HL7 FHIR Release 4.0.1 to make available certain data to their enrollees and beneficiaries (called "patients" in the CMS interoperability rule). These certain data include data concerning claims and encounters, with the intent to ensure access to their own health care information through third-party software applications. The rule also established new Conditions of Participation for Medicare and Medicaid participating hospitals and critical access hospitals (CAHs), requiring them to send electronic notifications to another healthcare facility or community provider or practitioner when a patient is admitted, discharged, or transferred if the hospital or CAH utilizes an electronic medical records system or other electronic administrative system which is conformant with the content exchange standard at 45 CFR 170.205(d)(2) (85 FR 25603). In the CY 2021 PFS final rule (85 FR 84472), we finalized a policy to align the certified EHR technology required for use in the Promoting Interoperability Programs and the MIPS Promoting Interoperability performance category with the updates to health IT certification criteria finalized in the ONC 21st Century Cures Act final rule. Under this policy, MIPS eligible clinicians, and eligible hospitals and CAHs participating in the Promoting Interoperability Programs, must use technology meeting the updated certification criteria for performance and reporting periods beginning in 2023 (85 FR 84825).
The use of APIs can also reduce longstanding barriers to quality measurement. Currently, health IT developers are required to implement individual measure specifications within their health IT products. The health IT developer must also accommodate how that product connects with the unique variety of systems within a specific care setting.[139] This may be further complicated by systems that integrate a wide range of data schemas. This process is burdensome and costly, and it is difficult to reliably obtain high quality data across systems. As health IT developers map their health IT data to the FHIR standard and related implementation specifications, APIs can enable these structured data to be easily accessible for quality measurement or other use cases, such as care coordination, clinical decision support, and supporting patient access.
We believe the emerging data standardization and interoperability enabled by APIs will support the transition to full digital quality measurement by 2025, and are committed to exploring and seeking input on potential solutions for the Start Printed Page 39341 transition to digital quality measurement as described in this RFI.
(2) Definition of Digital Quality Measures
In this section we seek to refine the definition of digital quality measures (dQMs) to further operationalize our objective of fully transitioning to dQMs by 2025. We previously noted dQMs use "sources of health information that are captured and can be transmitted electronically and via interoperable systems." (85 FR 84845) In this RFI, we seek input on future elaboration that would define a dQM as a software that processes digital data to produce a measure score or measure scores. Data sources for dQMs may include administrative systems, electronically submitted clinical assessment data, case management systems, EHRs, instruments (for example, medical devices and wearable devices), patient portals or applications (for example, for collection of patient-generated health data), health information exchanges (HIEs) or registries, and other sources. We also note that dQMs are intended to improve the patient experience including quality of care, improve the health of populations, and/or reduce costs.
We discuss one potential approach to developing dQM software in section IV.A.1.c of this proposed rule. In this section, we are seeking comment on the potential definition of dQMs in this RFI.
We also seek feedback on how leveraging advances in technology (for example, FHIR APIs) to access and electronically transmit interoperable data for dQMs could reinforce other activities to support quality measurement and improvement (for example, the aggregation of data across multiple data sources, rapid-cycle feedback, and alignment of programmatic requirements).
The transition to dQMs relies on advances in data standardization and interoperability. As providers and payers work to implement the required advances in interoperability over the next several years, we will continue to support reporting of eCQMs through CMS quality reporting programs and through the Promoting Interoperability programs.[140] These fully digital measures continue to be important drivers of interoperability advancement and learning. As discussed in the next section, CMS is currently re-specifying and testing these measures to use FHIR rather than the currently adopted Quality Data Model (QDM) in anticipation of the wider use of FHIR standards. CMS intends to apply significant components of the output of this work, such as the re-specified measure logic and the learning done through measure testing with FHIR APIs, to define and build future dQMs that take advantage of the expansion of standardized, interoperable data.
(3) Use of FHIR for Current eCQMs
Since we adopted eCQMs in our hospital and clinician quality programs, we have heard from stakeholders about the technological challenges, burden, and related costs of reporting eCQM data. The CMS eCQM Strategy Project engaged with stakeholders through site visits and listening sessions with health systems and provider organizations to learn about their experiences. This stakeholder feedback identified recommendations to improve processes related to alignment; development; implementation and reporting; certification; and communication, education, and outreach. Over the past 2 years, we have focused on opportunities to streamline and modernize quality data collection and reporting processes, such as exploring FHIR® (http://hl7.org/fhir) as a framework for measure structure and data submission for quality reporting programs, specifically for eCQMs. FHIR is a free and open source standards framework (in both commercial and government settings) created by Health Level Seven International (HL7®) that establishes a common language and process for all health information technology. FHIR allows systems to communicate and information to be shared seamlessly, with a lower burden for hospitals, providers, clinicians, vendors, and quality measurement stakeholders. Specifically, for quality reporting, FHIR enables representing the data in eCQMs as well as provides a structure for eCQMs and reporting, using FHIR as the standard for all. Whereas today, multiple standards being used to report eCQMs is challenging and burdensome.
We are working to convert current eCQMs to the FHIR standard. We are currently testing the exchange of data elements represented in FHIR to CMS through ongoing HL7 Connectathons and integrated system testing by using and refining implementation guides. Submitting data through FHIR APIs has the potential to improve data exchange by providing consistent security, performance, scalability, and structure to all users. In addition, development of FHIR APIs could decrease provider burden by automating more of the measure data collection process. We continue to explore and expand potential applications of the FHIR standard and testing with eCQM use cases, and we are considering a transition to FHIR-based quality reporting with the use of the FHIR standard for eCQMs in quality and value-based reporting programs. As we move to an all-dQM format for quality programs, we are depending on testing results and community readiness to improve interoperability, reduce burden, and facilitate better patient care. We will continue to consider how to leverage the interoperability advantages offered by the FHIR standards and API-based data submission, including digital quality measurement.
(4) Changes Under Consideration To Advance Digital Quality Measurement: Potential Actions in Four Areas To Transition to Digital Quality Measures by 2025
Building on the advances in interoperability and learning from testing of FHIR-converted eCQMs, we aim to move fully to dQMs, originating from sources of health information that are captured and can be transmitted electronically via interoperable systems, by 2025.
To enable this transformation, we are considering further modernization of the quality measurement enterprise in four major ways: (1) Leverage and advance standards for digital data and obtain all EHR data required for quality measures via provider FHIR-based APIs; (2) redesign our quality measures to be self-contained tools; (3) better support data aggregation; and (4) work to align measure requirements across our reporting programs, other federal programs and agencies, and the private sector where appropriate.
These changes would enable us to collect and utilize more timely, actionable, and standardized data from diverse sources and care settings to improve the scope and quality of data used in quality reporting and payment programs, reduce quality reporting burden, and make results available to stakeholders in a rapid-cycle fashion. Data collection and reporting efforts would become more efficient, supported by advances in interoperability and data standardization. Aggregation of data from multiple sources would allow assessments of costs and outcomes to be measured across multiple care settings for an individual patient or clinical conditions. We believe that aggregating data for measurement can incorporate a more holistic assessment of an individual's health and health care and produce the rich set of data needed to enable patients and caregivers to make Start Printed Page 39342 informed decisions by combining data from multiple sources (for example, patient reported data, EHR data, and claims data) for measurement.
Perhaps most importantly, these steps would help us deliver on the full promise of quality measurement and drive us toward a learning health system that transforms healthcare quality, safety, and coordination and effectively measures and achieves value-based care. The shift from a static to a learning health system hinges on the interoperability of healthcare data, and the use of standardized data. dQMs would leverage this interoperability to deliver on the promise of a learning health system wherein standards-based data sharing and analysis, rapid-cycle feedback, and quality measurement and incentives are aligned for continuous improvement in patient-centered care. Similarly, standardized, interoperable data used for measurement can also be used for other use cases, such as clinical decision support, care coordination and care decision support, which impacts health care and care quality.
We are requesting comments on four potential future actions that would enable transformation to a fully digital quality measurement enterprise by 2025.
(a) Leveraging and Advancing Standards for Digital Data and Obtaining All EHR Data Required for Quality Measures via Provider FHIR-Based APIs
We are considering targeting the data required for our quality measures that utilize EHR data to be data retrieved via FHIR-based APIs based on standardized, interoperable data. Utilizing standardized data for EHR-based measurement (based on FHIR and associated implementation guides) and aligning where possible with interoperability requirements can eliminate the data collection burden providers currently experience with required chart-abstracted quality measures and reduce the burden of reporting digital quality measure results. We can fully leverage this advance to adapt eCQMs and expand to other dQMs through the adoption of interoperable standards across other digital data sources. We are considering methods and approaches to leverage the interoperability data requirements for APIs in certified health IT set by the ONC 21st Century Cures Act final rule to support modernization of CMS quality measure reporting. As discussed previously, these requirements will be included in certified technology in future years (85 FR 84825) including availability of data included in the USCDI via standards-based APIs, and CMS will require clinicians and hospitals participating in MIPS and the Promoting Interoperability Programs, respectively, to transition to use of certified technology updated consistent with the 2015 Cures Edition Update (85 FR 84825).
Digital data used for measurement could also expand beyond data captured in traditional clinical settings, administrative claims data, and EHRs. Many important data sources are not currently captured digitally, such as survey and PGHD. We intend to work to innovate and broaden the digital data used across the quality measurement enterprise beyond the clinical EHR and administrative claims. Agreed upon standards for these data, and associated implementation guides will be important for interoperability and quality measurement. We will consider developing clear guidelines and requirements for these digital data that align with interoperability requirements, for example, requirements for expressing data in standards, exposing data via standards-based APIs, and incentivizing technologies that innovate data capture and interoperability.
High quality data are also essential for reliable and valid measurement. Hence, in implementing the shift to collect all clinical EHR data via FHIR-based APIs, we would support efforts to strengthen and test the quality of the data obtained through FHIR-based APIs for quality measurement. We currently conduct audits of electronic data submitted to the Hospital IQR Program with functions including checks for data completeness and data accuracy, confirmation of proper data formatting, alignment with standards, and appropriate data cleaning (82 FR 38398 through 38402). These functions would continue and be applied to dQMs and further expanded to automate the manual validation of the data compared to the original data source (for example, the medical record) where possible. Analytic advancements such as natural language processing, big data analytics, and artificial intelligence, can support this evolution. These techniques can be applied to validating observed patterns in data and inferences or conclusions drawn from associations, as data are received, to ensure high quality data are used for measurement.
We are seeking feedback on the goal of aligning data needed for quality measurement with interoperability requirements and the strengths and limitations of this approach. We are also seeking feedback on the importance of and approaches to supporting inclusion of PGHD and other currently non-standardized data. We also welcome comment on approaches for testing data quality and validity.
(b) Redesigning Quality Measures To Be Self-Contained Tools
We are considering approaches for including quality measures that take advantage of standardized data and interoperability requirements that have expanded flexibility and functionality compared to CMS' current eCQMs. We are considering defining and developing dQM software as end-to-end measure calculation solutions that retrieve data from primarily FHIR-based resources maintained by providers, payers, CMS, and others; calculate measure score(s), and produce reports. In general, we believe to optimize the use of standardized and interoperable data, the software solution for dQMs should do the following:
- Have the flexibility to support calculation of single or multiple quality measure(s).
- Perform three functions—
++ Obtain data via automated queries from a broad set of digital data sources (initially from EHRs, and in the future from claims, PRO, and PGHD);
++ Calculate the measure score according to measure logic; and
++ Generate measure score report(s).
- Be compatible with any data source systems that implement standard interoperability requirements.
- Exist separately from digital data source(s) and respect the limitations of the functionality of those data sources.
- Be tested and updated independently of the data source systems.
- Operate in accordance with health information protection requirements under applicable laws and comply with governance functions for health information exchange.
- Have the flexibility to be deployed by individual health systems, health IT vendors, data aggregators, and health plans; and/or run by CMS depending on the program and measure needs and specifications.
- Be designed to enable easy installation for supplemental uses by medical professionals and other non-technical end-users, such as local calculation of quality measure scores or quality improvement.
- Have the flexibility to employ current and evolving advanced analytic approaches such as natural language processing.
- Be designed to support pro-competitive practices for development, maintenance, and implementation, as well as diffusion of quality measurement and related quality Start Printed Page 39343 improvement and clinical tools through, for example, the use of open-source core architecture.
We seek comment on these suggested functionalities and other additional functionalities that quality measure tools should ideally have particularly in the context of the possible expanding availability of standardized and interoperable data (for example, standardized EHR data available via FHIR-based APIs).
We are also interested whether and how this more open, agile strategy may facilitate broader engagement in quality measure development, the use of tools developed for measurement for local quality improvement, and/or the application of quality tools for related purposes such as public health or research.
(c) Building a Pathway to Data Aggregation in Support of Quality Measurement
Using multiple sources of collected data to inform measurement would reduce data fragmentation (or, different pieces of data regarding a single patient stored in many different places). Additionally, we are considering expanding and establishing policies and processes for data aggregation and measure calculation by third-party aggregators that include, but are not limited to, HIEs and clinical registries. Health IT vendors that meet the requirements of a Qualified Clinical Data Registries (QCDRs) and qualified registries that report quality measures for eligible clinicians in the MIPS program are potential examples[141] at section IV.A.3.g. of this proposed rule and can also support measure reporting.
We seek feedback on aggregation of data from multiple sources to inform measurement and potential policy considerations. We also seek feedback on the role data intermediaries can and should play in CMS quality measure reporting in collaboration with providers, and how we can best facilitate and enable aggregation.
(d) Potential Future Alignment of Measures Across Reporting Programs, Federal and State Agencies, and the Private Sector
We are committed to using policy levers and working with stakeholders to solve the issue of interoperable data exchange and to transition to full digital quality measurement. We are considering the future potential development and multi-staged implementation of a common portfolio of dQMs across our regulated programs, agencies, and private payers. This common portfolio would require alignment of: (1) Measure concepts and specifications including narrative statements, measure logic, and value sets; and (2) the individual data elements used to build these measure specifications and calculate the measure logic. Further, the required data elements would be limited to standardized, interoperable data elements to the fullest extent possible; hence, part of the alignment strategy will be the consideration and advancement of data standards and implementation guides for key data elements. We would coordinate closely with quality measure developers, federal and state agencies, and private payers to develop and to maintain a cohesive dQM portfolio that meets our programmatic requirements and that fully aligns across federal and state agencies and payers to the extent possible.
We intend for this coordination to be ongoing and allow for continuous refinement to ensure quality measures remain aligned with evolving healthcare practices and priorities (for example, PROs, disparities, and care coordination), and track with the transformation of data collection, alignment with health IT module updates including capabilities and standards adopted by ONC (for example, standards to enable APIs). It would focus on the quality domains of safety, timeliness, efficiency, effectiveness, equitability, and patient-centeredness. It would leverage several existing federal and public-private efforts including our Meaningful Measures 2.0 Framework; the Federal Electronic Health Record Modernization (Department of Defense and Veterans Affairs [DoD/VA]); the Agency for Healthcare Research and Quality's Clinical Decision Support Initiative; the Centers for Disease Control and Prevention's Adapting Clinical Guidelines for the Digital Age initiative; Core Quality Measure Collaborative, which convenes stakeholders from America's Health Insurance Plans (AHIP), CMS, National Quality Forum (NQF), provider organizations, private payers, and consumers and develops consensus on quality measures for provider specialties; and the NQF-convened Measure Applications Partnership (MAP), which recommends measures for use in public payment and reporting programs. We would coordinate with HL7's ongoing work to advance FHIR resources in critical areas to support patient care and measurement such as social determinants of health. Through this coordination, we would identify which existing measures could be used or evolved to be used as dQMs, in recognition of current healthcare practice and priorities.
This multi-stakeholder, joint federal, state, and industry effort, made possible and enabled by the pending advances towards true interoperability, would yield a significantly improved quality measurement enterprise. The success of the dQM portfolio would be enhanced by the degree to which the measures achieve our programmatic requirements for measures as well as the requirements of other agencies and payers.
We seek feedback on initial priority areas for the dQM portfolio given evolving interoperability requirements (for example, measurement areas, measure requirements, tools, and data standards). We also seek to identify opportunities to collaborate with other federal agencies, states, and the private sector to adopt standards and technology-driven solutions to address our quality measurement priorities across sectors.
(5) Solicitation of Comments
As noted previously, we seek input on the future development of the following:
(a) Definition of Digital Quality Measures
We are seeking feedback on the following as described in section IV.A.1.c. of this proposed rule:
- Do you have feedback on the dQM definition?
- Does this approach to defining and deploying dQMs to interface with FHIR-based APIs seem promising? We also welcome more specific comments on the attributes or functions to support such an approach of deploying dQMs.
(b) Use of FHIR for Current eCQMs
We are seeking feedback on the following as described in section IV.A.1.c. of this proposed rule:
- Do you agree that a transition to FHIR-based quality reporting can reduce burden on health IT vendors and providers? Please explain if you do not agree.
- Would access to near real-time quality measure scores benefit your practice? How so?
- What parts of the current CMS QRDA IGs cause the most burden (please explain the primary drivers of burden)? Start Printed Page 39344
- In what ways could a CMS FHIR Reporting IG be crafted to reduce burden on providers and vendors?
(c) Changes Under Consideration To Advance Digital Quality Measurement
Actions in Four Areas to Transition to Digital Quality Measures by 2025.
- We are seeking feedback on the following as described in section IV.A.1.c. of this proposed rule:
++ Do you agree with the goal of aligning data needed for quality measurement with interoperability requirements? What are the strengths and limitations of this approach? Are there specific FHIR Implementation Guides suggested for consideration?
++ How important is a data standardization approach that also supports inclusion of PGHD and other currently non-standardized data?
++ What are possible approaches for testing data quality and validity?
- We are seeking feedback on the following as described in section IV.A.1.c. of this proposed rule:
++ What functionalities, described in Section (4)(b) or others, should quality measure tools ideally have in the context of the pending availability of standardized and interoperable data (for example, standardized EHR data available via FHIR-based APIs)?
++ How would this more open, agile strategy for end-to-end measure calculation facilitate broader engagement in quality measure development, the use of tools developed for measurement for local quality improvement, and/or the application of quality tools for related purposes such as public health or research?
- We seek feedback on the following as described in section IV.A.1.c. of this proposed rule:
++ What are key policy considerations for aggregation of data from multiple sources being used to inform measurement?
++ What role can or should data aggregators play in CMS quality measure reporting in collaboration with providers? How can CMS best facilitate and enable aggregation?
- We seek feedback on the following as described in section IVA.1.c. of this proposed rule:
++ What are initial priority areas for the dQM portfolio given evolving interoperability requirements (for example, measurement areas, measure requirements, tools)?
++ We also seek to identify opportunities to collaborate with other federal agencies, states, and the private sector to adopt standards and technology-driven solutions to address our quality measurement priorities and across sectors.
Commenters should consider provisions in the CMS Interoperability and Patient Access final rule (85 FR 25510), CMS CY 2021 PFS final rule (85 FR 84472), and the ONC 21st Century Cures Act final rule (85 FR 25642).
We plan to continue working with other agencies and stakeholders to coordinate and to inform any potential transition to dQMs by 2025. While we will not be responding to specific comments submitted in response to this Request for Information in the CY 2022 PFS final rule, we will actively consider all input as we develop future regulatory proposals or future subregulatory policy guidance. Any updates to specific program requirements related to quality measurement and reporting provisions would be addressed through separate and future notice-and-comment rulemaking, as necessary.
d. Closing the Health Equity Gap in CMS Clinician Quality Programs—Request for Information (RFI)
Persistent inequities in health care outcomes exist in the United States, including among Medicare patients.[142] In recognition of persistent health disparities and the importance of closing the health equity gap, we request information on revising several related CMS programs to make reporting of health disparities based on social risk factors and race and ethnicity more comprehensive and actionable for hospitals, providers, and patients. The following is part of an ongoing effort across CMS to evaluate appropriate initiatives to reduce health disparities. Feedback will be used to inform the creation of a future, comprehensive, RFI focused on closing the health equity gap in CMS programs and policies (86 FR 25554 through 255561).
Belonging to a racial or ethnic minority group; living with a disability; being a member of the lesbian, gay, bisexual, transgender, and queer (LGBTQ+) community; living in a rural area; or being near or below the poverty level, is often associated with worse health outcomes.[143 144 145 146 147 148 149 150] Such disparities in health outcomes are the result of number of factors, but importantly for CMS programs, although not the sole determinant, poor access and provision of lower quality health care contribute to health disparities. For instance, numerous studies have shown among Medicare beneficiaries, racial and ethnic minority individuals often receive lower quality of care, report lower experiences of care, and experience more frequent hospital readmissions and procedural complications.[151 152 153 154 155 156]
We are committed to achieving equity in health care outcomes for Medicare beneficiaries by supporting providers in quality improvement activities to reduce health inequities, enabling them to make more informed decisions, and promoting provider accountability for health care disparities.[157] For the purposes of this rule, we are using a definition of equity established in Executive Order 13985, issued on January 25, 2021, as "the consistent and Start Printed Page 39345 systematic fair, just, and impartial treatment of all individuals, including individuals who belong to underserved communities who have been denied such treatment, such as Black, Latino, and Indigenous and Native American persons, Asian Americans and Pacific Islanders and other persons of color; members of religious minorities; lesbian, gay, bisexual, transgender, and queer (LGBTQ+) persons; persons with disabilities; persons who live in rural areas; and persons otherwise adversely affected by persistent poverty or inequality."[158] We note this definition was recently established and provides a useful, common definition for equity across different areas of government, although numerous other definitions of equity exist.
Our ongoing commitment to closing the equity gap in CMS quality programs is demonstrated by a portfolio of programs aimed at making information on the quality of health care providers and services, including disparities, more transparent to consumers and providers. The CMS Equity Plan for Improving Quality in Medicare outlines a path to equity which aims to support Quality Improvement Network Quality Improvement Organizations (QIN-QIOs); federal, state, local, and tribal organizations; providers; researchers; policymakers; beneficiaries and their families; and other stakeholders in activities to achieve health equity.[159] The CMS Equity Plan for Improving Quality in Medicare focuses on three core priority areas which inform our policies and programs: (1) Increasing understanding and awareness of health disparities; (2) developing and disseminating solutions to achieve health equity; and (3) implementing sustainable actions to achieve health equity.[160] The CMS Quality Strategy[161] and Meaningful Measures Framework[162] also include elimination of racial and ethnic disparities as central principles. Our efforts aimed at closing the health equity gap to date have included providing transparency of health disparities, supporting providers with evidence-informed solutions to achieve health equity, and reporting to providers on gaps in quality as follows:
- The CMS Mapping Medicare Disparities Tool which is an interactive map which identifies areas of disparities and is a starting point to understand and investigate geographic, racial and ethnic differences in health outcomes for Medicare patients.[163]
- The Racial, Ethnic, and Gender Disparities in Health Care in Medicare Advantage Stratified Report, which highlights racial and ethnic differences in health care experiences and clinical care, compares quality of care for women and men, and looks at racial and ethnic differences in quality of care among women and men separately for Medicare Advantage plans.[164]
- The Rural-Urban Disparities in Health Care in Medicare Report which details rural-urban differences in health care experiences and clinical care.[165]
- The Standardized Patient Assessment Data Elements for certain post-acute care Quality Reporting Programs, which now includes data reporting for race and ethnicity and preferred language, in addition to screening questions for social needs (84 FR 42536 through 42588).
- The CMS Innovation Center's Accountable Health Communities Model which includes standardized collection of health-related social needs data.
- The Guide to Reducing Disparities which provides an overview of key issues related to disparities in readmissions and reviews set of activities which can help hospital leaders reduce readmissions in diverse populations.[166]
- The CMS Disparity Methods which provide hospital-level confidential results stratified by dual eligibility for condition-specific readmission measures currently included in the Hospital Readmissions Reduction Program (see 84 FR 42496 through 42500 for a discussion of using stratified data in additional measures).
These programs are informed by reports by the National Academies of Science, Engineering and Medicine (NASEM)[167] and the Office of the Assistant Secretary for Planning and Evaluation (ASPE)[168] which have examined the influence of social risk factors on several of our quality programs. In this RFI, we discuss initiatives specific to further bridging the health equity gap within the MIPS track of the Quality Payment Program.
In Appendix 2: Improvement Activities of this proposed rule, we discuss a proposed improvement activity titled "create and implement an anti-racism plan". This improvement activity acknowledges it is insufficient to gather and analyze data by race, and document disparities by different population groups. Rather, it emphasizes systemic racism is the root cause for differences in health outcomes between socially defined racial groups. Further, we also propose to modify five existing improvement activities to address health equity. We note that some improvement activities within our current Inventory already aim to improve equity. We believe further modifying them can more explicitly link the activity to health equity without changing the core activity. In other cases, our proposals to modify an activity fundamentally shifts the activity to focus on health equity specifically.
Additionally, in section IV.A.3.e.(2) of this proposed rule, we are proposing to update the complex patient bonus formula. We specifically refer to ASPE's second report, Social Risk and Performance in Medicare's Value-Based Purchasing Programs, which was publicly-released in May 2020.[169] The second report builds on the analyses included in the initial report and provides additional insight for addressing risk factors in MIPS and other value-based payment programs. More specifically, the report has a 3- Start Printed Page 39346 pronged strategy approach to: Measure and report quality; set high, fair quality standards; and reward and support better outcomes for beneficiaries with social risk. As a part of this 3-pronged strategy, the report supports use of the complex patient bonus in MIPS, explaining that it is well supported because this policy gives additional points to clinicians with a higher share of medically and socially complex patients and does not lower the standard of care. Hence, although, ASPE's reports to Congress support the use of a complex patient bonus at the final score level, we respond to other findings reported in other literature studies by identifying ways to make the complex patient bonus more targeted for clinicians caring for high risk and complex patients and to mitigate differences in resources that affect MIPS scores. Hence, the proposed formula is based on standardized scores and to reward only those clinicians who fall in higher quintiles in order to focus the bonus on those serving a higher proportion of more complex and vulnerable patients.
Lastly, we acknowledge that small practices within the MIPS program often face challenges in many ways. More specifically, as noted in section IV.A.3.e.(2) of this proposed rule, the Quality Payment Program gives an advantage to large organizations because such organizations have more resources invested in the infrastructure required to track and report measures to MIPS (82 FR 53776). In response to the feedback on the potential burden on small practices, we have established special policies available for small practices including the small practice bonus and special scoring policies. For example, in the CY 2018 QPP final rule (82 FR 53682 through 53683), we established a significant hardship exception for small practices for the Promoting Interoperability performance category. To further alleviate the burden on small practices and reduce this disparity between large and small practices, we propose in section IV.A.3.d.(4) to automatically redistribute the Promoting Interoperability performance category weight for any small practice that does not submit data for the performance category, and in section IV.A.3.e.(2), we propose different redistribution weights for small practices.
We are committed to advancing health equity by improving data collection to better measure and analyze disparities across programs and policies.[170] We have been considering, among other things, expanding our efforts to provide stratified data for additional social risk factors and measures, optimizing the ease-of-use of the results, enhancing public transparency of equity results, and building towards provider accountability for health equity. We are seeking public comment on two potential future expansions of the CMS Disparity Methods, including: (1) Future potential stratification of quality measure results by race and ethnicity, and (2) improving demographic data collection.
(1) Future Potential Stratification of Quality Measure Results by Race and Ethnicity
The Administration's Executive Order on Advancing Racial Equity and Support for Underserved Communities Through the Federal Government directs agencies to assess potential barriers that underserved communities and individuals may face to enrollment in and access to benefits and services in federal programs. As summarized previously, studies have shown that among Medicare beneficiaries, racial and ethnic minority persons often experience worse health outcomes, including more frequent hospital readmissions and procedural complications. We are considering expanding the disparity methods to include stratification of the condition/procedure-specific readmission measures by race and ethnicity. The 1997 Office of Management and Budget (OMB) Revisions to the Standards for the Collection of Federal Data on Race and Ethnicity, outlines the racial and ethnic categories which may potentially be used for reporting the disparity methods, which we note are intended to be considered as social and cultural, and not biological or genetic.[171] The 1997 OMB Standard lists five minimum categories of race: (1) American Indian or Alaska Native; (2) Asian; (3) Black or African American; (4) Native Hawaiian or Other Pacific Islander; (5) and White. In the OMB standards, Hispanic or Latino is the only ethnicity category included, and since race and ethnicity are two separate and distinct concepts, persons who report themselves as Hispanic or Latino can be of any race.[172] Another example, the "Race & Ethnicity—CDC" code system in PHIN Vocabulary Access and Distribution System (VADS)[173] permits a much more granular structured recording of a patient's race and ethnicity with its inclusion of over 900 concepts for race and ethnicity. The recording and exchange of patient race and ethnicity at such a granular level can facilitate the accurate identification and analysis of health disparities based on race and ethnicity. Further, the "Race & Ethnicity—CDC" code system has a hierarchy that rolls up to the OMB minimum categories for race and ethnicity and, thus, supports aggregation and reporting using the OMB standard. ONC includes both the CDC and OMB standards in its criterion for certified health IT products.[174] For race and ethnicity, a certified health IT product must be able to express both detailed races and ethnicities using any of the 900 plus concepts in the "Race & Ethnicity—CDC" code system in the Public Health Information Network (PHIN) Vocabulary Access and Distribution Systems (VADS), as well as aggregate each one of a patient's races and ethnicities to the categories in the OMB standard for race and ethnicity. This approach can reduce burden on providers recording demographics using certified products.
Self-reported race and ethnicity data are the gold standard for classifying an individual according to race or ethnicity. However, CMS currently does not consistently collect self-reported race and ethnicity for the Medicare program, but instead gets the data from the Social Security Administration (SSA) and the data accuracy and comprehensiveness have proven challenging despite capabilities in the marketplace via certified health IT products. Historical inaccuracies in federal data systems and limited collection classifications have also contributed to the limited quality of race and ethnicity information in our administrative data systems.[175] In recent decades, to address these data quality Start Printed Page 39347 issues, we have undertaken numerous initiatives, including updating data taxonomies and conducting direct mailings to some beneficiaries to enable more comprehensive racial and ethnic identification.[176 177] Despite those efforts, studies reveal varying data accuracy in identification of racial and ethnic groups in Medicare administrative data, with higher sensitivity for correctly identifying white and Black individuals, and lower sensitivity for correctly identifying individuals of Hispanic ethnicity or of Asian/Pacific Islander (API) and American Indian/Alaskan Native race.[178] Incorrectly classified race or ethnicity may result in overestimation or underestimation in the quality of care received by certain groups of beneficiaries.
We continue to work with public and private partners to better collect and leverage data on social risk to improve our understanding of how these factors can be better measured in order to close the health equity gap. Among other things, we have developed an Inventory of Resources for Standardized Demographic and Language Data Collection[179] and supported collection of specialized International Classification of Disease, 10th Edition, Clinical Modification (ICD-10-CM) codes for describing the socioeconomic, cultural, and environmental determinants of health, and sponsored several initiatives to statistically estimate race and ethnicity information when it is absent.[180] The Office of the National Coordinator for Health Information Technology (ONC) included social, psychological, and behavioral standards in the 2015 Edition health information technology certification criteria (2015 Edition), providing interoperability standards (LOINC [Logical Observation Identifiers Names and Codes] and SNOMED CT [Systematized Nomenclature of Medicine—Clinical Terms]) for financial strain, education, social connection and isolation, and others. Additional stakeholder efforts underway to expand capabilities to capture additional social determinants of health data elements include the Gravity Project to identify and harmonize social risk factor data for interoperable electronic health information exchange for EHR fields, as well as proposals to expand the ICD-10 (International Classification of Diseases, Tenth Revision) z-codes, the alphanumeric codes used worldwide to represent diagnoses.[181]
While development of sustainable and consistent programs to collect data on social determinants of health can be considerable undertakings, we recognize that another method to identify better race and ethnicity data is needed in the short term to address the need for reporting on health equity. In working with our contractors, two algorithms have been developed to indirectly estimate the race and ethnicity of Medicare beneficiaries (as described further in the next section). We believe that using indirect estimation can help to overcome the current limitations of demographic information and enable timelier reporting of equity results until longer term collaborations to improve demographic data quality across the health care sector materialize. The use of indirect estimated race and ethnicity for conducting stratified reporting does not place any additional collection or reporting burdens on hospitals as these data are derived using existing administrative and census-linked data.
Indirect estimation relies on a statistical imputation method for inferring a missing variable or improving an imperfect administrative variable using a related set of information that is more readily available.[182] Indirectly estimated data are most commonly used at the population level (such as the hospital or health plan-level) where aggregated results form a more accurate description of the population than existing, imperfect data sets. These methods often estimate race and ethnicity using a combination of other data sources which are predictive of self-identified race and ethnicity, such as language preference, information about race and ethnicity in our administrative records, first and last names matched to validated lists of names correlated to specific national origin groups, and the racial and ethnic composition of the surrounding neighborhood. Indirect estimation has been used in other settings to support population-based equity measurement when self-identified data are not available.[183]
As described earlier, we previously supported the development of two such methods of indirect estimation of race and ethnicity among Medicare beneficiaries. One indirect estimation approach developed by our contractor uses Medicare administrative data, first name and surname matching, derived from the U.S. Census and other sources, with beneficiary language preference, state of residence, and the source of the race and ethnicity code in Medicare administrative data to reclassify some beneficiaries as Hispanic or API.[184] In recent years, we have also worked with another contractor to develop a new approach, the Medicare Bayesian Improved Surname Geocoding (MBISG), which combines Medicare administrative data, first and surname matching, geocoded residential address linked to the 2010 U.S. Census, and uses both Bayesian updating and multinomial logistic regression to estimate the probability of belonging to each of six racial/ethnic groups.[185]
The MBISG model is currently used to conduct the national, contract-level, stratified reporting of Medicare Part C and D performance data for Medicare Advantage Plans by race and Start Printed Page 39348 ethnicity.[186] Validation testing reveals concordance of 0.88-0.95 between indirectly estimated and self-report among individuals who identify as White, Black, Hispanic and API for the MIBSG version 2.0 and concordance with self-reported race and ethnicity of 0.96-0.99 for these same groups for MBISG version 2.1[187 188] The algorithms under consideration are considerably less accurate for individuals who self-identify as American Indian/Alaskan Native or multiracial.[189] Indirect estimation can be a statistically reliable approach for calculating population-level equity results for groups of individuals (such as the hospital-level) and is not intended, nor being considered, as an approach for inferring the race and ethnicity of an individual.
However, despite the high degree of statistical accuracy of the indirect estimation algorithms under consideration, there remains the small risk of unintentionally introducing measurement bias. For example, if the indirect estimation is not as accurate in correctly estimating race and ethnicity in certain geographies or populations it could lead to some bias in the method results. Such bias might result in slight overestimation or underestimation of the quality of care received by a given group. We feel this amount of bias is considerably less than would be expected if stratified reporting was conducted using the race and ethnicity currently contained in our administrative data. Indirect estimation of race and ethnicity is envisioned as an intermediate step, filling the pressing need for more accurate demographic information for the purposes of exploring inequities in service delivery, while allowing newer approaches, as described in the next section, for improving demographic data collection to progress. We are interested in learning more about, and soliciting comments about, the potential benefits and challenges associated with measuring hospital equity using an imputation algorithm to enhance existing administrative data quality for race and ethnicity until self-reported information is sufficiently available.
(2) Improving Demographic Data Collection
Currently self-reported race and ethnicity data are the gold standard for classifying an individual according to race or ethnicity. The CMS Quality Strategy outlines our commitment to strengthening infrastructure and data systems by ensuring that standardized demographic information is collected to identify disparities in health care delivery outcomes.[190] Collection and sharing of a standardized set of social, psychological, and behavioral data by clinicians, including race and ethnicity, using electronic data definitions which permit nationwide, interoperable health information exchange, can significantly enhance the accuracy and robustness of our equity reporting.[191] This could potentially include expansion to additional social factors, such as language preference and disability status, where accuracy of administrative data is currently limited. We are mindful that additional resources, including data collection and staff training may be necessary to ensure that conditions are created whereby all patients are comfortable answering all demographic questions, and that individual preferences for non-response are maintained.
We note that clinicians participating in the Medicare Promoting Interoperability Program must use certified EHR technology (CEHRT) that has been certified to the 2015 Edition of health IT certification criteria. As noted previously, the certification criterion for Demographics under the 2015 Edition (at 45 CFR 170.315(a)(5)) supports collection of data using both the OMB standards for collecting data on race and ethnicity as well as the more granular "Race & Ethnicity—CDC" standard. In the 2020 ONC 21st Century Cures Act final rule, ONC also adopted a new framework for the core data set which certified health IT products must exchange, called the United States Core Data for Interoperability (USCDI) (85 FR 25669). The USCDI incorporates the demographic data and associated code sets finalized for the 2015 Edition certification criteria.
As noted previously, ONC also finalized a certification criterion in the 2015 Edition which supports a certified health IT products ability to collect social, psychological, and behavioral data (at 45 CFR 170.315(a)(15)). However, this functionality is not included as part of the certified EHR technology required by the Promoting Interoperability performance category. While the technical functionality exists to achieve the gold standard of data collection, we understand challenges and barriers exist in using the technologies with these capabilities.
We are interested in learning about, and are soliciting comments on, current data collection practices by hospitals to capture demographic data elements (such as race, ethnicity, sex, sexual orientation and gender identity (SOGI), language preference, tribal membership, and disability status). Further, we are interested in potential challenges facing clinicians with collecting a minimum set of demographic data elements in alignment with national data collection standards (such as the standards finalized by the Affordable Care Act[192] ) and standards for interoperable exchange (such as the United States Core Data for Interoperability incorporated into certified health IT products as part of the 2015 Edition of health IT certification criteria[193] ). Advancing data interoperability through collection of a minimum set of demographic data collection, and incorporation of this demographic information into quality measure specifications, has the potential for improving the robustness of the disparity method results, potentially permitting reporting using more accurate, self-reported, information, such as race and ethnicity, and expanding reporting to additional dimensions of equity, including stratified reporting by disability status.
Therefore, based on our current and newly proposed policies, we seek comments on other efforts we can take within the MIPS program to further bridge the equity gap. We plan to continue working with ASPE, clinicians, the public, and other key stakeholders on this important issue to identify policy solutions to achieve the Start Printed Page 39349 goals of attaining health equity for all patients and minimizing unintended consequences. We look forward to receiving feedback on these topics and note for readers that responses to the RFI will not directly impact payment decisions. We also note our intention for additional RFI or rulemaking on this topic in the future. While we will not be responding to specific comments submitted in response to this Request for Information in the CY 2022 PFS final rule, we will actively consider all input as we develop future regulatory proposals or future subregulatory policy guidance.
2. Definitions
At § 414.1305, we are proposing definitions of the following terms:
- Collection type (revision).
- Meaningful EHR user for MIPS (revision).
- MIPS determination period (revision).
- MIPS eligible clinician (revision).
- Multispecialty group (addition).
- MVP Participant (addition).
- Population health measure (addition).
- QCDR measure (addition).
- Single specialty group (addition).
- Special status (addition).
- Subgroup (addition).
- Submission type (revision).
These terms and definitions are discussed in detail in the relevant sections of this proposed rule.
3. MIPS Program Details
a. MIPS Eligibility
(1) MIPS Eligible Clinician Definition
In the CY 2017 Quality Payment Program final rule (81 FR77040 through 77041), we defined a MIPS eligible clinician at § 414.1305, as identified by a unique billing TIN and NPI combination used to assess performance, as any of the following (excluding those identified at § 414.1310(b)): A physician (as defined in section 1861(r) of the Act), a PA, NP, and CNS (as such terms are defined in section 1861(aa)(5) of the Act), a certified registered nurse anesthetist (as defined in section 1861(bb)(2) of the Act), and a group that includes such clinicians. We established at § 414.1310(b) and (c) that the following are excluded from this definition per the statutory exclusions defined in section 1848(q)(1)(C)(ii) and (v) of the Act: (1) QPs; (2) Partial QPs who choose not to report on applicable measures and activities that are required to be reported under MIPS for any given performance period in a year; (3) low-volume threshold eligible clinicians; and (4) new Medicare-enrolled eligible clinicians. In accordance with sections 1848(q)(1)(A) and (q)(1)(C)(vi) of the Act, we established at § 414.1310(b)(2) that eligible clinicians (as defined at § 414.1305) who are not MIPS eligible clinicians have the option to voluntarily report measures and activities for MIPS. Additionally, we established at § 414.1310(d) that in no case will a MIPS payment adjustment apply to the items and services furnished during a year by eligible clinicians who are not MIPS eligible clinicians, as described in § 414.1310(b) and (c), including those who voluntarily report on applicable measures and activities specified under MIPS. In this proposed rule, we are proposing to amend § 414.1305 to revise the definition of a MIPS eligible clinician, as identified by a unique billing TIN and NPI combination used to assess performance, to include certified nurse-midwives (as defined in section 1861(gg)(2) of the Act) and clinical social workers (as defined in section 1861(hh)(1) of the Act).
Section 1848(q)(1)(C)(i)(II) of the Act provides the Secretary with discretion, beginning with the 2021 MIPS payment year, to specify additional eligible clinicians (as defined in section 1848(k)(3)(B) of the Act) as MIPS eligible clinicians. Such clinicians may include physical therapists, occupational therapists, or qualified speech-language pathologists; qualified audiologists (as defined in section 1861(ll)(3)(B) of the Act); certified nurse-midwives (as defined in section 1861(gg)(2) of the Act); clinical social workers (as defined in section 1861(hh)(1) of the Act); clinical psychologists (as defined by the Secretary for purposes of section 1861(ii) of the Act); and registered dietitians or nutrition professionals. Therefore, in the CY 2019 PFS proposed rule (83 FR 35883 through 35884), we proposed to amend § 414.1305 to revise the definition of a MIPS eligible clinician, as identified by a unique billing TIN and NPI combination used to assess performance, to mean any of the following (excluding those identified at § 414.1310(b)): A physician (as defined in section 1861(r) of the Act); a PA, NP, CNS (as such terms are defined in section 1861(aa)(5) of the Act); a certified registered nurse anesthetist (as defined in section 1861(bb)(2) of the Act); beginning with the 2021 MIPS payment year, a physical therapist, occupational therapist, clinical social worker (as defined in section 1861(hh)(1) of the Act), and clinical psychologist (as defined by the Secretary for purposes of section 1861(ii) of the Act. In addition, we requested comments on specifying qualified speech-language pathologists, qualified audiologists, certified nurse-midwives, and registered dietitians or nutrition professionals as MIPS eligible clinicians beginning with the 2021 MIPS payment year.
After consideration of comments, we received we finalized to revise our proposal in the CY 2019 PFS final rule (83 FR 59722 through 59727) and amend § 414.1305 to revise the definition of a MIPS eligible clinician, as identified by a unique billing TIN and NPI combination used to assess performance, to mean any of the following (excluding those identified at § 414.1310(b)): A physician (as defined in section 1861(r) of the Act); a PA, NP, CNS (as such terms are defined in section 1861(aa)(5) of the Act); a certified registered nurse anesthetist (as defined in section 1861(bb)(2) of the Act); beginning with the 2021 MIPS payment year, a physical therapist, occupational therapist, qualified speech-language pathologist; qualified audiologist (as defined in section 1861(ll)(3)(B) of the Act); clinical psychologist (as defined by the Secretary for purposes of section 1861(ii) of the Act); and registered dietician or nutrition professional; and a group that includes such clinicians.
In this proposed rule, we are proposing to amend § 414.1305 to revise the definition of a MIPS eligible clinician, as identified by a unique billing TIN and NPI combination used to assess performance, to include certified nurse-midwives (as defined in section 1861(gg)(2) of the Act) and clinical social workers (as defined in section 1861(hh)(1) of the Act). The new definition would mean any of the following (excluding those identified at § 414.1310(b)): A physician (as defined in section 1861(r) of the Act); a PA, NP, CNS (as such terms are defined in section 1861(aa)(5) of the Act); a certified registered nurse anesthetist (as defined in section 1861(bb)(2) of the Act); beginning with the 2021 through 2023 MIPS payment years, a physical therapist, occupational therapist, qualified speech-language pathologist; qualified audiologist (as defined in section 1861(ll)(3)(B) of the Act); clinical psychologist (as defined by the Secretary for purposes of section 1861(ii) of the Act); and registered dietician or nutrition professional; beginning with the 2024 MIPS payment year, certified nurse-midwives (as defined in section 1861(gg)(2) of the Act); clinical social workers (as defined in section 1861(hh)(1) of the Act); and a group that includes such clinicians. Start Printed Page 39350
In order to assess whether these additional eligible clinicians (certified nurse-midwives and clinical social workers) could successfully participate in MIPS, we evaluated whether there would be sufficient measures and activities applicable and available for each of the additional eligible clinician types. We finalized in the CY 2018 Quality Payment Program final rule (82 FR 53780), that having sufficient measures for the quality performance category, means having sufficient measures applicable and available that we can calculate a quality performance category percent score for the MIPS eligible clinician because at least one quality measure is applicable and available to the clinician. For the improvement activities performance category, we believe that all MIPS eligible clinicians will have sufficient activities applicable and available, as they are broadly applicable. We focused our analysis on the quality and improvement activities performance categories because these performance categories require submission of data. For the Promoting Interoperability performance category, we do not believe that clinical social workers would have sufficient and available measures available. We refer readers to section IV.A.3.d.(4)(h) of this proposed rule, where we are proposing a policy to automatically assign a zero percent weighting for the Promoting Interoperability performance category for the clinical social workers. However, for the certified nurse-midwives we do believe they would have sufficient and available measures as many of them have participated in the Medicaid EHR Incentive Program and have experience with the adoption or use of CEHRT (81 FR 77243). Therefore, the certified nurse-midwives score would not be reweighted for the Promoting Interoperability performance category. However, it should be noted that if a clinician believes they are under undue hardship they may apply for a Hardship Application. We did not focus as part of our analysis on the cost performance category because we are only able to assess cost performance for a subset of eligible clinicians—specifically, those who are currently eligible as a result of not meeting any of the current exclusion criteria. We do not believe there are cost measures that would apply to the care that clinical social workers or certified nurse-midwives tend to provide. The current set of episode-based measures in the cost performance category focuses on a range of acute inpatient medical conditions and procedures, and the two population-based cost measures assess inpatient and primary care. Therefore, we anticipate the cost category would be reweighted for the majority of these clinician types. The impact of the cost performance category for these additional eligible clinicians would continue to be considered but is currently not a decisive factor for successful participation in MIPS. From our analysis, we found that improvement activities would generally be applicable and available for each of the additional eligible clinician types. For the quality performance category, we found that the additional eligible clinician types would have sufficient MIPS quality measures applicable and available. Since the CY 2019 PFS final rule, we have increased the quality measures that we believe are applicable to clinical social workers to 15 quality measures, which includes 2 outcome measures and 8 high priority measures. In the CY 2021 PFS final rule (85 FR 85069 through 85071), we finalized a Clinical Social Worker Specialty Measure Set. For certified nurse-midwives we believe there are 7 quality measures which includes 2 outcome measures and 5 high priority measures available for reporting in performance year 2022. In Appendix 1, Table Group BA of this proposed rule, we are proposing a Certified Nurse-Midwives Specialty Set. In addition, we received correspondence from the clinical social workers national associations requesting to be included in MIPS. Finally, amending the definition of a MIPS eligible clinician to include clinical social workers and certified nurse-midwives would align with § 414.1305 definition of an eligible clinician utilized by MIPS APMs for eligibility determinations.
We request comments on our proposal to amend § 414.1305 to modify the definition of a MIPS eligible clinician, as identified by a unique billing TIN and NPI combination used to assess performance, to mean any of following (excluding those identified at § 414.1310(b)): For the 2019 and 2020 MIPS payment years, a physician (as defined in section 1861(r) of the Act); a PA, NP, or CNS (as such terms are defined in section 1861(aa)(5) of the Act); a certified registered nurse anesthetist (as defined in section 1861(bb)(2) of the Act); for the 2021 through 2023 MIPS payment years, a physical therapist, occupational therapist, qualified speech-language pathologist; qualified audiologist (as defined in section 1861(ll)(3)(B) of the Act); clinical psychologist (as defined by the Secretary for purposes of section 1861(ii) of the Act); and registered dietician or nutrition professional; for the 2024 MIPS payment year and future years, a certified nurse-midwives (as defined in section 1861(gg)(2) of the Act); clinical social workers (as defined in section 1861(hh)(1) of the Act); and a group that includes such clinicians.
(2) MIPS Performance Period
In the CY 2019 PFS final rule (83 FR 59745 through 59747) we finalized to amend § 414.1320(d)(1) that for purposes of the 2022 MIPS payment year and future years, the performance period for the quality and cost performance categories would be the full calendar year (January 1 through December 31) that occurs 2 years prior to the applicable MIPS payment year. In addition, we finalized at § 414.1320(d)(2) that for purposes of the 2022 MIPS payment year and future years, the performance period for the improvement activities performance category would be a minimum of a continuous 90-day period within the calendar year that occurs 2 years prior to the applicable MIPS payment year, up to and including the full calendar year.
In the CY 2021 PFS final rule (85 FR 84873), we finalized the performance period for the quality and cost performance categories at § 414.1320(d)(1) as follows: Beginning with the 2023 MIPS payment year, the performance period for the quality and cost performance categories is the full calendar year (January 1 through December 31) that occurs 2 years prior to the applicable MIPS payment year, except as otherwise specified for administrative claims-based measures in the MIPS final list of quality measures described in § 414.1330(a)(1). However, the quality, cost, and improvement activities performance period for the 2022 MIPS payment year, formerly at § 414.1320(d), was inadvertently deleted, and the amended language regarding administrative claims measures was not expressly retroactive. We recognize that the application of this policy for the 2020 MIPS performance period would be retroactive. To the extent that the application of this policy for the 2020 MIPS performance period would be retroactive, section 1871(e)(1)(A)(ii) of the Act provides for retroactive application of a substantive change to an existing policy when the Secretary determines that failure to apply the policy change retroactively would be contrary to the public interest. We believe that failure to reinstate the inadvertently deleted language retroactively would be contrary to the public interest because the performance period establishes the timespan for the Start Printed Page 39351 collection of performance data, assessment of performance, and computation of the MIPS payment adjustment, to which clinicians have already committed valuable time and resources. In addition, many of the MIPS policies such as the MIPS determination period and the low-volume threshold determinations utilize the performance period as an integral part of the policy, without which we would be unable to operate the MIPS program as required by statute. Therefore, we are requesting comments on our technical amendment to reinstate the inadvertently deleted language, with a modification to state "For purposes of . . ." rather than "Beginning with . . .". The proposed text would state, for purposes of the 2022 MIPS payment year, the performance period for: (1) The quality and cost performance categories is the full calendar year (January 1 through December 31) that occurs 2 years prior to the applicable MIPS payment year; and (2) The improvement activities performance categories is a minimum of a continuous 90-day period within the calendar year that occurs 2 years prior to the applicable MIPS payment year, up to and including the full calendar year. Lastly, we propose to redesignate current § 414.1320(d) through (g) to § 414.1320(e) through (h), respectively.
(3) Modifications to Small Practice Groups Reporting Medicare Part B Claims Measures
In the CY 2019 PFS final rule (83 FR 59753), we established that beginning with the 2019 performance period, Medicare Part B Claims would be an available collection type and submission type for the quality performance category for small practices reporting as individuals or a group. We also stated that in circumstances where only Medicare Part B claims were submitted, that we intended on calculating the quality performance category for the practice as both a group and as individuals and apply the quality performance category score that is the greater of the two. We considered requiring an election for assessment as a group but believed this would be unduly burdensome on small practices (83 FR 59752).
Although we stated we would take the highest of the individual or group score for MIPS eligible clinicians in small practices, we now recognize that this policy has had an unintended impact for clinicians in a small practice who did not submit Medicare Part B quality claims and would not otherwise be eligible for MIPS. Once we receive a Medicare Part B submission, both an individual score and a group score is created. Once a group score is created, a clinician who was individually excluded from MIPS for being under the low-volume threshold, may now be eligible if the group exceeds the low-volume threshold. These clinicians would receive the MIPS final score based on the Medicare Part B submissions, even if the group did not intend to report to MIPS as a group. While we still perform an analysis to only provide to the clinicians the highest final score available, clinicians who are only MIPS eligible by the act of exceeding the low volume threshold as a group are receiving final scores that are unintended. This issue will continue to be further exacerbated as the performance threshold continues to increase, so does the likelihood that a final score from the quality performance category alone (or quality and cost as cost does not have submission requirements) could be below the performance threshold for a group. We therefore now believe it is important for the group to clearly signal its intention to report to MIPS as a group before we expand potential eligibility to other members of the group.
We have existing policies under MIPS that require clinicians to indicate to us when to utilize a group submission. For example, in the CY 2019 PFS final rule (83 FR 59862), we stated that submission of data on improvement activities or Promoting Interoperability measures would indicate that the clinicians in that group wanted to be scored as a group for the purposes of facility-based measurement. Therefore, we believe a similar policy would be appropriate for small practices to indicate they wish to submit Medicare Part B claims for a group quality performance category score. We are proposing that starting with the CY2022 MIPS performance period/2024 MIPS payment year, small practices, excluding those participating in MIPS as part of a virtual group, must submit data as a group in any performance category to indicate that they wish to be scored as a group for Medicare Part B claims. This means a group would need to submit data as a group to the improvement activities, Promoting Interoperability performance categories, or to the quality performance category via another submission mechanism as a group (for example, a group that submits MIPS CQMs in addition to Medicare Part B claims data). Once the group submits data to MIPS as a group, we would consider any available Medicare Part B claims measure submissions in calculating their quality performance category score.
We believe using the choice to submit data as a group would indicate the group's intention to participate and be measured as a group. The proposal would preserve and respect the choices made by clinicians and groups by not inadvertently expanding eligibility unwittingly to other clinicians. We note that this proposal would not apply to small practices participating in MIPS as part of a virtual group, because clinicians signal their intent to be scored as a virtual group through the virtual group election process.
We request public comments on our proposal.
b. Transforming MIPS: MIPS Value Pathways
(1) Overview
We are moving to MIPS Value Pathways (MVPs) to improve value, reduce burden, inform patient choice in selecting clinicians, and reduce barriers to facilitate movement into APMs (84 FR 40732 through 40734 and 85 FR 84844 through 84845). We intend to promote high value care by paying for health care services by linking performance on cost, quality, and the patient's experience of care. The MVP framework will move MIPS forward on the path to value by connecting the MIPS performance categories, better informing and empowering patients to make decisions about their healthcare, and by helping clinicians to achieve better outcomes using robust and accessible healthcare data and interoperability.
Stakeholders have supported the MVP framework and our MVP guiding principles, which aim to reduce complexity and burden, move towards more meaningful measurement, capture the patient voice, and move to higher value care (84 FR 62946 and 85 FR 84845). We believe MVP reporting will reduce selection burden with choosing MIPS quality measures and improvement activities to submit; reduce reporting burden by requiring submission of fewer MIPS quality measures than the traditional MIPS participation method; and further align across performance categories the measures and activities identified by specialists and patients as being meaningful and relevant. We believe MVPs developed in coordination with stakeholders with an established process in which clinician and patient perspectives are incorporated (85 FR 84850) can result in more meaningful performance data, reduced complexity Start Printed Page 39352 of the MIPS program, and lowered clinician burden to participate.
MVPs will make MIPS more meaningful by allowing a more cohesive participation experience; by standardizing performance measurement of a specialty, medical condition, or episode of care; and reducing the siloed nature of the traditional MIPS participation experience. We intend for MVPs to drive value and help clinicians and practices prepare to take on and manage financial risk, as in Advanced APMs, as they build out their quality infrastructure components (measurement tracking, performance improvement processes, interoperability and data information systems) that align with the MIPS performance categories and gain experience with cost measurement (84 FR 40733). Performance measure reporting for specific populations, such as in MVPs, encourages practices to build an infrastructure with capabilities to compile and analyze population health data, a critical capability in assuming and managing risk. The experience with MVPs, in which there is aligned measurement of quality (of care and experience of care) and cost, continuous improvement/innovation within the practice, and efficient management and transfers of information will help clinicians deliver higher value care and remove barriers to APM participation. Combining linked performance measures and activities with more standardization of measures in MVPs will produce data that can better assist patients in comparing clinician performance and selecting clinicians from which to seek care.
Further, MVPs will provide multispecialty groups a way to report performance information which is meaningful to various specialties and teams within the group through the proposed future subgroup reporting option discussed in section IV.A.3.b.(3) of this proposed rule. Multispecialty groups, especially those groups with many specialties and clinicians, often provide an array of services that may not be captured in a single set of measures or in a single MVP. Subgroup reporting would allow increased comprehensiveness of multispecialty group performance data as more services, including specialty services, can be represented since a group and subgroup may be able to report more than one MVP. The subgroup performance data would provide more detailed and clinically relevant information than the group reporting option available under traditional MIPS. The subgroup performance data would also assist patients in selecting clinicians because the data would be more relevant and specific to the care provided by clinicians in the subgroup. We refer readers to section IV.A.3.b.(3) of this proposed rule for our proposals on MVP subgroup reporting.
Under traditional MIPS, we understand clinicians and their administrative staff spend time and resources sifting through large inventories of measures and activities and depending upon how their practice decides to participate, may ultimately submit unrelated measures and activities. While we have attempted to streamline this process through the development of quality specialty sets and user-friendly tools on our website (see measure selection tool at https://qpp.cms.gov/mips/explore-measures?tab=qualityMeasures&py=2020#measures), we still hear concerns from stakeholders about not having relevant measures and activities (January 7, 2021 Town Hall (85 FR 74729 through 74730) feedback). MVPs are being developed to focus on a given specialty, condition, and/or episode of care (85 FR 84851). As more MVPs become available, we intend to continue to offer tools to clinicians that meet the real needs of the users, based on human-centered design considerations[194] that will be helpful as they select and submit MVPs, such as including MVPs in the MIPS shopping cart. MVPs would also bring the opportunity for clinicians to participate as subgroups, which would address the issue that exists under traditional MIPS of limited multispecialty group performance reporting. Subgroups formed within a multispecialty group would be able to submit performance measures and activities that are specific to their services and the associated patient conditions or health priorities. Additionally, clinician performance data, which is more meaningful to the services provided by subgroup participants, will be more readily available to be used by clinicians to improve quality and value of their services. As more clinicians have applicable MVPs, the performance data available to patients will expand, and in the future, information for specialists in multispecialty groups will become more available on our Compare Tools, enabling patients to make more informed choices for their care.
We continue our efforts to improve the healthcare of Medicare patients by allowing clinicians to focus on providing care for their patients and report on measures and activities that best reflect their care. As we propose MVP implementation policies, we are considering the critical factors that will contribute to and demonstrate MVP success and the characteristics of the overall MVP portfolio. We look forward to continuing to work with stakeholders to improve the program and implement the vision of MVPs.
(2) MVP Framework and Implementation Considerations
As discussed in the CY 2020 PFS proposed and CY 2021 PFS final rules (84 FR 40732 through 40734, 85 FR 50279 and 85 FR 84844 through 84845 respectively), our MVP framework calls for linking the MIPS quality, cost, and improvement activities performance categories with a foundation of the Promoting Interoperability and population health claims-based measures. We are considering how to best implement an MVP portfolio that balances our MVP goals for transformative change and our five MVP guiding principles (85 FR 84845) within current capabilities. We note there are constraints related to the ability to implement significant program changes including statutory restrictions on the structure of MIPS, and limitations of the current quality and cost measure inventory.
(a) MVP Transition
Stakeholders have urged us to allow sufficient transition time for MVP implementation (85 FR 84859). We recognize that the complete transition to MVPs should account for clinicians' readiness for change, the current state of measure development, CMS's operational limitations, and stakeholder capacity for developing and implementing MVPs. We refer readers to our 2022 PFS Proposed Rule Timeline: Transition from Traditional MIPS to MVPs graphic at https://qpp-cm-prod-content.s3.amazonaws.com/uploads/1501/2022%20PFS%20Proposed%20Rule_MVP%20Timeline%20Graphic.pdf and section IV.A.3.b.(2)(d) of this proposed rule for discussion of our MVP transition timeline which outlines our response to stakeholder concerns about adequate transition time and our plans for a gradual incremental transition to MVPs.
Stakeholders have largely supported our MVP goals, but a few have voiced concerns regarding whether our goals to drive value, reduce burden, and derive comparative data can be achieved via the MVP framework (85 FR 84845 through 84847). We have received comments stating MVPs should utilize more innovative approaches (85 FR Start Printed Page 39353 84850). Regarding utilization of innovative approaches, we note that the statutory requirements at section 1848 of the Act may constrain our ability to adopt certain changes. These requirements include but are not limited to: The use of four MIPS performance categories (quality, cost, improvement activities and Promoting Interoperability); setting the performance threshold; the call for measures and annual quality measure selection process; and the prescribed performance category weights. Conversely, the statute does provide limited flexibilities in some other areas, so we are interested in exploring any existing MIPS flexibilities that will assist us in implementing MVPs. As we begin MVP implementation, the portfolio of MVPs will be developed with a focus on our end goals while adhering to statutory requirements.
We request public comment on innovative ideas that can help achieve our desired MVP results. MVPs aim to improve value, reduce burden, help patients compare clinician performance to inform patient choice in selecting clinicians, and reduce barriers to movement into APMs.
Additional performance measures that support targeted MVP clinical areas may be needed to develop MVPs for all clinicians and to ensure they have meaningful measures, which include the patient perspective, care outcomes, and to support linkages between cost and quality. In section IV.A.3.d.(2)(c) of this proposed rule, we seek comment on challenges that stakeholders may encounter, and ways to ensure that stakeholder-developed cost measures meet certain standards and are consistent with the goals of MIPS and MVPs. Additionally, in section IV.A.1.(d) of this proposed rule, we discuss our request for information on closing the health equity gap in CMS clinician quality programs, potential future stratification of quality measures, and request comments on other efforts we can take within the MIPS program to further bridge the health equity gap.
While we aim to shift towards the ideal MVP state, we have data submission limitations slowing our ability to reach our objective of reporting burden reduction. Ultimately, we envision that the future goal of the Quality Payment Program, particularly with MIPS and MVPs, is to ensure there is more granular data available for patients, clinicians, and other stakeholders. We envision an end state where technology will allow for the submission of discrete data elements. CMS will be able to calculate measure performance for clinicians, subgroups, and groups, rather than having measure performance aggregated and calculated at a group or subgroup level prior to reporting. We anticipate more granular data will be available for patients, clinicians, and other stakeholders through an approach of future mandatory subgroup reporting (as discussed in section IV.A.3.b.(3)(h) of this proposed rule). We also look forward to broad use of standards-based APIs that leverage the FHIR standard within EHRs (as discussed in section IV.A.3.d.(4)(j)(i) of this proposed rule) and the creation and use of dQMs (as discussed in section IV.A.1.c.(5) of this proposed rule). See section IV.A.1.c. of this proposed rule for our Advancing to Digital Quality Measurement and the Use of Fast Healthcare Interoperability Resources (FHIR®) in Physician Quality Programs RFI, which addresses: (1) A refined definition of digital quality measures, (2) the use of FHIR® for eCQMs, and (3) changes under consideration to advance digital quality measurement with the intent of transitioning to digital quality measures by 2025.
We held a Town Hall meeting on January 7, 2021(recording available at https://www.youtube.com/watch?v=7CjQeuD3eFE&feature=youtu.be) to obtain stakeholder feedback on MVP considerations for MVP design and implementation (85 FR 74729 through 74730). We have received commenter concerns from previous MVP rulemaking and MIPS MVP Town Hall about fragmented care under specialty focused MVPs with a few commenters voicing support for a care-coordination focus. We do not want to restrict MVP development to only the concepts already presented. For example, we envision that some MVPs would be reported primarily by a single specialty and other MVPs would include measures and activities relevant to a broad range of clinicians. We are interested in MVPs that target a focused episode of care, as well as MVPs that measure the patient journey and care experience longitudinally. We would like to explore how MVPs could best measure the value of multi-disciplinary team-based care. See section IV.A.3.b.(4)(b)(i)(A) of this proposed rule, for discussion of MVP development areas and broad and team-based holistic MVP approaches.
Our approach to developing the portfolio of MVPs must balance objectives for having MVPs available for the diverse range of MIPS eligible clinicians, the variety of health conditions affecting Medicare patients, and the patient's needs for relevant, meaningful information. We seek stakeholder feedback on the types of MVPs and quality and cost measures required to meet those objectives.
We request public comment on the concepts outlined above.
(b) MVP Guiding Principles
(i) Overview of the Guiding Principles
In the CY 2021 PFS final rule (85 FR 84845 through 84849), we updated the MVP guiding principles from the CY 2020 PFS proposed rule (84 FR 40734) to incorporate RFI comments and the evolution of the MVP framework.
The guiding principles for MVPs are as follows:
1. MVPs should consist of limited, connected complementary sets of measures and activities that are meaningful to clinicians. This will reduce clinician burden, align scoring, and lead to sufficient comparative data.
2. MVPs should include measures and activities that result in providing comparative performance data, which is valuable to patients and caregivers in evaluating clinician performance and making choices about their care. MVPs will enhance this comparative performance data as they allow subgroup reporting that comprehensively reflects the services provided by multispecialty groups.
3. MVPs should include measures selected using the Meaningful Measures approach and, wherever possible, the patient voice must be included, to encourage performance improvements in high priority areas.
4. MVPs should reduce barriers to APM participation by including measures that are part of APMs where feasible, and by linking cost and quality measurement.
5. MVPs should support the transition to digital quality measures.
(ii) Implementation of MVP Guiding Principles and Practical Considerations
The MVP guiding principles will help move us towards our goals of transforming healthcare. Stakeholders have supported the guiding principles and have indicated their interest in further understanding how we intend to implement the MVP Guiding Principles (85 FR 84845). As we introduce MVPs and address operational policies, we are focused on the guiding principles and the concrete steps needed to implement the principles at both the individual MVP level and the MVP portfolio level. We acknowledge certain tensions between our intent to simplify MIPS through increased performance measurement standardization versus clinician's desire for flexibility and choice of performance measure and Start Printed Page 39354 activities. We also acknowledge tensions between our intent to obtain comparable clinician performance data and include meaningful measures for all clinician types and specialties while minimizing burden. We want to provide patients with valuable and comparable clinician performance data to assist patients and caregivers when selecting a clinician or group. At the same time, we must consider clinician burden and performance measurement aspects such as measure reliability and attribution methodologies. The availability of the subgroup reporting option, proposed in section IV.A.3.b.(3) of this proposed rule, would move in the direction of facilitating more comprehensive performance data for multidisciplinary groups.
We want to connect cost measures to quality measures and improvement activities in newly developed MVPs as stated in guiding principle 1 (85 FR 84849 through 84854). However, as we look to develop MVPs for all MIPS eligible clinicians, we are hampered by the limited availability of cost measures. Also, the siloed cost measures and quality measures development processes can present a degree of challenge in forming cohesive measure sets in MVPs as cost and quality measures are often developed independently of one another, addressing different patient populations and care conditions. Because improving value requires the ability to measure quality and cost of care, we are concerned with the limited number of cost measures currently available. We are proposing five cost measures for implementation into MIPS in section IV.A.3.d.(2)(b) of this proposed rule. We also want to expand our ability to have cost measures available for MVPs. We refer readers to section IV.A.3.d.(2)(c) of this proposed rule, which discusses our proposal for external cost measure development by stakeholders.
Given these tensions and challenges, we plan to continue balancing the MVP framework approaches and our incremental MVP introduction while focusing on our overarching goals and considerations of current and future developments to help us implement the MVP guiding principles.
(iii) Implementing MVP Guiding Principles
In this rule we propose several policies that begin to implement the MVP guiding principles. We also outline several challenges to implementing the guiding principles and, in multiple sections of this rule, request public comment to guide us in future rulemaking.
We are proposing seven initial MVPs in section IV.A.3.b.(4)(c) of this proposed rule for implementation in CY 2023 performance period. The proposed MVPs contain related cost and quality measures and improvement activities. The proposed MVPs also limit the number of quality measures and improvement activities from which clinicians would choose to report and require fewer reported quality measures than in traditional MIPS. Both cost and population health measures are calculated from claims data and do not have to be submitted by clinicians. The proposed MVPs represent concrete progress toward implementing the guiding principles.
For example:
- Requiring the submission of fewer quality measures and a lessened measure selection effort reduces clinician burden.
- Hearing from stakeholders ensures that measures are relevant to clinicians.
- The limited numbers of cost and quality measures in an MVP will support greater numbers of clinicians being scored on the same measures, leading to improved comparative data.
- The MVPs address a Meaningful Measure domain and contain measures that are clinically appropriate to the clinicians and care settings for whom the MVP is focused.
In section IV.A.3.b.(5) of this proposed rule, we implement the guiding principle 1 concept to "align scoring" by proposing MVP scoring policies that mirror traditional MIPS scoring in MVPs while moving away from earlier transitional policies that may have masked performance differences or inflated performance scores. Our scoring policies aim to spur improvements, drive higher value care, and promote fairness. We propose to maintain scoring policies finalized in traditional MIPS for MVPs to leverage meaningful scoring policies and retain stable scoring for APM Participants.
The subgroup reporting option outlined in section IV.A.3.b.(3) of this proposed rule operationalizes guiding principle 2 and, as noted above, will increase the number of clinicians reporting and better serve specialty clinicians who want to be scored on performance measures relevant to their specialty and services provided. Public reporting of MVP and subgroup information as proposed in section IV.A.3.b.(3)(g) of this rule will further guiding principle 2 goal of providing comparative information that is valuable to patients and caregivers.
We request public comment on innovative approaches to measuring value that might include APM performance measurement approaches and using a single patient population both for MVP cost and quality measures in the future.
We have also discussed our intent to provide more robust clinician performance data feedback for MVP submissions (84 FR 40733 through 40734). Receiving more meaningful feedback through MVPs would help prepare clinicians to meet APM goals for managing patient populations. While clinicians support more robust data feedback, the current timing of data submission after the performance year ends is a barrier to providing more timely data feedback to clinicians during the performance year. As we move to dQMs and utilization of standards-based APIs to retrieve data from provider data sources, earlier, more frequent reporting, and more granular data (as in subgroup reporting) may be possible without additional clinician burden, allowing us to provide more timely clinician performance data feedback. See section IV.A.3.b.(5)(d) of this proposed rule for discussion of MVP clinician data feedback.
During our January 7, 2021, MVP Town Hall meeting we sought feedback on how to best coordinate and align MVPs and APMs (85 FR 74729 through 74730). A few Town Hall commenters suggested that MVPs serve as a long-term performance-based option to improve physicians' experience in MIPS and as an on-ramp for clinicians from MIPS to APMs. A few commenters supported the development of MVPs for areas where APMs are absent, with a few stakeholders supporting an initial focus on developing MVPs around existing specialty measures sets before transitioning the MVP into a bridge for clinicians who do not have an applicable APM. We also received a comment that there may be scenarios in which it may be challenging to use the same measures in an MVP as an APM, as the commenter stated APM participants have more legal flexibility and APM models often use Innovation Center waiver authority (section 1115A of the Act). We continue to explore the ideal MVP relationship with APMs, and how to best drive value and align performance measurement given differences in payment, performance measurement methods (such as prospective and retrospective measure attribution), patient population (all payer versus fee-for-service), and data submission.
While the proposals in this proposed rule referenced above demonstrate important progress toward realizing the MVP guiding principles, challenges remain for CMS and stakeholders in Start Printed Page 39355 developing a portfolio of MVPs that fully implement the guiding principles and achieve our vision for MVPs. As we propose to introduce MVPs and implement our guiding principles, we continue to strive to fully implement MVPs and the overall portfolio to drive value, obtain comparative performance data, and elevate the patient voice while reducing clinician burden.
(c) MVP Participant
(i) MVP Participant Definition
As we look ahead to implementing MVPs, we believe it is important to clearly define who can participate in MIPS through MVPs. We believe that defining MVP participation will help stakeholders better understand how our policies affect them, as well as provide clarity and simplicity for readers.
At § 414.1305 we have previously finalized definitions for a MIPS eligible clinician, group, and APM Entity. While we are not proposing to change these definitions, and are using these existing terms, we seek to clarify who can participate in MVPs. We are proposing a new opportunity for clinicians to participate in MVPs, as a subgroup. We refer readers to section IV.A.3.b.(3) and § 414.1305 of this proposed rule, where we propose the definition for a subgroup. In addition, we believe it would be helpful to distinguish the types of groups that participate in MIPS, and how they could participate in MVPs. Therefore, we refer readers to section IV.A.3.b.(3) and § 414.1305 of this proposed rule, where we propose definitions for single specialty group, multispecialty group, and special status, to provide further clarity for stakeholders as they seek to understand how they can participate in MVPs.
In keeping with MVPs broader aim of cohesive participation, at § 414.1305 we are proposing the term MVP Participant to mean: An individual MIPS eligible clinician, multispecialty group, single specialty group, subgroup, or APM Entity that is assessed on an MVP in accordance with § 414.1365 for all MIPS performance categories. For the CY 2025 MIPS performance period/2027 MIPS payment year and future years, MVP Participant means an individual MIPS eligible clinician, single specialty group, subgroup, or APM Entity that is assessed on an MVP in accordance with § 414.1365 for all MIPS performance categories. The proposed definition of MVP Participant accounts for the gradual transition to requiring multispecialty groups to form subgroups if they want to report MVPs. We believe this is important because multispecialty groups report on the same set of measures, which may not be relevant or meaningful to all specialists that participate within the multispecialty group, to make improvements in the care they provide to patients. We refer readers to section IV.A.3.b.(2)(d)(ii) of this proposed rule for discussion of subgroup implementation, including multispecialty groups forming subgroups to report MVPs beginning with the CY 2025 MIPS performance period/2027 MIPS payment year. Table 30 serves to summarize our proposals, specifically which MVP Participants can report an MVP in the future:

We recognize that in some limited instances, there are specific policy proposals that are more narrow or expansive than the term MVP Participant allows for. In those cases, we will continue to clarify which specific participants a given policy applies to, rather than using the new term. For example, if we have policies regarding what is required during subgroup registration, as discussed below, we would specify that these policies would be specific to subgroups rather than use the term MVP Participants. In another example, in section IV.A.3.b.(5)(b)(iv) of this proposed rule, we propose Promoting Interoperability performance category scoring policies that apply to individual MIPS eligible clinicians, groups, and APM Entities, but do not apply to subgroups. In this example, we would clarify that the policy applies to MVP Participants, except subgroups. In addition, if we determine a given policy proposal is applicable to groups, regardless of whether they are single specialty or multispecialty, we may simply refer to them as groups. We believe stakeholders would welcome the simplicity that using the term MVP Participant would provide. It is an important step forward for the program that would promote clarity and consistency of policy drafting and compliance by stakeholders. We request public comment on the proposal.
(ii) Opt-In Participants, Voluntary Participants, and Virtual Groups
As discussed above, we are proposing that for the implementation of MVPs, certain clinicians would not be able to participate. These include, voluntary reporters, opt-in eligible clinicians, and virtual groups, who would have their participation in MVPs delayed. We refer readers to section IV.A.3.b.(3)(d)(iv)(C) of this proposed rule for discussion of the participation rates of opt-in and voluntary participants. Similar to our request for comments on whether Opt-In and voluntary participants should be allowed to join subgroup reporting in a future state, we also request comment on whether opt-in participants, voluntary participants, and virtual groups should be allowed to report MVPs as MVP Participants in a future state.
(d) MVP and Subgroup Implementation Timeline
(i) MVP Implementation Timeline
Since the finalization of the MIPS Value Pathways framework through the CY 2020 PFS final rule (84 FR 62946 through 62949), stakeholders have provided feedback on our implementation timeline through multiple methods, including public comment through rulemaking, meetings, and the MVP Town Hall that held in January 2021. Associated resources related to the MVP Town Hall are available for stakeholder review through the Quality Payment Program Resource Library are available at https://qpp.cms.gov/resources/webinars.
Through the MVP Town Hall, we have heard stakeholders encourage MVPs be implemented through a Start Printed Page 39356 gradual process that provides MVP participants and third party intermediaries with time to adapt to the changes in policy, requirements, and programming updates that would need to occur in technological systems. Therefore, we believe it is appropriate to delay the implementation and availability of the proposed MVPs, described in Appendix 3: MVP Inventory of this proposed rule until the 2023 performance period/2025 MIPS payment year, of the MIPS program. We propose at § 414.1365(a)(1), that for the 2023 MIPS performance period/2025 MIPS payment year, and future years, we use MVPs included in the MIPS final inventory of MVPs established by CMS through rulemaking to assess performance for the quality, cost, improvement activities, and Promoting Interoperability performance categories.
In addition to proposing a timeline in which MVPs would be first available, we also believe it is important to be transparent with the agency's current vision and request public comment on the timing of how long MVP reporting should be voluntary, the transition to mandatory MVP reporting, and the timing for when we should sunset traditional MIPS.
While we have heard from stakeholders their request for us to maintain both reporting methods, traditional MIPS and MIPS Value Pathways, we believe it is not a feasible option long term, because of the operational burden, complexity, and costs associated with simultaneously maintaining both versions of the program.
We have also heard from stakeholders (through the MVP Town Hall and from Health Affairs[195] ) the importance in continuing this shift to value through MVPs, and doing so by providing as much transparency as possible. We agree, and believe that providing transparency with our thinking (in terms of a transition timeline) and seeking public comment will serve to provide stakeholders with important information to make informed decisions about their eventual transition to MVP reporting. We believe it is critical to establish a timeline for the awareness of all stakeholders (such as MVP participants, third party intermediaries, and health systems) so they can plan their work accordingly to coincide with this timeline.
As such, we outline a timeline in which MVP implementation could occur. As stated above, we are proposing at § 414.1365(a) that the first year MVP reporting be available is the CY 2023 MIPS performance period/2025 MIPS payment year. Based on the discussion above, we are proposing for the CY 2023 MIPS performance period/2025 MIPS payment year, MVP reporting is voluntary. We request comments on this proposal.
Through the remainder of the timeline outlined in Table 31, we seek to lay out our thinking for the future of the MIPS program, for purposes of transparency, and to request public comment. We believe moving forward with voluntary MVP reporting in the initial years would provide MVP participants sufficient time to prepare for mandatory MVP reporting. Therefore, as outlined below, we are considering MVP reporting would be voluntary for the CY 2023 through the CY 2027 MIPS performance periods/2025 through the 2029 MIPS payment years. Furthermore, we plan for potential future mandatory MVP reporting to coincide with the sunset of traditional MIPS.

As previously described, maintaining both traditional MIPS and MVPs is not a feasible long-term approach for the agency. As such, we are thinking of sunsetting traditional MIPS by the end of the CY 2027 performance period/2029 MIPS payment year. We would like to note that we are not proposing the timeframe in which MVP reporting would no longer be voluntary (by the end of the CY 2027 performance period/2029 MIPS payment year), and the future sunset of traditional MIPS at this time; any proposal to sunset traditional MIPS would be made in future rulemaking. Our discussion of the MVP implementation timeline is an effort to be transparent with our long-term vision of the MIPS program.
We request public comments on this incremental timeline to transition to mandatory MVP reporting, including the timing of the sunset of traditional MIPS. Specifically, are there concerns with this timeline? Is there an alternative timeline we should consider and why? In addition, what factors should CMS monitor to determine stakeholders readiness to sunset traditional MIPS and transition to MVPs? We understand that some clinicians who participate in MIPS practice in highly specialized clinical Start Printed Page 39357 areas and subspecialties, where they may believe there is not an MVP applicable to their highly specialized practice. Therefore, we also request comment on what should happen in instances where highly specialized clinicians cannot identify an applicable and relevant MVP.
We request public comments on this approach.
(ii) Subgroup Implementation Timeline
In the CY 2021 PFS final rule (85 FR 84845), we signaled our intent to implement subgroup reporting by finalizing modifications to the MVP guiding principles. We refer readers to section IV.A.3.b.(3) of this proposed rule for detailed discussion of subgroup proposals; and to section IV.A.3.b.(3)(c) of this proposed rule and § 414.1305 for the definitions of groups, multispecialty groups, single specialty groups, and subgroups.
From our understanding, groups may be made up of a single specialty or of multiple specialties. We do not believe that single specialty groups, should be required to form subgroups in order to report MVPs. In this scenario, we believe that a single specialty group would be able to report on the same set of relevant and applicable measures for all clinicians within the group, and would be able to ascertain results that may lead to improvements in the patient care provided. Therefore, for now, we do not anticipate the need to require single specialty groups to form subgroups in order to report an MVP.
The intent of the subgroup reporting proposals is to move away from large multispecialty groups reporting on the same set of measures, which may not be relevant or meaningful to all specialists that participate within a multispecialty group. In addition, we have heard from stakeholders over the past few years that large multispecialty groups tend to submit data that is not necessarily representative of all the clinicians that make up that group. For example, a group from a large hospital system, may include various specialties such as primary care, oncology, surgery, anesthesia, and radiology that submit data to CMS on primary care quality measures. We are concerned that these type of group submissions do not accurately reflect the performance of all clinicians within the group, and does not provide all clinicians with results that leads to quality improvement in the care provided. In addition, we do not believe that the other specialties within the group can make data-driven improvements in the quality of patient care provided, when only primary care measure data is submitted to CMS; and the results of that data submission is only relevant to the primary care clinicians. From the patient and caregiver perspective, only receiving information on primary care measures when searching for a specialist is not helpful. Data submitted at the subgroup level would provide increased data granularity that patients and caregivers could use in making data-driven decisions regarding the involvement of specialists in their care. In addition, we believe that transitioning multispecialty groups to subgroup reporting will address some of the inherent gaming risks that are apparent when we have multi-specialty groups report on measures that are not necessarily representative of the care provided by all clinicians within the group, where clinicians in a group may rely on the performance of other clinicians (of a different specialty) within the group to meet quality reporting requirements. We anticipate that multispecialty groups would need some time to familiarize and prepare themselves for subgroup reporting.
We refer readers to section IV.A.3.b.(2)(c)(i) of this proposed rule, where we discuss the proposed MVP Participant definition. Through the proposed MVP Participant definition, multispecialty groups and single specialty groups may report as groups or choose to form subgroups to report MVPs for the CY 2023 and CY 2024 performance period/2025 and 2026 MIPS payment year. We believe that the delayed implementation of subgroups for the CY 2023 MIPS performance period/2025 MIPS payment year provides third party intermediaries with sufficient time to adapt to the changes in policy, requirements, and programming updates that would need to occur in technological systems to support subgroup reporting. We encourage the early adoption of subgroup reporting to allow groups to gain experience with the future state of the program.
In addition, beginning with the CY 2025 MIPS performance period/2027 MIPS payment year, we propose through the MVP Participant definition to no longer allow multispecialty groups to report MVPs. This would mean that if a multispecialty group would like to report MVPs, beginning with the CY 2025 MIPS performance period/2027 MIPS payment year, they could only do so if they form subgroups. We believe this 2-year span of time would give multispecialty groups time to familiarize themselves and prepare for subgroup reporting. We strongly encourage multispecialty groups to monitor the implementation of MVPs to determine when to adopt subgroup reporting and transition to MVPs prior to the CY 2025 MIPS performance period/2027 MIPS payment year. We encourage groups to adopt MVP and subgroup reporting as early as possible to provide some time to work through any inadvertent operational issues they may encounter as MVP participants prepare for the future of the MIPS program. While we understand that groups may choose between MVP reporting and continuing to participate through traditional MIPS, we highly encourage groups to submit via subgroups if applicable in the first few years of MVP reporting. We believe early adoption of MVPs and subgroup reporting is important for stakeholders, as this would allow clinicians to acclimate to MVP reporting in the event we sunset traditional MIPS in the future.
We understand that some clinicians practice utilizing a team-based care approach, through a multispecialty group. We believe that MVP reporting can continue to foster the utilization of team-based care through subgroup reporting. As such, we describe in section IV.A.3.b.(4)(b)(i)(A) of this proposed rule, that MVPs may be developed to reflect the team-based care approach used during an episode of care. A proposed timeline to implement subgroup reporting is outlined in Table 32.
Start Printed Page 39358

As we continue to expand the portfolio of MVPs available over the next few years, MIPS eligible clinicians, groups, and APM entities that do not have a relevant MVP for reporting could continue to report through traditional MIPS. We plan to time the sunset of traditional MIPS with the implementation of an appropriate portfolio of MVPs that are relevant to specialists that participate in the MIPS program. Until that time, there may be instances where some clinicians in a multispecialty group may have a relevant MVP available for reporting, while other clinicians within that same multispecialty group may not. In this scenario, the clinicians within the multispecialty group that have an MVP available may form a subgroup to report the MVP, while the group continues to report traditional MIPS. We refer readers to section IV.A.3.b.(3) of this proposed rule for additional discussion of subgroup proposals.
We believe there is a need for multispecialty groups to transition to subgroup reporting in order to align with the goals of MVP reporting. That is, to provide more direct attribution of quality measure data and results to all clinicians that participate in the program rather than relying on quality reporting results that can only be attributed to a few clinicians within the group. This direct attribution would lead to more valuable, meaningful, and actionable results that contribute to patient care and improvement. We refer readers to sections IV.A.3.b.(3) and IV.A.3.b.(4)(d) of this proposed rule for a detailed discussion of subgroup proposals and MVP reporting requirement proposals.
(e) Subgroups Reporting the APM Performance Pathway (APP)
In the CY 2021 PFS final rule (85 FR 84859 through 84866), we finalized the availability of the APM Performance Pathway beginning with the CY 2021 performance period. Specifically, we finalized that individual MIPS eligible clinicians who are participants in MIPS APMs may report through the APP at the individual level (85 FR 84860). Furthermore, we finalized that groups and APM Entities may report through the APP on behalf of constituent MIPS eligible clinicians (85 FR 84860). Because we already identify the MIPS eligible clinicians who are MIPS APM participants based on Participation Lists for each APM, it is unnecessary to require MIPS APM participants to register as subgroups for purposes of reporting the APP. We use Participation Lists to identify each individual APM participant for purposes of MIPS APM participation, as well as application of the Improvement Activities credit for APM participants; beginning with performance year 2023, we will use Participation Lists to identify the MIPS eligible clinicians within a group TIN that should be included in the subgroup of APM participants for purposes of reporting the APP.
(f) Catalyst for Reporting MVPs
(i) Background
Through the MIPS Value Pathways framework, finalized in the CY 2020 PFS final rule (84 FR 62946 through 62949), stakeholders provided feedback, specifically questioning what incentives would MVP Participants have to report on MVPs, when they have the choice to report traditional MIPS instead. We have heard these questions raised through multiple methods, including public comment through rulemaking, meetings, and the MVP Town Hall that was held in January 2021. Through this rule, we have proposed MVP policies that we believe act as catalysts to encourage MVP Participants to transition to MVP reporting. This includes reduced reporting requirements, as described in section IV.A.3.b.(4)(d) of this proposed rule, allowing MVP Participants to report on a smaller, more cohesive subset of measures and activities that are relevant to a given clinical topic, condition, procedure, or episode of care. In addition, as described in section IV.A.3.b.(5)(d) of this proposed rule, we propose to provide MVP Participants who report on MVPs with enhanced performance feedback that allows for meaningful comparison to similar clinicians and provides more useful information to make improvements in the care provided.
Additionally, we understand that clinicians have other requirements that must be met to maintain their licensure and as appropriate board certification status. In many instances, clinicians must comply with Continuing Medical Education (CME) requirements and/or Maintenance of Certification (MOC) requirements. We believe that any alignment between what clinicians must do to maintain their licensures/board certifications and reporting MVPs would be beneficial by reducing burden in terms of the various requirements clinicians must comply with. Therefore, in some cases, it seems possible that offering CME credit or credit towards MOC could be connected with MVPs. We encourage accrediting organizations such as specialty societies, to work with MVP submitters and consider whether CME credit or credit towards MOC could be offered for reporting MVPs. We believe by allowing clinicians to receive CME credit for MVP reporting, there is potential for there to be a reduction in the administrative burden clinicians face when trying to balance meeting CMS program requirements with the requirements of medical licensing or certification.
Proposing incentives for clinicians to report on MVPs in lieu of traditional MIPS may encourage early adoption of MVPs and allows those clinicians to gain experience with the future state of the program. We believe that creating incentives to report MVPs may help MVP participants familiarize themselves with MVP reporting requirements, particularly in cases where clinicians identify an available MVP as relevant to their practice.
(ii) Public Reporting of MVP Data
We have heard from stakeholders who expressed hesitancy to partake in the initial transition to MVP reporting citing concerns with what results may be publicly reported. We refer readers to Start Printed Page 39359 section IV.A.3.i. of this proposed rule for discussion of public reporting proposals related to MVP data and subgroup reporting.
(3) Subgroup Composition
(a) Overview
In the CY 2021 PFS final rule, we finalized updates to MVP guiding principles (85 FR 84844 through 84849), including the addition of subgroup reporting to enhance comparative performance data, and MVP development criteria and process (85 FR 84845 through 84849) that guide MVP implementation. Through this proposed rule, we are proposing to establish subgroup reporting as an option for MVP Participants and for those individuals and entities who choose to report the APP. In this section, we propose: (1) Definitions for subgroup reporting, single specialty group, multispecialty group, and special status designation; (2) subgroup eligibility requirements; and (3) application of low-volume threshold and special status designations for subgroups. In this section, we also have a request for information on the future direction of subgroup reporting. Additionally, we refer readers to section IV.A.3.b.(4) of this proposed rule, where we detail our proposals on: (1) Subgroup reporting requirements; (2) subgroup election process; and (3) subgroup identification. In section IV.A.3.b.(5) of this proposed rule, we detail our proposals on subgroup scoring.
(b) Background
Section 1848(q)(1)(D)(i) of the Act requires that the Secretary establish and apply a process that includes features of the provisions of section 1848(m)(3)(C) of the Act for group practice reporting for the quality performance category and provides that the Secretary may establish such a process for the other MIPS performance categories. At § 414.1305, a group is defined as a single TIN with two or more eligible clinicians (including at least one MIPS eligible clinician), as identified by their individual NPI, who have reassigned their billing rights to the TIN. In the CY 2021 PFS final rule, we finalized updates to MVP guiding principles (85 FR 84844 through 84849), including the addition of subgroup reporting to enhance comparative performance data, and MVP development criteria and process (85 FR 84849 through 84853) that guide MVP implementation. In section IV.A.3.b.(2)(d)(ii) of this proposed rule, we propose to allow voluntary MVP reporting beginning with the CY 2023 MIPS performance period/2025 MIPS payment year and are considering mandatory MVP reporting to coincide with the sunset of traditional MIPS beginning with the CY 2028 MIPS performance period/2030 MIPS payment year. We believe one important element of transitioning to MVPs is allowing clinicians the ability to report and be assessed on measures and activities which are meaningful to their practice. Currently, within the MIPS program, we offer clinicians many opportunities to participate, including as an individual and as a group; we have found most clinicians choose the group reporting option. We anticipate some groups may consist of clinicians who all practice under a single-specialty or clinical focus and are able to successfully select an MVP whose measures and activities are applicable and meaningful to all or a significant majority of their patients or practice. On the other hand, some groups encompass 20 or more different specialties, contain many clinicians, and often provide an array of services that may not be captured in a single set of measures or in a single MVP. For instance, we estimated in the CY 2021 PFS final rule RIA that among the 863,627 clinicians who submitted data, 510,057 were in practices with more than 100 clinicians (85 FR 85019). This represents 59 percent of the total MIPS eligible clinician population estimated for the CY 2023 payment year using 2019 submissions data (85 FR 85019).
In the 2017 Quality Payment Program final rule (81 FR 77058), commenters had noted interest in CMS providing additional flexibility to allow clinicians to submit information that would represent reporting for some portion, but not the entirety, of a group or TIN. In the CY 2018 Quality Payment Program final rule (82 FR 53593), we stated that in future rulemaking we intend to explore the feasibility of establishing group-related policies which would permit participation in MIPS at a subgroup level and create such functionality through a new identifier. Prior to the introduction of MVPs, in the CY 2019 PFS proposed rule (83 FR 35891), we again acknowledged the overarching themes from stakeholders that we should make an option available to groups which would allow a portion of a group to report as a separate subgroup on measures and activities which are more applicable to the subgroup and be assessed and scored accordingly based on the performance of the subgroup. We solicited comment on specific options and questions for implementation of subgroup level reporting in future years. However, as we noted in the CY 2019 PFS final rule (83 FR 59742), because there are numerous operational challenges with implementing a subgroup option, we did not propose any changes to our established reporting policies regarding the use of a subgroup identifier.
In the CY 2020 PFS proposed rule (84 FR 40740 through 40741), we sought comment on MVP policies for multispecialty practices. Overall, we heard from commenters that subgroup reporting should be offered as an additional reporting option where subgroup reporting would provide more specific information for patients and clinicians rather than having multispecialty groups report on multiple MVPs at the group level. We also sought comment in the CY 2020 PFS proposed rule (84 FR 40740 through 40741) on whether we should use an approach in which groups submitted data on multiple MVPs reflecting their diverse specialties as an alternative to subgroup reporting to more comprehensively capture the range of items and services furnished by a group practice. Several commenters voiced concerns related to tradeoffs between the burden of reporting multiple MVPs and having more comprehensive performance data. Many commenters urged CMS to allow for subgroups and did not see reporting on multiple MVPs by the entire group as an alternative to subgroup reporting. Some commenters recommended allowing subgroup reporting in lieu of MVPs, while others recommended subgroup reporting for either the traditional MIPS program rules or for MVPs. A few commenters recommended steps we could take to identify subgroups, including creating a subgroup identifier and allowing the formation of subgroups through an election process at the Quality Payment Program website (qpp.cms.gov) that would function similarly to CMS Web Interface or CAHPS for MIPS registration. A few commenters suggested that allowing subgroup reporting may be necessary to implement MVPs and help groups, particularly multispecialty practices, meet data completeness criteria.
Additionally, in response to our request for comment on whether we could use the MVP approach as an alternative to subgroup reporting to more comprehensively capture the range of items and services furnished by a group practice, we heard concerns that subgroup reporting may deter group practices from utilizing a team approach to patient care, may make departments more competitive, may not improve care for patients, and may increase errors, Start Printed Page 39360 costs, stress, and administrative burdens to implementation across various departments. In considering these concerns, we have continued to work towards meaningful subgroup reporting that balances the hurdles of a new reporting option with the benefits of more comprehensive and granular data available for patients and clinicians.
We held the MVP Town Hall on January 7, 2021 (85 FR 74729) and publicly shared the MVP Town Hall Preparation Guide (https://qpp-cm-prod-content.s3.amazonaws.com/uploads/1233/MVP%20Town%20Hall%20Preparation%20Guide.pdf) to aid MVP Town Hall participants in understanding the direction we may take with MVPs and subgroups. We sought public comment on the MVP Town Hall topics and the approaches outlined in the MVP Town Hall Preparation Guide (recording available at https://www.youtube.com/watch?v=7CjQeuD3eFE&feature=youtu.be). Overall, several commenters supported the option of voluntary subgroup reporting and recognized the value for patient care and research. Several commenters suggested CMS to allow sufficient time for CMS and stakeholders to make software changes (that include coding, development, testing, production, training, and education tasks) to support subgroup reporting, ultimately recommending CMS to slow down implementation. Additionally, we have solicited comment from stakeholders beginning in the CY 2018 PFS proposed rule (82 FR 30027) through the MVP Town Hall (85 FR 74729) on the ways in which participation in MIPS at the subgroup level could be established. In considering stakeholder feedback—in conjunction with the introduction of MVPs, technical innovations, and working through operational issues within CMS—we are ready to propose subgroup reporting. We acknowledge some clinicians in multispecialty groups are currently not able to report as a team for the measures that are meaningful to them because the group reports on behalf of all clinicians within the practice. Even with the introduction of MVP reporting, we anticipate this problem could persist if subgroup reporting is not offered as a reporting option for MIPS. Therefore, subgroup reporting would provide an avenue for clinical teams within a larger group to be able to submit MVPs that are clinically relevant to them. It would also be a first step in allowing for more granular clinician information to be made available to patients.
We thank commenters for their feedback. After considering the feedback, in section IV.A.3.b.(2)(d)(ii) of this rule, we propose voluntary subgroup reporting in MIPS beginning with the CY 2023 MIPS performance period/2025 MIPS payment year for MIPS eligible clinicians and groups who participate in MIPS through MVP reporting. As discussed in section IV.A.3.b.(3)(d)(iv)(B) of this proposed rule, APM Entities can report to MIPS through MVPs but may not break out into subgroups. As discussed in section IV.A.3.b.(2)(d)(ii) of this proposed rule, we are proposing that beginning in the CY 2025 MIPS performance period/2027 MIPS payment year, multispecialty groups would be required to form subgroups in order to report MVPs.
(c) Definitions of a Single Specialty Group, Multispecialty Group, Subgroup, and Special Status
A group is currently defined at § 414.1305 as a single TIN with two or more eligible clinicians (including at least one MIPS eligible clinician), as identified by their individual NPI, who have reassigned their billing rights to the TIN. As discussed in section IV.A.3.b.(3)(d) of this proposed rule, we are proposing to use certain characteristics of the group to determine eligibility and special status of the clinicians in the subgroup. To provide clarity, we are proposing definitions for single specialty groups, multispecialty groups, subgroups, and special status.
(i) Proposed Single Specialty and Multispecialty Groups Definitions
We propose to add to § 414.1305 to include that a single specialty group is a group as defined at § 414.1305 that consists of one specialty type as identified by eligible clinicians in the Medicare Provider Enrollment, Chain, and Ownership System (PECOS) (https://pecos.cms.hhs.gov/). We believe that using clinician specialty information from PECOS would allow us to align data sources and create greater consistency within the program given that PECOS specialty information is publicly reported on Care Tools.[196] We refer readers to our comment solicitation in section IV.A.3.b.(3)(h)(iv) of this proposed rule, where we request information on the threshold that subgroups would have to meet in order to be defined as a single specialty (such as having at least 75 percent of clinicians sharing the same specialty in a subgroup).
We also propose to add to § 414.1305 to include that a multispecialty group is a group as defined at § 414.1305 that consists of two or more specialty types as identified by eligible clinicians in the Medicare Provider Enrollment, Chain, and Ownership System (PECOS). We refer readers to our comment solicitation in section IV.A.3.b.(3)(h)(iv) of this proposed rule, where we request information on setting the threshold that subgroups would have to meet in order to be defined as a multispecialty group. Beginning in the CY 2025 MIPS performance period/CY 2027 MIPS payment year and discussed in section IV.A.3.b.(2)(d)(ii) of this proposed rule, multispecialty groups would be required to form subgroups in order to report MVPs.
Additionally, we recognize that individual eligible clinicians may practice or be a part of multiple specialties as a part of their scope of practice and that eligible clinicians may have more than one specialty designation in PECOS. These clinicians could participate in multiple subgroups and report on multiple MVPs or clinicians could join a subgroup that is most applicable to their scope of practice and report on one MVP. We anticipate that at a future time when subgroup reporting is mandatory, that there will need to be criteria to determine which specialty is a primary specialty of clinicians. At this time, we do not have limitations on which specialty will be considered the primary specialty. However, we refer readers to section IV.A.3.b.(3)(h)(iv) of this proposed rule, where we request information on the identification of a primary specialty in scenarios where an eligible clinician may have more than one.
We request public comment on these proposals.
(ii) Proposed Subgroup Definition
We propose to define a subgroup at § 414.1305 as a subset of a group which contains at least one MIPS eligible clinician and is identified by a combination of the group TIN, the subgroup identifier, and each eligible clinician's NPI. Groups would identify their affiliated subgroups, and those subgroups would submit data on the MVPs which are clinically meaningful to MIPS eligible clinicians within a subgroup or their patients. We propose at § 414.1318(b) to state that except as provided under § 414.1317(b), each MIPS eligible clinician in the subgroup receives a final score based on the subgroup's combined performance assessment. Additionally, we propose to amend § 414.1310(e)(1) to state that except as provided under §§ 414.1315(a)(2), 414.1317(b), Start Printed Page 39361 414.1318(b), and 414.1370(f)(2) each MIPS eligible clinician in the group receives a final score based on the group's combined performance assessment. With the inclusion of the exception provided under § 414.1318(b), this would allow for an exception for subgroups to receive a final score based on the subgroup's combined performance.
We note that in these proposed additions to and amendments of the regulation text, we refer to the final score instead of the MIPS payment adjustment factor (or additional MIPS payment adjustment factor) because we believe this phrasing is more precise. It is possible that more than one final score could be associated with a TIN/NPI for a performance period, and in those situations, we apply a final score hierarchy for purposes of determining the MIPS payment adjustment for that TIN/NPI (see, for example, the discussion in the CY 2021 PFS final rule (85 FR 84917 through 84919)). We believe allowing and, beginning with the CY 2025 MIPS performance period/CY 2027 MIPS payment year, requiring subgroup reporting for MVPs would offer clinicians the opportunity to participate in MIPS more meaningfully and would allow patients to have more granular and meaningful information when selecting an eligible clinician.
Measuring performance at the subgroup level would still allow for groups to practice team-based care, with groups having the ability to self-define which clinicians participate in which subgroups. Team-based health care is defined by the National Academy of Medicine as "the provision of health services to individuals, families, and/or their communities by at least two health providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care."[197] As discussed in section IV.A.3.b.(3)(d)(iii) of this proposed rule, we request comments on how to establish criteria around the composition of subgroups; criteria may include clinical relevance, scope of care, and patient population.
As discussed in section IV.A.3.b.(2)(d)(ii) of this proposed rule, MIPS eligible clinicians in groups who do not have an MVP available and applicable to their practice would participate in MIPS through group reporting or as an individual. If their group reports through traditional MIPS or an MVP, the clinicians could receive their group's score, if their group submits data. If the group chooses not to report, a MIPS eligible clinician can report as an individual and receive their individual score. While subgroup reporting of MVPs would be voluntary through the CY 2024 MIPS performance period/CY 2026 MIPS payment year, groups will continue to report to MIPS for the eligible clinicians (as identified by NPI) under their TIN, including clinicians reporting through subgroups, which is discussed in section IV.A.3.b.(2)(d)(i) of this proposed rule.
We request public comment on this proposal.
(iii) Proposed Special Status Definition
In the CY 2018 Quality Payment Program final rule, we finalized definitions for special status determinations for ambulatory surgical center (ASC)-based MIPS eligible clinicians, facility-based MIPS eligible clinicians, Health Professional Shortage Areas (HPSA), hospital-based MIPS eligible clinicians, non-patient facing MIPS eligible clinicians, rural area, or small practice status and codified at § 414.1305 definitions for each (82 FR 53479 through 53586). We often refer informally to these as "special status"; however, we have not previously defined what "special status" means. Therefore, we propose to add to § 414.1305 and define that special status means that a MIPS eligible clinician: (1) Meets the definition of an ASC-based MIPS eligible clinician, facility-based MIPS eligible clinician, hospital-based MIPS eligible clinician, non-patient facing MIPS eligible clinician, or is in a small practice; or (2) is located in an HSPA or rural area. We believe that defining special status will help clinicians better understand the application of subgroup policies.
We request public comment on this proposal.
(d) Subgroup Eligibility
As described in section IV.A.3.b.(3)(c)(ii) of this proposed rule, we are proposing voluntary subgroup reporting for clinicians beginning with the CY 2023 MIPS performance period/CY 2025 MIPS payment year and to define subgroup as a subset of a group which contains at least one MIPS eligible clinician and is identified by a combination of the group TIN, the subgroup identifier, and each eligible clinician's NPI.
During the MVP Town Hall held in January 2021 (85 FR 74729 to 74730), stakeholders expressed concern about creating separate eligibility criteria and associated policies which would lead to confusion and additional burden for clinicians in subgroups and associated groups. These stakeholders recommended that eligibility for subgroup reporting be based on group eligibility. Additionally, during the initial years of MVP implementation, we recognize that there may be an inadequate number of MVPs available for clinicians to participate as subgroups. Therefore, in this proposed rule, we are proposing: (1) Application of a low-volume threshold; (2) application of special status designation; and (3) subgroup inclusions and exclusions. Additionally, we seek comment on subgroup composition and limitations.
(i) Proposed Application of Low-Volume Threshold
We considered whether a low-volume threshold for clinicians participating in subgroup reporting should be calculated at the group or subgroup level. In consideration of stakeholder feedback and to minimize changes in eligibility determination for clinicians, we believe it would be optimal to determine the low-volume threshold for clinicians participating in a subgroup at the group level. As we implement subgroup reporting and as clinicians and groups familiarize themselves with this new participation option, we believe we should limit the complexity of the program to the extent that is feasible.
At § 414.1305, one of the ways we determine MIPS eligibility is by defining how the low-volume threshold is applied to individual clinicians and groups. We determine eligibility for MIPS during two different eligibility periods, which include an assessment of: (1) Those who have allowed charges for covered professional services less than or equal to $90,000; (2) those who provide covered professional services to 200 or fewer Part B-enrolled individuals; and (3) those who provide 200 or fewer covered professional services to Part B-enrolled individuals (83 FR 59735) provided by the clinician and group during that time-period. Therefore, we propose at § 414.1318(a)(1) that except as provided under § 414.1318(a)(2), for a MIPS payment year, determinations of meeting the low-volume threshold criteria and special status for subgroups are determined at the group level as provided under §§ 414.1305 and 414.1310.
We request public comment on this proposal. As MVPs continue to evolve, we anticipate increased opportunities for clinician participation in subgroups, Start Printed Page 39362 and we also request feedback from stakeholders if we should reevaluate, in the future, MIPS eligibility for clinician participation in subgroups at the subgroup level.
(ii) Proposed Application of Special Status Designation
Groups in MIPS could have their data submission requirements and scoring affected by special statuses outside of their underlying eligibility for MIPS. Each of these special statuses, described in section IV.A.3.b.(3)(c)(iii) of this proposed rule, are determined at the time of eligibility determinations.
We propose at § 414.1318(a)(1) for a MIPS payment year, determinations of meeting the low-volume threshold criteria and special status, as defined at § 414.1305, for subgroups is determined at the group level as provided under § 414.1310. We believe it is necessary to explain how special status determinations would work in the context of subgroup reporting. For example, a large, multispecialty group may include subgroups of clinicians that meet the requirements for small practice status, or non-patient facing status, or facility-based status. While we are certain some existing groups could have subgroups that could be eligible at the subgroup level for special status designation as described in section IV.A.3.b.(3)(c)(iii) of this proposed rule, we do not believe that this determination should be made at the subgroup level at this time. We want to deter construction of subgroups that would inappropriately create special status exemptions, such as subgroups of 15 or fewer clinicians in a large group. Overall, we believe this should help limit the complexity of the program as we implement this new participation option.
Additionally, at this time, we are not planning to establish limits on the number of subgroups that a clinician can be part of. For example, a primary care clinician who is part of a multispecialty group may choose to participate in a subgroup. This subgroup may choose to report through two MVPs because they believe both were relevant to the scope of care provided by the clinicians in their subgroup and under this example, the subgroup would be allowed to report and be assessed on both MVPs if they choose to do so. While we acknowledge the potential for clinicians to participate in multiple subgroups for reporting MVPs, we also recognize the burden for groups to submit data for an unlimited number of subgroups for each clinician, but we anticipate this will be an uncommon scenario. For instance, we do not anticipate that a group of 20 specialties would want to form 100 subgroups and be assessed on all the applicable MVPs. We believe clinician participation in subgroup reporting will be based on the scope of clinical care provided or a relevant specialty type and clinicians would not use subgroup reporting to game the MIPS program. We will monitor the ways in which clinicians form subgroups and will revisit this issue in future rulemaking if we discover that clinician participation in multiple subgroups is not what we intended.
We request public comments on this proposal.
(iii) Subgroup Composition Limitations
We are not proposing to require any criteria for the composition of subgroups at this time; however, as discussed in section IV.A.3.b.(3)(h) of this proposed rule, we are seeking comment on criteria that we could consider in the future, such as in the CY 2023 PFS rulemaking cycle. In developing the voluntary subgroup reporting proposal, we also considered whether we should limit the opportunity for clinicians to participate as a subgroup based on certain characteristics for the first year of voluntary subgroup reporting and in the future under mandatory subgroup reporting. As we have noted in this section of the rule, many stakeholders requested that we create an opportunity to participate via subgroups and suggested that participation in subgroups would allow clinicians across different specialties within a practice to report.
We considered whether we should limit the composition of a subgroup to clinicians of the same specialty or a related specialty. We are also aware clinicians providing patient care as part of a clinical team may practice in different locations under the same group (TIN) and may be interested in reporting through different subgroups. These clinicians may practice in specific clinical settings, such as dialysis centers or urgent care settings, and may use different third-party intermediaries, such as different EHR vendors or qualified registries. Alternatively, we considered if we should establish limits on the size of a subgroup, such as setting minimum and maximum thresholds for the number of clinicians that could participate as a subgroup. Similarly, we considered establishing a threshold where 75 percent of the eligible clinicians in a group or subgroup would have to be of the same specialty or a related specialty to report a given MVP, and thus form a subgroup. We also considered if we should establish criteria based on the number of clinicians practicing in a group, specialty mix assessed through analyzing previous billing patterns within a group, practice location, type of clinical setting, patient population, or scope of care provided. Additionally, we also considered establishing restrictions on the type of clinicians who can report a given MVP. In considering these potential restrictions, we are concerned we would be unable to anticipate the different methods by which groups may wish to utilize subgroup reporting without seeking public comment on the best approach.
As MVPs evolve and subgroup reporting becomes mandatory in the future, we plan to explore the options for establishing criteria for composition of subgroups. We recognize that MVPs will continue to be developed by the time subgroup reporting is mandatory for MVP reporting by multispecialty groups and that there will be clinicians in multispecialty groups who do not have an available MVP, and thus, would be unable to form subgroups. We believe it is valuable for multispecialty group practices to gain experience with subgroup reporting while MVPs continue to be developed. We also note that as described below in this section, APM Entities cannot be broken down into subgroups because APM Entities are often composed of multiple TINs. We refer readers to section IV.A.3.b.(4)(d) of this proposed rule for additional details on MVP and subgroup reporting requirements for APM Entities.
Therefore, we request comment from stakeholders on the options for multispecialty groups to participate as subgroups for reporting MVPs for the first year of voluntary subgroup reporting, beginning in the CY 2023 MIPS performance period/2025 MIPS payment year. We also request feedback from stakeholders on whether restrictions should be applied in the future for the composition of subgroups and any associated criteria that need to be established without needlessly limiting flexibility for clinicians involved in team-based care. We request public comment on the criteria which should be used to define what types of groups are required to report more than one MVP.
(iv) Proposed Subgroup Inclusions and Exclusions
(aa) Background
We recognize MIPS eligible clinicians participating in subgroups may be part of a group that has a portion of its Start Printed Page 39363 clinicians participating in MIPS as part of a virtual group, MIPS APM, or clinicians that do not attain QP or Partial QP status in an Advanced APM. Eligible clinicians may participate in MIPS if they meet at least one of the low-volume threshold criteria and choose to opt-in to MIPS reporting (83 FR 59740). Eligible clinicians (as defined at § 414.1305) who are not MIPS eligible, have the option to voluntarily report on applicable measures and activities for MIPS and these clinicians will not be subject to MIPS payment adjustment (81 FR 77041). Additionally, Partial QPs in Advanced APMs will have the option to elect whether or not to report under MIPS, which determines whether or not they will be subject to MIPS payment adjustments (81 FR 77062). We believe eligible clinicians in MIPS APMs, clinicians that do not attain QP or Partial QP status in Advanced APMs, or who are opt-in eligible may desire to participate as subgroups for reporting MVPs and may meet the proposed criteria for definition of a subgroup as described in section IV.A.3.b.(3)(c)(ii) of this proposed rule.
(bb) Proposed Subgroup Eligibility—Participants in MIPS APMs
In the CY 2021 PFS final rule (85 FR 84860), we finalized a policy to allow groups and APM Entities to report through the APP on behalf of their constituent MIPS eligible clinicians. We believe that an APM Entity should not be eligible to form subgroups for reporting MVPs or the APP because APM Entities are often composed of multiple TINs and may use multiple EHR systems. We believe that the definition of a subgroup consisting of one TIN, as proposed in section IV.A.3.b.(3)(c)(ii) of this rule, would not include APM Entities comprised of more than one TIN, which would result in exclusion of APM Entities from forming subgroups for reporting MVPs or the APP. We anticipate that eligible clinicians in APM Entities comprised of multiple TINs could choose to form subgroups through their affiliated TIN. We refer readers to section IV.A.3.b.(4)(d)(vi)(B) of this rule where we seek comment on whether there are strategies that CMS should consider to allow the formation of subgroups for clinicians in APM Entities comprised of multiple billing TINs. We also note that, in cases where an APM Entity is itself comprised of a single TIN, the single TIN could form subgroups under the TIN, rather than under the APM Entity ID.
(cc) Proposed Subgroup Exclusions—Opt-In Eligible Clinicians and Voluntary Participants
Based on historical data, a significantly low number of clinicians have utilized the following participation options in MIPS: Virtual groups; opt-in eligible clinicians; and voluntary reporters. For example, if the number of opt-in eligible clinicians remains the same as estimated in the CY 2021 PFS final rule (85 FR 85015), we anticipate that an estimated 0.3 percent of the total number of MIPS eligible clinicians would fit into this category. We believe that there are several operational considerations, such as implementation burden for stakeholders and CMS, value of subgroup reporting for these clinicians versus burden, scoring policies, etc. that must be addressed prior to allowing clinicians in these categories to participate as subgroups for reporting MVPs. Additionally, we believe that the definition of a subgroup consisting of one TIN, as proposed in section IV.A.3.b.(3)(c)(ii) of this rule, would not be applicable for clinicians in a virtual group because a virtual group is a combination of two or more TINs, resulting in exclusion of clinicians in virtual groups from participating as subgroups for reporting MVPs.
Therefore, beginning in the CY 2023 MIPS performance period/2025 MIPS payment year, we are proposing at § 414.1318(a)(2) that an individual clinician or group electing to participate in MIPS as an eligible clinician in accordance with § 414.1310(b)(1)(iii)(A) or § 414.1310(b)(2) is not eligible to participate as a subgroup. As we consider transitioning to MVPs and retiring traditional MIPS, we will revisit subgroup eligibility for opt-in eligible clinicians, voluntary participants and clinicians in virtual groups in future years. We also seek feedback from stakeholders on whether clinicians in these categories should be allowed to form subgroups in future years, and if there are additional criteria that should be established.
We seek public comment on this proposal.
(e) Subgroup Examples
In Appendix 3: MVP Inventory of this proposed rule, we are proposing seven MVPs for implementation in the CY 2023 MIPS performance period/2025 MIPS payment year. We have provided examples below to show how eligible clinicians could choose to participate as subgroups for reporting MVPs if these MVPs are finalized. These examples are not intended to be exhaustive of the eligible clinician types that could participate as subgroup.
Example 1: A group is composed of all anesthesiologists. In this example, all the clinicians in the group have the same primary specialty designation, which is an example for a single-specialty group. We would not anticipate that they would wish to form subgroups but could report the Patient Safety and Support of Positive Experiences with Anesthesia MVP as a group.
Example 2: Table 33 illustrates an example of subgroup reporting for a group consisting of anesthesiologists, orthopedic surgeons, and certified registered nurse anesthetists (CRNAs). In this example, the group could form a total of three subgroups. The anesthesiologists and CRNAs could form either one or two subgroups for reporting the proposed Patient Safety and Support of Positive Experiences with Anesthesia MVP as described in Table G: Proposed Patient Safety and Support of Positive Experiences with Anesthesia MVP Beginning with the CY 2023 MIPS Performance Period/2025 MIPS Payment Year of Appendix 3: MVP Inventory of this proposed rule. We believe the measures and activities included in this MVP would be most applicable to clinicians who provide anesthesia services to patients within the surgical setting, are considered anesthesiologists, or are other qualified anesthesia professionals. For instance, the anesthesiologists and the CRNAs could form separate subgroups for reporting on applicable measures and activities in the MVP. Alternatively, the CRNAs and the anesthesiologists could report on the applicable measures and activities in the MVP as one subgroup if this aligns better with how the subgroup would practice and they all report the same measures and activities. The orthopedic surgeons in the group could then form a separate subgroup to report the applicable measures and activities in the proposed Improving Care for Lower Extremity Joint Repair MVP.
Start Printed Page 39364

(f) Third-Party Intermediaries for Subgroup Reporting
As described in section IV.A.3.h.(2)(b) of this proposed rule, we are proposing at § 414.1400(a)(1) for third-party intermediaries to implement MVPs and subgroup reporting options for MIPS eligible clinicians starting with the CY 2023 MIPS performance period/CY 2025 MIPS payment year. Since subgroups will be implemented concurrently with MVPs, we believe that it is important that all third-party intermediaries support subgroup reporting in order for clinicians to meaningfully report MVPs. We refer readers to section IV.A.3.h.(2)(b) of this proposed rule for Start Printed Page 39365 additional details on requirements for third-party intermediaries supporting MVPs and subgroups.
(g) Public Reporting of Subgroup Performance Information
As described in section IV.A.3.i.(1) of this proposed rule, we propose to delay public reporting of subgroup performance information by an additional year. Our proposal would result in the public reporting of subgroup performance information beginning with the CY 2024 MIPS performance period/2026 MIPS payment year and each performance period/MIPS payment year thereafter. We refer readers to section IV.A.3.i.(1) of this proposed rule for additional details on the proposed policies related to public reporting of subgroup performance information on the compare tool.
(h) Future Vision of Subgroups
(i) Overview
Given the delay of subgroup and MVP implementation until the CY 2023 MIPS performance period/2025 MIPS payment year, we recognize there are additional policy nuances which need to be worked through during future rulemaking, and we would like to share our vision and request information to help us craft policy solutions. We believe team-based care is an essential element to providing high-quality care to patients and acknowledge some of the subgroup policies may be construed to create competition within groups. It is not our intention to create this competition, rather, we believe as MVPs continue to be created and evolve, this will also include MVPs that are focused on team-based care for some specialties. We share this vision below along with a request for information on the future of subgroup reporting. We welcome feedback and potential alternatives ideas we could consider ensuring the success of subgroup and MVP reporting.
(ii) Vision for Data Granularity
Ultimately, we envision that a future goal of the Quality Payment Program, particularly with MIPS and MVPs, is to ensure there is more granular data available for patients, clinicians, and other stakeholders. We envision an end state where technology will allow for the submission of discrete data elements and allow us to calculate measure performance for clinicians, subgroups, groups, and APM Entities, rather than having measure performance aggregated and calculated at a group or subgroup level prior to reporting. We anticipate more granular data will be available for patients, clinicians, and other stakeholders through a three-pronged approach of mandatory subgroup reporting, broad use of standards-based APIs that leverage the FHIR standard within EHRs (as discussed in section IV.A.1.c.(4)(a) of this proposed rule), and the creation and use of dQMs (as discussed in section IV.A.1.c.(2) of this proposed rule). We believe this would give patients specific and meaningful information which can better inform their choices when selecting a clinician and offer more targeted feedback to clinicians. We request information on the vision for data granularity.
(iii) Sunsetting Traditional MIPS
Given our goals for increasing the level of data that is available to clinicians and patients, we envision a future state where all multispecialty groups would participate in MIPS through subgroup reporting. As additional MVPs are developed and eligible clinicians are given the opportunity to report on MVPs, including reporting via subgroups, we believe that clinicians will have even more meaningful ways to participate in MIPS at a more discrete level. As discussed in section IV.A.3.b.(2)(d) of this proposed rule, we are considering retiring traditional MIPS, where it would no longer be available by the CY 2028 MIPS performance period/2030 MIPS payment year but would make any proposal to do so in a future rulemaking.
If we sunset traditional MIPS beginning in the 2028 MIPS performance period/2030 MIPS payment year, we anticipate that groups, particularly large multispecialty groups, would have had the opportunity to gradually ramp up their reporting on MVPs, gaining a few years of experience reporting on more than one MVP. This allows for clinicians to be assessed on information that is clinically meaningful to their scope of practice and to publicly report that information. At a high-level, if we finalize the proposal as described in section IV.A.3.b.(2)(d)(ii) of this rule, we anticipate that multispecialty groups would report more than 1 MVP beginning in the CY 2025 MIPS performance period/2027 MIPS payment year. We believe that clinicians in multispecialty groups should be assessed on measures and activities that are related to the scope of care that they provide. We believe that in order to meet the goals of MVPs and provide enhanced performance feedback to clinicians and to ensure more granular information is publicly available for patients, multispecialty groups must form subgroups to report additional information. Additionally, we do not believe that there will be an MVP that will be applicable to all types of clinicians within multispecialty groups. We refer readers to section IV.A.3.b.(2)(d) of this rule for additional details on the proposed timeline for MVPs and subgroup implementation.
(iv) Limiting Subgroup Composition to Single-Specialty
We are also considering placing limits around how clinicians can participate and be assessed as subgroups, particularly if clinician participation in subgroups must be restricted to a single specialty. As a central part of the MVP goals, we believe that the value of subgroup reporting would be for clinicians to be assessed and scored on measures and activities that are applicable to their scope of care while also allowing patients to have greater access to clinician information. We are concerned that we if do not place limitations on how subgroups can be constructed, subgroups could be formed in a way that would result in different types of clinicians assessed on measures that are only applicable to a small subset. We believe that without establishing limitations to subgroup composition prior to implementation, we would not meet the desired programmatic goals of MVPs and in many ways would replicate our concerns with the current state with traditional MIPS. One approach we could consider would be to limit clinicians in multispecialty groups to participate through single-specialty subgroups. Under this approach, we would determine specialty designation as defined by PECOS and are considering if it is feasible for this to be determined at the time of MIPS eligibility determination. We recognize many clinicians have more than one specialty designation in PECOS and may even have multiple PECOS profiles, which contain different specialty designations. We also recognize for many clinician types, the primary specialty designation is related to their clinical degree and not to the type of care they provide (such as PAs, NPs, etc.). To account for differences in care, we could set a threshold to be met in order for a subgroup to be considered a single-specialty subgroup. To align with other thresholds in the Quality Payment Program, such as the requirements for facility-based and hospital-based clinicians, we are considering requiring that 75 percent of clinicians in a subgroup have the same PECOS primary specialty designation or specialty codes on Medicare Part-B claims. This would mean that 75 percent of clinicians in subgroup would need to have the same Start Printed Page 39366 primary specialty designation. We believe that this would offer simplicity for clinicians and help assure that clinicians are being assessed with like clinicians within their subgroup on the same measures and activities.
For instance, if anesthesiologists are a part of multispecialty group, we anticipate a future state where the anesthesiologists would form a subgroup and report the Patient Safety and Support of Positive Experiences with Anesthesia MVP if it is finalized (detailed in this proposed rule under Table G: Proposed Patient Safety and Support of Positive Experiences with Anesthesia MVP Beginning with the CY 2023 MIPS Performance Period/2025 MIPS Payment Year in Appendix 3: MVP Inventory). Under this scenario, the scope of applicable measures in the MVP is narrow and we anticipate that this MVP would be appropriate for limited clinician types, thus a single-specialty subgroup. In another example, if the Optimizing Chronic Disease Management MVP (detailed in Appendix 3: MVP Inventory of this proposed rule) is finalized, we could anticipate that a group that included family physicians and cardiologists could form two subgroups, one single-specialty subgroup each for the family physicians and cardiologists, with each subgroup reporting the Optimizing Chronic Disease Management MVP. In this scenario, the family physician subgroup and cardiology subgroup would both be reporting on the same MVP, but they could be selecting different measures/activities from within the MVP as applicable to their scope of practice, as the Optimizing Chronic Disease Management MVP is more broadly applicable to a wider range of clinicians.
We do recognize that there may be issues that need to be resolved with this approach. We have concerns about some of the limitations of PECOS, especially for clinician types such as PAs and NPs, whose specialty in PECOS is not related to the scope of care they provide but rather the degree received. We believe that all clinician types are essential to team-based care and request comment on ways to comprehensively categorize clinician specialty. We believe setting a high but not absolute threshold would allow additional flexibilities for subgroups to accurately reflect the care they provide. We do have concerns that this may leave gaps in data because it would not require everyone under a given specialty to report together, may exclude clinicians given the limitations intrinsic to the PECOS system and Medicare Part-B claims data, and could result in clinicians being unable to report in a subgroup based on their specialty designation. For future consideration, we seek comment on setting a threshold for single-specialty subgroups and ways to overcome our concerns. We would also like to consider potential approaches to validating and auditing specialty information. Specialty information could be validated at the time of eligibility determination, during subgroup and MVP registration, or even through attestation. We seek comment on ways we can validate specialty information in a low burden, streamlined manner for future consideration.
Alternatively, we are considering whether subgroup composition could be determined by a different data source. We are interested in ways that we could provide guardrails for subgroups that do not use PECOS or use PECOS information to categorize specialties into specialty families or teams of clinicians who practice in relevant specialties for a given MVP. For ease of readability, we will refer to this concept as specialty families for the remainder of this discussion. Under this alternative, we anticipate that during the subgroup and MVP registration period, a practice administrator or the clinicians in a particular subgroup would attest that the clinicians in a subgroup practice similar scopes of care. We welcome feedback on how specialty families could be identified and what criteria would need to be established for us to set requirements on subgroup formation. For example, specialty families could be constructed similarly to how the Aligned Other Payer Medical Home Model[198] defines primary care focus through identifying multiple specialties to include the following Physician Specialty Codes: 01 General Practice; 08 Family Medicine; 11 Internal Medicine; 16 Obstetrics and Gynecology; 37 Pediatric Medicine; 38 Geriatric Medicine; 50 Nurse Practitioner; 89 Clinical Nurse Specialist; and 97 Physician Assistant. As a third alternative, we have also considered whether we should analyze claims data to identify the primary clinician specialty based on their billing patterns. We believe that this could be a way to help validate subgroup composition for clinicians who practice in more than one specialty. We request comment on these three approaches to setting limitations around the composition of subgroups for future consideration.
As an alternative to establishing limits on how subgroups could be formed, we are also considering adding to the MVP specifications an approved list of specialties and clinician types permitted to report each MVP. Instead of directly limiting the composition of the subgroup, this would limit who can report a given MVP. We believe this option offers additional benefit of not being specific to subgroup reporting but possibly standardizing MVP reporting and impacting clinicians and groups as well. We believe this approach may also promote team-based care and further ensure that MVPs are relevant to those who report them. However, we do have concerns this could have unforeseen consequences in that certain MVPs may be appropriate for specialties not on the designated list, and we would not want to inadvertently place artificial limitations on how clinicians provide care and report to MIPS. We also request comment on this approach for our future consideration.
(v) Request for Information on the Future Vision of Subgroup Reporting
As we look towards future rulemaking, we also request feedback on:
- If the determination of specialty composition should be made during the MVP registration process, as discussed in section IV.A.3.b.(4)(f) of this proposed rule.
- Additional approaches we should consider to incentivize team-based care as we move towards MVP and subgroup implementation.
- If there are other approaches or data sets, in addition to PECOS, that should be considered to classify the scope of care clinicians provide.
- If individual clinicians or groups should attest to their specialty during MVP and subgroup registration.
- If there may be ways to group clinicians in like specialties who may provide similar care and would be interested in reporting the same measures and activities under a given MVP.
- If we should establish criteria or set a threshold for groups to be deemed multispecialty.
- If there are concepts other than specialty that could demonstrate that a subgroup is composed of clinicians who provide care relevant to the MVP the subgroup intends to report.
Overall, we request public comments on how subgroups should be structured, assessed, and scored in a future state as clinicians gain familiarity with the program, more MVPs are developed, and technological advancements allow for low-burden reporting. Start Printed Page 39367
(4) MVP Requirements
(a) Overview
In the CY 2020 PFS final rule (84 FR 62948), we finalized at § 414.1305 that MIPS Value Pathway means a subset of measures and activities established through rulemaking. We describe our vision for MVPs to connect the four performance categories while using a foundational layer of population health claims-based measures and interoperability, on which to build, quality, cost, and improvement activity linkages. In the CY 2021 PFS final rule (85 FR 84849 through 84859), we finalized a set of MVP development criteria and a process to receive MVP candidates from stakeholders. Through this proposed rule, we are proposing to establish additional MVP related policies to support the implementation and availability of MVPs. In this section, we propose: (1) Refinements to the MVP development criteria; (2) a maintenance process for established MVPs; (3) MVP reporting requirements; and (4) the MVP registration process.
(b) MVP Development and Maintenance
(i) MVP Development Criteria
(A) General MVP Structure
From the time the CY 2021 PFS final rule published, we have solicited feedback from several stakeholders who have submitted MVP candidates for CMS consideration utilizing the MVP candidate solicitation process (85 FR 84854 through 84856). Through this feedback, we have understood that the quality and patient improvement priorities of specialists may differ based on the way they practice. There are clinicians who practice utilizing a team-based approach, involving several clinicians of different specialties working together and for that reason, find quality reporting that reflects that approach more meaningful. Team-based health care is defined by the National Academy of Medicine as "the provision of health services to individuals, families, and/or their communities by at least two health providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care."[199] Other clinicians may be specialized in a manner where they focus on a limited number of procedures.
For these reasons, we believe there are various ways to approach MVP development, and the method utilized would be dependent on the topic measured by the MVP. One method is to construct MVPs in a manner that is broad, for example, addressing cancer care comprehensively versus the creation of MVPs for each unique diagnosis of cancer care. Another method is to construct MVPs in a more granular manner, for example, addressing a specific procedure, such as hip and knee arthroplasty. A third approach is to structure MVPs in a manner that reflects a team-based healthcare model. This approach considers the patient's care from a holistic perspective, involving various clinicians as needed. One such example is around surgical care, which involves several clinician types, such as surgeons and anesthesiologists. We believe this approach captures the patient experience and outcomes in a manner that is meaningful, that would result in patient improvement. In the CY 2021 PFS final rule (85 FR 84850), we finalized MVP development criteria that accounts for the development of MVPs collaboratively by multiple specialties for this reason. We believe that the team-based healthcare model has an impact to patient outcomes and encourage the use of this approach, as feasible, when developing MVPs.
In section IV.A.3.b.(4)(b)(ii) of this proposed rule, we discuss a proposed maintenance process for MVPs. In instances where an MVP is initially implemented, for example, to address a specific procedure and there is opportunity to evolve the MVP over time to reflect the team-based healthcare model, we would strongly encourage that transition.
However, we do understand there is not a "one size fits all" MVP structure that is suitable for all specialties and believe the use of one of the structure methodologies is appropriate for MVP development.
(B) Selection of Measures and Improvement Activities Within an MVP
As described above, in the CY 2021 PFS final rule (85 FR 84849 through 84850), we established a set of criteria for use in the development and selection of MVPs. Specifically, we had finalized that we were not prescriptive on the number of quality measures that are included in an MVP (85 FR 84850). Through this rulemaking cycle, we are proposing reporting requirements for MVPs, and discuss the allowance of clinician choice in selecting which quality measures and improvement activities to report, as described in detail below in section IV.3.b.(4)(d) of this proposed rule. We believe that it is important to provide clarity in our expectations of the number of quality measures and improvement activities that are available for an MVP Participant to choose.
Generally, an MVP should include a sufficient number of quality measures and improvement activities to allow MVP Participants to select measures and report them to meet the reporting requirements outlined in section IV.3.b.(4)(d) of this proposed rule. To the extent feasible, MVPs should include a maximum of 10 quality measures and 10 improvement activities, to offer MVP Participants some choice without being overwhelming. However, we understand that the total number of measures and activities available in an MVP would depend on the MVP structure. For example, in Appendix 3: MVP Inventory, we are proposing the Optimizing Chronic Disease Management MVP that includes 9 quality measures and 12 improvement activities. Chronic disease can broadly encompass several conditions; therefore, we have selected measures and improvement activities that are closely aligned to the topic and offer clinicians some choice. We refer readers to Appendix 3: MVP Inventory for discussion of our proposed MVPs.
(aa) Requirement of Outcomes or High Priority Measures
In section IV.3.b.(4)(d)(ii) of this proposed rule, we propose MVP quality reporting requirements, that are similar to the requirements of traditional MIPS under § 414.1335. We discuss a proposal to require the reporting of one outcome measure or high priority measure (if an outcome measure is not available). Accordingly, we believe it is important to modify the previously finalized MVP development criteria (85 FR 84849 through 84859), where we describe the criteria for including quality measures in an MVP. We believe we need to update the criteria to ensure MVPs are developed in a manner that accounts for this proposed quality reporting requirement.
(AA) Proposed Outcomes Measures Requirement
Therefore, we propose that beginning with the CY 2022 MIPS performance period/2024 MIPS payment year, MVPs must include at least one outcome measure that is relevant to the MVP topic, so MVP Participants are measured on outcomes that are meaningful to the Start Printed Page 39368 care they provide. In addition, beginning with the CY 2022 MIPS performance period/2024 MIPS payment year, each MVP that is applicable to more than one clinician specialty should include at least one outcome measure that is relevant to each clinician specialty included. This is important since MVPs are proposed to be constructed in a manner that may include one or more clinician specialties, as described above in section IV.3.b.(4)(b)(i)(B)(AA) of this proposed rule, and there should be outcome measures included in the MVP that are relevant to each clinician specialty.
We anticipate over the next few years, there may be opportunities where outcomes-based measures are developed and can be reported utilizing the administrative claims collection type. For example, in the CY 2021 PFS final rule (85 FR 85049 through 85051), we finalized the Risk-standardized complication rate (RSCR) following elective primary total hip arthroplasty (THA) and/or total knee arthroplasty (TKA) for Merit-based Incentive Payment Systems (MIPS) outcome-based administrative claims measure. In addition, in Appendix 1: MIPS Quality Measures of this proposed rule, we propose at Table A.4. the Risk-Standardized Acute Unplanned Cardiovascular-Related Admission Rates for Patients with Heart Failure for the Merit-based Incentive Payment System, which is also an outcome-based administrative claims measure. We propose to allow the inclusion of outcomes-based administrative claims measures within the quality component of an MVP. We believe these measures can be used to meet the outcome measure requirement discussed under the MVP reporting requirements in section IV.A.3.b.(4)(d)(ii) of this proposed rule. We request comments on these proposals.
(BB) Proposed Exception When None Are Available
As described in the CY 2021 PFS final rule (85 FR 84850), we are aware that not all specialties and subspecialties may have outcome measures currently available to them in the MIPS program. We are aware of this measurement gap, and believe it is appropriate to allow for the use of high priority measures when outcome measures are not available.
Therefore, we propose that beginning with the CY 2022 MIPS performance period/2024 MIPS payment year, in instances when outcome measures are not available, each MVP must include at least one high priority measure that is relevant to the MVP topic, so MVP Participants are measured on high priority measures that are meaningful to the care they provide. In addition, beginning with the CY 2022 MIPS performance period/2024 MIPS payment year, each MVP must include at least one high priority measure that is relevant to each clinician specialty included. This is important since MVPs are proposed to be constructed in a manner that may include one or several clinician specialties, as described above in section IV.3.b.(4)(b)(i)(A) of this proposed rule. As previously established at § 414.1305, we define high priority measures to include outcome (including intermediate-outcome and patient-reported outcome), appropriate use, patient safety, efficiency, patient experience, care coordination, or opioid-related quality measures.
We continue to encourage stakeholders to utilize our established pre-rulemaking processes, such as the Call for Measures: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/Pre-Rulemaking, to develop outcome measures relevant to their specialty if outcome measures currently do not exist and for eventual inclusion in an MVP. We encourage, to the extent feasible, the inclusion of several outcome and/or high priority measures, if available and relevant to the MVP topic. The inclusion of several measures would allow clinicians to have some choice in selecting the most relevant outcome or high priority measure that is meaningful to their specific practice. We request comments on these proposals.
(bb) Encouragement To Include Patient-Centered Measures
In the CY 2021 PFS final rule (85 FR 84850), we finalized MVP development criteria that takes into consideration the patient voice. Specifically, we finalized MVP development and selection criteria that considers the inclusion of (to the extent feasible), patient-reported outcome measures, patient experience measures, and/or patient satisfaction measures. Through interactions with stakeholders and presentations, we have referred to these measures as patient-centered measures.
We clarify that we are not proposing any revisions to our previously finalized policy, however, we believe it is important that we rely on a consistent understanding of patient-centered measures. Health Affairs[200] stated the following with respect to such measures, "Measures should be patient-centered and incorporate new approaches to assessing patient health status and patient experience. Such measures include assessment of clinical outcomes, patient-reported outcome measures, as well as new approaches to evaluation of patient experience." We request comment on whether there are other aspects of patient measurement that should be considered as a part of the patient-centered measures definition.
We acknowledge that our existing portfolio of patient reported outcome measures is limited and may not be applicable to all specialties and subspecialties. We continue to encourage stakeholders to utilize our established pre-rulemaking processes, such as the Call for Measures, described in the CY 2020 PFS final rule (84 FR 62953 through 62955) to develop patient reported outcome measures relevant to their specialty. In addition, we encourage measure stewards of new and existing quality measures in MIPS to consider updating their measures to include the patient centered approach through the measure maintenance cycle or the development of new measures.
(cc) Requirements for QCDR Measures Considered for an MVP
In the CY 2021 PFS final rule (85 FR 84857 through 84859), we finalized that QCDR measures that were approved in the previous year may be considered for inclusion within an MVP. In addition, we finalized at § 414.1400(b)(3)(v)(C)(4) that QCDR measures should be fully tested at the clinician level prior to the QCDR measure being included in an MVP. We refer readers to the CY 2021 PFS final rule (85 FR 84857 through 84859) for the specific policies that were previously finalized. Through this proposed rule, we seek to clarify when we would expect a QCDR to prove that their QCDR measure is fully tested before it is implemented within an MVP. QCDRs must self-nominate as a QCDR and submit QCDR measures for CMS consideration within the 60-day self-nomination period that begins on July 1st of the calendar year prior to the applicable performance period and ending on September 1 of the same year. In order to determine whether a QCDR measure may be finalized within an MVP, we will need to receive QCDR measure testing data for review by the end of the self-nomination period, that is no later than September 1 of the year prior to the applicable performance Start Printed Page 39369 period. We encourage, as feasible, that QCDRs share testing data for their fully tested QCDR measures at the time of MVP candidate submission which may be prior to the September 1st deadline. If a QCDR is unable to submit testing data to demonstrate that their QCDR measure is fully tested at the clinician level by end of the self-nomination period (September 1st) or does not otherwise meet our requirements, we will not finalize the inclusion of the QCDR measure within an MVP.
(C) Foundational Layer
In the CY 2020 PFS final rule (84 FR 62947 through 62948), we establish that the implementation of a foundational population health core measure set using administrative claims-based quality measures that can be broadly applied to communities or populations can result in MVPs that provide more uniformity in how the program measures population health, reduce clinician reporting burden, focuses on important public health priorities, and increases the value of MIPS performance data. In addition, we discuss our beliefs that interoperability is also a foundational element that would apply to all clinicians, regardless of MVP, for whom the Promoting Interoperability performance category is required. Furthermore, we also discuss the importance of the integration of population health measures and Promoting Interoperability measures into MVPs, as they provide a degree of standardization across all clinician types and promotes an infrastructure on which to assess and improve value-based care.
(aa) Population Health Measure
In the CY 2021 PFS final rule, we discuss the inclusion of population health measures calculated from administrative claims-based data as a part of the foundational layer of MVPs, in an effort to improve patient outcomes, reduce reporting burden and costs, and better align with clinician quality improvement efforts. We refer readers to the CY 2021 PFS final rule (85 FR 84856 through 84857) where we discuss population health. Through this proposed rule, we propose: (1) To define the term population health measure; and (2) update the population health measure inventory.
(AA) Proposed Definition
In the 2020 CMS Quality Measure Development Plan—2020 Population Health Environmental Scan and Gap Analysis Report (https://www.cms.gov/files/zip/2020-mdp-population-health-e-scan.zip), we conducted an environmental scan to identify gaps in population health measurement within MIPS, specifically for use in the foundational layer of MVPs. Through this environmental scan and gap analysis, we have settled on a definition of "population health measure". In addition, as described in the "Roadmap for Promoting Health Equity and Eliminating Disparities": https://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=86046, developed by the National Quality Forum, health equity continues to be a priority for the agency, we believe it is important to include the measurement of health disparities when measuring population health.
In this proposed rule, we propose to codify this at § 414.1305, such that a population health measure means a quality measure that indicates the quality of a population or cohort's overall health and well-being, such as, access to care, clinical outcomes, coordination of care and community services, health behaviors, preventive care and screening, health equity, or utilization of health services. We request comments on this proposal.
(BB) Population Health Measures Inventory
In the CY 2021 PFS final rule (85 FR 84856 through 84857), we finalized the inclusion of the Hospital-Wide, 30-day, All-Cause Unplanned Readmission (HWR) Rate for the Merit-Based Incentive Payment System Program (MIPS) Eligible Clinician Groups as a part of the population health measures within the foundational layer of MVPs.
As described in Appendix 1: MIPS Quality Measure Inventory of this proposed rule, we are proposing to include the Clinician and Clinician Group Risk-standardized Hospital Admission Rates for Patients with Multiple Chronic Conditions in the MIPS program. This measure is calculated from administrative claims and measures the annual risk-standardized rate of acute, unplanned hospital admissions among Medicare FFS patients aged 65 years and older with multiple chronic conditions (MCCs). We refer readers to Appendix 1: MIPS Quality Measure Inventory of this proposed rule for additional details on this measure. If finalized, this measure would be an additional population health measure for MVP reporters to submit as part of the Foundational Layer. We have heard from stakeholders the need for more specialty relevant population health measures. We encourage stakeholders to utilize the aforementioned 2020 CMS Quality Measure Development Plan—2020 Population Health Environmental Scan and Gap Analysis Report as a resource to explore population health measure development. Furthermore, we continue to encourage stakeholders to utilize our established Call for Measures process, described in the CY 2020 PFS final rule (84 FR 62953 through 62955) to submit fully developed population health measures for our consideration.
(bb) Promoting Interoperability
In the CY 2021 PFS final rule (85 FR 84849 through 84850), as a part of the MVP development criteria, we had finalized that MVPs must include the full set of Promoting Interoperability measures. Any updates made to the set of Promoting Interoperability measures through traditional MIPS will apply to the MVPs. Therefore, we refer readers to section IV.A.3.d.(4) of this proposed rule where we discuss Promoting Interoperability performance category proposed policies and updates.
(D) Health Equity Measures in MVPs—Request for Information (RFI)
In section IV.A.1.c.(5)(d) of this proposed rule, we discuss our request for information on closing the health equity gap in CMS clinician quality programs, describing our current efforts as an agency, and asking specifically what other efforts can we take within the MIPS program to further bridge the equity gap. We believe there is potential to address health equity specifically through MVPs. As described in Appendix 3: The MVP Inventory, we are proposing improvement activities related to health equity in all 7 proposed MVPs. We also believe there is value in including quality measures that capture health equity in each MVP. However, in evaluating our current measure inventory, we acknowledge that we lack the availability of health equity measures. We intend on prioritizing the development of health equity measures through future cycles of measure development, and would also encourage stakeholders to do the same by utilizing our established processes, such as the Call for Measures: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/Pre-Rulemaking, to develop health equity measures for consideration of the MIPS program and potential inclusion in an MVP. Health equity continues to be a high priority for the agency and for the MIPS program. We envision a future state where health equity measures would be included in all MVPs. We request information on the following:
- Should health equity measures be developed in a manner to be broadly Start Printed Page 39370 applicable to the various specialties and subspecialties that participate in MIPS?
- Is there value in the development of more specialty specific health equity measures?
- Considering MIPS and MVPs includes several specialties and subspecialties, what factors should be considered when developing a health equity measure?
- Should we include a health equity measure in the foundational layer of all MVPs, as a required measure, in the future? If not, why not?
(ii) Proposed Maintenance Process for MVPs
We believe it is important that we implement a maintenance process for established MVPs. Independent of the implementation of MVPs; the individual measures typically undergo annual updates and maintenance for several reasons. These updates may include technical coding updates, changes to clinical guidelines, or modifications to various aspects of the measure specification (such as the numerator or denominator). It will be important that we monitor when changes are made to individual measures to ensure that the updated measure is relevant and should be maintained within the MVP.
Therefore, beginning with the CY 2023 MIPS performance period/2024 MIPS payment year, we propose an annual maintenance process for finalized MVPs. In order to ensure that various stakeholder perspectives are also considered, we propose a solicitation process to solicit stakeholder recommendations for potential updates to established MVPs. Under this proposal, beginning in January of the year prior to the performance period, stakeholders could submit their recommendations to revise established MVPs. We would accept stakeholder input on a rolling basis. Any changes to MVPs would be addressed through future notice and comment rulemaking, for example, suggesting the addition or removal of a quality measure or improvement activity. If changes are made to existing individual measures and activities, they would be made under the traditional MIPS performance category policies and criteria for measures and activities and those changes would be reflected within the MVP. We would be unable to communicate with a stakeholder about whether or not their recommendations are accepted ahead of rulemaking. Additional logistical information, such as where to submit recommendations would be provided through the QPP resource library and listserv messaging, prior to the opening of the solicitation process. However, CMS would consult with the stakeholders who originally nominated the MVP about any publicly recommended changes to that MVP. We would be unable to communicate with a stakeholder whether or not their recommendations would be accepted ahead of rulemaking and CMS would ultimately decide whether updates to the established MVPs should be made. To be clear, the annual maintenance process for finalized MVPs would be separate from the new MVP candidate solicitation process that was described in the CY 2021 PFS final rule (85 FR 84854 through 84856). We request comments on these proposals.
(c) Establishing a Portfolio of MVPs
In this proposed rule, we are proposing seven MVPs on the following topics: Rheumatology, Stroke Care, Ischemic Heart Disease, Chronic Disease Management, Emergency Medicine, Lower Extremity Joint Repair, and Anesthesia. We refer readers to Appendix 3: MVP Inventory for a full description of each MVP, proposal rationale, and where we are soliciting comments.
We anticipate that the portfolio of MVPs would continue to grow over the next few years. Through the review of data received through MIPS reporting for the 2019 performance period, we have identified the ten specialties who have the most participants in the MIPS program. These specialties include primary care, emergency medicine, diagnostic radiology, anesthesiology, cardiology, obstetrics and gynecology, orthopedic surgery, psychiatry, general surgery, and ophthalmology. We believe it is important to develop MVPs that address these specialties, amongst the other specialties that participate in the program. We are, however, aware of the limited availability of relevant cost measures for all specialties and subspecialties and intend to address this concern through this proposed rule. We also refer readers to section IV.A.3.d.(2)(c) of this proposed rule, for our proposal on Cost measure development by stakeholders, where we discuss a potential strategy to mitigate the issue of a limited inventory of cost measures that would potentially remove barriers for MVP implementation.
(d) Proposed MVP Reporting Requirements
(i) Overview
We have reviewed the existing reporting requirements under traditional MIPS and believe that by changing the reporting requirements for MVPs, we would reduce reporting burden. We believe MVP reporting would allow for measurement that is more meaningful by requiring clinicians to report on measures and activities that comprehensively reflect an episode of care or clinical condition. We have heard from stakeholders the importance of having a choice when reporting. In this section, we discuss our proposals for MVP reporting requirements and subgroup reporting limitations for the Quality performance category; Cost performance category; Improvement Activities performance category; and the foundational layer-, which consists of the Promoting Interoperability performance category, and the population health measures.
(ii) Proposed Quality Reporting Requirements in MVPs
Since MVPs would include cohesive and complementary subsets of measures and activities that are relevant and to a given specialty, we believe that MVP Participants would report on measures that provide more meaningful and actionable results. MVP Participants would have the opportunity to select from a subset of measures within an MVP. Furthermore, as discussed above, we believe it is important to continue to require the reporting of outcome and high priority measures in MIPS. Therefore, at § 414.1365(c)(1), we propose that except as provided in paragraph § 414.1365(c)(1)(i), an MVP Participant must select and report, if applicable, 4 quality measures, including 1 outcome measure (or, if an outcome measure is not available, 1 high priority measure, included in the MVP, excluding the population health measure required under paragraph (c)(4)(ii). We discuss in section IV.A.3.b.(4)(b)(i)(B)(aa)(AA) of this proposed rule, that there may be instances where MVPs are developed to include outcomes-based administrative claims measures within the quality component of an MVP, where those measures are not considered to be population-health based. In such instances, we believe it would be appropriate to allow MVP Participants to select to be calculated on the outcomes-based administrative claims measure, at the time of MVP registration, and to allow that measure to meet the outcome measure requirement of MVP quality reporting.
In addition, we have concerns about the ability of small practices to report all required measures in the MVP quality performance category when they select Medicare Part B claims measures as a collection type. In cases when an MVP includes fewer than 4 Medicare Part B claims measures, an MVP Participant in Start Printed Page 39371 a small practice would need to report an additional collection type which would add reporting burden. We are concerned that small practices do not have the same resources to meet the quality reporting requirement of 4 measures if the MVP does not include 4 Medicare Part B claims measures. We want to establish policy that does not penalize a small practice for submitting an MVP. Therefore, we propose at § 414.1365(c)(1)(i) that paragraph § 414.1365(c)(1), does not apply to a small practice that reports on an MVP that includes fewer than 4 Medicare Part B claims measures, provided that the small practice reports each such measure that is applicable.
We request comments on these proposals. We refer readers to section IV.A.3.b.(5)(b)(i) of this proposed rule for details on the MVP quality scoring proposals.
(iii) Proposed Cost Reporting Requirements in MVPs
As MVPs are implemented and available for reporting, each MVP would only include cost measures that are relevant and applicable to the MVP topic. Therefore, the number of cost measures in a given MVP may vary depending on the clinical topic of the MVP. An MVP may include the episode-based cost measures that are relevant to the topic, total per capita cost measure (TPCC), and/or Medicare Spending Per Beneficiary Clinician (MSPB Clinician) measure. As such, we propose at § 414.1365(c)(2) that an MVP Participant is scored on the cost measures included in the MVP they select and report. To be clear, MVP Participants would not submit data for the cost measures; they would be calculated by CMS using administrative claims data, as in traditional MIPS. We request comments on this proposal.
In addition, we refer readers to section IV.A.3.b.(5)(b)(ii) of this proposed rule for details on the MVP cost scoring proposals.
(iv) Proposed Improvement Activity Requirements in MVPs
Similar to the quality performance category within MVPs, we also believe the improvement activities performance category should provide clinicians with an opportunity to select from a subset of improvement activities within an MVP that are relevant to the clinical topic being measured. Therefore, at § 414.1365(c)(3), we propose that MVP Participant who reports an MVP, must report one of the following: Two medium-weighted improvement activities; one high-weighted improvement activity; or participation in a certified or recognized patient-centered medical home (PCMH) or comparable specialty practice as described at (82 FR 53652) and at § 414.1380(b)(3)(ii). We note that these proposed MVP improvement activity requirements are reduced in comparison to what is required in traditional MIPS (82 FR 53652) under which we generally require two high-weighted activities, one high-weighted and two medium-weighted activities, four medium-weighted activities, or participation in a certified or recognized patient-centered medical home (PCMH) or comparable specialty practice. We believe reduced reporting requirements are necessary to support adoption of and reduce burden for implementation of MVPs. We request comments on this proposal and refer readers to section IV.A.3.b.(5)(b)(iii) of this proposed rule for proposals related to MVP improvement activities scoring and discussion of why improvement activities are double-weighted under MVP reporting.
(v) Proposed Reporting Requirements for the Foundational Layer
(A) Promoting Interoperability
(aa) Proposed Reporting Requirements
As described in the CY 2021 PFS final rule (85 FR 84849 through 84853), all MVPs should include the entire set of Promoting Interoperability measures, as a part of the foundational layer. We do not intend to establish different reporting requirements for Promoting Interoperability for MVPs from what is established under traditional MIPS. Therefore, we propose at § 414.1365(c)(4)(i) that an MVP Participant, is required to meet the Promoting Interoperability performance category reporting requirements described at § 414.1375(b). We request comments on this proposal and refer readers to section IV.A.3.b.(5)(b)(iv) of this proposed rule for details on the proposals for MVP Promoting Interoperability scoring and reweighting.
(bb) Subgroup Limitations
As noted in section IV.A.3.b.(3) of this proposed rule, we believe that subgroups should be assessed using subgroup level data to the extent that it is operationally feasible. However, through the MVP Town Hall (85 FR 84846), we heard from stakeholders that some clinicians would need additional time to resolve operational challenges, including challenges related to configuration of EHR systems. Given these operational challenges, as well as other considerations specific to the Promoting Interoperability performance category, we believe that each subgroup should submit their affiliated group's data for the Promoting Interoperability performance category and receive a score based on that data. We acknowledge that requiring each subgroup to submit their affiliated group's data could result in duplicative reporting of the same data if their affiliated group also reports as a group for the Promoting Interoperability performance category. However, we believe that this approach is the most appropriate way to address the operational challenges identified by stakeholders and other issues specific to the Promoting Interoperability performance category. For instance, requiring clinicians to report Promoting Interoperability by subgroup may initially disincentivize clinicians from choosing to report MVPs as it may exacerbate the reporting burden and use of resources by a smaller cohort of clinicians. Furthermore, the Promoting Interoperability measures are applicable to many clinician types and are not designed to be specialty specific like the quality measures, therefore, it is unclear whether an advantage of assessing Promoting Interoperability performance on a subgroup of clinicians exists. Other performance categories include specialty specific measures, where assessment of performance at the subgroup level may be more meaningful. Therefore, we propose at § 414.1365(c)(4)(i)(A) that for the CY 2023 and 2024 MIPS performance periods/2025 and 2026 MIPS payment years, to require an MVP Participant that is a subgroup to submit its affiliated group's data for the Promoting Interoperability performance category. The submission of the affiliated group's data would be on the subgroup's behalf. If the affiliated group chooses to report as a group for the Promoting Interoperability performance category, the group still would be required to submit its own data separately and pursuant to the reporting rules for groups.
Alternatively, we considered proposing that for the 2023 and 2024 MIPS performance periods/2025 and 2026 MIPS payment years that each subgroup would be required to submit either their affiliated group's data or their subgroup data for the Promoting Interoperability performance category. We considered requiring subgroups to indicate whether they would be submitting data for the Promoting Interoperability performance category at the group or subgroup level. However, we believe that the advantages of Start Printed Page 39372 reporting Promoting Interoperability performance category data at the subgroup level are not clear at this time and such a proposal, may introduce additional and unnecessary complexity.
In a future state, we could reassess whether subgroups should be required to submit subgroup level performance data for the Promoting Interoperability performance category. We refer readers to section IV.A.1.c.(5) of this proposed rule for details on the Digital Quality Measurement Blueprint and the Fast Healthcare Interoperability Resources (FHIR)-standard. Some subgroups may prefer to have the ability to report Promoting Interoperability performance category data at the subgroup level.
We request public comment on whether subgroups should be allowed or required to submit subgroup level performance data for the Promoting Interoperability performance category beginning with the performance period in 2023/2025 MIPS payment year, or in the future. We also request comment on any technical challenges that clinicians may encounter in reporting on Promoting Interoperability measures for a subgroup using technology certified to the existing certification criteria for reporting and capturing this information at § 170.315(g)(1) and (2). In addition, we request public comment on the aforementioned issues that would give us a better understanding on whether we should reconsider subgroup reporting in the future.
As previously discussed in the CY 2020 PFS proposed rule (84 FR 40734), in future years we may consider customizing the Promoting Interoperability measures in each MVP and we are seeking comment on how the Promoting Interoperability performance category could evolve in the future to meet our goal of greater cohesion between the MIPS performance categories. We believe that eligible clinicians could benefit from more targeted approaches to assessing the meaningful use of certified EHR technology which aligns with clinically relevant MVPs cutting across the MIPS performance categories. For instance, we may consider how measures in the Promoting Interoperability performance could align with specific MVPs.
We may also consider developing Promoting Interoperability measures which are better tailored to specific MVPs, or that seek to assess the use of health IT associated with reporting measures in other categories and could be paired with these measures under an MVP. Finally, we may consider whether existing Promoting Interoperability measures could be tailored to specific populations addressed under an MVP. We invite comment on these concepts, as well as other suggestions for how the Promoting Interoperability performance category could align with the MVP framework in the future.
(B) Population Health Measures
As described in the CY 2017 Quality Payment Program final rule (81 FR 77130 through 77136) we had received public comment that not all population health measures are applicable or attributable to all specialties. In order to mitigate this concern, at § 414.1365(c)(4)(ii), we propose that an MVP Participant is scored on 1 population health measure in accordance with paragraph § 414.1365(d)(1). To be clear, the population health measure calculation does not contribute to the required reporting of four quality measures, as described at § 414.1365(c)(1) and in section IV.A.3.b.(4)(d)(ii) of this proposed rule. Since the aforementioned population health measures are administrative claims based, they do not require data submission from clinicians. Therefore, it is important that an election period is established in which MIPS eligible clinicians, groups, subgroups, and APM entities would identify which MVP and population health measure they intend to report. We refer readers to the proposed registration process below and intend to provide additional guidance through subregulatory means.
In crafting our proposal, we also considered the alternative where we wouldn't require MVP participants to select which population health measure to be calculated on. Under this alternative considered, we would require and calculate both population health measures and apply the higher score to the quality score. While we thought this approach would reduce some of the burden associated with requiring this selection at the time of MVP registration, we ultimately decided to propose to allow MVP participants to select which population health measure to be calculated on. As discussed above, this selection process is being proposed in an effort to mitigate some of the previously stated concerns stakeholders had with these measures.
We request comments on our proposal as discussed above and refer readers to section IV.A.3.b.(5) of this proposed rule for details on the scoring of population health measures.
(vi) Subgroup Reporting
(A) Subgroup Reporting Overview
As discussed in section IV.A.3.b.(3) of this proposed rule, subgroup reporting would provide an avenue for clinician teams within a larger group to be able to submit MVPs that are clinically relevant to them and would be a first step in allowing more granular clinician information to be made available to patients. To generate more clinically relevant and granular information about clinician performance, we believe that subgroups should be assessed using subgroup level data to the extent that it is operationally feasible. We anticipate more granular data would be available for patients, clinicians, and other stakeholders through a three-pronged approach of mandatory subgroup reporting, broad use of standards-based APIs that leverage the FHIR standard and the creation and use of dQMs as discussed in section IV.A.1.c.(5) of this proposed rule. We believe that subgroups should report data for the quality and improvement activities performance categories as a subgroup. The cost performance category does not require data submission; however, as described in section IV.A.3.b.(5)(b)(ii) of this proposed rule, we believe cost data should be assessed at the subgroup level as well.
(B) Proposed Subgroup Reporting Limits
As described in section IV.A.3.b.(2)(d)(i) of this proposed rule, we are proposing voluntary reporting of MVPs as a gradual approach to prepare stakeholders through the transition plan for MIPS before eventually requiring reporting through an MVP or the APP. As a part of the transition, we discuss our intention to continue to offer reporting through traditional MIPS at the group level, as discussed in section IV.A.3.b.(4)(d), to allow clinicians and groups additional time to continue reporting in traditional MIPS while we work expand the inventory of MVPs over the next few years.
While we intend to allow for this flexibility through the transition, we believe that groups should only form subgroups if they are reporting through an MVP or the APP and not through traditional MIPS. As such, we propose at § 414.1318(c)(2) that individual eligible clinicians that elect to participate in MIPS as a subgroup will have their performance assessed at the subgroup level across all of the MIPS performance categories based on an MVP in accordance with § 414.1365, and on the APP in accordance with § 414.1367, as applicable. Subgroups that are MVP Participants must adhere to an election process described in § 414.1365(b). This includes MIPS eligible clinicians who are APM participants that choose to report on an MVP as a subgroup. We believe Start Printed Page 39373 encouraging the subgroup reporting in MVPs is an important step to help MVP Participants transition to MVP reporting in the future.
As stated in the CY 2021 PFS final rule (85 FR 84846), we envisioned subgroup reporting would be implemented for multispecialty groups reporting MVPs. A subset of a TIN could form a subgroup if they are part of the same TIN, but could not form a subgroup if they are part of different TINs. For example, a group consisting of a single billing TIN that contains a number of participants in the same APM Entity, could form a subgroup to report an MVP or the APP. However, an APM Entity could not select eligible clinicians who are part of different TINs, based on their specialty, and report as a single subgroup. We refer readers to section IV.A.3.b.(2)(e) of this proposed rule, where we discuss subgroups reporting the APP. Due to operational and technical issues described above, we do not believe it is feasible to permit MIPS eligible clinicians in multiple TINs to form a subgroup to report MVPs or the APP. We request public comment on whether there are strategies we should consider to enable formation of subgroups comprised of MIPS eligible clinicians from multiple billing TINS to report MVPs or the APP.
We request public comment on this proposal.
(vii) Proposed MVP Reporting Requirements Summary
Table 34 summarizes the proposed MVP reporting requirements:

(e) Third Party Intermediaries Reporting MVPs
We believe it is also important to ensure that third party intermediaries have the capabilities to support MVPs. We refer readers to section IV.A.3.h. of this proposed rule for proposals related to requiring third party intermediaries to support MVP and subgroup reporting.
(f) MVP Participant Registration
We strive to limit administrative burden and offer as much flexibility as possible. With this principle in mind, we propose steps that an MVP Participant must take to inform CMS of their participation and submission options, with certain exceptions for when the method of collection requires the information in advance of the performance period or we do not have any discretion (such as in virtual groups). We believe that a registration process would be easiest and the most efficient option for MVP Participants and CMS to accurately capture: (1) MVP selection; (2) population health measure selection; (3) administrative claim-based quality measure selection; and (4) subgroup participation.
(i) Proposed Registration Timeline
(A) General Timeline
We considered whether registration should occur at the time of data submission (as described at § 414.1325(e)) or at a specific point during the performance period (for example, by July 1 of the applicable performance period to coincide with the CAHPS for MIPS registration period (81 FR 77072)). On January 7, 2021, we held the MVP Town Hall (85 FR 74729) and publicly shared the MVP Town Hall Preparation Guide,[201] as well as these two potential options and solicited feedback from stakeholders.
This first option would require registering at the time of performance data submission because: It would provide clinicians additional time and flexibility to submit the identification information; better account for TIN/NPI changes during the performance period; allow third-party intermediaries time to accommodate reporting; streamline Start Printed Page 39374 reporting; minimize burden; allow time to review APM participation lists and confirm QP status in order to determine the clinicians to be included in subgroups. Many commenters supported this. A few commenters recommended that the election process take place during the performance period in conjunction with the CAHPS for MIPS registration process and stated that the increased burden associated in enrolling clinicians in a subgroup during the performance period was worth it for subgroups to be scored on administrative claims quality measures and cost measures specific to their subgroup.
We also considered the second option: Having the registration window coincide with performance data submission given that this would provide the additional time and flexibility for clinicians and practices to identify the clinicians in a subgroup. Even though this option would not require a prior registration process to identify clinicians in a subgroup and would allow groups to internally account for clinicians joining or leaving their group throughout the performance period, it would also have significant tradeoffs such as not allowing enough time for CMS to provide enhanced performance feedback as proposed in section IV.A.3.b.(5)(d) of this proposed rule. We are also concerned that this option would result in subgroups that are assessed on less information due to insufficient operational time for CMS to pull and reconcile claims data and CAHPS beneficiary sampling for the subgroup that informs performance measurement of the subgroup. Furthermore, this option would also require clinicians in subgroups to be assessed on the overall group's administrative claims quality measures and cost measures instead of the subgroup's performance on those measures; this means that if subgroups would like to be assessed on the subgroup level for claims data and CAHPS, this would delay feedback and scoring.
In consideration of stakeholder feedback and to ensure timelines are feasible for CMS and stakeholders alike, we believe the first option is more appropriate—a registration period that begins on April 1st and ends November 30th of the applicable performance period (h). Therefore, we propose at § 414.1365(b)(1), that to report an MVP, an MVP Participant must register for the MVP, and if applicable, as a subgroup during a period that begins on April 1 and ends on November 30 of the applicable CY performance period or a later date specified by CMS. Under this proposal, to report the CAHPS for MIPS survey associated with an MVP, a group, subgroup, or APM entity must complete their registration by June 30 of such performance period or a later date specified by CMS.
We believe the benefits of aligning MVP, MVP population health measure, and subgroup registration during the performance period, outweigh the limitations of performance period registration. Through the MVP town hall, we have heard stakeholders indicate that a registration period that is held during the performance period is limiting because it provides clinicians with less time to decide which MVP they would like to report or make changes to their selection. However, we believe that this would encourage clinicians to identify important MVP topics early on in the performance period, in which they can focus their quality improvement efforts on. Also, this would allow us sufficient time to identify clinician participation in subgroups and provide more granular and meaningful subgroup performance feedback to inform quality improvement and patient choice resulting in clinician assessment on more information relevant to their subgroups, such as targeted administrative claims quality measures and cost measures.
In addition, we believe that the proposed registration period would allow more flexibility in the creation of subgroups that represent clinical alignment and to add or remove clinicians from the subgroup, or otherwise, make changes to their participation status in subgroups, before the end of the registration period.
We request public comment on our proposals as discussed above.
(B) Exception for MVP Participants That Want To Report the CAHPS for MIPS Survey Measure
Currently, as finalized in the CY 2017 Quality Payment Program final rule (81 FR 77072), groups that register to administer the CAHPS for MIPS survey measure prior to the registration deadline could cancel their registration or change their CAHPS for MIPS survey selection before the close of registration on June 30th. In this proposed rule, we propose at § 414.1365(b)(1) that in order for an MVP Participant to report the CAHPS for MIPS survey measure associated with an MVP, a group, subgroup, or APM entity would need to register by the same deadline as the CAHPS for MIPS registration, which is June 30 of the applicable 12-month performance period (81 FR 77072).
Under this proposal, clinicians participating in subgroups or groups reporting on the CAHPS for MIPS survey measure within an MVP would be unable to make any changes to their participation in the CAHPS for MIPS survey beginning July 1 of the applicable performance period. We note that clinicians in subgroups who do not intend to report the CAHPS for MIPS measure would still be able to make changes to their participation status in subgroups before the registration period ends on November 30th.
We request public comment on these proposals.
(ii) Proposed MVP Participant Registration Requirements
We believe there are certain elements of information that are important to include at the time of MVP registration. Specifically, we propose at § 414.1365(b)(2)(i) and (ii), that at the time of registration, an MVP Participant must submit the following information, as applicable: (1) Each MVP Participant must select an MVP, 1 population health measure included in the MVP, and if applicable, any outcomes-based administrative claims measure on which the MVP Participant intends to be scored; (2) Each subgroup must submit a list of each TIN/NPI associated with the subgroup which identifies each individual eligible clinician NPI in the applicable subgroup for the group TIN and a plain language name for the subgroup. The following subsections discuss each of these elements.
(A) MVP Selection
To accurately capture who is participating in MVP reporting, it is important to establish the use of identifiers to identify what is intended to be reported, and by whom. We intend to publish a list of MVPs that have been finalized in rulemaking in the prior year, with identifiers available for a given performance period on the QPP Resource Library, prior to the start of the registration period, along with registration guidance. Therefore, we propose that the MVP Participants must select a specific MVP, at the time of registration, as described at proposed § 414.1365(b)(2)(i). Under this proposal, MVP Participants would not be able to submit or make changes to the MVPs they select after the close of the registration period, and therefore, would not be allowed to report on an MVP they did not register for. We request comments on this proposal.
In addition, for our consideration for future rulemaking, we request public comment on whether MVP Participants would be interested in the ability to select multiple MVPs at the time of registration. If so, we would like to Start Printed Page 39375 understand what value MVP Participants might find with this allowance. Specifically, we would like to know if MVP Participants believe they will likely report on multiple MVPs. Also, we would like to know if MVP Participants would want to submit data on multiple MVPs. We also refer readers to section IV.A.3.b.(3)(h) of this proposed rule, where we discuss a similar comment request for subgroups.
(B) Population Health Measure Selection
Similarly, we plan to publish a list of the population health measures that have been finalized for a given performance period on the QPP Resource Library. We plan for this to occur prior to the start of the registration period, along with posting registration guidance. As proposed in section IV.A.3.b.(4)(d)(V)(B) of this proposed rule, we propose that MVP Participants who report an MVP, must submit one population health measure of their choice from the list of finalized population health measures within the foundational layer of the MVPs. The two proposed and previously finalized population health measures are both administrative claims based, and do not require physical data submission by clinicians. Therefore, in order for this selection to be tracked, we propose at § 414.1365(b)(4)(i) that MVP Participants would be required to select this population health measure at the time of registration. Under this proposal, MVP Participants would not be able to submit or make changes to the selected population health measure after the close of the registration period. In addition, MVP Participants would not be able to successfully register to report an MVP if they do not select a population health measure, as the registration would be considered incomplete. We request comments on this proposal.
(C) Outcomes-Based Administrative Claims Measure Selection
Within the MIPS quality performance category quality measure portfolio, there are some MIPS quality measures that are outcomes-based and utilize the administrative claims-based collection type. There are instances in which these measures are not identified as population health measures. For example, because the measure related to a specific procedure such as hip and knee arthroplasty we do not define this as population health since it does not necessarily impact the health of a population. While these measures may not be population health measures, they still reflect important clinical concepts and practices that are important to clinicians and lead to improved patient outcomes. Therefore, we believe it is important to not exclude these measures from MVPs. Depending on the MVP topic, these quality measures may be applicable and relevant to the topic being measured. In addition, administrative claims-based measures reduce reporting burden placed on clinicians because CMS calculates these measures utilizing administrative claims data. As such, we propose at § 414.1365(b)(2)(i) that the MVP Participant must select any outcomes-based administrative claims measures on which the MVP Participant intends to be scored. As proposed in section IV.A.3.b.(4)(d)(ii) above in this proposed rule and at § 414.1365(c)(1), an MVP Participant must select and report 4 quality measures, including 1 outcome measure (or, if an outcome measure is not available, 1 high priority measure), included in the MVP. As applicable, an outcomes-based administrative claims measure, may be selected at the time of MVP registration to meet the outcome measure requirement (excluding the population health measures required under § 414.1365(c)(4)(ii)). We request comments on this proposal.
(D) Subgroup Participants
As part of the registration process, to accurately capture all the clinicians participating in a subgroup, we propose at § 414.1365(b)(2)(ii) that each subgroup must submit: (1) A list of each TIN/NPI associated with the subgroup, which should identify each individual eligible clinician NPI in the applicable subgroup for the group TIN; and (2) the subgroup's name in a plain language manner.
We believe that the subgroup names would help communicate the specialty, location, or other relevant information which would be displayed on the Compare Tools, helping stakeholders differentiate between subgroups. We plan to provide additional guidance for the template in subregulatory guidance for the nomenclature of subgroups and intend to provide a template and guidance to clinicians and practices on the use of plain language for naming subgroups. For example, a subgroup which consists of oncologists in the Mayberry location of one overall group TIN who chooses to report the Oncology MVP could be called Mayberry Oncology. We considered an alternative option to allow flexibility for groups to choose their own naming convention for subgroups. We are concerned that this option may result in the use of naming conventions that may not be meaningful for patients. Additionally, we believe that this option may increase operational complexity for CMS and stakeholders due to the potential for duplicate naming of subgroups.
Upon successful registration submission, we would assign a unique subgroup identifier. This subgroup identifier would be separate from the individual NPI identifier, the group TIN identifier, and the MVP identifier, discussed in this proposed rule. We would maintain the same identifier year over year, as applicable. In scenarios where a subgroup's makeup changes, which will be identified at the time of registration, we will issue the subgroup a new identifier. We believe this identifier is also needed to allow third-party intermediaries to capture and submit performance data for clinicians participating in subgroup reporting as discussed in section IV.A.3.h.(2)(b) of this proposed rule.
We request public comment on these proposals. Additionally, we request feedback on if there are any additional operational considerations or recommendations for the implementation of this policy for future consideration.
(iii) Summary of the Overall Proposed Registration Process
Table 35 presents a comprehensive perspective of the overall proposed registration timeline:
Start Printed Page 39376

Table 36 presents a crosswalk of the various clinician types, the information expected at the time of registration, and a reminder of the MVP reporting requirements if our proposals are finalized as proposed.
Start Printed Page 39377

(5) Scoring MVP Performance
(a) Overview of MVP Scoring
In the CY 2020 PFS proposed rule, we requested comments on how we should address scoring policies as we transition to MVPs (84 FR 40741 through 40742). Generally, commenters indicated scoring for MVPs should include policies for simplification and alignment of the scores for measures and activities within the performance categories and the final score. We subsequently held an MVP Town Hall on January 7, 2021 (85 FR 74729 through 74730) and publicly shared the MVP Town Hall Preparation Guide (https://qpp-cm-prod-content.s3.amazonaws.com/uploads/1233/MVP%20Town%20Hall%20Preparation%20Guide.pdf) to aid MVP Town Hall participants in understanding the direction we may take with MVPs scoring. We received feedback opposing the use of policies that may differentiate the way we score measures and activities within MVPs compared to traditional MIPS because of concerns over adding complexity to the program. Stakeholders urged us to apply simple scoring rules for MVPs whenever possible to ease burden and help clinicians to predict and understand their MVP scores. Wherever possible, stakeholders requested that we align with traditional MIPS scoring. Start Printed Page 39378
In general, in this proposed rule, we propose to score MVPs similar to policies established for traditional MIPS, including without limitation the methodology to score MVP Participants based on their performance on measures and activities in the four performance categories; performance standards for each of the performance categories; calculation of achievement and improvement scores; and calculation of the final score. We strive to ensure our methodology to convert the scores of activities and measures into a final score balances the statutory requirements and goals of the program with the ease of use, stability, and meaningfulness to MIPS eligible clinicians (83 FR 59840). Our proposed scoring methodology would allow for accountability and alignment across the performance categories and minimizes burden on MIPS eligible clinicians (85 FR 50305). We believe these proposed scoring policies would ensure the meaningful evaluation of performance of MVP Participants based on measures and activities in the MVP. Several of the proposed MVP specific scoring policies would support our identified goal to simplify the program by offering MVPs that link clinically relevant measures and activities, which are meaningful to clinicians, patients, and the program.
We propose at § 414.1365(d)(1) that an MVP Participant that is not an APM Entity is scored on measures and activities included in the MVP in accordance with paragraphs § 414.1365(d)(1) through § 414.1365(d)(3). We also propose at § 414.1365(d)(1) that an MVP Participant that is an APM Entity is scored on measures and activities included in the MVP in accordance with § 414.1317(b). Additionally, we propose at § 414.1365(d)(2) that unless otherwise indicated in § 414.1365(d), the performance standards described at § 414.1380(a)(1)(i) through (iv) apply to the measures and activities included in the MVP. Lastly, we propose at § 414.1365(d)(3) that an MVP Participant is scored under MIPS in four performance categories.
We request public comments on these proposals.
In sections IV.A.3.b.(5)(b) and IV.A.3.b.(5)(c) we propose scoring policies for MVPs that address scoring population health measures within the quality performance category, scoring an outcomes-based administrative claims measure selected as an outcome measure at the time of registration, scoring only the cost measures specified in the MVP for the cost performance category, assigning 20 points for each medium-weighted and 40 points for each high-weighted improvement activity in the improvement performance category, scoring subgroups on the Promoting Interoperability performance category using the affiliated group score, circumstances in which we would reweight performance categories within an MVP, and application of the complex patient bonus to MVPs. We also propose using enhanced performance feedback in MVPs.
As discussed in section IV.A.3.b.(5)(b), we propose scoring policies that apply to MVP Participants. We do not anticipate the need for separate scoring policies for MVPs that might be reported by a single specialty, for example a group of specialists that perform the procedure addressed by an MVP, or for MVPs that could be reported by multiple specialties engaged in team-based care; we believe all scoring policies would apply in each scenario.
(b) Performance Category Scores
(i) Scoring the Quality Performance Category in MVPs
(A) Scoring Based on Achievement
We propose to maintain scoring policies finalized in traditional MIPS for MVPs to leverage meaningful scoring policies and retain stable scoring for MVP Participants. We refer readers to § 414.1380(b)(1)(i) for details on our policies for scoring performance on quality measures for traditional MIPS (81 FR 77276 through 77307, 82 FR 53694 through 53701, 83 FR 59841 through 59856, 84 FR 63011 through 63019, and 85 FR 84904 through 84906). Our proposed policies for scoring quality in traditional MIPS are described in further detail in section IV.A.3.e.(1)(c) of this proposed rule. We refer readers to section IV.A.3.e.(1)(c)(iii) of this proposed rule for our proposals to remove the 3-point floor for Class 1 and Class 2 measures for the 2024 MIPS payment year for traditional MIPS, and for Class 4 measures (new measures) to provide a score from 5 to 10 points in the first two performance periods a measure is used in MIPS. We propose to align with these policies as well to maintain consistency between MVPs and traditional MIPS.
We propose at § 414.1365(d)(3)(i) that, except as provided in paragraphs (d)(3)(i)(A)(1) and (B), the quality performance category score for MVP Participants is calculated in accordance with § 414.1380(b)(1) based on measures included in the MVP.
(B) Population Health Measures
Population health measures are administrative claims measures that are part of the foundational layer of MVPs, as described in section IV.A.3.b.(4)(b)(i)(C)(aa) of this proposed rule. As proposed in section IV.A.3.b.(4)(f)(ii)(B) of this proposed rule, MVP participants would select a population health measure during the registration process. We would score the selected measure as proposed at § 414.1365(d)(3)(i). Since we are proposing to adopt our scoring policies used in traditional MIPS for MVPs, we would exclude the measure from the total achievement points and the total available points if the administrative claims measure does not have a benchmark or meet the case minimum requirement in accordance with § 414.1380(b)(1)(i)(A)(2)(ii). We propose at § 414.1365(d)(3)(i)(A) that except as provided in paragraph (d)(3)(i)(A)(1) each selected population health measure that does not have a benchmark or meet the case minimum requirement is excluded from the MVP participant's total measure achievement points and total available measure achievement points.
We are concerned about the ability of subgroups to meet the case minimum for an administrative claims measure but are interested in including population health measures in the subgroup's score for the MVP. We propose at § 414.1365(d)(3)(i)(A)(1) that subgroups are scored on each selected population health measure that does not have a benchmark or meet the case minimum requirement based on their affiliated group score, if available. We believe this is appropriate because we believe it is important for subgroups to be scored on population health measures, and we believe that the groups score will be reflective of the subgroup's performance on population health measures. We also propose at § 414.1365(d)(3)(i)(A)(1) if the subgroup's affiliated group score is not available, each such measure is excluded from the subgroup's total measure achievement points and total available measure achievement points.
We request public comments on this proposal.
We note that we are also concerned about scoring individual clinicians on population health measures. Because population health measures have measured the quality of a population or cohort's overall health and well-being, we historically have required a minimum reliability standard of 0.4 which for most measures equates to a high case minimum in order to be Start Printed Page 39379 scored. We believe it will be common that a solo practitioner, or an individual clinician that is part of a group but chooses to be scored as an individual clinician will not be scored on population health measures. In these scenarios, we would remove the population health measure from the denominator in accordance with § 414.1380(b)(1)(i)(A)(2)(ii). Historically, we have not combined performance for the individual clinician with performance from the group and therefore have concerns with using the group score for individual clinicians who cannot be scored for population health measures. We understand there may be concerns from stakeholders on scoring individual clinicians on broad population health measures. However, we request comment on approaches to scoring individual clinicians on population health measures given the importance of these measures.
We request public comments on this proposal.
(C) Outcomes-Based Administrative Claims Measures
We want to clarify that MVPs may include outcomes-based administrative claims measures that are identified as outcome measures but are not population health measures as defined at § 414.1305. As described in section IV.A.3.b.(4)(f)(ii)(C) of this proposed rule, clinicians will be able to select outcomes-based administrative claims measures as their required outcome measure during MVP registration, if an outcomes-based administrative claims measure is available within the MVP. For example, if a clinician selects an outcomes-based administrative claims measure during registration to be used as an outcome measure and submits three additional measures, and the outcomes-based administrative claims measure has a benchmark and meets the case minimum, all four measures will be scored. If the clinician selects an outcomes-based administrative claim measure to fulfill their outcome measure requirement, and the outcomes-based administrative claims measure does not meet the benchmark or meet the case minimum. Therefore, we propose at § 414.1365(d)(3)(i)(B) that MVP Participants receive zero measure achievement points for each selected outcomes-based administrative claims measure that does not have a benchmark or meet the case minimum requirement. This scoring aligns with our proposal at IV.A.3.e.(1)(c) of this proposed rule to score Class 2 measures in traditional MIPS. If the clinician selects the outcomes-based administrative claims measure, which can be calculated and submits more than three measures, including an additional outcome measure, scores from the highest four measures will be used to determine the quality performance category score.
Below is an example of scoring for a MIPS eligible clinician who selects an outcomes-based administrative claims measure as the required outcome measure, does not meet case minimum for the measure, and does not submit an additional outcome measure to meet the outcome measure requirement:
- Measure #1: 10/10 points.
- Measure #2: 10/10 points.
- Measure #3: 10/10 points.
- Outcomes-based administrative claims measure: Does not meet case minimum, no other outcome measure submitted: 0/10 points.
- Total quality performance category points: 30/40.
We request comment on this proposal.
(D) Scoring for MVP Participants That Do Not Meet the Quality Performance Category Requirements
We believe it is important that MVP Participants submit the required quality measures specified in the MVP they selected to report. For traditional MIPS, we implemented a validation process to determine if measures are available and applicable (81 FR 77290 through 77291, and 82 FR 30108 through 30109). We have received feedback from stakeholders that this process may be confusing for clinicians. We do not believe we need a validation process to determine the availability and applicability of measures for MVP Participants because MVPs will focus on a condition or specialty, and we believe MVPs will be selected and reported because of the MVP applicability to their practice and patients.
(E) Scoring Example
Table 37 includes examples of scoring within the quality performance category for MVPs. Quality scoring example #1 includes a group that reports four measures and has one population health measure automatically calculated for them and receives 2 percentage points for improvement scoring for an MVP. For quality scoring example #2, the group is a small practice who reports all the Medicare Part B claims measures in an MVP. Because there are only three Medicare Part B claims measures available in this example, the group has a reduced denominator, so the group is not penalized for not reporting four quality measures specified in MVP reporting requirements. The second example also includes 2 percentage points for improvement scoring.
Start Printed Page 39380

(ii) Scoring the Cost Performance Category in MVPs
We propose to use the methodology established for traditional MIPS to score the cost performance category for MVPs, including the proposed revisions to that methodology described in section IV.A.3.e.(1)(d) of this proposed rule. We refer readers to § 414.1380(b)(2)(i) through (v) for our policies to score the cost performance category for traditional MIPS based on achievement and improvement when the case minimum specified under § 414.1350(c) is met or exceeded and CMS has determined a benchmark. We propose at § 414.1365(d)(3)(ii) that the cost performance category score is calculated for an MVP Participant using the methodology at § 414.1380(b)(2)(i) through (v) and the cost measures included in the MVP that they select and report.
Although we expect MVP Participants will submit MVPs that align clinically with their practice, we are concerned clinicians could use the reporting of MVPs to avoid being measured on clinically appropriate cost measures. For example, we are concerned larger groups could attempt to have the cost performance category reweighted by splitting into smaller subgroups that might not meet the case minimums for the cost measures within the MVP they choose to report. We intend to monitor the reporting of MVPs to ensure the use of MVPs reflects the clinical nature of the MVP Participants that report.
We request public comments on this proposal.
(iii) Scoring the Improvement Activities Performance Category in MVPs
Under traditional MIPS, we scored improvement activities by assigning each improvement activity a weight, either high-weight or medium-weight, and by assigning 10 points for each medium-weighted improvement activity and 20 points for each high-weighted improvement activity. We refer readers to § 414.1380(b)(3) for details on scoring the improvement activities performance category for traditional MIPS. Additionally, we refer the reader to §§ 414.1317(b)(3) and 414.1380, which indicates that MIPS eligible clinicians participating in APMs receive a score of at least 50 percent in the improvement activities performance category. As a result, APM entities that report an MVP will receive an improvement activities performance category score of at least 50 percent.
We believe we should continue scoring high-weighted and medium-weighted improvement activities that support the linked activities and measures specified within the MVP using a similar scoring methodology. However, for MVPs, we are proposing to assign 20 points for each medium-weighted improvement activity and 40 points for each high-weighted improvement activity to align with the reporting requirements proposed in section IV.A.3.b.(4)(d)(iv) of this proposed rule, that require that MVP Participants report on one high weighted improvement or two medium weighted improvement activities. We believe that this would help to incentivize clinicians to report on MVPs versus traditional MIPs by reducing reporting burden, since fewer submissions are required to receive a full score.
Therefore, we propose at § 414.1365(d)(3)(iii) that the improvement activities performance category score is calculated based on the submission of high- and medium-weighted improvement activities. We are also proposing that MVP Participants would receive 20 points for each medium-weighted improvement activity and 40 points for each high-weighted improvement activity required under § 414.1360 on which data is submitted in accordance with § 414.1325 or for participation in a certified or recognized patient-centered medical home (PCMH) or comparable specialty practice, as described at § 414.1380(b)(3)(ii). Therefore, to receive a score of 40 points, or full credit, an MVP Participant must submit one high-weighted improvement activity or two medium-weighted improvement activities included in the MVP.
We request public comments on this proposal.
(iv) Scoring the Promoting Interoperability Performance Category in MVPs
We propose to use the scoring methodology established for the Promoting Interoperability performance category in traditional MIPS, and as proposed to be revised in section IV.A.3.d.(4) of this proposed rule, for MVP Participants, except for subgroups who would be scored based on their Start Printed Page 39381 affiliated group's Promoting Interoperability performance category data. The Promoting Interoperability performance category is a foundational layer of MVPs that uses limited, connected complementary sets of measures that are meaningful to clinicians. The scoring methodology for the Promoting Interoperability performance category recognizes the importance of promoting adoption and use of CEHRT to support quality improvement, interoperability, and patient engagement and provides an important approach to scoring that we propose to use for MVPs. We propose at § 414.1365(d)(3)(iv) to calculate the Promoting Interoperability performance category score for an MVP Participant using the methodology at § 414.1380(b)(4), except as provided at § 414.1365(d)(3)(iv)(A).
As discussed in section IV.A.3.b.(4), we proposed at § 414.1365(c)(4)(i)(A) to require subgroups to submit their affiliated group's data for the Promoting Interoperability performance category. We propose at § 414.1365(d)(3)(iv)(A) that if a subgroup does not submit its affiliated group's data for the Promoting Interoperability performance category, the subgroup will receive a score of zero for the Promoting Interoperability performance category.
We request public comments on these proposals.
(v) Facility-Based Scoring
We believe facility-based MIPS eligible clinicians and groups should have the same opportunities to submit MVPs as other MIPS eligible clinicians and groups. Please refer to 83 FR 59856 through 59865, regulation text at § 414.1380(e), and section IV.A.3.e.(2)(b)(v) of this proposed rule for our finalized and proposed policies for facility-based scoring. We propose at § 414.1365(e)(3) if an MVP Participant, that is not an APM Entity, is eligible for facility-based scoring, a facility-based score will also be calculated in accordance with § 414.1380(e). In this case, we would use the highest final score as proposed in section IV.A.3.e.(2)(b)(v) of this proposed rule and at § 414.1380(e)(6)(vi).
(c) Calculating the Final Score in MVPs
(i) Final Score Calculation
We propose at § 414.1365(e) that the final score is calculated for an MVP Participant using the same scoring methodology at § 414.1380(c) unless otherwise indicated in § 414.1365(e). This includes what is established for traditional MIPS, including proposed updates section IV.A.3.e.(2) of this proposed rule.
We request public comment on the proposal.
(A) General Performance Category Weights
We propose at § 414.1365(e)(1) to use the performance category weights established for traditional MIPS and described at § 414.1380(c)(1) to calculate the final score for an MVP Participant that is not an APM Entity. We also propose at § 414.1365(e)(1) to use the performance category weights established for APM Entities and described at § 414.1317(b) to calculate the final score for an MVP Participant that is an APM Entity. We refer readers to section IV.A.3.d.(5)(c) of this proposed rule where we propose additions to the regulation text at § 414.1317(b)(2).
We request public comments on these proposals.
(B) Flexibility for Weighting Performance Categories
(aa) Reweighting Performance Categories for MVPs
For MVP Participants, we are proposing reweighting policies that generally align with our current policies for traditional MIPS with a few minor modifications. We propose at § 414.1365(e)(2)(i) that for an MVP Participant that is not an APM Entity, a scoring weight different from the weights described at § 414.1380(c)(1) will be assigned to a performance category, and its weight as described at § 414.1380(c)(1) will be redistributed to another performance category or categories, in the circumstances described at § 414.1380(c)(2)(i)(A)(2) through (9), and § 414.1380(c)(2)(i)(C). We also propose at § 414.1365(e)(2)(i) that for an MVP Participant that is an APM Entity, the performance category weights will be redistributed in accordance with § 414.1317(b) (see section IV.A.3.d.(5)(c) of this proposed rule for further information on proposed additions to the regulation text at § 414.1317(b)(2)).
As discussed in section IV.A.3.b.(5)(b)(i) of this proposed rule, for MVP Participants, we do not believe there will be cases where no measures in the quality performance category are available and applicable and can be scored. Therefore, we do not believe the traditional MIPS policy for reweighting the quality performance category as specified at § 414.1380(c)(2)(i)(A)(1) should be applicable to MVP Participants. We believe MVP Participants should select an MVP with four quality measures which can be scored based on achievement (unless clinicians report the Medicare Part B collection type and fewer than four measures are included in the MVP for that reporting option). We do not believe we should reweight the quality performance category if no quality measures in the MVP can be scored.
We propose at § 414.1365(e)(2)(ii) that for an MVP Participant that is a subgroup, any reweighting applied to its affiliated group will also be applied to the subgroup. In addition, we propose at § 414.1365(e)(2)(ii) that if reweighting is not applied to the affiliated group, the subgroup may receive reweighting independent of the affiliated group in the following circumstances. We believe a subgroup may be subject to extreme and uncontrollable circumstances that do not impact the entire affiliated group. For example, a subgroup might represent a single practice location that is affected by a natural disaster, while the affiliated group's other practice locations are not affected. Accordingly, we propose at § 414.1365(e)(2)(ii)(A) that a subgroup may submit an application to CMS demonstrating that it was subject to extreme and uncontrollable circumstances and receive reweighting in accordance with § 414.1380(c)(2)(i)(A)(6) and (c)(2)(i)(C)(2). Under this proposal, we propose that in the event that a subgroup submits data for a performance category, the scoring weight described at § 414.1380(c)(1) would be applied and its weight would not be redistributed.
We are also concerned that subgroups, independent of their affiliated groups, may be subject to data that are inaccurate, unusable or otherwise compromised. We propose to add at § 414.1365(e)(2)(ii)(B) that, a subgroup will receive reweighting if CMS determines, based on information known to the agency prior to the beginning of the relevant MIPS payment year, that data for the subgroup are inaccurate, unusable or otherwise compromised due to circumstances outside of the control of the subgroup and its agents, in accordance with § 414.1380(c)(2)(i)(A)(9) and (c)(2)(i)(C)(10).
We request public comments on these proposals.
(C) Redistributing Performance Category Weights
We propose to redistribute the performance category weights for MVPs in accordance with the redistribution policies we propose for traditional MIPS in section IV.A.3.e.(2)(b)(iii) of this proposed rule. We propose at Start Printed Page 39382 § 414.1365(e)(2)(iii) that for an MVP Participant that is not an APM Entity, a scoring weight different from the weights described at § 414.1380(c)(1) will be assigned to a performance category, and its weight as described at § 414.1380(c)(1) will be redistributed to another performance category or categories, in accordance with § 414.1380(c)(2)(ii). We also propose at § 414.1365(e)(2)(iii) that for an MVP Participant that is an APM Entity, the performance category weights will be redistributed in accordance with § 414.1317(b) (see section IV.A.3.d.(5)(c) of this proposed rule for further information on proposed additions to the regulation text at § 414.1317(b)(2)).
We request public comments on this proposal.
(D) Complex Patient Bonus
We refer the reader to § 414.1380(c)(3) and section IV.A.3.e.(2)(a) of this proposed rule for our previously established and proposed policies on applying a complex patient bonus. We propose at § 414.1365(e)(4) to add a complex patient bonus to the final score for an MVP Participant in accordance with § 414.1380(c)(3).
We request public comments on these proposals.
(E) Scoring Example
Table 38 includes an example of calculation of the final score for MVPs. The example continues the example #1 from the quality scoring example in Table 37.
We note that the small practice bonus may constitute a larger proportion of the quality performance category score as compared with MIPS eligible clinicians reporting under traditional MIPS with a quality performance category denominator of 60 points, where the small practice bonus would add 10 percent to the quality performance category score. In cases where the quality performance category denominator is 50 points, as a result of 4 quality measures and one population health measure, a small practice bonus of 6 points would add 12 percent to the quality performance category score. We note that within traditional MIPS the quality performance category denominator can sometimes be less than 60 points (in cases where there are not 6 quality measures are relevant to the MIPS clinician, for example), and therefore, the small practice bonus is sometimes greater than 10 percent. We believe this variability is appropriate and believe that modifying the value of the small practice bonus for MVPs would cause unnecessary confusion.

Start Printed Page 39383
(d) Enhanced Performance Feedback in MVPs
(i) Background
In the CY 2018 Quality Payment Program final rule (82 FR 53799 through 53801), we finalized that under section 1848(q)(12)(A)(i) of the Act, on an annual basis, we will provide confidential feedback to MIPS eligible clinicians and groups on their performance. Currently, in traditional MIPS, clinicians are not required to submit data throughout the performance period. Instead, data is submitted through the submission period that follows the performance period, as described at § 414.1325(e). In addition, current performance feedback includes measure-level performance data and scores, activity-level scores, and category comparison.
In the CY 2020 PFS final rule, we indicated a commitment to the transformation of MIPS to allow for streamlined, cohesive reporting through MVPs that would result in enhanced and timely feedback (84 FR 62945). Through previous rulemaking cycles, we have heard from stakeholders that clinicians are interested in receiving feedback reports from CMS throughout the year rather than annually to allow clinicians to review and make improvements where appropriate. Other stakeholders have expressed interest in receiving feedback which includes comparative data to other practices of similar size, location, and specialty. Stakeholders have indicated this is also key to put performance in perspective, particularly if performance evaluation and payment adjustments are contingent on the performance of other clinicians (84 FR 63057 through 63058).
(ii) Proposed Enhanced Performance Feedback in MVPs
A goal of enhanced performance feedback would be to compare similar clinicians to one another. For example, we could compare clinicians within a group or subgroup that practice in similar ways. We considered two options for providing enhanced performance feedback. The first option is to provide comparative performance feedback, comparing the performance of like clinicians who report on the same MVP, which provides more granular comparison than the current performance feedback under traditional MIPS. This method does not require clinicians to submit data earlier than they currently do. In addition, we anticipate that we could provide comparative performance feedback at the time of annual performance feedback. The second option is to provide performance feedback during the performance period. We acknowledge stakeholders would like to receive more timely and actionable feedback during the performance period. However, there are complexities that need mitigation to pursue this option. These complexities include requiring clinicians to submit data earlier to provide enhanced performance feedback during the performance period, and requiring a significant investment of resources, including both time and money for CMS, third party intermediaries, and clinicians.
Therefore, beginning with the CY 2023 performance period, we propose the first option described—to include comparative performance feedback within the annual performance feedback we provide for MVP Participants, comparing the performance of similar clinicians who report on the same MVP. The comparative feedback would only be available to those who report on MVPs, and would be incorporated into the annual performance feedback that we currently provide in traditional MIPS.
We request public comments on this proposal.
(iii) Request for Information for Future Consideration
As described in the CY 2020 PFS proposed rule (84 FFR 40733 through 40734), stakeholders have requested that they be provided with actionable feedback. To gain a better understanding, we request comments from stakeholders to elaborate on what they consider to be "actionable". Would this include CMS identifying in the annual performance feedback areas of improvement based on how a clinician scores on a measure? Is there unintended burden to stakeholders such as third party intermediaries and EHR vendors associated with receiving "actionable" feedback? For example, this could include financial burden from system changes or operational burden on changes to workflows.
We request public comments on the above.
b. APM Performance Pathway
(1) Overview
In the CY 2021 PFS final rule (85 FR 84859), we finalized the APM Performance Pathway (APP), which was designed to provide a predictable and consistent MIPS reporting option to reduce reporting burden and encourage continued APM participation. The APP is available for reporting by any submitter type, with the exception of Virtual Groups.
(2) MIPS Performance Category Scoring
(a) Quality Performance Category
In the CY 2021 PFS final rule, we finalized our proposal to use the measures listed in Table 39 for purposes of quality performance category scoring for the APP.
Start Printed Page 39384

In the CY 2021 PFS final rule, we finalized the inclusion of the CMS Web Interface as an option for Shared Savings Program ACOs to report quality for the 2021 performance period only, with such quality reporting option no longer available beginning with the CY 2022 performance period. However, since the CY 2021 PFS final rule, we have received stakeholder feedback that the transition away from reporting the CMS Web Interface measures to the reporting of eCQMs/MIPS CQMs is more technologically difficult for some ACOs than originally anticipated, particularly under the extraordinary circumstances of the PHE for COVID-19. In light of this feedback, we are proposing to extend the CMS Web Interface as a means of reporting quality under the APP for Shared Savings Program ACOs for performance years 2022 and 2023.
Under this proposal, for performance year 2022, Web Interface reporting would work in the same manner as for performance year 2021, where ACOs would have the option of reporting either the CMS Web Interface, the APP eCQM/MIPS CQM measure set, or both.
In addition, we are proposing that for the 2023 performance year, we would only score Web Interface submissions for ACOs that have also submitted at least one eCQM/MIPS CQM measure from the APP measure set. While we understand that there may be barriers to ACOs transitioning away from the CMS Web Interface along the timeline Start Printed Page 39385 originally contemplated, we believe it is important to continue to encourage and incent that transition. By extending the CMS Web Interface for the 2022 and 2023 performance years, as proposed, we would give ACOs additional time to familiarize themselves with the eCQM/MIPS CQM measures and the data aggregation and submission processes. However, we believe that by also proposing to limit the continued use of the CMS Web Interface in the 2023 performance year only to those ACOs that also attempt an eCQM/MIPS CQM submission, we would continue to move these ACOs and their ACO participants towards CMS' goal of more complete and uniform reporting requirements for all MIPS participants.
We note that for both performance year 2022 and performance year 2023, ACOs would continue to have the opportunity to report on both the eCQMs/MIPS CQMs and the CMS Web Interface measures, and to have their MIPS quality performance category score based on the higher submission. We believe these proposed policies will help to encourage ACOs to move towards eCQM/MIPS CQM reporting in a low-risk environment where they will have the opportunity to continue to rely on measures reported through the CMS Web Interface for purposes of quality performance scoring as they become familiar with the eCQM/MIPS CQM submission and scoring process.
We seek comment on these proposals.
For the CY 2021 MIPS performance period, we limited the use of the Risk-standardized, All-cause Unplanned Admissions for Multiple Chronic Conditions for ACOs (MCC for ACOs) measure to ACOs because, at that time, we were still investigating the question of whether it would be appropriate to include the Risk-standardized, All-cause Unplanned Admissions of Multiple Chronic Conditions for MIPS (MCC for MIPS) measure in the generally applicable MIPS quality measure set. However, we are proposing to add the MCC for MIPS measure into the MIPS quality measure set beginning with the CY 2022 MIPS performance period, as discussed in Appendix 1 of this proposed rule.
We are also proposing to replace the MCC for ACOs measure with the MCC for MIPS measure within the APP beginning with the 2022 MIPS performance period. This change would continue our transition towards alignment of quality measure data reported by MIPS eligible clinicians who are not participants in APMs and those who are, as discussed in the CY 2021 PFS final rule (85 FR 84859). We believe the MCC for MIPS measure is a valuable tool in assessing quality performance, with no additional reporting burden, and is therefore an asset to the APP measure set as well. By replacing the MCC for ACOs measure with the MCC for MIPS measure, we would have the opportunity to capture performance on this measure for additional MIPS eligible clinicians who are not participants in ACO-based APMs.
We also believe it is important to remove the MCC for ACOs measure from the APP in order to reduce the potential for confusion around performance scores and feedback for MIPS eligible clinicians who might otherwise have been scored on both measures with differing results.
We seek comment on our proposal to include the measures listed in Table 40 in the quality measure set for the APP for the 2022 MIPS performance period.
Start Printed Page 39386

d. MIPS Performance Category Measures and Activities
(1) Quality Performance Category
(a) Background
We refer readers to §§ 414.1330 through 414.1340 and the CY 2018 Quality Payment Program final rule (82 FR 53626 through 53641) for our previously established policies regarding the quality performance category.
In this proposed rule, we propose to:
- Maintain the data completeness criteria threshold of at least 70 percent for 2021 and 2022 MIPS performance periods (2023 and 2024 MIPS payment years), and increase the data completeness criteria threshold to at least 80 percent for the 2023 MIPS performance period (2025 MIPS payment year).
- Extend the availability of the CMS Web Interface as a collection and submission type for the 2022 MIPS performance period.
- Make changes to the MIPS quality measure set as described in Appendix 1 of this proposed rule, including addition of new measures, updates to specialty sets, removal of existing measures, and substantive changes to existing measures.
- Establish criteria for determining whether a measure change is considered substantive starting with 2022 MIPS performance period. Start Printed Page 39387
- Beginning with the 2021 MIPS performance period CAHPS for MIPS survey, Medicare Shared Savings Program (Shared Savings Program) Accountable Care Organizations (ACOs) are required to administer the CAHPS for MIPS Survey and report via the Alternative Payment Model (APM) Performance Pathway (APP). We are proposing refinements to our policies for administration of the CAHPS for MIPS survey to align with certain policies that previously applied to the CAHPS for ACOs survey.
(b) Data Submission Criteria
(i) Submission Criteria for Quality Measures Excluding the CMS Web Interface and CAHPS for MIPS
In the CY 2017 QPP final rule, we established the submission criteria for quality measures (excluding the CMS Web Interface measures and the CAHPS for MIPS survey measure) at § 414.1335, which requires a MIPS eligible clinician, group, or virtual group that is reporting on Qualified Clinical Data Registry (QCDR) measures, MIPS clinical quality measures (MIPS CQMs), electronic CQMs (eCQMs), or Medicare Part B claims measures to submit data on at least 6 measures, including at least 1 outcome measure (81 FR 77100 through 77114). If an applicable outcome measure is not available, then a MIPS individual eligible clinician, group, or virtual group would report on 1 other high priority measure. If there are fewer than 6 measures that apply to a MIPS eligible clinician, group, or virtual group, then reporting on each applicable measure is required. For MIPS eligible clinicians, groups, and virtual groups that report on a specialty or subspecialty measure set (as designated in the MIPS final list of quality measures established by CMS through rulemaking), they are required to submit data on at least 6 measures within the set, including at least 1 outcome measure. If an applicable outcome measure is not available, then a MIPS individual eligible clinician, group, or virtual group would report on 1 other high priority measure. If a specialty or subspecialty measure set contains fewer than 6 measures or if fewer than 6 measures within the measure set apply to a MIPS eligible clinician, group, or virtual group, then reporting on each applicable measure is required. In addition to the assessment of performance based on submitted data for at least 6 measures (all measures if there are fewer than 6 measures that are applicable), performance is also assessed on administrative claims measures. CMS automatically evaluates and calculates administrative claims measures for individual MIPS eligible clinicians, groups, and virtual groups if the case minimum requirement of the measure is met.
With each year of program implementation, we continue to assess means for creating a more cohesive and meaningful participation experience in MIPS that improves value and reduces clinician burden. As the program evolves, we want to enable a seamless transition from participation in traditional MIPS to the preliminary onset of voluntary participation in MVPs to the required participation in MVPs. Transitioning from traditional MIPS to MVPs improves the participation experience of MIPS by having the program be more relevant to a clinician's scope of practice and meaningful to patient care. One element that we assessed in preparation for the transition from traditional MIPS to MVPs regards the utilization of outcomes-based administrative claims measures to reduce the reporting burden under MIPS, particularly the allowance of outcome-based administrative claims measures to be applied as the required outcome measure requirement under traditional MIPS (in general, 6 measures, including 1 outcome measure) and MVPs (in general, 4 measures as outlined in section IV.A.3.b.(4)(d)(ii) of this proposed rule).
Since the inception of MIPS under the Quality Payment Program starting with the 2017 MIPS performance period, we established administrative claims measures that are automatically evaluated and calculated for individual MIPS eligible clinicians, groups, and virtual groups if the case minimum requirement of the measure is met (81 FR 77130 through 77136). With CMS conducting the assessment and calculations of administrative claims measures, the reporting burden for individual MIPS eligible clinicians, groups, and virtual groups is reduced (81 FR 77134). A subset of the administrative claims measures are outcome-based measures (and in some cases, are also population health measures), which focus on the improvement of patient health outcomes. As more outcome-based administrative claims measures are implemented in MIPS, we would like to further reduce the reporting burden by allowing such measures (not applicable to administrative claims measures that are considered population health measures) to fulfill the outcome measure requirement, when applicable. For the 2022 MIPS performance period, we are proposing the following outcome-based administrative claims measure under MIPS: Risk-Standardized Acute Unplanned Cardiovascular-Related Admission Rates for Patients with Heart Failure for the Merit-based Incentive Payment System (see Table Group A of Appendix 1 of this proposed rule).
In section IV.A.3.b.(4)(d)(ii) of this proposed rule, we are proposing at § 414.1365(c)(1) to allow an administrative claims measure that is outcome-based (not applicable to administrative claims measures that are population health measures), if applicable, to be selected at the time of MVP registration as a measure to meet the outcome measure requirement starting with the 2023 MIPS performance period. The outcomes-based administrative claims measure would be applicable and relevant to the specific MVP, and as a result, included as 1 of the measures available within an MVP. Once an outcomes-based administrative claims measure is selected during the MVP registration process, the measure would meet the outcome-based measure reporting requirement and count as 1 of the 4 minimum required measures if the MVP Participants meet the case minimum requirement for the administrative claims measure; otherwise, the administrative claims measure would receive a score of zero points and the MVP Participants would not meet the minimum reporting requirement of 4 measures. However, if the MVP Participants select an outcomes-based administrative claims measure available within the MVP and report on 4 measures, the MVP Participants would meet the minimum reporting requirement of 4 measures if it was determined that the case minimum requirement for the outcomes-based administrative claims measure was not met. We believe that such approach reduces reporting burden and allows for a more cohesive and meaningful participation experience that focuses on measures that are more relevant to a clinician's scope of practice while preventing gaming/misuse of selecting an outcomes-based administrative claims measures to be assessed and scored on for purposes of MVP participation.
As we analyzed the allowance and utilization of outcome-based administrative claims measures (not applicable to administrative claims measures that are population health measures) to be applied to fulfill the outcome measure requirement within traditional MIPS, it became apparent that the implementation of such a policy would pose challenges and obstacles. We assessed 3 options. For the first Start Printed Page 39388 option, we assessed the potential implementation of outcomes-based administrative claims measures to fulfill the outcome requirement utilizing a registration system, similar to the proposal for MVPs. For traditional MIPS, a registration process would require the individual MIPS eligible clinicians, groups, and virtual groups to elect to have an outcomes-based administrative claims measure be calculated and scored to fulfill the outcome measure requirement as part of the 6 minimum required measures. Prior to an individual MIPS eligible clinician, group, or virtual group making such an election via a registration process, it would be imperative for a registration process to only permit an individual MIPS eligible clinician, group, or virtual group to register if eligible (meets case minimum requirement) for an outcome-based administrative claims measure (not applicable to administrative claims measures that are population health measures) to be calculated and scored. Such registration process would need to be able to identify which individual MIPS eligible clinicians, groups, and virtual groups would be eligible for an outcome-based administrative claims measure to fulfill the outcome measure requirement as part of the required minimum of 6 measures, which would prevent the potential for gaming and enable individual MIPS eligible clinicians, groups, and virtual groups to know in advance of the submission period if they would need to report on a minimum of 5 measures instead of a minimum of 6 measures; we believe that this approach would reduce burden.
In addition, for cases in which the case minimum of the outcomes-based administrative claims measure would not be met and therefore, could not be calculated, we have considered whether the denominator should be reduced and performance assessment and scoring would be based on 5 measures instead of 6 measures (or less measures if, initially, there were fewer than 6 applicable measures or fewer than 6 measures available within a measure set), which would reduce the reporting burden under traditional MIPS. However, we recognize that if our policy—an election process, via a registration system, to have an outcome-based administrative claims measure as 1 of the required minimum of 6 measures—includes an element for a denominator reduction, we believe that our policy would pose the potential risk for gaming. We would want to prevent the potential for gaming—knowingly selecting an outcome-based administrative claims measures during registration that is not applicable to a practice or specialty, which would result in not meeting the case minimum requirement to be calculated and scored on such measure, and thus, having performance assessed on 5 measures instead of 6 measures (or less measures if, initially, there were fewer than 6 applicable measures or fewer than 6 measures available within a measure set).
As we assessed such approach, we believed that there would not be sufficient parameters/safeguards to ensure that only individual MIPS eligible clinicians, groups, and virtual groups eligible for an outcome-based administrative claims measure calculation would be able to register. We would seek to prevent the potential for gaming by minimizing the number of individual MIPS eligible clinicians, groups, and virtual groups not eligible for an outcome-based administrative claims measure calculation to make such election. However, as we conducted our assessment, we determined that it would not be technically possible to develop a registration system based on applicable outcomes based administrative claims measure data for the applicable performance period to identify if a MIPS eligible clinician, group, or virtual group is eligible for an outcome-based administrative claims measure calculation given that such data would not be readily available until several months after the end of an applicable MIPS performance period. Without having an ability to identify MIPS eligible clinicians, groups, and virtual groups eligible for an outcome-based administrative claims measure calculation for registration purposes based on data from the applicable performance period in order to prevent gaming, we believed that the implementation of a policy to allow for an outcome-based administrative claims measure to be applied as 1 of the minimum required 6 measures would pose risk to the integrity of the program. For the second option, we assessed the potential for automatically calculating an outcome-based administrative claims measure, if applicable, for individual MIPS eligible clinicians, groups, and virtual groups participating in traditional MIPS. For the implementation of such a policy, we would use the status quo of requiring the submission of the minimum of 6 measures (or less measures if fewer than 6 measures were applicable or fewer than 6 measures were available within a measure set) (81 FR 77100 through 77114) in addition to automatically calculating an applicable outcome-based administrative claims measure. We would calculate a score for a total of 7 measures (6 required measures and outcome-based administrative claims measure), but performance for the quality performance category would be based on 6 measures with the highest score, which would include an outcome-based measure.
For option 2, we would not be reducing the reporting burden given that the reporting requirements would remain as status quo, but instead of us applying all administrative claims to all MIPS eligible clinicians as an addition to their quality performance category denominator, we would be replacing the outcome measure requirement, with an available outcomes-based administrative claims measure, if it can be applied. Under this approach, we would not be able to objectively decipher the intent of a MIPS eligible clinician, group, or virtual group (without a formal process to signify an election) as to whether or not they would want to have the outcome-based administrative claims measure automatically calculated and applied as 1 of the 6 minimum required measures. To not objectively know the intension of a MIPS eligible clinician, group, or virtual group, the following scenario could arise under option 2. For example, a MIPS eligible clinician, group, or virtual group submitted 5 measures, it is unclear if it was intentional to only submit 5 measures with the expectation that the MIPS eligible clinician, group, or virtual group sought to be evaluated on the outcomes-based administrative claims measure or if the submission of 5 measures was a result of a MIPS eligible clinician, group, or virtual group of not meeting the minimum of 6 required measures and thus, receive zero points for 1 of the 6 required measures.
After assessing the first 2 aforementioned options, we assessed a third option that would address concerns regarding the first 2 options. For option 3, we assessed the utilization of historical data (for example, previous MIPS performance period data) given that applicable performance period data would not be readily available to be included as part of a registration process. The use of historical data for an outcome-based administrative claims measure as the means for eligibility determinations within a registration system would only permit individual MIPS eligible clinicians, groups, and virtual groups identified as eligible for the calculation of an outcome-based administrative claims measure. Such option would allow an individual MIPS eligible clinician, group, or virtual Start Printed Page 39389 group to select the application of an outcome-based administrative claims measure during a registration process, which would allow CMS to identify the individual MIPS eligible clinicians, groups, and virtual groups electing to have such measure applied as 1 of the minimum-required 6 measures that meets the outcome-based measure requirement. We believe that historical data would be able to adequately and reliably identify individual MIPS eligible clinicians, groups, and virtual groups eligible for an outcome-based administrative claims measure calculation, which we anticipate could be available during the applicable MIPS performance period, as technically feasible. We believe that by providing this historical information to individual MIPS eligible clinicians, groups, and virtual groups would provide pertinent information to make a determine if they would be selecting an outcome-based administrative claims measure during a registration process to be applied as 1 of the minimum-required 6 measures. We believe this approach could minimize gaming and individual MIPS eligible clinicians, groups, and virtual groups would know that they would need to select a minimum of 5 (instead of 6) other measures to meet the reporting requirements for the quality performance category. The potential drawback to this option is it would only be available for individual MIPS eligible clinicians, groups, and virtual groups who participated in MIPS for a prior performance period, so not all clinicians would benefit under this option. However, if technically feasible, the utilization of historical data from the outcome-based administrative claims measure would be able to reduce the reporting burden and would align with the similar proposed process for MVPs. Although we are not making a proposal for the implementation of such policy, we would like to obtain feedback from stakeholders regarding how the automatic calculation of an outcome-based administrative claims measure and have it applied as 1 of the minimum 6 required measures, particularly the outcome-based measure requirement, and if such option is a policy that would be advantage for them as they participate in traditional MIPS. We actively seek the engagement of our stakeholders as we assess means for reducing the reporting burden and enhance the experience of participating in traditional MIPS.
Thus, we are seeking public comment on the means for being able to implement such a policy. Are there other options that we should consider in determining how to implement such a policy? Are there other ways we would be able to identify which individual MIPS eligible clinicians, groups, and virtual groups are eligible for an outcomes-based administrative measure calculation to ensure that only those that are eligible are able make such an election? Should we consider the use of historical data that would allow the predetermination and identification of MIPS eligible clinicians, groups, and virtual groups eligible for an outcome-based administrative claims measure? How would we be able to determine if a MIPS eligible clinician, group, or virtual group would like to have an automatic calculation of an outcome-based administrative claims measure conducted on their behalf outside of a registration process? Are there other challenges that we should be aware of as we continue to assess a means for developing and implementing such a policy?
(c) Data Completeness Criteria
(i) 2021 MIPS Performance Period (2023 MIPS Payment Year)
In the CY 2020 PFS final rule, we established the data completeness criteria at § 414.1340(a)(3) and § 414.1340(b)(3) for the 2020 MIPS performance period (2022 MIPS payment year), which determined that MIPS eligible clinicians and groups submitting quality measures data on QCDR measures, MIPS CQMs, eCQMs, or Medicare Part B claims measures must submit data on at least a 70 percent of the MIPS eligible clinician or group's patients that meet the measure's denominator criteria, regardless of payer. In regard to the data completeness criteria established for Medicare Part B claims measures for the 2020 MIPS performance period (2022 MIPS payment year), we found that the policy established at § 414.1340(b)(3) erroneously reflected the data completeness criteria only applicable to QCDR measures, MIPS CQMs, and eCQMs, which requires data submission to pertain to patients that meet the measure's denominator criteria, regardless of payer (all-payer). It is not possible for Medicare Part B claims data to include all-payer patients; the submission of data for Medicare Part B claims measures can only account for Medicare Part B patients. Since the implementation of MIPS, the data completeness criteria for Medicare Part B claims measures has pertained to the applicable Medicare Part B patients seen during an applicable MIPS performance period. The issue with the policy established at § 414.1340(b)(3) in the CY 2020 PFS final rule for Medicare Part B claims measures only pertains to the type of patient population for data submission purposes and not the threshold established for data completeness of at least 70 percent. Thus, we are proposing to modify the data completeness threshold criteria established at § 414.1340(b)(3) for the 2020 MIPS performance period (2022 MIPS payment year) retroactively, effective January 1, 2020, in accordance with section 1871(e)(1)(A)(ii) of the Act. We believe that failure to apply the change retroactively would be contrary to the public interest because it would require individual eligible clinicians, groups, and virtual groups to meet data completeness criteria (the submission of patient data for all-payers) for Medicare Part B claims measures that is not possible. We believe that it is imperative for individual eligible clinicians, groups, and virtual groups to be certain as to the true criteria used to measure data completeness for Medicare Part B claims measures. For the 2021 MIPS performance period (2023 MIPS payment year), we are proposing to modify the data completeness criteria established at § 414.1340(b)(3) to be as follows: MIPS eligible clinicians and groups submitting quality measures data on Medicare Part B claims measures must submit data on at least 70 percent of the applicable Medicare Part B patients seen during the performance period to which the measure applies for MIPS payment year 2022.
In the CY 2021 PFS proposed and final rules, we inadvertently omitted a proposal that would have otherwise extended our existing policy to determine the data completeness criteria for the 2021 MIPS performance period (2023 MIPS payment year); we only included a reference to the data completeness criteria of at least 70 percent for the 2021 MIPS performance period (2023 MIPS payment year) as it relates to the scoring policies for class 1 measures as outlined in Table 49 of the CY 2021 PFS proposed and final rules (85 FR 50309 and 85 FR 84906). Thus, we are proposing to establish the data completeness criteria for the 2021 MIPS performance period (2023 MIPS payment year) retroactively, effective January 1, 2021, in accordance with section 1871(e)(1)(A)(ii) of the Act. We believe that failure to apply the change retroactively would be contrary to the public interest because it could be construed as permitting the submission of incomplete, inaccurate, or otherwise comprised data, which would have a detrimental effect on the performance data used for calculating MIPS payment Start Printed Page 39390 adjustments and public reporting. For the 2021 MIPS performance period (2023 MIPS payment year), we are proposing: At § 414.1340(a)(3) to maintain the data completeness criteria threshold of at least 70 percent, in which MIPS eligible clinicians and groups submitting quality measures data on QCDR measures, MIPS CQMs, or eCQMs would need to submit data on at least a 70 percent of the MIPS eligible clinician or group's patients that meet the measure's denominator criteria, regardless of payer; and at § 414.1340(b)(3) to establish the data completeness criteria threshold of at least 70 percent, in which MIPS eligible clinicians and groups submitting quality measures data on Medicare Part B claims measures must submit data on at least 70 percent of the applicable Medicare Part B patients seen during the 2021 MIPS performance period to which the measure applies for MIPS payment year 2023.
We believe that it is imperative to establish the data completeness criteria for the 2023 MIPS payment year in this proposed rule and any failure to apply the updated data completeness criteria retroactively would be contrary to the public interest as such omission presents ambiguity and a potential notion for an array of interpretations. We believe that it is in the public interest to retroactively apply the updated data completeness threshold as it would ensure that all MIPS eligible clinicians participating in MIPS for the 2021 MIPS performance period (2023 MIPS payment year), whether at the individual, group, or virtual group levels, would be aware that there is a definitive data completeness criteria for the 2021 MIPS performance period and any data submitted for the quality performance category would need to meet the data completeness criteria. We believe that such approach would: Establish the data completeness criteria prior to the timeframe in which data submission would occur (first 3 months of CY 2022), which would enable MIPS eligible clinicians participating in MIPS at the individual, group, or virtual group levels to prepare their data submission to meet the updated data completeness criteria; and ensure that all data submitted for the quality performance category would meet the same criteria (that is, specific data completeness threshold of at least 70 percent of a MIPS eligible clinician or group's patient population that meets the measure's denominator criteria for QCDR measures, MIPS CQMs, and eCQMs; or specific data completeness threshold of at least 70 percent of the applicable Medicare Part B patients seen during the 2021 MIPS performance period for Medicare Part B claims measures) versus an unspecified, interpretive data completeness threshold that could result in various threshold ranges and inconsistent reported patient populations such as portion of submitted data be a representative of all patient (all-payer) data while the remaining portion of submitted data be a representative of only Medicare patient data, which would provide data integrity, usability, and reliability to assess the performance of MIPS eligible clinicians at the individual, group, or virtual group level in a manner that is consistent and enable performance data to be comparable to the applicable historical benchmarks that have been established for the various measures.
(ii) 2022 MIPS Performance Period (2024 MIPS Payment Year)
In the CY 2017 and CY 2018 Quality Payment Program final rules, we note that we would increase the data completeness criteria threshold over time (81 FR 77121 and 82 FR 53632). Starting with the 2020 MIPS performance period (2022 MIPS payment year), we increased the data completeness criteria from at least 60 percent to at least 70 percent and as noted above, we are proposing to maintain the data completeness criteria threshold of at least 70 percent for the 2021 MIPS performance period (2023 MIPS payment year). We continue to believe that it is important to incrementally increase the data completeness criteria as MIPS eligible clinicians, groups, and virtual groups gain experience with MIPS. However, with the COVID-19 PHE that started during the 2020 MIPS performance period (2022 MIPS payment year) and continued into the 2021 MIPS performance period (2023 MIPS payment year), we believe that it would be appropriate to continue to maintain the data completeness criteria of at least 70 percent for the 2022 MIPS performance period (2024 MIPS payment year) as healthcare systems across the country have been overwhelmed and strained by the COVID-19 PHE.
In order to not place further undue burden as MIPS eligible clinicians, groups, and virtual groups navigate through the COVID-19 pandemic, we are proposing:
- At § 414.1340(a)(3) to maintain the data completeness criteria threshold of at least 70 percent, in which MIPS eligible clinicians and groups submitting quality measures data on QCDR measures, MIPS CQMs, or eCQMs would need to submit data on at least a 70 percent of the MIPS eligible clinician or group's patients that meet the measure's denominator criteria, regardless of payer, for the 2022 MIPS performance period (2024 MIPS payment year); and
- At § 414.1340(b)(3) to maintain the data completeness criteria threshold of at least 70 percent, in which MIPS eligible clinicians and groups submitting quality measures data on Medicare Part B claims measures must submit data on at least 70 percent of the applicable Medicare Part B patients seen during the 2022 MIPS performance period to which the measure applies for MIPS payment year 2024.
(iii) 2023 MIPS Performance Period (2025 MIPS Payment Year)
We believe that the incorporation of higher data completeness thresholds in future years ensure a more accurate assessment of a MIPS eligible clinician's performance on quality measures and avoid any selection bias. We have strongly encouraged all MIPS eligible clinicians to perform the quality actions associated with the quality measures on their patients. The data submitted for each measure is expected to be representative of the individual MIPS eligible clinician, group, or virtual group's overall performance for that measure. The data completeness threshold of less than 100 percent is intended to reduce burden and accommodate operational issues that may arise during data collection during the initial years of the program.
Since the inception of the program, we have provided notice to MIPS eligible clinicians, groups, and virtual groups in order for them to take the necessary steps to prepare for higher data completeness thresholds in future years. In a similar manner, we are providing advance notice that we intend to increase the data completeness criteria threshold for the 2023 MIPS performance period (2025 MIPS payment year). We are proposing: At § 414.1340(a)(4) to increase the data completeness criteria threshold from at least 70 percent to at least 80 percent, in which MIPS eligible clinicians and groups submitting quality measures data on QCDR measures, MIPS CQMs, eCQMs, or Medicare Part B claims measures would need to submit data on at least a 80 percent of the MIPS eligible clinician or group's patients that meet the measure's denominator criteria, regardless of payer, for the 2023 MIPS performance period (2025 MIPS payment year); and at § 414.1340(b)(4) to increase the data completeness Start Printed Page 39391 criteria threshold from at least 70 percent to at least 80 percent, in which MIPS eligible clinicians and groups submitting quality measures data on Medicare Part B claims measures must submit data on at least 80 percent of the applicable Medicare Part B patients seen during the 2023 MIPS performance period to which the measure applies for MIPS payment year 2025. We believe that MIPS eligible clinicians, groups, and virtual groups would be provided with adequate time to prepare for the data completeness criteria threshold to increase.
We seek public comment on our proposals to maintain the data completeness criteria threshold of at least 70 percent for the 2021 and 2022 MIPS performance periods (2023 and 2024 MIPS payment years), and increase the data completeness criteria threshold from at least 70 percent to at least 80 percent for the 2023 MIPS performance period (2025 MIPS payment year).
(d) Groups and Virtual Groups Reporting via the CMS Web Interface
At § 414.1335(a)(2), the CMS Web Interface measures is a collection type in which groups and virtual groups with 25 or more eligible clinicians are able to report data on a set of pre-determined quality measures. For the 2021 MIPS performance period, the total number of CMS Web Interface measures required to complete reporting on is 10 CMS Web Interface measures (83 FR 59713 through 79715 and 59756). In the CY 2021 PFS final rule, at § 414.1325(c)(1) et seq., the CMS Web Interface was sunset and removed as an available collection and submission type under MIPS starting with the 2022 MIPS performance period (85 FR 84870). In addition, starting with the 2022 MIPS performance period, the definition of the terms collection type and submission type would no longer include the CMS Web Interface measures as an available option. It was our belief that the sunset and removal of the CMS Web Interface as a collection and submission type starting with the 2022 MIPS performance period would reduce the potential burden experienced by groups and virtual groups during the COVID-19 PHE. Based on the public comments received during the notice-and-comment rulemaking process for the CY 2021 PFS proposed rule, we believed that the 1-year delay to sunset and remove the CMS Web Interface as a collection and submission type would provide stakeholders utilizing the CMS Web Interface sufficient time to prepare and transition to an alternative collection and/or submission type starting with the 2022 MIPS performance period.
However, following the close of the submission period for the 2020 MIPS performance period (March 31, 2021), stakeholders utilizing the CMS Web Interface contacted CMS to convey concerns regarding the technological challenges and resource limitations they face that would prevent them from being able to adequately transition to implementing and utilizing an alternative collection and/or submission type starting with the 2022 MIPS performance period. Also, they emphasized that some practices around the country continue to endure a fiscal impact resulting from the COVID-19 pandemic and would need additional time to prepare for a transition to an alternative collection and/or submission type. They indicated that if the availability of the CMS Web Interface was extended for an additional year (the 2022 MIPS performance period), they would have sufficient time to address technological challenges such as the implementation of processes to aggregate data within one EHR system or across multiple EHR systems to align with the reporting requirements of another collection type (that is, MIPS CQMs or eCQMs), build and integrate new health IT infrastructures and systems, implement workflows, and train staff on new health IT systems.
We recognize that an adequate and sufficient timeframe is a critical factor in the success of a group or virtual group transitioning to an alternative collection and/or submission type, particularly with such transition occurring amidst the mitigation of the COVID-19 pandemic. It seems that as CMS Web Interface users began the actualization of preparing and taking steps to transition to utilizing a different collection and/or submission type, the timeframe identified by most CMS Web Interface users (starting with the 2022 MIPS performance period) during the notice-and-comment rulemaking process for the CY 2021 PFS proposed rule, which is reflective of our policy to sunset the CMS Web Interface starting with the 2022 MIPS performance period, would not have provided an adequate or sufficient timeframe for CMS Web Interface users to fully transition to an alternative collection and/or submission type. In considering the concerns expressed by CMS Web Interface users such as the technological challenges that they would need to overcome, their inability to update systems and workflows in time for the 2022 MIPS performance period, and the fiscal implications they endure from mitigating and responding the COVID-19 PHE, we believe it is appropriate to reduce the burden of groups and virtual groups at this juncture and postpone the sunset of the CMS Web Interface by extending the availability of the CMS Web Interface as a collection and submission type for the 2022 MIPS performance period. We recognize that groups and virtual groups are on a continuum with regard to technological readiness for transitioning to a different collection type, but we believe that the availability of the CMS Web Interface for the 2022 MIPS performance period would reduce burden by providing additional time, and enable more groups and virtual groups to be able to successfully transition to another collection type by the start of the 2023 MIPS performance period. Moreover, we want to ensure that groups utilizing the CMS Web Interface are prepared to participate in MIPS as it evolves from traditional MIPS to MVPs, in which such groups could begin voluntary participation in an MVP as the availability of MVPs become an option starting with the 2023 MIPS performance period.
We are proposing at § 414.1325(c)(1) et seq. to sunset the CMS Web Interface measures as a collection type/submission type starting with the 2023 performance period. Specifically, at § 414.1305, we are proposing to modify the definition of the terms collection type and submission type to remove the CMS Web Interface measures as an available option starting with the CY 2022 MIPS performance period/2024 MIPS payment year. We are proposing to modify the definition of "collection type" to mean a set of quality measures with comparable specifications and data completeness criteria, as applicable, including, but not limited to: Electronic clinical quality measures (eCQMs); MIPS clinical quality measures (MIPS CQMs); QCDR measures; Medicare Part B claims measures; for the CY 2017 through 2022 MIPS performance periods/2019 through 2024 MIPS payment years, CMS Web Interface measures; the CAHPS for MIPS Survey; and administrative claims measures. We are proposing to modify the definition of "submission type" to mean the mechanism by which the submitter type submits data to CMS, including, but not limited to: Direct; log in and upload; log in and attest; Medicare Part B claims; and for the CY 2017 through 2022 MIPS performance periods/2019 through 2024 MIPS payment years, the CMS Web Interface.
For the 2022 MIPS performance period, the total number of CMS Web Interface measures required to complete reporting on would be 10 CMS Web Start Printed Page 39392 Interface measures (83 FR 59713 through 79715 and 59756). In Table Group B of Appendix 1 of this proposed rule, we are proposing modifications to the CMS Web Interface measures and in Table Group D of Appendix 1 of this proposed rule, we are proposing substantive changes to the CMS Web Interface measures. We believe that it is necessary for the CMS Web Interface measures to be updated to reflect applicable substantive changes for the 2022 MIPS performance period given that the CMS Web Interface measures have remained the same for 3 consecutive (2019, 2020, and 2021) MIPS performance periods.
We solicit public comment on our proposals: To extend the availability of the CMS Web Interface as a submission and collection type for the 2022 MIPS performance period, which would sunset and remove the collection and submission type under MIPS starting with the 2023 performance period; and update the CMS Web Interface measures with substantive changes for the 2022 MIPS performance period as outlined in Table Group B of Appendix 1 of this proposed rule.
(e) Selection of MIPS Quality Measures
Previously finalized MIPS quality measures can be found in the CY 2021 PFS final rule (85 FR 85045 through 85377); CY 2020 PFS final rule (84 FR 63205 through 63513); CY 2019 PFS final rule (83 FR 60097 through 60285); CY 2018 Quality Payment Program final rule (82 FR 53966 through 54174); and in the CY 2017 Quality Payment Program final rule (81 FR 77558 through 77816). Proposed changes to the MIPS quality measure set as described in Appendix 1 of this proposed rule, include the following: Addition of new measures; updates to specialty sets; removal of existing measures, and substantive changes to existing measures. For the 2022 MIPS performance period, we are proposing a measure set of 195 MIPS quality measures.
The new MIPS quality measures proposed for inclusion in MIPS for the 2022 performance period and future years are found in Table Group A of Appendix 1 of this proposed rule. For the 2022 MIPS performance period, we are proposing 5 new MIPS quality measures, which includes 2 administrative claims measures. Also, in Table Group AA of Appendix 1, we outline 1 potential new MIPS quality measure, the COVID-19 Vaccination by Clinicians measure, which we intend to propose in a future rulemaking cycle. We refer readers to section IV.A.3.d.(1)(f) of this proposed rule for our request for information pertaining to the COVID-19 Vaccination by Clinicians measure specifications.
In addition to the establishment of new individual MIPS quality measures, we also develop and maintain specialty measure sets to assist MIPS eligible clinicians with selecting quality measures that are most relevant to their scope of practice. Our proposals for modifications to existing specialty sets and new specialty sets are outlined in Table Group B of Appendix 1 of this proposed rule. Specialty sets may include: New measures, previously finalized measures with modifications, previously finalized measures with no modifications, the removal of certain previously finalized quality measures, or the addition of existing MIPS quality measures. Please note that the specialty and subspecialty sets are not inclusive of every specialty or subspecialty.
On January 7, 2021, we announced that we would be accepting recommendations for potential new specialty measure sets or revisions to existing specialty measure sets for year 6 of MIPS under the Quality Payment Program.[203] These recommendations were based on the MIPS quality measures finalized in the CY 2020 PFS final rule, the 2020 Measures Under Consideration list, and provides recommendations to add or remove the current MIPS quality measures from existing specialty sets, or provides recommendations for the creation of new specialty sets. All specialty set recommendations submitted for consideration were assessed and vetted, and as a result, the recommendations that we agree with are being proposed in this proposed rule.
In addition to establishing new individual MIPS quality measures and modifying existing specialty sets and new specialty sets as outlined in Tables Group A and Group B of Appendix 1 of this proposed rule, we refer readers to Table Group C of Appendix 1 of this proposed rule for a list of quality measures and rationales for removal. For the 2022 MIPS performance period, we are proposing to remove 19 MIPS quality measures: 1 MIPS quality measure that is duplicative to another current MIPS quality measure; 9 MIPS quality measures that do not align with the Meaningful Measure Initiative; 5 MIPS quality measures that are no longer stewarded or maintained; and 4 MIPS quality measures that are under the topped out lifecycle. We have continuously communicated to stakeholders our desire to reduce the number of process measures within the MIPS quality measure set. We believe our proposal to remove the quality measures outlined in Table Group C will lead to a more parsimonious inventory of meaningful, robust measures in the program, and that our approach to remove measures should occur through an iterative process that will include an annual review of the quality measures to determine whether they meet our removal criteria.
Lastly, MIPS quality measures with proposed substantive changes can be found in Table Group D of Appendix 1 of this proposed rule. We are proposing substantive changes to 84 MIPS quality measures. On an annual basis, we review the established MIPS quality measure inventory to consider updates to the measures. Possible updates to measures may be minor or substantive. Section 1848(q)(2)(D)(i)(II)(cc) of the Act requires all substantive measure changes to be proposed and identified through notice-and-comment rulemaking. In the CY 2017 Quality Payment Program final rule (81 FR 77137), we determined that substantive changes to measures (that is, measure specifications, measure title, and domain modifications) would be identified during the rulemaking process while maintenance changes that do not substantively change the intent of the measure (that is, updated diagnosis and procedure codes, definitions, and changes to patient population exclusions) would not be included in the rulemaking process.
We are not proposing any changes to our current approach of identifying substantive measures changes during the rulemaking process. However, in order to more precisely distinguish between substantive measure changes and non-substantive measure changes, we are proposing to consider the following criteria for determining whether a measure change is substantive starting with 2022 MIPS performance period:
- Whether the change causes the measure to be more stringent;
- Whether the change modifies the collection and/or submission types applicable to the measure;
- Whether the change impacts the clinical action and/or outcome of the measure;
- Whether the change increases the burden of the measure;
- Whether the change modifies the premise and/or objective of the measure; Start Printed Page 39393
- Whether the change modifies the scope of the measure (such as patient population eligible for the measure or measurement period); and
- Other relevant criteria as may be identified by CMS on a case-by-case basis.
We note that any substantive change made to a measure would be proposed and identified through notice-and-comment rulemaking. For a substantive change to a measure, we only intend to propose and identify the substantive change as applicable to the appropriate elements (that is, only include substantive changes if it is applicable to the measure specifications, collection type(s), measure description, measure title, etc.) of the measure through notice-and-comment rulemaking. For example, if there is a substantive change to a measure in the measure specifications that changes the premise/overarching objective (that is, a screening measure that is changed to include treatment and follow-up) and/or clinical action of the measure, such substantive change would be proposed and identified through notice-and-comment rulemaking. We do not believe that it is necessary to propose or identify through notice-and-comment rulemaking changes to a measure that do not meet any of the above substantive change criteria for measures (for example, a modification to the title or domain that do not change or impact any element of the measure). We generally consider such changes to be non-substantive and would be published in subregulatory guidance (that is, release notes applicable to a measure). We believe that it is important to provide a clear delineation of substantive changes to be included in a rulemaking process versus our previous approach, which generally included any changes made to measure specifications, measure titles, and domain modifications (81 FR 77137). We found that many changes made to measures based on our previous approach were not substantive in nature and should not be classified as substantive changes. Thus, we believe that establishing the substantive change criteria for measures provides further clarity as to what we consider a substantive change, particularly as it relates to how a change affects and/or impacts a measure. We note that measures identified as having a substantive change would generally have an update to their applicable benchmark. For measures that meet the data completeness criteria, but do not a benchmark or meet case minimum (class 2 measures), they would be scored in accordance to our proposed scoring policy as outlined in section IV.A.3.e.(1)(c)(iii)(B) of this proposed rule.
In addition, we intend to align the utilization of terminology across CMS programs when appropriate and applicable for consistency purposes. Since the implementation of MIPS, we have referenced the term patient reported outcome as a type of measure, which is similar, but not exact to a measure type categorization reference in the CMS Blueprint. In order to align the categorization reference of such measure type under MIPS with the CMS Blueprint terminology, we are modifying how the term is referenced as a measure type under MIPS and will reference such measure type as patient-reported outcome-based performance measure (PRO-PM) starting with the 2022 MIPS performance period. We believe that such modification does not have any substantive implications, but is merely a minor technical change of semantics that enables the utilization of consistent terminology across CMS programs when referencing such measure type.
We seek public comment on our proposal to establish measure substantive change criteria that would be utilized by CMS to identify such measures.
(f) Request for Information Regarding the COVID-19 Vaccination by Clinicians Measure
As of July 7, 2021, the Centers for Disease Control and Prevention (CDC) reported that there are 33,582,352 cases of coronavirus disease 2019 (COVID-19) and 603,656 deaths[204] caused by COVID-19 at the time of publication of this proposed rule and subject to change. In 2020, COVID-19 was the third leading cause of death in the United States, exceeded only by cancer and heart disease.[205] Widespread vaccination to prevent COVID-19 will be critically important to stemming the morbidity and mortality caused by this disease. Three vaccines have received the FDA emergency use authorization (EUA) for the prevention of COVID-19 (Pfizer-BioNTech, Moderna, and Janssen) as of July 7, 2021. The EUA allows the Pfizer-BioNTech, Moderna, and Janssen COVID-19 vaccines to be distributed in the United States.[206] As of July 7, 2021, 331,651,464 vaccine doses have been administered.[207]
To address this urgent public health emergency, CMS began the development of the COVID-19 Vaccination by Clinicians measure for MIPS, which would assess the percentage of patients aged 18 years and older seen for a visit during the measurement period who have ever completed or reported having ever completed a COVID-19 vaccination series. The measure would be reported by MIPS eligible clinicians as a MIPS CQM to determine the percentage of patients seen for a visit during the measurement period who have ever completed or reported having ever completed a COVID-19 vaccination series, either from the submitting MIPS eligible clinician or another MIPS eligible clinician. The measure as specified (see Table Group AA of Appendix 1 of this proposed rule) allows clinicians to determine a patient's vaccination status and deliver a vaccine dose, if possible and appropriate. The measure is intended to capture whether or not clinicians take an appropriate step to ensure that their patients are vaccinated. Patients receiving hospice care at any time during the measurement period would be excluded from the patient population of measure. The measure allows for an exception if the COVID-19 vaccination series was not administered, as documented by a MIPS eligible clinician, due to patient contraindication, or vaccine availability.
Between November of 2020 and January of 2021, we solicited feedback on the measure from measure-specific multi-stakeholder expert workgroups, specifically the Measure Application Partnership (MAP) coordinated through the National Quality Forum.[208] While the MAP agreed that the COVID-19 Vaccination by Clinicians measure could be an important tool to: Support vaccine uptake by collecting valuable information from the field, provide feedback to clinicians, and help identify where to conduct targeted education and outreach to limit the spread of infections, the MAP expressed concerns regarding the following elements of the measure: The patient population that would be assessed to measure performance (the inclusion of assessing patients who received 1 dose of a COVID-19 vaccine versus only assessing patients who received a complete COVID-19 vaccination series), and lack of available evidence and clinical guidance for vaccine Start Printed Page 39394 administration (the feasibility of implementing the measure given the limited vaccine supply and availability, and the potential inconsistencies and discrepancies derived from the novelty of data collection and reporting for COVID vaccinations). We seek to mitigate such issues by obtaining further information and feedback from additional stakeholders. We intend to utilize the obtained information and feedback to inform measure specification improvements that would be implemented for a future performance period.
We seek public comment on the draft COVID-19 Vaccination by Clinicians measure specifications, which is available on the Quality Payment Program website in the Resource Library located at https://qpp-cm-prod-content.s3.amazonaws.com/uploads/1471/Draft%20COVID-19%20Vaccine%20Specs.pdf. Specifically, we are seeking feedback on the following questions. Should the measure assess whether or not patients completed a COVID-19 vaccination series to capture provision of effective clinical care and why? Given that there are differences in the age ranges for patients eligible to receive the various COVID-19 vaccinations (Moderna and Janssen COVID-19 vaccines are authorized for patients ages 18 years and older; Pfizer-BioNTech COVID-19 vaccine is authorized for patients ages 12 and older; and future COVID-19 vaccines may be approved for other age ranges that are implemented after the publication of this proposed rule), is 18 years and older an appropriate initial age threshold for this measure? Given the current COVID-19 PHE and the intent of the measure, should this measure be mandatory for reporting in a future year? If this measure would be mandated as a required measure for reporting purposes under MIPS, what issues or concerns would need to be considered and/or mitigated regarding the implementation of the measure in a future year? What are the potential unintended consequences associated with the potential future implementation of the measure as specified in Table Group AA of Appendix 1 of this proposed rule and applicable measure specifications? What are the feasibility challenges and barriers to implementing the measure? What are the potential options and/or recommendations that we should consider to address and/or mitigate the feasibility challenges and barriers to be experienced during the 2022 MIPS performance period that could be improved upon for the 2023 MIPS performance period? If this measure would be mandated, how would the collection of the measure data be useful after the 2023 MIPS performance period?
(g) Quality Data Submission Criteria
(i) CAHPS for MIPS Background
As part of the CY 2021 PFS final rule (85 FR 84718), we finalized the policy requiring Medicare Shared Savings Program (Shared Savings Program) Accountable Care Organizations (ACOs) to report quality data via the Alternative Payment Model (APM) Performance Pathway (APP). Beginning with the 2021 MIPS performance period, Shared Savings Program ACOs are required to field the Consumer Assessment of Healthcare Providers and Systems (CAHPS) for MIPS survey as part of the APP. We previously established in the CY 2019 PFS final rule that MIPS quality benchmarks will be based on collection type, from all available sources, including MIPS eligible clinicians and APM Entities, to the extent feasible, during the applicable baseline or performance period (83 FR 59842). Given that Shared Savings Program ACOs will now be required to field the CAHPS for MIPS survey as part of the APP, we note that beginning with the 2022 MIPS performance period CAHPS for MIPS survey, the CAHPS for MIPS benchmarks will be calculated based on summary survey measure (SSM) scores from MIPS groups and APM entities (including Shared Savings Program ACOs) that fielded the CAHPS for MIPS Survey in the applicable baseline or performance period. Furthermore, the CAHPS for MIPS SSM scores will be adjusted for patient case-mix using a single case-mix adjustment model that incorporates data from both MIPS groups and APM entities (including Shared Savings Program ACOs) that field the CAHPS for MIPS Survey.
Beginning with the 2022 MIPS performance period/2024 MIPS payment year for the CAHPS for MIPS survey, to further support the alignment of CAHPS for MIPS sampling and scoring procedures between Shared Savings Program ACOs and MIPS groups, we are proposing to adopt certain policies that had been part of the CAHPS for ACOs survey administration process, but have not previously been a part of CAHPS for MIPS. These policies fall into 3 broad categories: Sampling, case mix adjustment, and scoring; for policies related to certified survey vendors rendering the CAHPS for MIPS survey for subgroups, please see section IV.A.3.h. of this rule. We are requesting comment on the following proposals related to CAHPS for MIPS.
(ii) CAHPS for MIPS Sampling Specifications
The CAHPS for MIPS Survey is administered to a sample of eligible patients for all Shared Savings Program ACOs and for those MIPS groups that elect the measure. Prior to drawing the sample, patients are excluded from the pool of potential survey recipients (called the sampling frame) for a number of reasons, including if they are known to have died or are known to be institutionalized. Currently, patients are considered institutionalized if 100 percent of their primary care charges are associated with an institutionalized setting during the sampling period. Starting in the 2018 performance period, the CAHPS for ACOs survey additionally flagged patients as institutionalized if their last primary care visit during the sampling period was associated with an institutional setting. This change (called the "last primary care visit rule") was made to better identify and exclude from the sample, patients likely to be institutionalized at the time the survey is fielded, and by extension, to improve response rates on the survey. This was of particular importance for a few Shared Savings Program ACOs for which large portions of their assigned beneficiaries are in nursing homes. Analysis of the 2020 performance period CAHPS for MIPS sample found that among the 100 MIPS groups that fielded the survey, less than 1 percent of the survey sample would be lost, on average, due to the application of this additional criterion to identify institutionalized patients. Of the groups fielding the survey in 2020 performance period, only 1 would have been excluded from participating in the survey as a result of falling below the minimum sampling threshold due to the expanded definition of institutionalization. Given these findings, which suggest a minimal impact on MIPS group sample sizes and eligibility to field the survey, we propose beginning with the 2022 MIPS performance period for the CAHPS for MIPS survey to add the "last primary care visit rule" as an additional exclusion to sampling for the CAHPS for MIPS survey. We expect that this change will better identify and exclude from the sample those patients likely to be institutionalized at the time the survey is fielded, and by extension, will improve response rates on the survey.
Other CMS programs use different CAHPS surveys to gather information on patient experience in a variety of health Start Printed Page 39395 care settings. The In-Center Hemodialysis (ICH) CAHPS survey is fielded twice per year to patients receiving dialysis treatment at an ICH facility. Previously, patients sampled for the ICH CAHPS survey during the spring implementation were removed from the CAHPS for ACOs sampling frame in an effort to improve response rates to the ICH CAHPS Survey and to avoid burdening patients with multiple surveys. Analyses of the 2019 and 2020 performance periods CAHPS for MIPS sampling frames suggest that implementing ICH CAHPS deduplication in CAHPS for MIPS would have only minor impacts on most MIPS groups. Of the groups fielding the survey in the 2020 performance period, only 1 would have been excluded from participating in the survey due to falling below the minimum sampling threshold following ICH CAHPS deduplication (the same group that would have been excluded due to the "last primary care visit rule", above). For the 2019 performance period, no participating groups would have been excluded. Therefore, we propose beginning with the 2022 MIPS performance period for the CAHPS for MIPS survey to remove patients who were sampled for the Spring ICH CAHPS survey from the sampling frames for CAHPS for MIPS. We expect this change would have only a minor impact on the CAHPS for MIPS sampling frame, but would increase response rates to the ICH CAHPS Survey and avoid burdening patients with multiple surveys.
(iii) CAHPS for MIPS Case-Mix Adjustment Model
Under CAHPS for MIPS, we adjust summary survey measure scores for case-mix to promote meaningful comparison of the performance of MIPS groups despite differences in their patient populations (81 FR 77120). The case-mix adjustment model for CAHPS for MIPS includes the following case-mix adjustors: Age; education; self-reported general health status; self-reported mental health status; proxy response; Medicaid dual eligibility; and eligibility for Medicare's low-income subsidy. The CAHPS for ACOs Survey included an additional adjustor, Asian language survey, following prior literature that recommended adjustment for Asian language surveys to account for cultural differences that affect reporting. The CAHPS for MIPS case-mix adjustment model has historically not included this adjustor because no Asian language surveys have been administered. Because Shared Savings Program ACOs are fielding the CAHPS for MIPS survey as of the 2021 MIPS performance period, we propose beginning with the 2022 MIPS performance period CAHPS for MIPS survey to add use of an Asian language survey as a case-mix adjustor to the CAHPS for MIPS case-mix adjustment model. Use of an Asian language survey has been shown to be significantly associated with specific response patterns to a number of survey items that contribute to summary survey measures. In particular, Asian language survey respondents are generally less likely to use responses at the extremes of the scales, which tends to result in lower overall scores compared to patients who respond to English-language surveys. Therefore, it is important to retain use of Asian language survey as a case-mix adjustor for Shared Savings Program ACOs, and also appropriate to include it in the case-mix adjustment model for MIPS groups should Asian language surveys be completed for these groups in the future. Analysis of 2019 performance period CAHPS for MIPS data found that adding the Asian language survey case-mix adjustor and pooling data from MIPS groups and Shared Savings Program ACOs for the purposes of case-mix adjustment had only a minimal impact on mean scores for MIPS groups, with scores increasing slightly as a result.
(iv) Scoring CAHPS for MIPS Summary Survey Measures
The CAHPS for MIPS survey contains 10 summary survey measures (SSMs). Of these, 8 are benchmarked and scored, while the other 2 (Health Status and Functional Status and Access to Specialists) are unscored and included for informational purposes only. The latter 2 measures had previously been scored, but were changed to unscored starting with the 2018 performance period (82 FR 53720). While Health Status and Functional Status was changed to unscored because it assesses underlying characteristics of a group's patient population and is less of a reflection of patient experience of care with the group, the Access to Specialists SSM was changed to unscored due to historically low reliability and response rates. At the same time this change was made (2018 performance period), a shorter, streamlined version of the CAHPS for MIPS Survey was implemented (82 FR 53632). Since the implementation of the shortened survey, which included a reduction in the number of survey items that make up the Access to Specialists SSM, response rates and reliability for this SSM have improved dramatically, with over 80 percent of MIPS groups achieving acceptable reliability on this SSM in 2018, 2019, and 2020 performance period(s), compared to less than 20 percent in 2017 performance period. Therefore, because CMS no longer has analytic concerns about scoring the measure, we propose beginning with the 2022 MIPS performance period for the CAHPS for MIPS survey to once again benchmark and score the Access to Specialists measure, which would mean there would be 9 SSMs included in the CAHPS for MIPS scoring process, with 1 SSM remaining unscored.
We request comments on these proposed changes to the CAHPS for MIPS policies and scoring process.
(2) Cost Performance Category
(a) Background
We refer readers to the CY 2017 and CY 2018 Quality Payment Program final rules, and the CY 2019, CY 2020, and CY 2021 PFS final rules (81 FR 77162 through 77177, 82 FR 53641 through 53648, 83 FR 59765 through 59776, 84 FR 62959 through 62979, and 85 FR 84877 through 84881, respectively) for a description of the statutory basis and existing policies pertaining to the cost performance category.
In this proposed rule, we are proposing to add 5 new episode-based measures to the cost performance category beginning with the 2022 performance period, and to update the operational list of care episode and patient condition groups and codes. Additionally, we are proposing a new process for stakeholders to develop cost measures for MIPS. Finally, we are proposing to establish criteria for determining whether a cost measure change is considered substantive starting with the 2022 MIPS performance period. These proposals are discussed in more detail in the following sections.
(b) Addition of Episode-Based Measures
(i) Background
Under § 414.1350(a), we specify cost measures for a performance period to assess the performance of MIPS eligible clinicians on the cost performance category. We will continue to evaluate cost measures that are included in MIPS on an ongoing basis and anticipate that measures could be added, modified, or removed through rulemaking as measure development continues. Any substantive changes to a measure would be proposed for adoption in future years through notice and comment rulemaking, following review by the Measure Applications Partnership (MAP). The MAP is a multi-stakeholder partnership that provides guidance to Start Printed Page 39396 CMS on performance measures for use in federal health programs—more information is available at https://www.qualityforum.org/Setting_Priorities/Partnership/Measure_Applications_Partnership.aspx. The MAP provides an additional opportunity for an interdisciplinary group of stakeholders to provide feedback on whether they believe the measures under consideration are applicable to clinicians and complement program-specific statutory and regulatory requirements. Through its Measure Selection Criteria, the MAP focuses on selecting high-quality measures that address the National Quality Strategy (NQS)'s three aims of better care, healthy people/communities, and affordable care, as well as fill critical measure gaps and increase alignment among programs.
We would take all comments and feedback from both the public comment period and the MAP review process into consideration as part of the ongoing measure evaluation process. Some modifications to measures used in the cost performance category might incorporate changes that would not substantively change the measure. Examples of such non-substantive changes may include updated telehealth service codes, diagnosis or procedure codes or risk adjustors. While we address such changes on a case-by-case basis, we generally believe these types of maintenance changes are distinct from substantive changes to measures that result in what are considered new or different measures. However, as described in section 7 of the Blueprint for the CMS Measures Management System Version 16.0 (https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/Blueprint.pdf), if substantive changes to these measures become necessary, we expect to follow the pre-rulemaking process for new measures, including resubmission to the Measures Under Consideration (MUC) list and consideration by the MAP.
In section IV.A.3.d.(2)(b)(ii) of this proposed rule, we have summarized the timeline for measure development, including stakeholder engagement activities undertaken by the measure development contractor, an entity that oversees the development, implementation and maintenance of cost measures in accordance with a contract with CMS. In sections IV.A.3.d.(2)(b)(iii) through IV.A.3.d.(2)(b)(vii) of this proposed rule, we summarize the new measures that would be included in the cost performance category for the CY 2022 performance period and future performance periods. For the new chronic condition episode-based measures, we provide detail about the measure framework, which lets attributed clinicians or clinician groups know the cost of care that is clinically related to their management of a patient's chronic condition during an episode of care ("episode").
(ii) Overview of Measure Development Timeline for New Episode-Based Measures
We develop episode-based measures to represent the cost to Medicare and beneficiaries for the items and services furnished during an episode. Episode-based measures are developed to compare clinicians on the basis of the cost of care that is clinically related to their treatment and management of a patient and provided during the episode's timeframe. Specifically, we define cost based on the allowed amounts on Medicare claims, which include both Medicare trust fund payments and any applicable beneficiary deductible and coinsurance amounts.
The measure development contractor has continued to seek extensive stakeholder feedback during measure development, building on the processes outlined in the CY 2018 QPP final rule (82 FR 53644 through 53645) and discussed in detail in the CY 2019 PFS final rule (83 FR 59767 through 59769). These processes include the measure development contractor convening multiple panels for different purposes, conducting national field testing of the developed measures, and seeking input from clinicians and stakeholders through engagement activities. The technical expert panel (TEP) serves a high-level advisory role and provides guidance to the measure development contractor on the overall direction of development; clinical subcommittees provide the measure development contractor with recommendations on the measures to prioritize for development within specific clinical areas and on the scope for the measures; and measure-specific clinician expert workgroups provide the measure development contractor recommendations on clinical specifications for each measure. More information about the measure development process for the 5 measures we are proposing to include is available in the measure development process document located on the MACRA Feedback Page at https://www.cms.gov/files/document/macra-cmft-ebcm-process-2020.pdf. Summaries of the clinical subcommittee and clinician expert workgroup meetings organized by measure development contractor are also available on the MACRA Feedback Page under the "Wave 3 MACRA cost measure development (2019-2020)" section located at https://www.cms.gov/Medicare/Quality-Payment-Program/Quality-Payment-Program/Give-Feedback.
We provided stakeholders an opportunity to review their performance under the new episode-based measures through national field testing, from August 17, 2020, to September 18, 2020. Clinicians and clinician groups meeting a minimum threshold of episodes for each measure could review their field test report and an episode-level file with detailed information to understand the types of services that comprise a large or small share of their episode costs. In response to stakeholder input from previous field-testing periods, the field test reports were changed to a portable document format (PDF) and distributed through the Quality Payment Program portal, which clinicians routinely access to review their MIPS performance feedback reports. In addition, stakeholders could review a number of supporting documents, including draft measure specifications, an overview of the development process, a fact sheet and frequently asked questions (FAQ) document and summary statistics on each of the measures. These documents were available through the MACRA Feedback Page (https://www.cms.gov/Medicare/Quality-Payment-Program/Quality-Payment-Program/Give-Feedback). A summary of stakeholder feedback from the field testing period is also available on the website (https://www.cms.gov/Medicare/Quality-Payment-Program/Quality-Payment-Program/Give-Feedback).
During field-testing, the measure development contractor also collected person and family engagement (PFE) input from patients with lived experience of the condition in question. The measure development contractor summarized input about their experience of care, including clinicians who were part of their care team, the types of services they received, and areas they identified for improvement in their care. For more information, we refer readers to the Summary of Person and Family Engagement (PFE) and Input for Wave 3 Episode-Based Cost Measure Development, which summarizes PFE input that the measure development contractor gathered for the measures (https://www.cms.gov/files/document/summary-person-and-family-engagement.pdf).
Similar to previous years, the measure development contractor has continued Start Printed Page 39397 to engage clinicians and stakeholders, conducting extensive outreach activities. These activities included general informational email blasts, targeted email outreach to specialty societies, hosting office hours to gather information on additional opportunities for participation and outreach, and posting a pre-recorded MACRA Cost Measures Field Testing Webinar to provide information about the measure development process and field test reports (available at https://qpp.cms.gov/resources/webinars).
(iii) New Episode-Based Measures for CY 2022 and Future Performance Periods
In this section of the proposed rule, we discuss the 5 new episode-based measures, including 2 new chronic condition measures, which we propose to add for the CY2022 and future performance periods. These measures are listed in Table 41. The acute inpatient medical condition and procedural measures are based on the previously established framework for episode-based measures, which we described in detail in the CY 2019 PFS final rule (83 FR 59767 through 59773).
Chronic condition episode-based measures expand on the previously established framework for episode-based measures to address unique factors inherent to the continuous nature of chronic disease care management. In section IV.A.3.d.(2)(b)(vi) of this proposed rule, we provide detail about the proposed episode definition and attribution methodology for chronic condition episode-based measures. After chronic condition episodes are defined and attributed to a clinician group and, or individual clinician, we include items and services furnished during the episode that are clinically related to the care and management of a patient's chronic condition. Items and services may include treatment and diagnostic services, ancillary items (such as medical nutrition therapy and refining and maintenance of a portable pump for diabetes), services directly related to treatment, and those furnished as a consequence of care. The two chronic condition measures specified in this proposed rule are calculated using claims data from Medicare Parts A, B, and D. Part D costs are included to account for the full range of treatment options used to manage chronic conditions. As with Part A and B payment standardization, Part D costs are standardized to facilitate meaningful comparisons of resource use within the market-based Medicare Part D program by accounting for non-clinical variation in costs. For more detail, the Part D payment standardization methodology is available at https://resdac.org/articles/cms-price-payment-standardization-overview. The Medicare Parts A and B payment standardization methodology is available at https://resdac.org/articles/cms-price-payment-standardization-overview.
Similar to other episode-based measures, chronic condition measures include features intended to ensure a more accurate comparison of costs across clinicians. First, we stratify the patient population captured by the measure into smaller, clinically similar patient cohorts. For example, the Diabetes measure separates patients with type 1 and type 2 diabetes, and the risk adjustment model is assessed at the level of each stratification to ensure that only patients with similar case mixes are compared to each other. We note that the term "stratification" will be used to describe a portion of a group in relation to the cost performance category and that such term is synonymous with the term "episode sub-group" used in the cost measure specification documents and other documents related to the cost performance category. In general, unless otherwise indicated, the term "episode sub-group" used in the cost measure specification documents and other documents related to the cost performance category has a different meaning than the term "subgroup" that we propose to define under § 414.1305 in this proposed rule. Second, we standardize episode costs to limit observed differences in costs to those that may result from health care delivery choices. Third, we exclude unique groups of patients from episodes where it may be unreasonable to compare the costs of these patients to the whole cohort. Last, the measures account for patient characteristics that can influence spending and are outside of a clinician's control using risk adjustment. For example, the risk adjustment model is run separately for patients with and without enrollment in a Part D drug plan to account for differences in costs that we might observe between patients enrolled in Part D and those who are not. In addition, the risk adjustment model for chronic condition episode-based measures specified in this rule account for a patient's status as a dual Medicare and Medicaid enrollee. This was based on testing demonstrating that dual status had a notable impact on performance for the two measures. For more information on the chronic condition episode-based measure framework, we refer readers to the Chronic Condition Cost Measure Framework located at https://www.cms.gov/files/document/chronic-condition-cost-measure-framework-poster.pdf.
The proposed specifications for all 5 proposed episode-based measures are available at https://www.cms.gov/Medicare/Quality-Payment-Program/Quality-Payment-Program/Give-Feedback. The specifications documents for each measure consist of a methods document that describes the steps for constructing the measure and a measure codes list file that contains the medical codes used in that methodology. First, the methods document provides details about components of episode-based measures: Identifying patients receiving care, defining an episode-based measure, attributing episodes to clinicians and clinician groups, assigning costs, defining exclusions, risk adjusting, and calculating measure score. For each measure component, the methods document provides detailed methodology describing each logic step involved in constructing the measure. For the chronic condition episode-based measures, the specifications also include an appendix to the methods document which provides additional detail on particular components of the measure construction framework, including the sub-grouping methodology, episode construction and calculation, and attribution to individual clinicians. Second, the measure codes list contains the codes used in the measure specifications, including the episode triggers, attribution, sub-groups, assigned items and services, exclusions, and risk adjustors.
More information about the five proposed episode-based measures is available in the measure justification forms, the national summary data report, and the national summary data report addendum with risk adjustment regression results. These documents are available through the MACRA Feedback Page (https://www.cms.gov/Medicare/Quality-Payment-Program/Quality-Payment-Program/Give-Feedback).
We seek public comment on the proposed episode-based measures, which are listed in Table 41.
Start Printed Page 39398

(iv) Attribution
In this section of the proposed rule, we discuss the attribution methodology for the episode-based measures. In the CY 2020 PFS final rule (84 FR 62962), we established at § 414.1350(b)(8) that beginning with the 2020 performance period, each cost measure is attributed according to the measure specifications for the applicable performance period. For the proposed acute inpatient medical condition and procedural episode-based measures, we refer readers to the proposed measure specifications for the attribution methodology, available at https://www.cms.gov/Medicare/Quality-Payment-Program/Quality-Payment-Program/Give-Feedback. For the proposed chronic condition measures, we would use a new attribution framework for identifying and confirming a clinician-patient relationship, which we discuss below. For further detail regarding the specific attribution methodology for the proposed Asthma/COPD and Diabetes measures, we refer readers to the measure specifications for each measure, available at https://www.cms.gov/Medicare/Quality-Payment-Program/Quality-Payment-Program/Give-Feedback.
For chronic condition episode-based measures, we would attribute episodes to the clinician group that renders the services that constitute a trigger event, which is identified by the occurrence of two claims billed in close proximity by the same clinician group. Both claims must have a diagnosis code indicating the chronic disease captured by the measure (for example, type 1 or type 2 diabetes for the Diabetes episode-based measure). The first claim must have an evaluation and management (E/M) code for primary care services and the second claim must have either another E/M code for primary care services or a condition-related HCPCS/CPT code for procedure codes related to the treatment or management of the chronic condition. The trigger event opens a year-long attribution window from the date of the initial E/M primary care service, during which the same clinician group could reasonably be considered responsible for managing the patient's chronic disease. The initiation of the attribution window at the onset of the trigger event ensures that costs are attributed only after the start of the clinician-patient relationship. We may extend the initial attribution window and the clinician group's responsibility by another year each time we see additional E/M codes for primary care services or condition-related HCPCS/CPT codes for procedure codes related to the treatment or management of the chronic condition that indicate an ongoing clinician-patient relationship. Therefore, the resulting total attribution window can span multiple years and vary in length for different patients. Because the total attribution window can span multiple performance periods, we measure it incrementally and periodically by dividing it into segments of episodes, which we assess in the performance period in which they conclude. Dividing the total attribution window into episodes allows us to assign costs during the time-period in which the clinician group is responsible for the patient's chronic condition care management.
After we identify the attributed clinician group as described in the previous paragraph, we would attribute the episode to individual clinician(s). For individual clinicians, we would attribute episodes to each MIPS eligible clinician within an attributed clinician group that renders at least 30 percent of qualifying services during the episode. Qualifying services include E/M codes for primary care services or condition-related HCPCS/CPT codes with a relevant chronic condition diagnosis. We employ two additional checks to confirm the qualifying clinician's role in the ongoing management of the patient's chronic condition. First, we check to ensure that the qualifying clinician(s) have rendered at least one E/M code for primary care services or condition-related HCPCS/CPT code with a relevant diagnosis within 1 year prior to or on the episode start date. This ensures that clinicians are not attributed an episode before they have their first encounter with the patient. Second, we check whether the clinician(s) have written at least 2 condition-related prescriptions on different days to two different patients during the performance period plus a one-year lookback period. The use of these prescription billing patterns ensures that we are capturing the clinicians actually involved in providing ongoing chronic care management, rather than clinicians who may have only refilled a patient's prescription once, as a courtesy. MIPS eligible clinicians within an attributed clinician group that render at least 30 percent of qualifying services and meet the two additional checks are considered for attribution. The individual clinician's performance is based on all of the episodes attributed to the individual clinician, whereas the clinician group's performance is based on all of the episodes attributed to the clinician group. If a single episode is attributed to multiple clinicians in a single clinician group, the episode is only counted once toward the clinician group's performance. Additional detail for this attribution methodology is available in the proposed measures specifications for the Diabetes and Asthma/COPD measures located on the MACRA Feedback Page at https://www.cms.gov/Medicare/Quality-Payment-Program/Quality-Payment-Program/Give-Feedback.
To illustrate the proposed attribution rules for chronic condition episode-based measures, we are providing an example of a clinical scenario where 3 MIPS eligible clinicians (A, B, and C) are part of the same clinician group. A patient with type 2 diabetes presents to the clinician group to receive services related to their condition. Clinician A bills an initial E/M code for primary care services related to the patient's diabetes (for example, an office/outpatient visit related to the patient's diabetes). During a follow-up appointment two weeks later, Clinician A bills a HCPCS/CPT code for tests related to the patient's diabetes. We consider the occurrence of these two services to constitute a trigger event Start Printed Page 39399 indicating the start of a clinician-patient relationship. This trigger event opens a one-year attribution window from the date of the initial E/M service and the clinician group that rendered the trigger event services would be attributed the Diabetes episode. If in this example, there were a total of 10 clinically related services captured during the episode, and Clinician A rendered 5 of those services, Clinician B rendered 2, and Clinician C rendered 3 of those services, then Clinicians A and C would be considered for attribution since they would have rendered at least 30 percent of qualifying services for the patient. Clinician B would not be considered for attribution. Before attributing the episode to Clinicians A and C, we check (i) whether the clinicians billed at least 1 E/M code for primary care services or condition-related HCPCS/CPT code with a relevant diabetes diagnosis within 1 year prior to or on the episode start date and (ii) whether they wrote 2 diabetes-related prescriptions on different days for 2 different patients during the performance period plus a one-year lookback period. Assuming Clinician A met these two checks and Clinician C did not, then only Clinician A would be attributed this Diabetes episode. This episode would count towards the Diabetes measure's case minimum for Clinician A, but not for Clinicians B or C. At the group reporting level, the episode would be included in the calculation of the clinician group's measure score and would count towards the measure's case minimum for the clinician group.
(v) Discussion of MAP Recommendations for New Episode-Based Measures
In this section of the rule, we discuss the results of the 2020-2021 MAP review cycle for the 5 measures we are proposing and address the points raised by the MAP. Following the measure development process, we describe in section IV.A.3.d.(2)(b)(ii) of this proposed rule, the MAP Clinician Workgroup and the MAP Coordinating Committee reviewed the 5 measures in January 2021. The MAP conditionally supported Melanoma Resection and Colon and Rectal Resection contingent on NQF endorsement. We intend to submit these 2 measures to a future NQF endorsement cycle, as we have done for other episode-based measures. The MAP recommended "do not support with potential for mitigation" for the Sepsis, Diabetes, and Asthma/COPD measures; we address these mitigation points in turn, below in this section.
The mitigation factors across the 3 measures focused primarily on testing. Across the 3 measures, the MAP noted the following mitigation points: (1) Explore the correlations between the cost measure and quality measures; and (2) NQF endorsement. For the Asthma/COPD and Diabetes measures, the MAP noted the following points: (1) Explore the concern that good care may result in higher episode costs but with global cost savings; and (2) evaluate the connection between upstream interventions and downstream cost savings. For the Sepsis measure, the MAP's recommendation was contingent on an analysis into the potential for gaming associated with the over-diagnosis of sepsis. For all 3 measures, the MAP noted that should testing data show that the measure appropriately assesses episode-based cost and can be used alongside quality measures, the measures would be valuable to add to the program. The MAP's final recommendations are available at http://www.qualityforum.org/Publications/2021/03/MAP_2020-2021_Considerations_for_Implementing_Measures_Final_Report_-_Clinicians,_Hospitals,_and_PAC-LTC.aspx.
We appreciate the MAP's review and their comments regarding the measures. We believe that we have addressed the mitigating factors by sharing additional testing information and clarifying the measure construction and intent at the MAP Coordinating Committee meeting on January 25, 2021.
The first mitigation factor common to the Asthma/COPD, Diabetes, and Sepsis episode-based measures is to explore the correlations with quality measures to address concerns about the potential for care stinting. We presented empirical testing results to the MAP Coordinating Committee on January 25, 2021. The MAP's concern about care stinting would be evidenced by a strong, inverse cost-quality correlation between cost and quality measures; that result would indicate that good performance on cost would be associated with poor performance on quality and that variation in cost is solely reflecting variation in quality. We do not see this relationship in any of the correlations. The correlation results instead indicate a modest and generally positive correlation between the 3 cost measures and related quality measures demonstrating that there is variation in cost, regardless of quality level. These results suggest that performance on these 3 episode-based measures can be improved without negatively impacting quality. More information on the results of this exploration of the relationship between cost and quality measures is available in the supplemental testing document at https://www.cms.gov/files/document/testing-updates-wave-3.pdf.
The second mitigation factor common to the Asthma/COPD, Diabetes, and Sepsis episode-based measures is NQF endorsement. We intend to submit these measures to a future NQF endorsement cycle, as we have done with other cost measures. For example, the MAP in the 2018-2019 review cycle had recommended "do not support with potential for mitigation" for the total per capita cost measure. This measure was endorsed by NQF in December 2020.
For the Asthma/COPD and Diabetes measures, the MAP raised two points for further evaluation. The first point is to evaluate the relationship between condition-specific costs and global patient costs, as the MAP was concerned that there may be tension between these types of costs. We tested the correlation between the episode-based measures and NQF #3575 total per capita cost, a population-based cost measure that assesses the overall costs of care. The correlation results show a moderately positive correlation between Asthma/COPD and Diabetes and the total per capita cost measure, indicating that clinicians who have lower costs for the specific care of asthma/COPD and diabetes tend to also have lower overall global costs. These results suggest that there is no concern that high quality care results in higher episode-specific costs and lower overall costs. These results also show that there is no redundancy between the measures; for instance, a correlation of 1 would indicate duplication across the episode-based and population-based cost measures. The results of this correlation testing are available at https://www.cms.gov/files/document/testing-updates-wave-3.pdf.
For the Asthma/COPD and Diabetes measures, the MAP's second point was to evaluate the potential for actionability, providing the example of the connection between upstream medical interventions and downstream costs. The MAP's recommendations note that this example was clarified as cost measures do not attempt to dictate clinician practice; that would be outside of their scope. Rather, the cost measures aim to fairly capture costs related to a clinician's care and account for factors outside of their influence. Regarding the overall actionability, we selected these measures to develop based on the evidence from the literature and input from clinical experts which identified the care and management of asthma, COPD, and diabetes as important in the goal of making care affordable, due to their prevalence and costliness, and the nature of the care as clinicians can make Start Printed Page 39400 care decisions that reduce the likelihood of high costs. For example, opportunities for clinicians to take action in the Asthma/COPD episode-based measure identified through an environmental scan, clinician input, and input from persons with lived experience of the conditions include enhancing education programs, support, and care continuity; providing appropriate medication and encouraging adherence to these medications; promoting pulmonary rehabilitation through physical activity and exercise; and encouraging smoking cessation. For the Diabetes episode-based measure, actions to decrease the likelihood of high costs include providing self-management education and support, providing appropriate medication, screening for other impairments, and encouraging adherence to preventive treatment guidelines.
The MAP noted a mitigation point for the Sepsis measure to explore a concern related to over-diagnosis of sepsis which could result in potential gaming of the measure. The variation in diagnosis coding has been considered extensively throughout development, including by the Sepsis clinician expert workgroup convened by the measure development contractor. The specifications reflect these careful considerations by defining a homogenous patient cohort while retaining the breadth of coverage needed to address coding issues. The measure uses a risk adjustment approach and exclusions to ensure fair comparisons, for example by adjusting for source of infection and source of admission (to account for patients transferred from another facility), and stratifying the patient population based on severity (with and without septic shock). As such, the measure construction itself is designed to capture costs accurately to compare clinician cost performance. We also tested the relationship between cost measure performance and types of variation in coding, such as the attributed clinician's share of episodes triggered by MS-DRGs 870-872 for sepsis and share of episodes out of all hospitalizations with 24 MS-DRGs for other infectious disease. The results of these correlations show no meaningful relationship between cost measure scores and coding patterns, addressing the concern about the impact of diagnosis coding on measure scores.
We appreciate the MAP's comment that the Asthma/COPD, Diabetes, and Sepsis measures would be valuable to add to MIPS, should testing data show that the measures appropriate assess costs. We believe that the extensive empirical testing throughout and after development (beyond the testing referenced in this section), national field testing, and engagement with clinician experts and incorporation of the patient voice demonstrate that the measures appropriately capture costs related to the care of these conditions. For more testing information, see https://www.cms.gov/files/document/testing-updates-wave-3.pdf.
(vi) Proposed Revisions to the Operational List of Care Episode and Patient Condition Groups and Codes
Section 1848(r) of the Act specifies a series of steps and activities for the Secretary to undertake to involve the physician, practitioner, and other stakeholder communities in enhancing the infrastructure for cost measurement, including for purposes of MIPS and APMs. Section 1848(r)(2) of the Act requires the development of care episode and patient condition groups, and classification codes for such groups, and provides for care episode and patient condition groups to account for a target of an estimated one-half of expenditures under Parts A and B (with this target increasing over time as appropriate). Sections 1848(r)(2)(E) through (G) of the Act require the Secretary to post on the CMS website a draft list of care episode and patient condition groups and codes for solicitation of input from stakeholders, and subsequently, post an operational list of such groups and codes. Section 1848(r)(2)(H) of the Act requires that not later than November 1 of each year (beginning with 2018), the Secretary shall, through rulemaking, revise the operational list as the Secretary determines may be appropriate, and that these revisions may be based on experience, new information developed under section 1848(n)(9)(A) of the Act, and input from physician specialty societies and other stakeholders.
In December 2016, we published the Episode-Based Measure Development for the Quality Payment Program (https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/Draft-list-of-episode-groups-and-trigger-codes-December-2016.zip) and requested input on a draft list of care episode and patient condition groups and codes as required by sections 1848(r)(2)(E) and (F) of the Act. In accordance with section 1848(r)(2)(G) of the Act, in January 2018, we posted an operational list of 8 care episode groups and patient condition groups, which is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/2018-Operational-List-of-Care-Episode-and-Patient-Condition-Codes.zip. Under section 1848(r)(5)(A)(iii) of the Act, to evaluate the resources used to treat patients with respect to care episode and patient condition groups, the Secretary shall, as the Secretary determines appropriate, conduct an analysis of resource use with respect to care episode and patient condition groups. In accordance with this section, we used the 8 care episode groups and patient condition groups included in the operational list as the basis for the 8 episode-based measures that were finalized for use in MIPS in the CY 2019 PFS final rule (83 FR 59767 through 59773). In the CY 2020 PFS final rule (84 FR 62968 through 62969), in accordance with section 1848(r)(2)(H) of the Act, we revised the operational list beginning with CY 2020 to include 10 additional care episode and patient condition groups, which served as the basis for the 10 additional episode-based measures that were refined based on extensive stakeholder input and finalized for use in MIPS in that same final rule (84 FR 62979). The operational list as revised in the CY 2020 PFS final rule is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-Feedback.html.
Under section 1848(r)(2)(H) of the Act, we are proposing to revise the operational list beginning with CY 2022 to include 5 new care episode and patient condition groups, based on input from clinician specialty societies and other stakeholders. These 5 care episode and patient condition groups were included in the draft list that we posted in December 2016 and refined based on extensive stakeholder input as described in section IV.A.3.d.(2)(b)(ii) of this rule. The codes and logic used to define these episode groups are available on our MACRA Feedback Page at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-Feedback.html. These care episode and patient condition groups serve as the basis for the 5 new episode-based measures that we are proposing for the cost performance category in section IV.A.3.d.(2)(b)(iii) of this rule. We request comments on our proposal to revise the operational list to include these 5 new care episode and patient condition groups.
(vii) Reliability and Case Minimum
In this section of the rule, we discuss the case minima for the 5 proposed cost Start Printed Page 39401 measures, weighing up considerations of reliability standards, the tradeoffs between accuracy and reliability, and the implications of increasing case minima on the extent to which the measure can apply to clinicians participating in MIPS. Reliability is a metric that evaluates the extent that variation in a measure comes from clinician performance ("signal") rather than random variation ("noise"). Higher reliability suggests that a measure is effectively capturing differences between the clinician and their peer cohort.
In the CY 2017 Quality Payment Program final rule (81 FR 77169 through 77171), we identified reliability levels between 0.4 to 0.7 as moderate and reliability levels above 0.7 as high. In the CY 2017 Quality Payment Program final rule, we also identified a threshold of 0.4 for mean reliability to be applied for measures in the cost performance category to ensure moderate reliability. This aligned with the reliability threshold applied to measures under the Value Modifier program and previous analyses of reliability.[209] We appreciate the concerns commenters had raised that this may be too low and as we noted in the CY 2017 Quality Payment Program final rule (81 FR 77169 through 77171), we continue to work on developing measures with the highest level of reliability that is feasible within the MIPS program and have since continued to monitor the overall scientific evidence on reliability. There are many different interpretations of reliability and what these values represent. Studies have pointed to various standards to indicate sufficient, adequate, moderate, or good reliability across healthcare and other disciplines with performance measures and different methods of estimating reliability.[210 211 212 213 214 215 216] We also monitor the evaluation and standards applied throughout the measure endorsement processes, and note that the endorsement standards state there is no minimum threshold for reliability.[217] As such, we believe that the 0.4 threshold for mean reliability continues to be appropriate for moderate reliability.
Under section 1848(r)(5)(A) of the Act, to evaluate the resources used to treat patients (with respect to care episode and patient condition groups), the Secretary shall, as the Secretary determines appropriate, conduct an analysis of resource use (with respect to care episodes and patient condition groups of such patients) using codes reported on claims. Our approach to cost measurement focuses on defining clinically homogenous patient conditions and care episodes. This ensures that these measures accurately compare clinician performance without the results being solely driven by clinical differences across episodes. While limiting the measure scope to improve homogeneity improves the accuracy of assessing cost performance, this also reduces the number of episodes per clinician. Fewer episodes per clinician results in lower reliability compared with global population measures. However, episode-based measures balance this concern using selective service assignment; only including the costs of services that are clinically related to the condition or procedure in the measures' cost calculation improves reliability by keeping the "signal" while reducing the "noise."[218] Overall, these measures prioritize capturing clinically appropriate, homogeneous care episodes over achieving results on certain testing mechanisms to meet the statutory objective of episode-based resource measurement and create more actionable measures for clinicians. As such, we continue to evaluate cost measures on a broader range of testing, along with the details of measure construction. For this reason, we continue to caution against placing too much emphasis on reliability results in isolation as we noted in the CY 2018 Quality Payment Program proposed rule (82 FR 30050 through 30051).
As we discussed in the CY 2018 Quality Payment Program proposed rule (82 FR 30050 through 30051), while a higher case minimum generally improves measure reliability, these incremental increases must be considered against decreases in the coverage of the measure. There are several important implications for clinicians and the program. Increasing the case minimum reduces the number of clinicians that can have their performance assessed by that measure. This can limit the applicability of episode-based measures to larger group practices with sufficient case volume, leaving smaller practices and individual practitioners to be assessed only with population-based cost measures. In addition, for measures to have the potential to improve performance, they should apply to as many clinicians as can be reliably measured. Finally, it is important to recall that clinicians receive a cost performance category score which incorporates their scores across all applicable cost measures. Adding more measures that can be used in a category score increases the amount of data used to calculate the category score, which may improve the precision of overall assessment of cost performance. Additional measures also allows us to evaluate clinicians' cost category performance across a broader range of their care practice.
We examined the reliability of the 5 proposed episode-based measures, and Table 42 presents the percentage of TINs and TIN/NPIs that meet the 0.4 reliability threshold and the mean reliability for TINs and TIN/NPIs at our proposed case minimum for each of the episode-based measures. We previously established at § 414.1350(c)(4) a case minimum of 10 episodes for procedural episode-based measures and at Start Printed Page 39402 § 414.1350(c)(5) a case minimum of 20 episodes for acute inpatient medical condition episode-based measures in the CY 2019 PFS final rule (83 59773 through 59774). For both the proposed Melanoma Resection procedural measure and the Sepsis acute inpatient medical condition measure, we found that the mean reliability for groups and individual clinicians exceeds 0.4 and that the majority of groups and individual clinicians meet the 0.4 reliability threshold when applying the established case minimum for the respective measure types. For the Colon and Rectal Resection procedural measure, at the established 10-episode case minimum for procedural measures, we found that the mean reliability does not exceed 0.4 for individual clinicians and that the majority of groups and individual clinicians do not meet the 0.4 reliability threshold. However, as displayed in Table 42, when the measure's case minimum is raised to 20 episodes, the mean reliability exceeds 0.4 for both groups and individual clinicians, and the majority of groups and individual clinicians meet the 0.4 reliability threshold. As such, we propose to raise the case minimum for the Colon and Rectal Resection procedural measure to 20 episodes, and corresponding revisions to § 414.1350(c)(4). For the chronic condition measures, we propose at § 414.1350(c)(6), a case minimum of 20 episodes. At a 20-episode case minimum, the mean reliability for both measures exceeds 0.4 for both groups and individual clinicians, and the majority of groups and individual clinicians meet the 0.4 reliability threshold. We believe that calculating the episode-based measures with these case minimums would accurately and reliably measure the performance of a large number of clinicians and clinician group practices.

(c) Proposed Process for Cost Measure Development by Stakeholders
(i) Background
Since 2017, we have conducted extensive stakeholder engagement to develop episode-based measures that cover a wide range of procedures, conditions, and specialties. This measure development process, as described in the CY 2019 PFS final rule (83 FR 59770), involves the measure development contractor convening hundreds of clinician experts to provide information to prioritize, conceptualize, and specify clinically refined cost measures and conducting national field testing on an 18-month timeline. The process involves engagement activities conducted by the measure development contractor to solicit expert input for measure development, gather feedback from individuals with lived experiences of the conditions in question, and collect stakeholder feedback on draft measure specifications that can inform how the measures can be improved. This approach follows CMS' standardized approach for developing, implementing, and maintaining measures. There are currently 18 episode-based measures in the cost performance category (CY 2020 PFS final rule (84 FR 62979)) with 5 more proposed to be added in this rule. There are also 2 global or population-based measures, the Medicare Spending per Beneficiary Clinician measure and the total per capita cost measure which were most recently refined in the CY 2020 PFS final rule (84 FR 62969 through 62977).
Many stakeholders have expressed support for episode-based measurement and for a process that prioritizes clinician involvement (as noted in the CY 2018 QPP final rule (82 FR 53645)). In the CY 2021 PFS final rule (85 FR 84879), we noted that commenters expressed interest in expanding the limited inventory of cost measures available to assess cost performance applicable to specialties. Commenters believed that additional episode-based measures would address gaps in cost performance assessment for various specialties. Expanding the range of procedures, conditions, and specialties would enable more MIPS eligible clinicians from different specialties and sub-specialties to have their cost performance assessed under clinically relevant episode-based measures. An increase in the range of cost measures available in MIPS that can be linked with quality measures and improvement activities in future MVPs would support the assessment of clinician value in providing specific types of care.
A process outside of the current development process that would allow stakeholders to develop cost measures could expand the inventory of episode-based measures. However, this process must ensure that any cost measures developed are consistent with the goals of MIPS, align with CMS priorities, and consistent with the Meaningful Measures Framework (more information Start Printed Page 39403 about the Meaningful Measures Framework can be found at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/MMF/General-info-Sub-Page). Episode-based measures developed by stakeholders that meet the standards and criteria we propose below for selecting measures would contribute to the target of an estimated 50 percent of expenditures under Parts A and B, as described in section 1848(r)(2)(D)(i)(I) of the Act.
We propose to establish a process, beginning in CY 2022, for the development of cost measures by stakeholders that would ensure that the cost performance category has consistency across measures. The sections below outline the proposals for the measure prioritization criteria, standards for measure construction, pre-rulemaking submission process and development support, which altogether would comprise our proposed process of cost measure development by stakeholders.
(ii) Measure Prioritization Criteria
As described in section IV.A.3.d.(2)(b)(ii) of this rule, the current process for prioritizing cost measures for development involves the measure development contractor identifying candidate clinical areas and episode groups informed by a TEP, patient and family engagement perspective, and clinician stakeholders. Criteria reflecting TEP input have guided strategic decisions and informed clinical subcommittees' considerations for measure prioritization. These criteria include:
- Clinical coherence of measure concept (to ensure valid comparisons across clinicians).
- Impact and importance to MIPS (including cost coverage, clinician coverage, and patient coverage).
- Opportunity for performance improvement.
- Alignment with quality measures and improvement activities to ensure meaningful assessments of value.
To inform cost measure development by stakeholders, we propose to apply these criteria to an environmental scan to identify a list of priority areas and suggested measures for development. This would ensure that measures developed by stakeholders align with program needs, while also providing flexibility for stakeholders to apply their own clinical expertise when identifying the most important areas for value improvement within the criteria listed above. Stakeholders who choose to develop cost measures can access the Blueprint for the CMS Measures Management System at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/MMS-Blueprint and the Meaningful Measures Framework at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/MMF/General-info-Sub-Page for further information.
We seek public comment on the proposed measure prioritization criteria, as well as priority areas for future episode-based measure development, such as specialties, types of clinical care, or specific conditions or procedures that would support proposed or future MVPs.
(iii) Standards for Measure Construction
Our current rigorous cost measure development process has included a series of standards that ensure measures are effective in assessing clinician cost performance within MIPS. These standards have been developed and vetted over time by a standing TEP and further refined through discussions with clinical subcommittees and clinician expert workgroups convened by the measure development contractor around areas of care and specific measures, respectively. For further detail, we refer readers to our detailed discussion of the measure development process and framework in response to stakeholder comments in the CY 2019 PFS final rule (83 FR 59770). To ensure that cost measures developed by stakeholders meet the same standards applied during the current measure development and testing process, we propose to apply the following standards when considering stakeholder developed measures:
- Measures must assign services that accurately capture the role of attributed clinicians.
- Measures must have clear, ex ante attribution to clinicians.
- Measures must be based on episode definitions that have clinical face validity and are consistent with practice standards.
- Measures' construction methodology must be readily understandable to clinicians.
- Measures must hold clinicians accountable for only the costs they can reasonably influence.
- Measures must convey clear information on how clinicians can alter their practice to improve measured performance.
- Measures must demonstrate variation to help distinguish quality of care across individual clinicians.
- Measure specifications must allow for consistent calculation and reproducibility using Medicare claims data.
To implement these standards and to meet the methodology requirements of section 1848(r)(5) of the Act, we believe that it is necessary to ensure that measures within the cost performance category are consistent and share the same key features. Specifically, cost measures must be based on a standard set of measure components informed by the standards outlined above. These include: (1) Episode definition based on trigger codes that determine the patient cohort; (2) attribution; (3) service assignment; (4) exclusions; and (5) risk adjustment.
Regarding item (1) episodes must be defined based on trigger codes for services, which are identifiable on Medicare claims, indicate the occurrence of the episode, and determine the patient cohort. Trigger codes must be based on services, and can incorporate diagnosis and other service information to define an episode. The patient cohort may be stratified into mutually exclusive stratifications (or "episode sub-groups") for meaningful clinical comparison to ensure that measures fairly compare clinicians with similar patient case-mix. Regarding item (2), episodes must be attributed to MIPS eligible clinician groups and clinicians who render the trigger services and are responsible for the patient's care and management. It is important that the attribution methodology allows for the most appropriate clinicians who have a significant role in a patient's care to be attributed and receive actionable feedback on their performance. Regarding item (3), all services that are clinically related to the attributed clinician's role in managing patient care must be included. This includes cases where the clinician can influence the frequency or intensity of services. The measure must include enough services to allow the measure to demonstrate that it captures variation in clinician performance. To address any potential concern around care stinting, the measure must cover a sufficiently long timeframe and broad enough services to capture downstream services. This includes expected follow-up care, rehabilitation, post-acute care (required if inpatient hospitalizations are included) and other support services, as well as complications, readmissions, and other consequences of care. Clinically unrelated services must not be assigned to the measure. Regarding item (4), measures must include applicable exclusions, which can be applied to the patient cohort or the episodes. Certain patients must be Start Printed Page 39404 excluded for data cleaning or to ensure completeness of data. For example, patients who do not have Medicare as their primary payer or were not continuously enrolled in Medicare Parts A and B and not C must be excluded as we would not be able to observe their complete care. Certain episodes must be excluded to improve episode homogeneity and to remove unique groups of patients from the measure in cases where it may be impractical or unreasonable to compare the costs of caring for these patients to the costs of caring for the measure cohort as a whole. Regarding item (5), measures must be adjusted for patient risk. Risk adjustment aims to isolate variation in clinician costs to only the costs that clinicians can reasonably influence by accounting for risk factors. The determination of an appropriate risk adjustment approach should be based on empirical testing. A base risk adjustment model must include standard risk adjustors (Hierarchical Condition Category [HCC] codes, interaction variables for certain comorbidities, age, disability status, end-stage renal disease status, recent use of institutional long-term care), as well as additional measure-specific risk factors. Finally, measures must include payment standardized claims data.
We have outlined in section IV.A.3.d.(2)(b)(iv) of this proposed rule a new methodological framework for assessing the cost of care for chronic conditions. This new chronic condition framework meets all the standards we outline above, and could serve as a basis for chronic condition measures developed by stakeholders that would ensure consistency with other MIPS measures. We propose to apply the standards for measure construction and measure components outlined above when considering stakeholder-developed measures to ensure that these measures follow the same standards as cost measures currently used in MIPS.
We seek public comment on our proposed standards for measure construction and measure components, as well as the challenges that stakeholders may encounter in the development of cost measures along with any resources that would assist stakeholders in development.
(iv) Cost Measure Submission to the Measures Under Consideration (MUC) List and Development Support
We propose that cost measures developed by stakeholders for potential use in MIPS would undergo the pre-rulemaking process described in section 1890A(a) of the Act. More details on the pre-rulemaking process can be found at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/Pre-Rulemaking. As with the process for the call for quality measures, we propose that the submission process for cost measures developed by stakeholders would begin with a Call for Cost Measures, where stakeholders would be invited to submit their candidate cost measures. We refer readers to the CY 2018 Quality Payment Program final rule (82 FR 53635 through 53637) for details on the process for quality measure submissions and selection. During the Call for Cost Measures period, we would organize webinars and office hours to provide stakeholders with information about the process and be available to answer questions. We would also provide templates of written materials, such as the measure codes lists for stakeholders to use. At the end of this period, stakeholders would submit their candidate measures for review by completing the required data fields required for submission to the MUC list and if approved, measures would be placed on the final MUC List, which we publicly post on December 1 of every year. Measures submitted to the MUC List must be fully specified and tested for reliability and validity.
Submissions to the MUC list must include all required information and would be reviewed against a set of inclusion criteria identified below to determine whether they should be considered for use in MIPS. The inclusion criteria for cost measures developed by stakeholders are based on the criteria outlined in the MUC List submission template as well as relevant criteria that the MIPS quality performance category follows as indicated in the CY 2020 PFS final rule (84 FR 62953 through 62954). For purposes of our review, we propose that stakeholders who wish to submit measures must submit measure specification information, testing results, and related research to address the following inclusion criteria:
- Applicable: There is clinical coherence and comparability in clinician treatment; measures ensure alignment with quality indicators.
- Feasible: Measures use Medicare claims data; there is a high degree of data completeness and limited frequency of missing data.
- Scientifically acceptable: Measures are clinically valid assessments of cost performance; testing is available for reliability at different case minima; beta testing and statistical testing are conducted.
- Not be duplicative of existing measures: Measures assess opportunities and gaps based on CMS priorities and goals; there is an assessment of duplicate measures to see which would be the better measure.
- Fully developed: Measures are fully developed and ready for implementation at the time of submission.
- Consistent: The measure is constructed using a methodology to assess resource use that is consistent with sections 1848(r)(2) through (5) of the Act, consistent with other MIPS cost measures.
- Fulfill a clinical performance gap: Environmental scans and literature reviews show evidence for measures, performance gaps, and opportunities for improvement; there is evidence for measures' impact and importance to MIPS.
We seek comments on this proposed approach, and challenges stakeholders may encounter in the development of cost measures.
(d) Substantive Changes Criteria for Cost Measures
On an annual basis, we review the MIPS measures that have been adopted and consider updates to the cost measures. Changes to measures are an important part of the measure maintenance process to ensure that measures are continuing to function as intended, and may be substantive or non-substantive. Section 1848(q)(2)(D)(i)(II)(cc) of the Act requires all substantive changes to quality measures to be proposed and identified through notice-and-comment rulemaking. Although this section of the Act does not establish this requirement for cost measures, we believe that similar considerations should apply to cost measures. As discussed in prior rulemaking, examples of non-substantive changes to cost measures include maintenance changes such as updated diagnosis or procedure codes or changes to existing exclusions to the patient populations or definitions, while substantive changes to a measure are changes that result in what are considered new or different measures (83 FR 59767 and 84 FR 62961). As we evaluate existing cost measures to determine whether such measures need to be updated, we believe that it is important to establish criteria for determining whether a measure change is substantive. Thus, we are proposing to establish several criteria for determining whether a cost measure change is substantive starting with the 2022 MIPS performance period. The criteria include, but are not limited to, the following: Start Printed Page 39405
- Whether the change modifies the premise and/or objective of the measure;
- Whether the change modifies the scope of the measure (such as patient population eligible for the measure or a new category of costs); and
- Whether the change to the measure calculation significantly impacts how a measure is assessed.
Certain changes to a measure may affect multiple elements of a measure, requiring an overall assessment of the measure specifications. The following are some examples of potential substantive and non-substantive changes:
- Measure objective: The measure objective refers to what is being assessed; in general, changes to the measure objective would be considered substantive. The question of what is being assessed can generally be thought of as: What type of care is the measure assessing, who is providing this care, and who is in the patient cohort. Specifically, under this criterion, a change to the measure objective could include updates to the triggering logic, measure exclusions, attribution rules, or other aspects of the specifications. While the effect of such updates may also be relevant while considering whether the change is substantive or not, the effect is secondary to the intention behind changes to the measure. Consider the following example: A hypothetical episode-based measure focuses on major joint repair, and is updated to cover all joint procedures by adding a range of trigger codes. This likely would be a substantive change, as the measure would be evaluating different joints and procedures than the initial measure objective.
- Types of Costs being Assessed: In general, new rules about which costs are being captured by a measure would be considered substantive if they change which categories or types of costs are included in a measure, and non-substantive if they merely refine how an existing category is captured. For example, a change to an episode-based measure's service assignment rules which adds a new category of costs (for example, adding Part D costs to a measure that did not previously include any Part D costs) likely would be a substantive change. By contrast, a measure update to reflect new codes for existing types of codes, for instance, where a code is split into sub-codes for greater granularity, likely would be considered non-substantive.
- Risk Adjustment: The purpose of risk adjustment is to account for factors outside of the clinician's or clinician group's reasonable influence. In certain cases, it is necessary to make changes to the risk adjustment model and/or individual risk adjustors to ensure that the risk adjustment approach is working as intended. Generally, changes to risk adjustment variables and the mechanics of the regression would be considered non-substantive, if they continue to give effect to the measure's intent. However, some changes to the risk adjustment approach may be substantive, such as changes to the type of risk adjustors used (for example, the addition of non-claims-based variables when the model previously only used claims-based data), or changes to the stratification that modify the interpretation of what the measure score represents.
We note that there are degrees in any evaluation of whether a change is substantive. For instance, there may be important differences in the effect of adding one service or code compared to a suite of services and codes that we would also consider as part of determining whether a change is substantive or not. We believe the proposed substantive change criteria for cost measures would help us to determine whether a change to a cost measure should be made through notice-and-comment rulemaking before it is implemented in MIPS. We seek public comment on our proposed criteria for determining whether a change to a cost measure is substantive.
(3) Improvement Activities Performance Category
(a) Background
For previous discussions on the general background of the improvement activities performance category, we refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77177 through 77178), the CY 2018 Quality Payment Program final rule (82 FR 53648 through 53661), the CY 2019 PFS final rule (83 FR 59776 through 59777), the CY 2020 PFS final rule (84 FR 62980 through 62990), and the CY 2021 PFS final rule (85 FR 84881 through 84886). We also refer readers to 42 CFR 414.1305 for the definition of improvement activities and attestation, § 414.1320 for the performance period, § 414.1325 for the data submission requirements, § 414.1355 for the improvement activity performance category generally, § 414.1360 for data submission criteria, and § 414.1380(b)(3) for improvement activities performance category scoring.
In this proposed rule, beginning with the CY 2022 performance period and future years, we propose: (1) To revise group reporting requirements for the 50 percent threshold to address subgroups; (2) to revise timeframe for improvement activities nominated during a public health emergency; (3) to revise the required criteria for improvement activity nominations received through the Annual Call for Activities; (4) to suspend activities that raise possible safety concerns or become obsolete from the program when this occurrence happens outside of the rulemaking process; (5) to add 7 new improvement activities, modify 15 existing improvement activities, and remove 6 previously adopted improvement activities for the CY 2022 performance period and future years; (6) to revise the "Drug Cost Transparency to include requirements for use of real-time benefit tools" improvement activity; and (7) to add the COVID-19 "Clinical Data Reporting with or without Clinical Trial" improvement activity for CY 2022 performance period and future years.
(b) Group Reporting
In the CY 2020 PFS final rule (84 FR 62981 through 62988), we revised § 414.1360(a)(2) to state that, beginning with the 2020 performance year, each improvement activity for which groups and virtual groups submit a yes response in accordance with paragraph (a)(1) of this section must be performed by at least 50 percent of the NPIs billing under the group's TIN or virtual group's TINs, as applicable; and the NPIs must perform the same activity during any continuous 90-day period within the same performance year.
In the CY 2021 PFS final rule (85 FR 84844 through 84849), we finalized to update the MIPS Value Pathways guiding principle #2 as follows: "2. MVPs should include measures and activities that would result in providing comparative performance data that is valuable to patients and caregivers in evaluating clinician performance and making choices about their care; MVPs will enhance this comparative performance data as they allow subgroup reporting that comprehensively reflects the services provided by multispecialty groups."
In this proposed rule, we are proposing the details of subgroup reporting for MVPs. We refer readers to section IV.A.3.b. of this proposed rule for further details. In order to implement group requirements in relation to subgroup reporting, we need to modify our policy regarding group reporting for improvement activities. We continue to believe that a 50 percent threshold is achievable and appropriate because, if a group or virtual group has implemented an improvement activity, the activity should be recognized and adopted throughout much of the practice to improve clinical practice, Start Printed Page 39406 care delivery, and outcomes. Similarly, we believe that it makes sense to allow subgroups to perform and attest to their improvement activities separately and apply the 50 percent threshold within their subgroup. Our policy codified at § 414.1360 does not currently include a subgroup option. Therefore, we are proposing to revise § 414.1360(a)(2) to state that, beginning with the 2022 performance year, each improvement activity for which groups and virtual groups submit a yes response in accordance with paragraph (a)(1) of this section must be performed by at least 50 percent of the NPIs that are billing under the group's TIN or virtual group's TINs or that are part of the subgroup, as applicable; and the NPIs must perform the same activity during any continuous 90-day period within the same performance year.
We receive many inquiries through the Quality Payment Program help desk requesting clarification on how to apply the 50 percent threshold to groups. Many commenters requested that their groups be allowed to account for the 50 percent threshold by specialty or as a subgroup as they have more in common when considering applicable improvement activities. The Quality Payment Program help desk tracks, documents, and resolves inquiries submitted by MIPS eligible clinicians and groups. Stakeholders may submit inquiries to the help desk via 1-866-288-8292 (Monday-Friday 8 a.m.-8 p.m. ET) or email QPP@cms.hhs.gov. We believe the ability to attest to improvement activities at the subgroup level responds to this stakeholder concern, as this proposal, if finalized, would allow eligible clinicians the ability to compose subgroups by specialty for reporting MVPs.
For example, if a TIN that includes 100 clinicians and decides that they will be participating in MIPS as a group, at least 50 percent (in this example, at least 50 clinicians) would need to attest to the same improvement activity for a continuous 90 days within the current performance year. However, if among this group, there are 30 clinicians that represent the orthopedic specialty, they may decide to form a subgroup to report measures and activities, more closely linked to their improvement goals. We refer readers to section IV.A.3.b. of this proposed rule for requirements when forming a subgroup. If the 30 clinicians that represent the orthopedic specialty register as a subgroup, at least 15 of these orthopedic clinicians would be required to complete an improvement activity for the required performance period at some point during the performance year to receive full credit for the subgroup. The 70 clinicians from the original group would need at least 50 percent (in this example, at least 35 clinicians) to complete a given improvement activity for this group to receive credit for the improvement activity.
We request public comments on our proposal.
(c) Improvement Activities Inventory
(i) Annual Call for Activities
In the CY 2017 Quality Payment Program final rule (81 FR 77190), for the transition year of MIPS, we implemented the initial improvement activities Inventory (81 FR 77817 through 77830) consisting of approximately 95 activities. We took several steps to ensure the Inventory was inclusive of activities in line with statutory and program requirements. We discussed that we had numerous interviews with highly performing organizations of all sizes, conducted an environmental scan to identify existing models, activities, or measures that met all or part of the improvement activities performance category, including the patient-centered medical homes, the Transforming Clinical Practice Initiative (TCPI), CAHPS surveys, and AHRQ's Patient Safety Organizations. In addition, we reviewed the CY 2016 PFS final rule with comment period (80 FR 70886) and the comments received in response to the MIPS and APMs RFI regarding the improvement activities performance category.
For Year 2, we provided an informal process for submitting new improvement activities or modifications for potential inclusion in the comprehensive improvement activities Inventory for the Quality Payment Program Year 2 and future years through subregulatory guidance (https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/Annual-Call-for-Measures-and-Activities-for-MIPS_Overview-Factsheet.pdf). In the CY 2018 Quality Payment Program final rule (82 FR 53656 through 53659), for Year 3 and future years, we finalized a formal Annual Call for Activities process for adding possible new activities or providing modifications to the current activities in the improvement activities Inventory, including information required to submit a nomination form similar to the one we utilized for Year 2 (82 FR 53656 through 53659). In order to submit a request for a new activity or a modification to an existing improvement activity the stakeholder must submit a nomination form available at www.qpp.cms.gov during the Annual Call for Activities.
(A) Timeframe for the Annual Call for Activities
We refer readers to the CY 2019 PFS final rule (83 FR 59781 through 59782) for our most recent policies with respect to the timeframe for the Annual Call for Activities. We are not proposing any changes to this policy in this proposed rule. However, we refer readers to section IV.A.3.d.(3)(c)(i) of this proposed rule where we are proposing changes to the timeframe for nominating new improvement activities during a public health emergency.
(B) Proposed Changes for Nominating New Improvement Activities
As discussed in the CY 2017 Quality Payment Program final rule (81 FR 77190), the initial improvement activities Inventory was not based on the established criteria; rather, it was compiled via stakeholder input, an environmental scan, MIPS and APMs RFI comments, and subsequent working sessions with AHRQ and ONC and additional communications with CDC, SAMHSA, and HRSA. In the CY 2018 Quality Payment Program final rule (82 FR 53656 through 53659), we finalized a formal Annual Call for Activities process for adding possible new activities or providing modifications to the current activities in the improvement activities Inventory that included establishing the required criteria. In the CY 2019 PFS final rule (83 FR 59778 through 59779), we adopted 1 new criterion and removed a criterion from the improvement activities nomination criteria. We also clarified our considerations in selecting improvement activities. In this proposed rule, we are proposing: (1) Changes to the timeframe for improvement activities nomination during a public health emergency (PHE); (2) 2 new improvement activities criteria; (3) to increase the minimum required minimum number of criteria that must be met for improvement activities nominations; and (4) to separate required from optional criteria for improvement activities nominations. These proposals are discussed in detail in section IV.A.3.d.(3)(c) of this proposed rule.
(aa) Proposed Changes to the Timeframe for Nominating New Improvement Activities During a Public Health Emergency
In the CY 2021 PFS final rule (85 FR 84882 through 84883), we finalized an exception stating that during public health emergencies (PHE) stakeholders Start Printed Page 39407 can nominate improvement activities outside of the established Annual Call for Activities timeframe. Instead of only accepting nominations and modifications submitted February 1st through July 1 each year, we adopted a policy to accept nominations for the duration of the PHE as long as the improvement activity is still relevant. No other aspects of the Annual Call for Activities process was affected (for example, criteria for nominating improvement activities, considerations for selection of improvement activities, or weighting policies would all still apply). We noted that we continue to believe it is important for stakeholders to be able to comment on improvement activities. Therefore, any improvement activity related to the PHE considered for inclusion in the Inventory would need be finalized through rulemaking.
In 2020, we used several interim final rules with comment period (IFCs) to propose necessary policies due to the PHE for COVID-19, including adding and modifying the COVID-19 Clinical Data Reporting with or without Clinical Trial (IA_ERP_3) for implementation in the same year. However, we want to be clear that we are not limited to IFCs and those vehicles may not be the most timely or feasible for a particular situation. In a typical year, we use various fiscal and calendar year rules to implement policy (for example, the IPPS, Inpatient Psychiatric Facility Prospective Payment System (IPF PPS), OPPS, etc. rules). In order to best operationalize our policy for improvement activities nominated during a PHE, we are proposing to modify our policy such that these nominations should be submitted by January 5th, of the year in which the activity is targeted for implementation unless otherwise specified by CMS, in order to maximize the chance that a potential improvement activity could be implemented in the same year via the most timely rulemaking vehicle.
We request public comments on this proposal.
(bb) Currently Adopted Criteria
In the CY 2017 Quality Payment Program final rule (81 FR 77190 through 77195), we discussed guidelines for the selection of improvement activities. In the CY 2018 Quality Payment Program final rule, we formalized the Annual Call for Activities process for Year 3 and future years and added additional criteria; stakeholders should apply 1 or more of the below criteria when submitting nominations for improvement activities (82 FR 53660). In addition, in the CY 2019 PFS final rule (83 FR 59779) we finalized to add a "public health emergency as determined by the Secretary" and in the CY 2021 PFS final rule (85 FR 84883 through 84884) we finalized to add "Include activities which can be linked to existing and related MIPS quality and cost measures, as applicable and feasible" to the criteria below.
- Relevance to an existing improvement activities subcategory (or a proposed new subcategory);
- Importance of an activity toward achieving improved beneficiary health outcomes;
- Importance of an activity that could lead to improvement in practice to reduce health care disparities;
- Aligned with patient-centered medical homes;
- Focus on meaningful actions from the person and family's point of view;
- Support the patient's family or personal caregiver;
- Representative of activities that multiple individual MIPS eligible clinicians or groups could perform (for example, primary care, specialty care);
- Feasible to implement, recognizing importance in minimizing burden, especially for small practices, practices in rural areas, or in areas designated as geographic HPSAs by HRSA;
- Evidence supports that an activity has a high probability of contributing to improved beneficiary health outcomes;
- Include activities which can be linked to existing and related MIPS quality and cost measures, as applicable and feasible;
- Include a public health emergency as determined by the Secretary; or
- CMS is able to validate the activity.
(cc) Proposed 2 New Criteria
In addition to the aforementioned considerations, when considering improvement activities for possible inclusion in MIPS, we propose 2 new criteria beginning with the CY 2022 Annual Call for Activities MIPS improvement activities: (1) Should not duplicate other improvement activities in the Inventory; and (2) should drive improvements that go beyond standard clinical practices. Regarding the first proposed criterion, we believe that there should not be duplication in the Inventory as clinicians could get double credit for doing the same activity. As discussed in the CY 2017 Quality Payment Program final rule (81 FR 77185), while the minimum reporting period for one improvement activity is 90 days, the maximum frequency with which an improvement activity may be reported would be once during the 12-month performance period. It is important that stakeholders review the current Inventory to ensure there is not a broader improvement activity that clinicians could attest to for the same activity. Regarding the second proposed criterion, we believe that improvement activities should drive improvements that go beyond standard clinical practices and should be innovative, to have the potential for significant patient benefit when clinicians learn and implement the activities.
We request public comments on our proposals.
(dd) Proposed Minimum Requirement
We have received feedback from stakeholders through the Annual Call for Activities process and during the CY 2021 PFS rulemaking process that the nomination acceptance process is unclear, stakeholders are frustrated by nominating improvement activities that are not accepted, and our reasoning for not accepting their nomination is not clear.
Our current policy requires that stakeholders apply a minimum of 1 or more of the established criteria when submitting a nomination through the Annual Call for Activities process for a new improvement activity (82 FR 53660). Through past Annual Call for Activities, we have found that many of the nominations that we receive meet the minimum 1 criterion, but are not appropriate for inclusion in the Inventory for other reasons. We often receive submissions describing practices that are standard and submissions that are too specific to a particular specialty. We believe that when evaluating nominations, we should use multiple factors in deciding on the selection of submitted improvement activities, not all of which can be listed upfront. Ultimately, we must rely on our internal processes and expertise in determining whether or not a nominated improvement activity meets the criteria and needs of the program. However, in an effort to increase transparency, we have reevaluated our criteria and believe there are a number of significant criteria that improvement activity proposals should meet to be included in the Inventory. Therefore, beginning with nominations submitted during the 2022 nomination period for the Annual Call for Activities, we are proposing to increase the number of criteria stakeholders are required to meet when submitting an activity proposal, from a minimum of 1 to 8 criteria, which includes the 2 proposed criteria in section IV.A.3.d(3)(c)(i)(B)(cc) of this proposed rule should they be finalized.
We believe that the following 8 criteria, 6 of which are on the current list and 2 of which are proposed (in Start Printed Page 39408 italics), should be the foundation for all improvement activities:
1. Relevance to an existing improvement activities subcategory (or a proposed new subcategory) (82 FR 53660);
2. Importance of an activity toward achieving improved beneficiary health outcomes (82 FR 53660);
3. Feasible to implement, recognizing importance in minimizing burden, including, to the extent possible, for small practices, practices in rural areas, or in areas designated as geographic Health Professional Shortage Areas (HPSAs) by the Health Resources and Services Administration (HRSA) (82 FR 53660);
4. Evidence supports that an activity has a high probability of contributing to improved beneficiary health outcomes (82 FR 53660);
5. Can be linked to existing and related MIPS quality, Promoting Interoperability, and cost measures as applicable and feasible (85 FR 84884);
6. CMS is able to validate the activity (82 FR 53660);
7. Does not duplicate other improvement activities in the Inventory; and
8. Should drive improvements that go beyond purely common clinical practices.
While we previously finalized the first 6 criteria in prior rulemakings, we did not specifically require all. We are seeking to minimize improvement activity nominations that do not meet program standards, and to increase nominations that will substantively contribute to improved patient care and outcomes.
In crafting this proposal, we also considered whether any of the above proposed required criteria should be optional instead (see proposal in the next section (IV.A.3.d(3)(c)(i)(B))). Ultimately, we proposed the above criteria as required, because we believe these reflect the minimum standard for improvement activities in the Inventory and would help increase transparency in our decisions.
We request public comments on our proposal. In addition, for future rulemaking, we also request comment on any other potential required criteria that should be considered for future adoption.
(ee) Proposed Optional Factors
Additionally, we are proposing the remaining 6 previously established factors would serve as optional factors beginning with nominations submitted during the 2022 nomination period for the Annual Call for Activities, as we believe they may be relevant to certain nominations, but not all. Meeting 1 or more of the optional factors may increase a nomination's chances of being added to the Inventory. The proposed optional factors are:
1. Alignment with patient-centered medical homes (82 FR 53660);
2. Support for the patient's family or personal caregiver (82 FR 53660);
3. Responds to a public health emergency as determined by the Secretary (83 FR 59779);
4. Addresses improvements in practice to reduce health care disparities (82 FR 53660);
5. Focus on meaningful actions from the person and family's point of view (82 FR 53660); and
6. Representative of activities that multiple individual MIPS eligible clinicians or groups could perform (for example, primary care, specialty care) (82 FR 53660).
We request public comments on our proposal.
(C) Improvement Activity Removal
In the CY 2020 PFS final rule (84 FR 62988 through 62990), we finalized the factors for consideration in removing improvement activities. We stated that we would fully examine each activity prior to removal and that commenters would have an opportunity to provide their input during notice-and-comment rulemaking.
(aa) Proposed Improvement Activity Suspension Policy
A circumstance has come to our attention that requires we examine the specifics of the improvement activities removal of activities policy in relation to the performance period. Following the publication of the CY 2021 PFS proposed rule, we became aware that the underlying program for 1 of the improvement activities in the Inventory had expired on March 31, 2020. Therefore, clinicians could no longer complete the activity from April 1 through December 31, 2020. To avoid any potential confusion or incorrect attestation, we removed this activity in the CY 2021 PFS final rule (85 FR 84885). This occurrence alerted us to the potential that other activities could become obsolete or be impacted by clinical practice guideline changes that affect the activity and could potentially result in patient harm, and that we may not learn about this information during the rulemaking timeframes. Because changes made in rulemaking do not apply until the following performance year, this timing could affect an improvement activity that needs to be urgently addressed.
As a result, beginning with the 2022 performance period, we are proposing that in the case of an improvement activity for which there is a reason to believe that the continued collection raises possible patient safety concerns or is obsolete, we would promptly suspend the improvement activity and immediately notify clinicians and the public through the usual communication channels, such as listservs and Web postings. We would then propose to remove or modify the improvement activity as appropriate in the next rulemaking cycle.
We request public comments on our proposal.
(ii) Changes to the Improvement Activities Inventory
(A) Background
In the CY 2018 Quality Payment Program final rule (82 FR 53660), we finalized that we would establish improvement activities through notice-and-comment rulemaking. We refer readers to Table H in the Appendix of the CY 2017 Quality Payment Program final rule (81 FR 77177 through 77199), Tables F and G in the Appendix of the CY 2018 Quality Payment Program final rule (82 FR 54175 through 54229), Tables A and B in the Appendix 2 of the CY 2019 PFS final rule (83 FR 60286 through 60303), Tables A, B, and C in the Appendix 2 of the CY 2020 PFS final rule (84 FR 63514 through 63538), and Tables A, B, and C in the Appendix 2 of the CY 2021 PFS final rule (85 FR 85370 through 85377) for our previously finalized improvement activities Inventory. We also refer readers to the Quality Payment Program website under Explore Measures and Activities at https://qpp.cms.gov/mips/explore-measures?tab=improvementActivities&py=2020 for a complete list of the current improvement activities. In this proposed rule, we are proposing to add 7 new improvement activities, modify 15 existing improvement activities, and remove 6 previously adopted improvement activities for the CY 2022 performance period and future years. We refer readers to the below and Appendix 2 of this proposed rule for more details.
(B) Proposed Changes to Adopted Improvement Activities
(aa) Proposed Changes to the "Drug Cost Transparency To Include Requirements for Use of Real-Time Benefit Tools" Improvement Activity
In the CY 2020 PFS final rule (84 FR 63515), we adopted IA_BE_25, titled "Drug Cost Transparency to include requirements for use of real-time benefit Start Printed Page 39409 tools" beginning with the 2020 performance year and for subsequent years. This activity description reads as follows: To receive credit for this improvement activity, MIPS eligible clinicians must attest that their practice provides counseling to patients and/or their caregivers about the costs of drugs and the patients' out-of-pocket costs for the drugs. If appropriate, the clinician must also explore with their patients the availability of alternative drugs and patients' eligibility for patient assistance programs that provide free medications to people who cannot afford to buy their medicine. One source of information for pricing of pharmaceuticals could be a real-time benefit tool (RTBT), which provides to the prescriber, real-time patient-specific formulary and benefit information for drugs, including cost-sharing for a beneficiary. (CMS finalized in the Modernizing Part D and Medicare Advantage to Lower Drug Prices and Reduce Out of Pocket Expenses final rule (84 FR 23832, 23883) that beginning January 1, 2021 Medicare Part D plans will be required to implement one or more RTBT(s)). Thus, this activity allows a real-time benefit tool (RTBT) to be one source of information for pricing of pharmaceuticals, which provides to the prescriber, real-time patient-specific formulary and benefit information for drugs, including cost-sharing for a beneficiary.
The 2021 Consolidated Appropriations Act (H.R. 116-133, Pub. L. 116-260) B included section 119 in Division CC entitled "Increasing the use of real-time benefit tools to lower beneficiary costs." Subsection (c) of section 119 includes a provision called "Inclusion of Use of Real-Time Electronic Information in Shared Decision-Making Under MIPS." This provision amended section 1848(q)(2)(B)(iii)(IV) of the Act by adding text noting that this subcategory shall include as an activity, for performance periods beginning on or after January 1, 2022, use of a real-time benefit tool as described in section 1860D-4(o) of the Act. In addition, the Secretary may establish this activity as a standalone or as a component of another activity.
In accordance with this statutory requirement, in this proposed rule, we propose to modify this improvement activity such that beginning with the CY 2022 performance year and for subsequent years, the activity would require use of an RTBT. As previously finalized, use of an RTBT was optional. We refer readers to Appendix 2 of this proposed rule for additional details.
We request public comments on our proposal.
(bb) Proposed Changes to the COVID-19 Clinical Data Reporting With or Without Clinical Trial (IA_ERP_3) Improvement Activity
We refer readers to the March 31st IFC for COVID-19 (85 FR 19276 through 19277) and September 2nd COVID-19 IFC (85 FR 54848 through 54851) for a regulatory history of this improvement activity. In the September 2nd COVID-19 IFC (85 FR 54848 through 54851), we extended the modified COVID-19 Clinical Data Reporting with or without Clinical Trial improvement activity through the CY 2021 performance period due to the continued COVID-19 infection we were experiencing nationwide. We anticipated the need for COVID-19 clinical trials and data collection/sharing through registries to continue through CY 2021 at which time we would reassess whether there remains a need for additional data sharing or if preventive measures and clinical treatments have advanced to the point where these type of data are not needed.
In this proposed rule, we are proposing to extend the COVID-19 Clinical Data Reporting with or without Clinical Trial improvement activity for CY 2022 performance period and future years due to continued COVID-19 infections we are experiencing nationwide and the need for further research. Clinicians will continue to treat beneficiaries with COVID-19, and we anticipate the need for COVID-19 clinical trials and data collection/sharing through registries to continue through CY 2022 and future years. Each year, we intend to reassess whether there remains a need for additional data sharing or if preventive measures and clinical treatments have advanced to the point where these type of data are not needed. We believe it is important for eligible clinicians to be able to attest to this improvement activity if it is still pertinent. Further, we believe that participation in this improvement activity is likely to result in improved outcomes by improving the collection of data clinicians use for the care of their patients as they monitor and manage COVID-19. We will continue to reassess whether there remains a need for additional data sharing or if preventive measures and clinical treatments have advanced to the point where these type of data are not needed and would discontinue the activity through notice-and-comment rulemaking as needed. We also refer readers to Appendix 2 of this proposed rule for details on our proposals to add 7 new improvement activities, modify 15 previously adopted improvement activities, and remove 6 previously adopted improvement activities.
We request public comments on our proposal.
(4) Promoting Interoperability Performance Category
(a) Background
Section 1848(q)(2)(A) of the Act includes the meaningful use of certified electronic health record technology (CEHRT) as a performance category under the MIPS. As required by sections 1848(q)(2) and (5) of the Act, the four performance categories of the MIPS shall be used in determining the MIPS final score for each MIPS eligible clinician. In general, MIPS eligible clinicians will be evaluated under all four of the MIPS performance categories, including the Promoting Interoperability performance category.
(b) Promoting Interoperability Performance Category Performance Period
As finalized in the CY 2021 PFS final rule at § 414.1320(g)(1) (85 FR 84886), for the 2024 MIPS payment year, and each subsequent MIPS payment year, the performance period for the Promoting Interoperability performance category is a minimum of any continuous 90-day period within the calendar year that occurs 2 years prior to the applicable MIPS payment year, up to and including the full calendar year. Thus, for the 2024 MIPS payment year, the performance period for the Promoting Interoperability performance category is a minimum of any continuous 90-day period within CY 2022, up to and including the full CY 2022 (January 1, 2022 through December 31, 2022). We stated that we believe this would be an appropriate performance period because it would offer stability and consistency for MIPS eligible clinicians reporting for the Promoting Interoperability performance category. We are not proposing any changes to the Promoting Interoperability performance category performance period that we established under § 414.1320(g)(1) (85 FR 84886).
(c) Promoting Interoperability Performance Category Measures for MIPS Eligible Clinicians
(i) Proposed Changes to the Query of Prescription Drug Monitoring Program Measure Under the Electronic Prescribing Objective
(A) Measure Background
We have adopted a Query of Prescription Drug Monitoring Program (PDMP) measure under the Electronic Prescribing objective. For background Start Printed Page 39410 on this measure, we refer readers to the CY 2019 PFS final rule (83 FR 59800 through 59803) and the CY 2020 PFS final rule (84 FR 62992 through 62994). In the CY 2021 PFS final rule (85 FR 84887 through 84888), we finalized that the Query of PDMP measure will remain optional and eligible for 10 bonus points for the CY 2021 performance period/2023 MIPS payment year.
(B) State PDMPs' Progress and Previous Stakeholder Feedback
In the CY 2020 and CY 2021 PFS final rules (84 FR 62992 through 62994 and 85 FR 84887 through 84888), we described the concern expressed by stakeholders that they believed it was premature for the Promoting Interoperability performance category to require the Query of PDMP measure and score it based on performance. Feedback received from health IT vendors and MIPS eligible clinicians expressed that flexibility in the measure presents unintended challenges such as significant burden associated with IT system design and additional development needed to accommodate the measure and any future changes to it.
We understand that there is wide variation across the country in how health care providers are implementing and integrating PDMP queries into health IT and clinical workflows, and that it could be burdensome for health care providers if we were to narrow the measure to specify a single approach to PDMP-EHR integration at this time. At the same time, we have heard extensive feedback from EHR developers that effectively incorporating the ability to count the number of PDMP queries in the EHR would require more robust measurement specifications. These stakeholders stated that health IT developers may face significant cost burdens if they either fully develop numerator and denominator calculations for all the potential use cases and are required to change the specification at a later date. Stakeholders have noted that the cost of additional development will likely be passed on to health care providers without additional benefit as this development would be solely for the purpose of calculating the measure rather than furthering the clinical goal of the measure (for public comments discussed in last year's final rule, we refer readers to 85 FR 84887 through 84888).
In support of efforts to expand the use of PDMPs, there are currently a number of federally supported activities underway aimed at developing a more robust and standardized approach to EHR-PDMP integration. federal partners, including the CDC and ONC, and private sector stakeholders, are focused on developing and refining standard-based approaches to enable effective integration into clinical workflows, exploring emerging technical solutions to enhance access and use of PDMP data, and providing technical resources to a variety of stakeholders to advance and scale the interoperability of health IT systems and PDMPs. Moreover, a number of enhancements to PDMPs are occurring across the country, including enhancements to RxCheck, which is a federally supported interstate exchange hub for PDMP data.[219] The ONC Interoperability Standards Advisory describes monitoring of current and emerging standards related to PDMP and opioid use disorder (OUD) data capture and exchange that would allow a provider to request a patient's medication history from a state PMDP and for PDMP data to be exchanged between systems and states.[220] We believe these standards and technical approaches are likely to rapidly reach maturity to support exchange across health care system stakeholders.
The SUPPORT for Patients and Communities Act (Pub. L. 115-271), enacted in 2018, is an important investment in combating the opioid epidemic. Several of the provisions of the SUPPORT for Patients and Communities Act address opioid use disorder prevention, recovery, and treatment, including legislative changes specific to the Medicare and Medicaid programs intended to increase access to evidence-based treatment and follow-up care. Specifically, with respect to PDMPs, the SUPPORT for Patients and Communities Act included new requirements and federal funding for PDMP enhancement, integration, and interoperability, and established mandatory use of PDMPs by certain Medicaid providers to help reduce opioid misuse and overprescribing and to help promote the overall effective prevention and treatment of opioid use disorder beginning in October of 2021.
(C) Proposed Measure Changes
Given current efforts to improve the technical foundation for EHR-PDMP integration, the continued implementation of the SUPPORT for Patients and Communities Act (in particular, its provisions specific to Medicaid providers and qualified PDMPs), our ongoing review of alternative measure approaches, and stakeholder concerns about the current readiness across states for implementation of the existing measure, we believe that at least 1 more year is needed prior to potentially requiring the Query of PDMP measure.
While we appreciate the concerns that stakeholders have shared, we continue to believe that this measure can play an important role in helping to address the opioid crisis. By integrating PDMP data into the health record, health care providers can improve clinical decision making by utilizing this information to identify potential opioid use disorders, inform the development of care plans, and develop effective interventions. Maintaining it as an optional measure with bonus points signals to the MIPS eligible clinician and vendor community that this is an important measure to address a current gap that can help spur development and innovation in order to reduce barriers and challenges.
Therefore, we are proposing to maintain the Electronic Prescribing Objective's Query of PDMP measure as optional and worth 10 bonus points for the CY 2022 performance period/2024 MIPS payment year. We seek comments on this proposal.
(D) Health IT Updates and Measure Direction
Given recent progress in a variety of areas, we believe that there is now a clearer trajectory forward to potentially requiring the Query of PDMP measure. These developments include updated requirements for certified health IT, standards development activities around PDMPs, and other projects which can more tangibly inform future policy changes. For example, under final policies recently adopted in the CY 2021 PFS final rule (85 FR 84815 through 84828), participants in the Medicare Promoting Interoperability Program for eligible hospitals and critical access hospitals (CAHs) and the Promoting Interoperability performance category are scheduled to begin using certified EHR technology incorporating application programming interfaces (APIs) based on HL7® FHIR® standard version Release 4 in CY 2023 consistent with updates to certified health IT which were finalized in the "21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program" final rule (hereinafter referred to as the "ONC 21st Century Cures Act final rule"), published in the May 1, 2020 Federal Register (85 FR 25642 through 25961, Start Printed Page 39411 25740).[221] Updates to 2015 Edition health IT certification criteria in the ONC 21st Century Cures Act final rule also incorporated NCPDP SCRIPT standard version 2017071 for electronic prescribing. The availability of both standardized APIs and updated standards for e-prescribing within certified health IT could serve as a stepping stone to future technical approaches that enable more seamless exchange of data between CEHRT and PDMP systems.
A number of recent efforts have sought to improve interoperability between EHRs and PDMPs. In 2020, ONC completed work to map the NCPDP SCRIPT standard version 2017071, the Prescription Monitoring Information eXchange (PMIX) standard version 2, and the 2015 American Society for Automation in Pharmacy (ASAP) Prescription Monitoring Program Web Service standard version 2.1A to the Health Level Seven International (HL7®) Fast Healthcare Interoperability Resources (FHIR®) standard version Release 4.
ONC also began work in partnership with the CDC, the Department of Justice's Bureau of Justice Assistance, and the eHealth Exchange to develop a prototype to pilot an innovative technical solution for the delivery of patient medication histories across state lines via HL7® FHIR®. The eHealth Exchange is a network of networks that is active in all 50 states connecting federal and non-federal healthcare organizations to improve patient care and public health. To date, the prototype has been successfully tested in several states. Early prototype testing used synthetic data to evaluate system capacity to send and receive a patient's medication history request and response. The goal of the project is to allow any provider who is live on the eHealth Exchange to use that existing connection to query a patient's record on the RxCheck Hub, which routes the query to individual state PDMPs who are also live on RxCheck. This solution will enable health care providers to query PDMPs via existing connections to health information exchange (HIE) networks as a way to: (1) Leverage existing technology; (2) reduce burden associated with multiple, disparate system interfaces and workflows; and (3) allow for the exchange and full integration of data within allowable law from the point of exchange for medication reconciliation, allergy checks, and other forms of clinical decision support.
Based upon these developments, which are advancing enhanced certified functionality, effective functional data exchange, and the use of open, mature standards, we believe there is a much better informed roadmap for achieving better integration between PDMPs and EHRs with enhanced interoperability of controlled prescription data across states and systems. We believe that as these activities develop, they can help to address some of the previous concerns raised by stakeholders around this measure, and we will continue to work with ONC to monitor these activities.
While we believe the Query of PDMP measure is very important to avoid and address the over-prescribing of opioids, we also recognize that some states and systems may not be ready at this time to effectively exchange this data. In light of further work in this area and our stated goals for increasing the impact of this measure, we are seeking stakeholder comment on plans for requiring the Query of PDMP measure in the Promoting Interoperability performance category in the near future. To advance in this direction with both transparent proposals and informed guidance, we request public comment on the future direction for the measure, specifically:
- To what degree would all MIPS eligible clinicians be prepared to report on the current Query of PDMP measure (Yes/No response) in the near future? What additional considerations would need to be addressed before transitioning to a version of the measure that requires the submission of a numerator/denominator?
- Would changes to the Query of PDMP measure be necessary to accommodate other technical approaches that may be implemented in the future, such as exchange of information with a PDMP or with multiple PDMPs using HL7® FHIR®?
- What, if any, exclusions should be made available as part of the measure's specifications with regard to MIPS eligible clinicians?
- When will state PDMPs be ready to effectively exchange data with provider systems using HL7® FHIR® to support this measure? What are the most common standards and approaches used to access PDMP data through provider systems currently?
- What technical considerations exist for intrastate vs. interstate PDMP queries? How could health information exchange networks play a role in expanding access to PDMP data? In what ways could FHIR® applications be supported to safely share PDMP data within a clinician's workflow?
(ii) Proposed Changes to the Provide Patients Electronic Access to Their Health Information Measure Under the Provider to Patient Exchange Objective
(A) Background
In the CY 2019 PFS final rule (83 FR 59812 through 59815), we renamed the Patient Electronic Access Objective to the Provider to Patient Exchange Objective, which includes the Provide Patients Electronic Access to Their Health Information measure. For more information about the Provide Patients Electronic Access to Their Health Information measure, we refer readers to the following preamble discussions in prior rulemaking: 84 FR 62995 and 62999 through 63000, 83 FR 59812, 82 FR 53674, 81 FR 77228, 80 FR 62841 through 62851, 77 FR 54007, and 75 FR 44353.
(B) Proposed Data Availability Requirement for MIPS Eligible Clinicians
The Provide Patients Electronic Access to Their Health Information measure requires, for at least one unique patient seen by the MIPS eligible clinician: (1) The patient (or the patient-authorized representative) is provided timely access to view online, download, and transmit his or her health information; and (2) the MIPS eligible clinician ensures the patient's health information is available for the patient (or patient-authorized representative) to access using any application of their choice that is configured to meet the technical specifications of the Application Programming Interface (API) in the MIPS eligible clinician's CEHRT (84 FR 62999-63000 and 82 FR 53674). We are proposing to modify this measure to require MIPS eligible clinicians to ensure that patient health information remains available to the patient (or patient-authorized representative) to access indefinitely and using any application of their choice that is configured to meet the technical specifications of the API in the MIPS eligible clinician's CEHRT. MIPS eligible clinicians would be required to ensure this information remains available indefinitely (that is, not merely for a defined period of time). The proposed requirement would apply beginning with the performance period in 2022, and would include all patient health information from encounters on or after January 1, 2016.
Currently, the Provide Patients Electronic Access to Their Health Information measure does not specify how long MIPS eligible clinicians are required to make patient data available, or to ensure that patient data remain Start Printed Page 39412 available to patients in the event that a MIPS eligible clinician switches EHR vendors (81 FR 77228). In an effort to minimize stakeholder burden, we want to align the date under our proposal for making information about encounters available with the date of service start date (January 1, 2016) as finalized in the Patient Access and Interoperability final rule (85 FR 25528), and as proposed for the Promoting Interoperability Program for eligible hospitals and CAHs in the FY 2022 IPPS/LTCH proposed rule (86 FR 25631). As an alternative to our proposal, we considered different encounter start dates, such as encounters on or after January 1, 2012, or encounters on or after January 1, 2019. We believe, however, that a requirement for MIPS eligible clinicians to ensure patient health information remains available indefinitely, as well as an encounter start date of January 1, 2016, would provide the most benefit to patients when accessing their health information as compared to the burden and costs to MIPS eligible clinicians implementing these proposed requirements.
We are seeking public comment on our proposal to modify the Provide Patients Electronic Access to Their Health Information measure, as well as the alternatives we have considered.
(iii) Modifications to the Public Health and Clinical Data Exchange Objective
(A) Background
In the CY 2019 PFS final rule (83 FR 59795, 59815 through 59817), for the Public Health and Clinical Data Exchange Objective, we finalized that a MIPS eligible clinician must submit a yes/no response for two different public health agencies or clinical data registries for any of the five measures associated with the Public Health and Clinical Data Exchange objective (Syndromic Surveillance Reporting; Immunization Registry Reporting; Clinical Data Registry Reporting; Electronic Case Reporting; and Public Health Registry Reporting) to earn 10 points for the objective. Failure to report on two different public health agencies or clinical data registries or submitting a "no" response for a measure will earn a score of zero. If an exclusion is claimed for one measure, but the MIPS eligible clinician submits a "yes" response for another measure, they will earn the 10 points for the objective. If a MIPS eligible clinician claims exclusions for both measures they select to report on, the 10 points will be redistributed to the Provide Patients Electronic Access to Their Health Information measure under the Provider to Patient Exchange objective.
The Promoting Interoperability performance category for eligible clinicians has been an important mechanism for encouraging health care data exchange. But in an attempt to reduce burden, we previously stated our intention to propose in future rulemaking to remove the Public Health and Clinical Data Exchange objective and measures no later than CY 2022 (83 FR 59816). Many commenters opposed this potential policy change and noted that interoperability of public health data is still evolving and incentivizes MIPS eligible clinicians to share data with public health agencies (83 FR 59816). In response to these comments, we stated that we will continue to monitor the data we compile specific to the public health reporting requirements and take the commenters' concerns into consideration related to future actions (83 FR 59816).
Effective responses to public health events, such as the COVID-19 pandemic, require fast, accurate exchange of data between health care providers and federal, state, and local public health agencies (PHAs). Health care providers collect these data for patient care and PHAs need them to protect the public, whether to track an outbreak, initiate contact tracing, find gaps in vaccine coverage, or pinpoint the source of a foodborne outbreak.
While our current approach has encouraged health care systems to stand up some of these capabilities, significant gaps remain, and absent stronger incentives it will be difficult to stand up the comprehensive data exchange needed for future public health response. Thus, we believe that a more assertive approach is needed.
(B) Proposed Modifications to the Reporting Requirements for the Public Health and Clinical Data Exchange Objective
In this section, we are proposing to require two of the measures associated with the Public Health and Clinical Data Exchange Objective, beginning with the performance period in CY 2022: Immunization Registry Reporting; and Electronic Case Reporting. These two measures would put PHAs on better footing for future health threats and a long-term COVID-19 pandemic recovery by strengthening two important public health functions: (1) Vaccine uptake; and (2) case surveillance. Requiring these measures would enable automated case reporting for fast public health response; and local and national visibility on immunization uptake so PHAs can tailor vaccine distribution strategies.
(aa) Immunization Registry Reporting Measure
Immunizations are considered one of the ten great public health achievements and have resulted in declines in cases, hospitalizations, deaths, and health care costs associated with vaccine preventable diseases.[222] The benefits and value of immunizations are realized when public policy, health systems, and community-based intervention efforts are working in coordination. Ensuring the coordination of these efforts can achieve high immunization coverage is dependent on the availability of timely, accurate, and complete information on vaccinations received by individuals in a population.
Immunization registries (also called immunization information systems, or IIS) are powerful tools that allow collaboration between vaccine providers and public health agencies and enable coordination of population-based interventions. Immunization registries are confidential, population-based, computerized systems that record all vaccination doses administered by participating health care providers for individuals residing within a particular jurisdiction. At the point of clinical care, an immunization registry can provide consolidated immunization histories to assist vaccine providers in determining appropriate patient vaccinations. At the population level, immunization registries provide data on vaccination coverage assessment and program operations and in guiding public health action to improve vaccination rates.
Currently, 50 states, the District of Columbia, eight island territories, and three cities (New York City, Philadelphia, and San Diego) operate an immunization registry. CDC provides technical assistance and nationwide leadership to all state immunization registries to ensure the optimal use of immunization registries for determining vaccination coverage at local, state, and national levels. Immunization registries already have connections in place to capture administered doses in real-time for a substantial portion of the population, a process accelerated over the last 10 years by the Medicare and Medicaid Promoting Interoperability Programs. According to data from the most recent CDC IIS Annual Report (2019) available, immunization registries currently hold demographics and immunization data on 95 percent of Start Printed Page 39413 children 0-6 years, 82 percent of adolescents, and 60 percent of adults.[223] While each state Immunization registry currently coordinates with health care providers and EHR systems to achieve interoperability and facilitate immunization reporting, varying state reporting policies limit the completeness and timeliness of records in immunization registries and the optimal use of immunization registries for determining vaccination coverage.
We are proposing to make the Immunization Registry Reporting a required measure under the Public Health and Clinical Data Exchange objective of the Promoting Interoperability performance category beginning with the performance period in CY 2022 as it is critical for understanding vaccination coverage both at the jurisdiction level and nationwide and identifying where additional vaccination efforts are needed. For more information about the Immunization Registry Reporting measure, we refer readers to the preamble discussion in prior rulemaking at 81 FR 77230. Making standardized reporting to an immunization registry a required measure would provide an immediate benefit by increasing the COVID-19 vaccination records reported to these systems. Making the measure required would also improve the data quality of records in immunization registries and facilitate use of immunization registries for clinical decision support and tracking of vaccine administration and distribution.
We believe that making the Immunization Registry Reporting measure required would increase the reporting of immunization data by health care providers to public health agencies. Making the measure required is also critical for the COVID-19 vaccination response because it would provide a better view of the vaccines administered and distributed at national, state and local levels. This is a function immunization registries currently provide for all public vaccines, but is particularly important for COVID-19 vaccines. In addition to the COVID-19 vaccination response is the equally important need for routine vaccination coverage to increase. Fear of COVID-19 has caused deferrals of routine vaccinations as patients limit their interactions, including with their family doctors. More complete data in immunization registries as a result of the required measure would also optimize the use of immunization registries to determine who has not been vaccinated, pockets of under vaccination, and identifying where interventions should be focused for routine and emergency response vaccines. Requiring the measure would reduce the regulatory and administrative burden health care providers experience when exchanging information with immunization registries.
We are not proposing any changes to the description of the measure including any of the exclusions that we established in CY 2019 PFS final rule at 83 FR 59815 through 59817.
(bb) Electronic Case Reporting
Health care providers are required by state law to report certain diseases and conditions, a process called case reporting, which provides PHAs with data on approximately 120 diseases and conditions of public health significance.[224] Case reporting is a vital and longstanding tool that PHAs use to prevent the spread of infectious diseases. Case reporting serves as early notification to PHAs for potential outbreaks and includes information that enables PHAs to start contact tracing and other prevention measures. Case reports also include critical clinical information that would not be included in syndromic surveillance or laboratory reporting, and can help to illuminate the impact of comorbidities, treatments, and variable access to care. Information from the case reports can be used to further work on social determinants of health and ensure equal access to preventative care across populations. Electronic case reporting is the automated, real-time, bidirectional exchange of case report information between EHRs and PHAs. Electronic case reporting uses standard codes to trigger the transfer of relevant clinical data to PHAs for case investigation and follow-up. As of March 2021, most states do not require electronic submission of case reports as part of their regulations and case reporting often occurs through outdated manual methods (for example, fax, email, or phone), which results in delays, underreporting, and incomplete or inaccurate case data. Manual case reporting also imposes burdens on health care providers, taking staff time away from patients to submit case reports and comply with state reporting requirements. Electronic case reporting allows health care providers to fulfill mandated public health reporting requirements without imposing additional burden and disrupting the clinical workflow. This automated data exchange facilitates faster and more efficient disease tracking, case management, and contact tracing. Electronic case reporting provides more timely and complete data than manual reporting, including data on demographics, comorbidities, immunizations, medications, occupation, and other treatments.
Recent efforts by the CDC have sought to significantly improve the effectiveness of electronic case reporting through eCR Now, a strategic initiative that allows for rapid adoption and implementation of electronic case reporting for COVID-19 (https://www.cdc.gov/coronavirus/2019-ncov/hcp/electronic-case-reporting.html). As part of this initiative, CDC and its partners have developed an eCR Now FHIR® application (app) to establish electronic case reporting capability in EHR systems. EHR vendors can also implement the eCR functionality within their products to accomplish this reporting. The initiative also supports an electronic case reporting infrastructure that is helping to advance interoperability. This infrastructure supports the transmission of electronic case reports to a shared service platform, and not directly to a PHA, which means that any health care provider that has established an electronic case reporting connection also has a connection with every state PHA, many large local health departments, and some territories. This promotes nationwide interoperability and increases the availability of data for patients who may be traveling or spending time away from their home state. For example, if a patient is a resident of one state but seeks care in another state, this infrastructure will automatically route the case report to both states that would have jurisdiction over this report. This increases inter-jurisdictional reporting, allowing for more seamless case investigation at the national level. The interoperable infrastructure and the use of a standard data format also reduces the variability of case report forms across conditions and jurisdictions, streamlining reporting forms for EHR vendors and health care providers.
As a result of the CDC effort to scale up eCR Now for COVID-19, all 50 states, the District of Columbia, Puerto Rico and 12 large local jurisdictions have connected to the shared services platform and are currently receiving electronic case reports, with more than 8,800 healthcare facilities on board and 8.6 million reports for COVID-19 Start Printed Page 39414 received by PHAs as of June 28, 2021.[225] The eCR infrastructure is designed to rapidly scale for PHEs, such as COVID-19, and it is enabled to currently support data transmission for 99 reportable and notifiable conditions. While these are significant advancements, the piecemeal approach of encouraging adoption of these tools by individual health care providers has not been an effective or efficient means to quickly scale this effort nationally as has been needed for the COVID-19 PHE response.
We believe the uneven adoption of electronic case reporting creates a public health vulnerability. We are proposing to make the Electronic Case Reporting measure a required measure under the Public Health and Clinical Data Exchange objective of the Promoting Interoperability performance category beginning with the performance period in CY 2022. For more information about the Electronic Case Reporting measure, we refer readers to the preamble discussion in prior rulemaking at 81 FR 77229. We believe making this a required measure would accelerate development of electronic case reporting capabilities in EHR systems, reduce health care administrative burden of complying with state-mandated disease reporting requirements, provide regulatory clarity for EHR vendors, and improve the timeliness, completeness, and utility of case report data for PHAs. We believe that requiring the Electronic Case Reporting measure would be feasible and beneficial for MIPS eligible clinicians. This change would encourage EHR vendors to make electronic case reporting available to their customers, which would make adoption of this capability relatively straightforward for MIPS eligible clinicians. To meet the CEHRT definition when reporting on this measure, in our EHR Incentive Program Stage 3 and Modifications to Meaningful Use in 2015 through 2017 final rule (80 FR 62870 through 62885) we established that health care providers are required to use a health IT module certified to the "Transmission to public health agencies—electronic case reporting" certification criterion at 45 CFR 170.315(f)(5) that relates to how the health IT uses structured data within an EHR to trigger or indicate the generation of an electronic initial case report.[226] They may then transmit the report in the manner specified by the case reporting requirements of the entity to which they are transmitting a report.
We believe that requiring the Electronic Case Reporting measure would not only provide certainty to EHR vendors and facilitate an organized and industry-wide rollout of electronic case reporting capabilities, but would also help health care providers reduce their public health reporting burden.
We are not proposing any changes to the description of the Electronic Case Reporting measure and the exclusions that we established in the CY 2019 PFS final rule at 83 FR 59815 through 59817 will remain available.
(cc) Proposed Scoring of the Public Health and Clinical Data Exchange Objective
We are proposing that beginning with the performance period in CY 2022, a MIPS eligible clinician would receive 10 points for the Public Health and Clinical Data Exchange objective if they report a "yes" response for each of the following required measures: Immunization Registry Reporting; and Electronic Case Reporting. In the event that a MIPS eligible clinician is able to claim an exclusion for one or more of these required measures, we are proposing they would receive 10 points for the objective if they report a "yes" response for one measure and claim an applicable exclusion for which they qualify for the remaining measure. If the MIPS eligible clinician fails to report on any one of the two measures required for this objective or reports a "no" response for one or more of these measures, we are proposing that the MIPS eligible clinician would receive a score of zero for the Public Health and Clinical Data Exchange objective, and a total score of zero for the Promoting Interoperability performance category. If an MIPS eligible clinician claims applicable exclusions for which they qualify for both required measures, we propose to redistribute the points associated with the objective to the Provider to Patient Exchange objective.
We are proposing to retain the Public Health Registry Reporting, Clinical Data Registry Reporting, and Syndromic Surveillance Reporting measures, and to make them optional and available for bonus points beginning with the performance period in CY 2022. For more information about these measures, we refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77229) and the EHR Incentive Program Stage 3 and Modifications to Meaningful Use in 2015 through 2017 final rule (80 FR 62818 through 62825). We are proposing a MIPS eligible clinician may earn 5 bonus points if they report a "yes" response for either the Public Health Registry Reporting measure or the Clinical Data Registry Reporting measure or the Syndromic Surveillance Reporting measure. Reporting on more than one of these optional measures would not yield additional bonus points.
In connection with our proposal to make these measures optional, we are proposing to remove the three exclusions that we established in the CY 2019 PFS final rule at 83 FR 59815 through 59817 for the Public Health Registry Reporting measure, Clinical Data Registry Reporting measure, and the Syndromic Surveillance Reporting measure.
We are seeking comment on these proposals.
(d) SAFER Guides
(i) Background
ONC developed and released the Safety Assurance Factors for EHR Resilience Guides (SAFER Guides) in 2014, and later updated them in 2016. This series of nine user guides support the ability of health care providers to address EHR safety.[227] Collectively, the SAFER Guides help health care organizations at all levels, from small practices to multi-system chains and tertiary care facilities, to conduct self-assessments to optimize the safety and safe use of EHRs in the three areas listed in Table 43. The SAFER Guides are intended to be utilized by EHR users, developers, patient safety organizations, and those who are concerned with optimizing the safe use of health IT. Completing a self-assessment using the SAFER Guides is one of the first steps MIPS eligible clinicians can take to support a "culture of safety" within their organization, and ensure they are responsible operators of technology tools, including certified health IT products, which they utilize in the delivery of care. The SAFER Guides are based on the best evidence available at the time of publication, which included a literature review, expert opinion, and field-testing at a wide range of health care organizations, from small ambulatory care practices to large health systems.
In the case of system disruption, failure, natural disaster, the SAFER Guides provide recommended safety practices during planned or unplanned EHR unavailability, where end users are unable to access all or part of their EHR. Also included are back-up procedures to Start Printed Page 39415 prevent the potential loss of clinical and administrative data, and how to utilize paper charting during such downtime. We believe that conducting an annual self-assessment starting with the High Priority Practices Guide would support consistent safety practices for all EHR users.
The High Priority Practices SAFER Guide identifies "high risk" and "high priority" recommended safety practices, intended to optimize the safety and safe use of EHRs.[228] This guide broadly discusses EHR safety concerns that are described in greater detail in the subsequent 8 SAFER Guides. The High Priority Practices Guide is considered the first to be completed of the 9 SAFER Guides.

(ii) Proposed New SAFER Guides Measure
We are proposing to add a new SAFER Guides measure to the Protect Patient Health Information objective, beginning with the CY 2022 performance period/2024 MIPS payment year. For this measure, we are proposing that a MIPS eligible clinician must attest to having conducted an annual self-assessment using the High Priority Practices Guide (available at https://www.healthit.gov/topic/safety/safer-guides), at any point during the calendar year in which the performance period occurs, with one "yes/no" attestation statement accounting for the complete self-assessment using the guide. We propose that this measure would be required, but it would not be scored, and that reporting "yes" or "no" would not affect the total number of points earned for the Promoting Interoperability performance category. We believe this measure would further enable the electronic exchange of health information to improve the quality of health care, such as promoting care coordination, as described in section 1848(o)(2)(A)(ii) of the Act. We are also proposing to add corresponding regulatory text for this measure at § 414.1375(b)(2)(ii)(C).
In order to complete a "self-assessment" using the High Priority Practices Guide, we would expect that each MIPS eligible clinician would complete a review and mark the associated checkboxes (fully, partially, or not implemented) of recommended practices included at the beginning of the Guide. Detailed worksheets with the rationales for, and examples of how, to implement each recommended practice follows the checklist section of the Guide. These worksheets also include likely sources of information the practice can turn to in order to complete their assessment of a recommended practice, as well as fillable note fields to record follow-up actions.
We understand that every organization faces unique circumstances, and will implement a particular practice differently. As a result, some of the specific examples in the SAFER Guides for recommended practices may not be applicable to every organization. We note that a "self-assessment" does not require an organization to confirm that it has implemented "fully in all areas" each practice described in a particular SAFER guide, nor will an organization be scored on how many of the practices the organization has fully implemented. Rather, the intent of this proposed requirement is for MIPS eligible clinicians to regularly assess their progress and status on important facets of patient safety.
The recommended practices in the SAFER Guides are intended to be useful for all EHR users. However, we recognize that the individuals responsible for the proposed annual assessment may vary across organizations. An optimal team for completing an annual review of the SAFER Guides might include clinicians (including physicians, nurses, pharmacists, and allied health staff), and the technical staff responsible for implementing and maintaining a practice's EHR as well as data connections with external partners (for example, an HIE). Regarding the frequency of completing the self-assessment for the High Priority Practices Guide, we are proposing that a MIPS eligible clinician must attest to completing their assessment using the High Priority Practices Guide on an annual basis, following an initial completion of the assessment (some clinicians may have already completed an assessment using the SAFER Guides prior to implementation of this requirement, if finalized). We would expect MIPS eligible clinicians to revisit this assessment to determine whether any changes have occurred for their organization. We believe that requiring MIPS eligible clinicians to periodically review this self-assessment as proposed would support a stronger culture of change management within organizations, and would assist organizations in actively understanding and addressing potential safety vulnerabilities, which may significantly impact an organization's safety posture. We recognize that organizations may be at different stages in their progress towards assessing patient safety vulnerabilities, and that MIPS eligible clinicians vary in the resources that they could devote to an annual review of the High Priority Practices Guide. Gathering this information may be time consuming for some, and others may not have the expertise available on staff to complete all of the requirements. For MIPS eligible clinicians with less experience in these areas, we note that there are a number of resources available, which Start Printed Page 39416 may be able to assist with completing a self-assessment.
We are inviting public comment on these proposals.
(e) Incrementation of the Numerator and Denominator for Promoting Interoperability Performance Category Measures
In the CY 2019 PFS final rule (83 FR 59799), we summarized a comment we received in response to proposals we had made in the CY 2019 PFS proposed rule concerning the measures for the Promoting Interoperability performance category beginning with the performance period in 2019. The commenter indicated that for some measures, MIPS eligible clinicians and group practices should be able to get credit for actions that are taken outside of the 90-day performance period. We responded to the comment by stating that since the inception of the Quality Payment Program, we have limited the ability to increment the numerator and denominator of measures to actions occurring during the performance period chosen, with the exception of the Security Risk Analysis measure, for which the relevant actions may occur any time during the calendar year. We now understand that our response to this comment may have caused confusion, and we wish to clarify our response. Instead of referring to the inception of the Quality Payment Program, we should have stated that the measures we proposed beginning with the performance period in 2019 would limit the ability to increment the numerator and denominator to actions occurring during the performance period chosen, with the exception of the Security Risk Analysis measure, for which the relevant actions may occur any time during the calendar year. We note an additional exception would be the SAFER Guides measure (as proposed in section IV.A.3.d.(4)(d) in this proposed rule) because the relevant actions may also occur at any time during the calendar year.
(f) Changes to the Scoring Methodology for the 2022 Performance Period
For ease of reference, Table 44 lists the objectives and measures for the Promoting Interoperability performance category for the performance period in CY 2022 as revised to reflect the proposals made in this proposed rule. Table 45 lists the 2015 Edition certification criteria required to meet the objectives and measures.
Start Printed Page 39417

Start Printed Page 39418

Start Printed Page 39419

Start Printed Page 39420

Start Printed Page 39421

Start Printed Page 39422

Table 46 reflects the scoring methodology for the Promoting Interoperability performance category for the performance period in CY 2022, if the proposed changes discussed earlier in this section are adopted as final, including the continuation of the optional Query of PDMP measure worth 10 bonus points for CY 2022, changes to the Provide Patients Electronic Access to Their Health Information Measure under the Provider to Patient Exchange objective, the adoption of a SAFER Guides measure, and modified requirements for the Public Health and Clinical Data Exchange objective.
Start Printed Page 39423

We also refer readers to section IV.A.3.e. of this proposed rule, where we are proposing changes to the regulatory text for scoring the Promoting Interoperability performance category at § 414.1380(b)(4)(ii).
(g) Actions To Limit or Restrict the Compatibility or Interoperability of CEHRT
(i) Background
Section 106(b)(2) of the Medicare Access and CHIP Reauthorization Act of 2015 (Pub. L. 114-10) (MACRA) includes the heading "Preventing Blocking The Sharing Of Information." Section 106(b)(2)(A) amended section 1848(o)(2)(A)(ii) of the Act to require that, to be a meaningful EHR user, a clinician demonstrates (through a process specified by the Secretary, such as the use of an attestation) that the clinician has not knowingly and willfully taken action (such as to disable functionality) to limit or restrict the compatibility or interoperability of the certified EHR technology. To implement these provisions, we established and codified at § 414.1375(b)(3)(ii) attestation requirements for the Promoting Interoperability performance category to support the prevention of information blocking, which consist of three statements containing specific representations about a MIPS eligible clinician's implementation and use of CEHRT. For further discussion on these requirements, we refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77028 through 77035). The attestation statements finalized for MIPS eligible clinicians at § 414.1375(b)(3)(ii) are:
- Statement A: Did not knowingly and willfully take action (such as to disable functionality) to limit or restrict the compatibility or interoperability of certified EHR technology.
- Statement B: Implemented technologies, standards, policies, practices, and agreements reasonably calculated to ensure, to the greatest extent practicable and permitted by law, that the certified EHR technology was, at all relevant times: (1) Connected in accordance with applicable law; (2) compliant with all standards applicable to the exchange of information, including the standards, implementation specifications, and certification criteria adopted at 45 CFR part 170; (3) Implemented in a manner that allowed for timely access by patients to their electronic health information; and (4) Implemented in a manner that allowed for the timely, secure, and trusted bi-directional exchange of structured electronic health information with other health care providers (as defined by 42 U.S.C. 300jj(3)), including unaffiliated providers, and with disparate certified EHR technology and health IT vendors.
- Statement C: Responded in good faith and in a timely manner to requests to retrieve or exchange electronic health information, including from patients, health care providers (as defined by 42 U.S.C. 300jj(3)), and other persons, regardless of the requestor's affiliation or technology vendor.
Section 4004 of the 21st Century Cures Act added section 3022 to the Public Health Service Act (PHSA) (the "PHSA information blocking provision,"), which describes practices by health care providers, health IT developers, and HIEs and networks, that constitute information blocking, and provides for civil monetary penalties and other disincentives for those who engage in information blocking.
In the ONC 21st Century Cures Act final rule published in the May 1, 2020 Federal Register, ONC finalized a definition of information blocking and identified reasonable and necessary activities ("exceptions") that do not constitute information blocking (85 FR 25642). For health care providers (as defined in 42 U.S.C. 300jj), information blocking means a practice that, except as required by law or covered by an exception, is likely to interfere with access, exchange, or use of electronic health information; and if conducted by a health care provider, such provider knows that such practice is unreasonable and is likely to interfere with, prevent, or materially discourage access, exchange, or use of electronic health information (45 CFR 171.103).
The 21st Century Cures Act provides for civil monetary penalties for any individual or entity that is a developer, network, or exchange that has committed information blocking (see section 3022(b)(2)(A) of the PHSA). Regarding health care providers, the 21st Century Cures Act provides that "Any [health care provider] determined by the [HHS] Inspector General to have committed information blocking shall be referred to the appropriate agency to be subject to appropriate disincentives using authorities under applicable Start Printed Page 39424 federal law, as the Secretary sets forth through notice and comment rulemaking" (section 3022(b)(2)(B) of the PHSA).
(ii) Proposed Changes to the Attestation Statements
Although there could be some degree of overlap between conduct described in the attestation statements under § 414.1375(b)(3)(ii) and conduct that could be considered information blocking under section 3022 of the PHSA and ONC's implementing regulations at 45 CFR 171.103, it is important to note these are separate and distinct authorities. For instance, the ONC 21st Century Cures Act final rule finalized a definition for what constitutes information blocking, and exceptions to information blocking that are not reflected in the previously finalized attestation statements under § 414.1375(b)(3)(ii). While we previously stated in the 2017 QPP final rule that these attestations statements did not impose "unnecessary or unreasonable requirements" on health care providers (81 FR 77029), after careful review of these attestation statements in light of the information blocking regulations at 45 CFR part 171, we believe that statements B and C are no longer necessary. Thus, beginning with the performance period in CY 2022, we are proposing to no longer require statements B and C. We believe that the similarities between practices described under statements B and C, and the practices that could constitute information blocking under section 3022 of the PHSA and ONC's implementing regulations will create confusion for stakeholders. To this point, the practices that could constitute information blocking under 45 CFR part 171 are much broader than those described in the attestation statements. We discuss specific instances of potential confusion below.
Statement B requires attestation to a series of statements regarding the use of certified technology and a designated manner for implementing certified technology. For instance, attestations to the implementation of technology compliant with the standards for certified health IT at 45 CFR part 170, and use of functionality to support health information exchange with other health care providers. However, as noted above, the definition of information blocking finalized in the ONC 21st Century Cures Act final rule is not specific to, nor limited to the use of certified technology, which is compliant with certain standards or the use of certain functionality. Under the ONC 21st Century Cures Act final rule, a health care provider may still be determined to have engaged in practices likely to interfere with access, exchange, or use of electronic health information (information blocking) regardless of whether they are using certified technology.
Regarding statement C, we stated in the 2017 QPP final rule that "technical, legal, and other practical constraints may prevent a health care provider from responding to some requests to access, exchange, or use electronic health information in a health care provider's certified EHR technology" (81 FR 77033). Subsequently, in the ONC 21st Century Cures Act final rule, ONC established a set of reasonable and necessary activities that are not considered information blocking when responding to a request for EHI. The reasonable and necessary activities established under the ONC 21st Century Cures Act final rule now provide more specific direction to health care providers when responding to a request for EHI than the general "technical, legal, and other practical constraints," which we described in the CY 2017 QPP final rule. Accordingly, we believe that continuing to require statement C may introduce confusion for those health care providers who are obligated to comply with the regulations finalized in the ONC 21st Century Cures Act final rule when responding to a request for EHI.
In order to distinguish the attestation required by section 106(b)(2)(A) of MACRA from information blocking under section 3022 of the PHSA, we are proposing to modify the headings of §§ 414.1375(b)(3) and (b)(3)(ii), add § 414.1375(b)(3)(iii), and modify the definition of "meaningful EHR user for MIPS" under § 414.1305 to specify that the clinician does not knowingly and willfully take action (such as to disable functionality) to limit or restrict the compatibility or interoperability of CEHRT, which reflects the language used in section 106(b)(2)(A) of MACRA. In addition, as discussed, we are proposing to no longer require attestation statements B and C beginning with the performance period in CY 2022, and we are proposing corresponding regulatory text amendments at § 414.1375(b)(3)(ii) and (iii). We made a similar proposal for the Medicare Promoting Interoperability Program in the FY 2022 IPPS/LTCH PPS proposed rule (86 FR 25639 through 25641).
We invite public comment on our proposal.
(h) Additional Considerations
(i) Reweighting the Promoting Interoperability Performance Category for MIPS Eligible Clinicians in Small Practices
We previously established under § 414.1380(c)(2)(i)(C)(9) a significant hardship exception for MIPS eligible clinicians in small practices as defined in § 414.1305. In the CY 2018 Quality Payment Program final rule (82 FR 53682 through 53683), we established that we will reweight the Promoting Interoperability performance category to zero percent of the MIPS final score for MIPS eligible clinicians who qualify for this hardship exception. We established that a MIPS eligible clinician seeking to qualify for this exception must submit an application to us demonstrating that there are overwhelming barriers that prevent them from complying with the requirements for the Promoting Interoperability performance category, and that the exception is subject to annual renewal. In the CY 2018 Quality Payment Program final rule (82 FR 53579 through 53581), we also established that we will determine the size of small practices by utilizing claims data. This policy was further modified in the CY 2019 PFS final rule (83 FR 59727 through 59730) so that beginning with the 2021 MIPS payment year, a small practice is a TIN consisting of 15 or fewer eligible clinicians during the MIPS determination period.
In the CY 2018 Quality Payment Program proposed rule (82 FR 30076), we stated that we believed that special consideration should be available for MIPS eligible clinicians in small practices based on concerns previously identified by commenters, including small practices not being able to afford the upfront investments (including investments in EHR technology), and small practices not adopting EHRs due to the administrative and financial burden. Although some commenters requested that we automatically apply the proposed hardship exception to clinicians in small practices, at the time we disagreed and stated that we believed that many small practices will be able to successfully report for the Promoting Interoperability performance category (82 FR 53683).
We have been monitoring the submission of data for the Promoting Interoperability performance category by small practices and individual clinicians who are part of a small practice, and the numbers remain low despite the no-cost technical assistance we offered small practices through the Small, Underserved, and Rural Support Initiative. Data from CY 2019 revealed Start Printed Page 39425 that of the 49,278 clinicians in small practices who were scored as an individual for MIPS, 84 percent of them did not submit Promoting Interoperability performance category data and did not apply for a small practice hardship exception application even though they may have qualified for the exception. Among clinicians who did not qualify for Promoting Interoperability performance category reweighting, only 29 percent of small practices compared to 61 percent of practices with more than 15 clinicians billing under the practice's TIN submitted data for the Promoting Interoperability performance category. Although we had expected many small practices would be able to successfully report data for the Promoting Interoperability performance category, we are concerned to see such low numbers of them either reporting data or applying for the small practice hardship exception, as such inaction would result in a score of zero for this performance category.
We want to support small practices and help them successfully participate in MIPS. As our analysis of the data suggests that successfully participating in the Promoting Interoperability performance category may be particularly challenging for small practices, we are proposing a modification of our policy. Beginning with the CY 2022 performance period/CY 2024 MIPS payment year, we are proposing to no longer require an application for clinicians and small practices seeking to qualify for the small practice hardship exception and reweighting. We are proposing instead to assign a weight of zero percent to the Promoting Interoperability performance category and redistribute its weight to another performance category or categories (as discussed further in section IV.A.3.e. of this proposed rule) in the event no data is submitted for any of the measures for the Promoting Interoperability performance category by or on behalf of a MIPS eligible clinician in a small practice. We are proposing that if data is submitted for a MIPS eligible clinician in a small practice, they would be scored on the Promoting Interoperability performance category like all other MIPS eligible clinicians, and the performance category would be given the weight prescribed by section 1848(q)(5)(E) of the Act. We are proposing the small practice significant hardship exception still would be subject to annual renewal, and we would verify whether a practice meets the definition of a small practice under § 414.1305 on an annual basis. We are proposing corresponding revisions to § 414.1380(c)(2)(i)(C)(9).
While we are proposing this policy at this time, it is not our intention that this policy be in place for the long term, but rather only for a few years, as we would like to increase participation of small practices in the Promoting Interoperability performance category. We would like to facilitate small practices successfully reporting data for the Promoting Interoperability performance category and are therefore seeking comment on potential options to increase small practice participation in the future. We are also seeking comment on why small practices that have not successfully reported for the Promoting Interoperability performance category not applied for the small practice hardship exception. Are practices choosing not to apply due to the requirement that they must have overwhelming barriers that prevent them from complying with the requirements for the Promoting Interoperability performance category? Is there confusion about what would be considered an overwhelming barrier? Are they aware that the small practice hardship exception is available? We are also interested in hearing about barriers that exist that prevent the adoption of CEHRT and/or the ability to submit Promoting Interoperability performance category measures. Are small practices wanting to adopt CEHRT but lack the resources?
Have practices previously adopted CEHRT but face barriers in upgrading to the edition of certified health IT currently required to meet the CEHRT definition for the Quality Payment Program? Alternatively, have these practices consciously chosen not to adopt CEHRT because of impending retirement or other factors? Are there other policies that we could pursue to remove barriers to or incentivize participation in the Promoting Interoperability performance category by small practices?
We are proposing that in the case of an APM Entity that also meets the definition of a small practice, we would continue applying the Promoting Interoperability performance category reporting and exception requirements at the group level, as described at § 414.1317. However, if the APM Entity is composed of a single TIN which itself meets the definition of a small practice, all TINs within the APM Entity (that is, the single TIN) would be eligible for this exception, and therefore the Promoting Interoperability performance category would be reweighted for the APM Entity and the performance category reweighting described above would be applied.
We are seeking comments on this proposal.
(ii) Nurse Practitioners, Physician Assistants, Clinical Nurse Specialists, and Certified Registered Nurse Anesthetists
We established a policy at § 414.1380(c)(2)(i)(A)(5) for the performance periods in 2017 through 2021 under section 1848(q)(5)(F) of the Act to assign a weight of zero to the Promoting Interoperability performance category in the MIPS final score if there are not sufficient measures applicable and available to NPs, PAs, CRNAs, and CNSs. We will assign a weight of zero only in the event that an NP, PA, CRNA, or CNS does not submit any data for any of the measures specified for the Promoting Interoperability performance category, but if they choose to report, they will be scored on the Promoting Interoperability performance category like all other MIPS eligible clinicians and the performance category will be given the weighting prescribed by section 1848(q)(5)(E) of the Act.
As in past years, we intend to use data from prior performance periods to further evaluate the participation of NPs, PAs, CRNAs, and CNSs in the Promoting Interoperability performance category and consider for subsequent years whether the measures specified for this category are applicable and available to these MIPS eligible clinicians. We have analyzed the data submitted for the 2017 performance period for the Promoting Interoperability performance category and have discovered that the vast majority of MIPS eligible clinicians submitted data as part of a group. Although we are pleased that MIPS eligible clinicians utilized the option to submit data as a group, it does limit our ability to analyze data at the individual NPI level. For the 2017 performance period, approximately 4 percent of MIPS eligible clinicians who are NPs, PAs, CRNAs, or CNSs submitted data individually for MIPS, and more than two-thirds of them did not submit data for the Promoting Interoperability performance category. For the 2018 performance period, we reported that of the MIPS eligible clinicians who are NPs, PAs, CRNAs, or CNSs and submitted data individually, approximately 34 percent submitted data for the Promoting Interoperability performance category. However, after further review and the refinement of our analytics it was revealed that the percentage was not 34 percent but was 24 percent of MIPS eligible clinicians Start Printed Page 39426 who are NPs, PAs, CRNAs, or CNSs that submitted data individually for the Promoting Interoperability performance category. For the 2019 performance period, of the MIPS eligible clinicians who are NPs, PAs, CRNAs, or CNSs and submitted data individually, approximately 30 percent submitted data individually for the Promoting Interoperability performance category, a modest increase from 2018. We continued our reweighting policy in 2020 although we do not yet have data from submissions for the CY 2020 performance period and do not expect it to be available prior to the release of this proposed rule.
We believe that having these clinician types using CEHRT and submitting data for the Promoting Interoperability performance category is important for increased interoperability and data exchange. We are exploring the possibility that these clinician types are able to submit data but are choosing not due to our current reweighting policies. In the future we may use other factors besides the submission data to determine whether to continue to reweight the Promoting Interoperability performance category for these clinicians. We are requesting comments as to whether these clinician types are using CEHRT and are able to submit data on the measures for the Promoting Interoperability performance category.
While we are encouraged by the increasing numbers of NPs, PAs, CRNAs, and CNSs submitting data for the Promoting Interoperability performance category, we believe that the low numbers warrant the continued reweighting the Promoting Interoperability performance category for NPs, PAs, CRNAs, and CNSs for the performance period in 2022. Thus, we are proposing to continue the existing policy for the 2022 performance period/2024 MIPS payment year and are proposing to revise § 414.1380(c)(2)(i)(A)(5), which is being redesignated as § 414.1380(c)(2)(i)(A)(4)(ii), to reflect the proposal.
We request comments on this proposal.
(iii) Physical Therapists, Occupational Therapists, Qualified Speech-Language Pathologists, Qualified Audiologists, Clinical Psychologists, and Registered Dieticians or Nutrition Professionals
In the CY 2020 PFS final rule (84 FR 63003 through 63004), we adopted a policy at § 414.1380(c)(2)(i)(A)(4) to apply the same policy we adopted for NPs, PAs, CNSs, and CRNAs to other types of MIPS eligible clinicians who are NPPs (physical therapists, occupational therapists, qualified speech-language pathologist, qualified audiologists, clinical psychologists, and registered dieticians or nutrition professionals) for the performance period in 2020. We stated that because many of these clinician types were or are not eligible to participate in the Medicare or Medicaid Promoting Interoperability Program, we have little evidence as to whether there are sufficient measures applicable and available to them under the Promoting Interoperability performance category. We extended this policy for the performance period in 2021 (85 FR 84895). As these clinicians were first eligible to participate in 2020, we do not have data to rely on to modify our current policy and do not anticipate it being available prior to the release of this proposed rule. Therefore, we are proposing to continue the existing policy of reweighting the Promoting Interoperability performance category for physical therapists, occupational therapists, qualified speech-language pathologist, qualified audiologists, clinical psychologists, and registered dieticians or nutrition professionals for the 2022 performance period/2024 MIPS payment year. We propose to revise § 414.1380(c)(2)(i)(A)(4), which is being redesignated as § 414.1380(c)(2)(i)(A)(4)(i), to reflect this proposal.
We request comments on this proposal.
(iv) Clinical Social Workers and Certified Nurse-Midwives
In section IV.A.3.a. of this proposed rule, we are proposing to add clinical social workers and certified nurse-midwives to the definition of a MIPS eligible clinician. These clinician types were not eligible to participate in the Medicare Promoting Interoperability Program to earn incentive payments for meaningful use of CEHRT or receive reduced Medicare payments for failing to meaningfully use CEHRT. Clinical social workers were not eligible for Medicaid EHR incentive payments and thus may lack experience with the adoption or use of CEHRT. Certified nurse-midwives were eligible for the Medicaid EHR incentive payments, and the majority did earn incentives. For the CY 2022 performance period/CY 2024 MIPS payment year, we are proposing to apply the same Promoting Interoperability reweighting policy we adopted previously for NPs, PAs, CNSs, CRNAs, and other types of MIPS eligible clinicians to clinical social workers as we believe that there may not be sufficient Promoting Interoperability performance category measures that are applicable and available to clinical social workers. We would assign a weight of zero only in the event that a clinical social worker does not submit data for any of the measures specified for the Promoting Interoperability performance category. We are proposing to add § 414.1380(c)(2)(i)(A)(4)(iii) to reflect this proposal for clinical social workers.
We believe there are sufficient measures applicable and available to certified nurse-midwives under the Promoting Interoperability performance category because of their experience with the Medicaid Promoting Interoperability Program. Many of them have adopted CEHRT and earned a Medicaid incentive payment, and the measures for the Medicaid Promoting Interoperability Program generally are the same or slightly modified versions of the Promoting Interoperability performance category measures. Thus, we are not proposing to apply the same Promoting Interoperability reweighting policy we adopted previously for NPs, PAs, CNSs, CRNAs, and other types of MIPS eligible clinicians to certified nurse-midwives. However, we are requesting comment on whether there are in fact sufficient measures applicable and available to certified nurse-midwives under the Promoting Interoperability performance category, and whether barriers exist that prevent certified nurse-midwives from complying with the requirements of the Promoting Interoperability performance category and may warrant reweighting. Like other types of MIPS eligible clinicians, a certified nurse-midwife may be able to qualify for a significant hardship exception from and reweighting of the Promoting Interoperability performance category under the existing policies at § 414.1380(c)(2)(i)(C), depending on their circumstances.
We request comments on this proposal.
(i) Technical Corrections to the Regulations
In the CY 2019 PFS final rule (83 FR 59798 through 59817), we adopted objectives and measures for the Promoting Interoperability performance category that would apply beginning with the performance period in 2019. The requirement for MIPS eligible clinicians to report on these objectives and measures can be found under § 414.1375(b)(2). In the CY 2021 PFS rulemaking, we inadvertently neglected to update this provision of the regulation text, although our intention was a continuation of the policy we established for the 2021 and 2022 MIPS Start Printed Page 39427 payment years. We are proposing a technical correction to § 414.1375(b)(2)(ii) to specify that the reporting requirements apply beginning with the 2021 MIPS payment year. We request comments on this proposal.
(j) Requests for Information
(i) Request for Information on Additional Objectives Adopting FHIR®-Based API Standards
Fast Healthcare Interoperability Resources (FHIR®) (http://hl7.org/fhir) is a free and open-source standards framework (in both commercial and government settings) created by Health Level Seven International (HL7®) that establishes a common language and process for all health IT, it allows systems to communicate and information to be shared seamlessly with a lower burden on stakeholders. Through the HL7® FHIR® standard, cost and burden for health care providers and patients are reduced since it simplifies implementation without sacrificing information integrity, establishes fast, efficient, and flexible health data exchange as a stand-alone standard or combined with existing standards. Essentially, HL7®'s FHIR® standard framework provides an interoperable platform for a variety of health care data by defining a standard way to structure this information as `resources' and allows the developer-friendly automated data-exchange to occur via APIs. The use of APIs utilizing the FHIR® standard has the potential to improve data exchange by providing consistent security, performance, scalability, and structure to all users. Given the progress of such emerging health IT innovation standards to promote interoperability at large, we see increased adoption of approaches utilizing the latest HL7® FHIR® standard as an opportunity to consider how these approaches can support other program goals.
In the CY 2021 PFS final rule, we finalized alignment of the CEHRT definition for the Promoting Interoperability programs with updates to 2015 Edition certification criteria as finalized in the ONC 21st Century Cures Act final rule. As part of the ONC 21st Century Cures Act final rule, ONC finalized a new certification criterion "Standardized API for patient and population services" at 45 CFR 170.315(g)(10) which supports the availability in certified health IT of an API using the FHIR® Release 4 standard and other implementation specifications. We noted that technology certified to this criterion will be used to support the API requirements in the Provide Patients Access to their Health Information objective. Regarding the bi-directional HIE measure finalized for MIPS eligible clinicians in the CY 2021 PFS final rule (85 FR 84888 through 84893), we also noted that the standards-based API criterion at 45 CFR 170.315(g)(10) could be used to support connections to an HIE in order to complete the measure's actions.
We are seeking comments on our intention to align additional Promoting Interoperability performance category objectives with approaches utilizing HL7® FHIR® standard Release 4-based API functionality (or the appropriately evolved standard), specifically targeting the Health Information Exchange as well as the Public Health and Clinical Data Exchange objectives. Throughout this ongoing developmental process, we are partnering with ONC and continuing to strengthen collaboration on the implementation of the 21st Century Cures Act final rule.
We are interested in public comments on how these two program objectives could be furthered through the use of FHIR®-based API solutions. Specifically, we are interested in the following questions:
- To what degree are stakeholders currently using or interested in using APIs to exchange information in support of the numerator/denominator measures under the HIE objective? What revisions to the measures under the HIE objective should CMS explore to facilitate use of standards-based APIs in health IT modules certified under the 2015 Edition Cures Update?
- How could technical approaches utilizing the FHIR® standard enhance existing data flows required under the public health measures? What are promising FHIR®-based approaches to public health reporting use cases that ONC and CMS should explore for potential future consideration as part of the Promoting Interoperability performance category and the ONC Health IT Certification Program?
- To what degree are PHAs and individual states currently exploring API-based approaches to conducting public health registry reporting? What other factors do stakeholders see as critical factors to adopting FHIR®-based approaches?
- What potential policy and program changes in CMS and other HHS programs could reduce health care provider and health IT developer burden related to measures under the Health Information Exchange and the Public Health and Clinical Data Exchange objectives?
(ii) Request for Information on a Patient Access Outcomes Measures
The evolution of EHRs has created a greater and more seamless flow of information within a digital health care infrastructure which allows for comprehensive records to be made available wherever and whenever they are needed in the clinical setting. These advances have led to: (1) Improved patient care; (2) increased patient participation; (3) improved care coordination; (4) greater practice efficiencies and cost savings; and (5) improved diagnostics and patient outcomes.[229] Much research effort has been dedicated to looking at the implementation of health IT in practice settings with its wide array of potential benefits, but equally important to the success of this EHR-advancement is better understanding the patient's role as an active end-user as well.
Several large, nationally representative surveys have been completed annually in order to collect and evaluate the public's access and use of health information. One of these endeavors operated by the National Cancer Institute (with support from ONC) is The Health Information National Trends Survey (HINTS)[230] that produces a plethora of key utilization data specifically pertaining to consumers' access and use of their online medical records via patient portals. The HINTS results point to an overall year-over-year rise in the number of Americans who are not only accessing their medical records online (from 51 percent in 2018 to 58 percent in 2019)[231] but are increasingly doing so to perform meaningful actions such as to view lab test results, transmit their data to a third-party, and to securely message their health care provider. While sources like the HINTS survey are revealing preferential trends, habits, and other key utilization points, the data also show some strong barriers associated with patients accessing CEHRT and continue to stress the need for further work in understanding these users' access outcomes.
We believe a strong partnership between EHR vendors, health care providers, and beneficiary users' outcomes is critical to improving the future of health care and furthering interoperability. Therefore, we are seeking comments surrounding changes Start Printed Page 39428 to the Promoting Interoperability performance category and related efforts which could better target patient access outcomes related to use of patient portals or third-party application(s). This request for information is an opportunity to garner general interest, solicit stakeholder feedback on how to best evaluate issues of patient behavior, and to explore additional key outcome variables to capture for measurement.
Specifically, we are looking for feedback on the following questions:
- What do stakeholders believe would be useful ways to measure patients' access to their electronic health information using health IT methods such as patient portals and/or third-party applications? What actionable figures related to users' medical record behavior, including but not limited to, the frequency of logins, number of messages sent, or lab results viewed could be captured?
- How effectively is the Promoting Interoperability performance category currently measuring the use of health IT-enabled processes to improve patient outcomes? What measures in the current performance category are most relevant to patient outcomes?
- Should we consider requiring health care providers to maintain a record of third-party applications which patients have used to access their patient health information through APIs incorporated within certified technology so that this information could be used to assess patient usage of these applications?
- What are specific technologies, capabilities, or system features (beyond those currently addressed in the Promoting Interoperability performance category) that can increase patient utilization of tools to access their health information? How do these technologies and features support improved access or usability within EHR systems and other applications (for instance, alternate authentication technologies that can simplify consumer logon)? How could CMS reward health care providers for higher adoption rates and use of these available technologies?
- What are key administrative processes that could benefit from more efficient electronic workflows? How could CMS measure and reward participating MIPS eligible clinicians for either greater uptake of patient portal access or subsequent health outcomes?
(iii) Request for Information on Clinical Notes
OpenNotes is an international movement aimed to spread and study the effects of transparent communication among patients, families, and clinicians.[232] With more than 50 million patients in the U.S. and Canada having gained access to their clinical notes, the push for patient engagement and transparent communication continues to grow.[233] Alongside this movement, "Clinical notes" have been regarded as highly desirable data necessary for the interoperable exchange of health information and patient access. Comprised of structured and unstructured data, clinical notes may include the assessment, diagnosis, plan of care and evaluation of plan, patient teaching, and other relevant data (85 FR 25674).
While the ability to share clinical notes has been previously supported for certified health IT in different ways, ONC took additional steps to ensure this important patient information is available as part of the recent ONC 21st Century Cures Act final rule (85 FR 25674 through 25677). In the rule, ONC finalized eight types of "clinical notes" required under the USCDI version 1: (1) Discharge Summary Note; (2) History & Physical; (3) Progress Note; (4) Consultation Note; (5) Imaging Narrative; (6) Laboratory Report Narrative; (7) Pathology Report Narrative; and (8) Procedure Note.[234]
As previously discussed in the CY 2021 PFS final rule (85 FR 84825), we finalized to align the CEHRT definition under the Promoting Interoperability performance category with the timelines established under the ONC 21st Century Cures Act final rule for implementation of the 2015 Edition Cures Update. This alignment includes updates to several certification criteria to refer to the USCDI and the expanded support for clinical notes specified in the USCDI version 1 standard. New and updated certification criteria incorporating the USCDI, include the "view, download, and transmit" criterion at 45 CFR 170.315(e)(1), and the "Standardized API for patient and population services" criterion at 45 CFR 170.315(g)(10). Once EHR developers and MIPS eligible clinicians have completed implementation of these updates, certified health IT required for participation in the Promoting Interoperability performance category will support availability of clinical notes as part of the data set made available to patients under the Provide Patients Access to their Health Information measure. According to the policy finalized in the CY 2021 PFS final rule, MIPS eligible clinicians may begin using updated technology as soon as it is available from their vendors (effective upon the effective date of the CY 2021 PFS final rule), with updated technology being required for performance periods beginning in CY 2023.
Under this RFI, we are seeking stakeholder feedback on changes we can make that will better support the goals of the OpenNotes movement to ensure that clinical notes are widely available to patients. Given the implementation of updates to certified technology described above that support the Provide Patients Access to their Health Information measure, are there additional changes to this measure, or other program guidance, which could further facilitate ensuring clinical notes are available to patients consistent with the goals of the OpenNotes movement? We are also seeking feedback on the development of a required and independently scored measure for the Promoting Interoperability performance category to allocate points for the use of "clinical note" types supported by certified health IT. Finally, we are seeking comment on the types of clinical notes that are commonly sought, but not easily accessible to patients. We included a similar RFI under the Medicare Promoting Interoperability Program in the FY 2022 IPPS/LTCH proposed rule (86 FR 25654).
(5) APM Entity Level Participation for MIPS Eligible Clinicians Participating in MIPS APMs
(a) Overview
In the CY 2021 PFS final rule (85 FR 84896), we finalized our policy to terminate the APM scoring standard effective January 1, 2021, and to retain certain APM Entity group reporting policies that were established and finalized for reporting and scoring under MIPS beginning with the CY 2021 MIPS performance period. Therefore, we redesignated, in part, the regulation that describes APM Entity group determinations, from § 414.1370(e) to § 414.1317, and titled that section "APM Entity Groups."
(b) APM Entity Level Reporting of Facility-Based Measures
In the CY 2021 PFS final rule (85 FR 84896), we finalized a policy to allow APM Entities to report to traditional MIPS using the same quality measures available to other groups, according to all applicable MIPS quality scoring policies. It has been brought to our attention that we did not make it clear Start Printed Page 39429 whether APM Entities may be eligible for facility-based scoring. We would like to clarify that because facility-based measures are not submitted, but rather collected by CMS using group-level participation scores used for the Hospital Value Based Purchasing Program, it would be impossible for CMS to calculate a score for a facility-based measure that represents the performance of an APM Entity. Therefore, facility-based scoring is not available to APM Entities under MIPS quality scoring rules, as described at § 414.1330. We note that participants in APM Entities that are eligible for facility based scoring at the individual or group level would still be eligible to receive these scores for purposes of individual or group MIPS scoring.
(c) APM Entity Performance Category Weights
In the CY 2021 PFS final rule (85 FR 84896), we finalized a policy to weight the cost performance category at zero percent of the final score for APM Entities in MIPS APMs. We codified the weight of the cost performance category at § 414.1317(b)(2), but we did not discuss in the CY 2021 PFS final rule how the weight that otherwise would have applied to the cost performance category will be redistributed among the other performance categories. For purposes of clarity, we propose to add to § 414.1317(b)(2) that the performance category reweighting scenarios under § 414.1380(c)(2) apply to an APM Entity.
Because APM Entities are participating in traditional MIPS and generally are being scored according to traditional MIPS scoring rules at § 414.1380 unless otherwise specified, the performance category reweighting scenarios under § 414.1380(c)(2) are applicable to APM Entities. Using the 2021 MIPS performance period/2023 MIPS payment year as an example, if the cost performance category is the only performance category weighted at zero percent for an APM Entity, the performance category weights would be as follows under § 414.1380(c)(2)(ii)(E): Quality 55 percent, cost zero percent, improvement activities 15 percent, and promoting interoperability 30 percent. If both cost and promoting interoperability are weighted at zero percent, then quality would be 85 percent and improvement activities would be 15 percent. For the remaining reweighting scenarios for the 2021 MIPS performance period/2023 MIPS payment year, we refer readers to Table 6 under § 414.1380(c)(2)(ii)(E). The reweighting scenarios applicable to APM Entities for the 2022 MIPS performance period/2024 MIPS payment year can be found in Table 7 under § 414.1380(c)(2)(ii)(F). We refer readers to section IV.A.3.e.(2)(a) of this proposed rule where we propose to apply the reweighting policy finalized for the 2022 MIPS performance period/2024 MIPS payment year at § 414.1380(c)(2)(ii)(F) to the 2025 MIPS payment year and each subsequent MIPS payment year. In the event we establish additional reweighting scenarios, they would also apply to APM Entities.
d. MIPS Final Score Methodology
(1) Performance Category Scores
(a) Background
For the CY 2022 performance period/2024 MIPS payment year, we intend to continue to build on the scoring methodology we finalized for prior years. The scoring methodology allows for accountability and alignment across the performance categories and minimizes burden on MIPS eligible clinicians. We are updating many of our scoring policies, focusing on removing transition policies. Specifically, we are proposing to—
- Change certain terminology related to scoring.
- Amend our scoring flexibility policy to include quality measures with omitted or deactivated codes in the finalized measure specifications.
- Implement benchmarking and topped out scoring policies that are responsive to potential low reporting rates for the CY 2020 performance period/2022 MIPS payment year due to the national PHE for COVID-19 and establishing a benchmark when measures are suppressed in the baseline period.
- Amend policies for scoring quality measures based on achievement and measures that do not meet case minimum or have a benchmark and introduce scoring policies for new measures.
- Amend the minimum case requirement policy.
- End the high priority and end to end reporting bonuses in the quality performance category.
- Continue improvement scoring in the quality performance category.
- Implement a scoring flexibility policy for changes that impact cost measures during the performance period.
- Revise certain provisions of the regulation text for the Promoting Interoperability performance category.
We are not proposing changes to scoring policies for the improvement activities performance category.
We have maintained our approach that MIPS eligible clinicians are scored against performance standards for each performance category and receive a final score, comprised of their performance category scores, and calculated according to the final score methodology. We refer readers to § 414.1380 for policies on scoring.
(b) Terminology Updates
We are proposing updates to § 414.1380 in an effort to more clearly and concisely capture previously established policies. These proposed updates are not intended to be substantive in nature, but rather to bring more clarity to the regulatory text. We are proposing to change the term "performance category percent score" to "performance category score" in § 414.1380(b)(1)(vi)(C) and (E) related to improvement scoring, § 414.1380(b)(1)(vii) related to scoring the quality performance category score, § 414.1380(b)(2)(iii) and (v) related to scoring the cost performance category, § 414.1380(c) and (c)(2)(ii)(A) on calculating the final score and final score reweighting, and § 414.1380(e)(6)(iv) and (v) related to facility-based scoring. Again, these changes are not intended to change the underlying policies reflected in the regulation text. Initially, the quality and cost performance categories used the term "performance category percent score" because those categories had improvement scoring and have a slightly different approach to calculation than the Promoting Interoperability and improvement activities performance category. However, the terms "performance category percent score" and "performance category score" have been used in the same way. For that reason, we are proposing to consolidate our language and use only the latter aforementioned term.
(c) Scoring the Quality Performance Category for the Following Collection Types: Medicare Part B Claims Measures, eCQMs, MIPS CQMs, QCDR Measures, the CAHPS for MIPS Survey Measure and Administrative Claims Measures
We refer readers to § 414.1380(b)(1) for our policies regarding quality measure benchmarks, calculating total measure achievement and measure bonus points, calculating the quality performance category percent score, including achievement and improvement points, and the small practice bonus (81 FR 77276 through 77308, 82 FR 53716 through 53748, 83 FR 59841 through 59855, 84 FR 63011 through 63018, 85 FR 84898 through 84913). We propose to amend policies Start Printed Page 39430 finalized in prior years to simplify scoring in MIPS as we transition to MVPs.
(i) Scoring Flexibility for Changes That Impact Quality Measures During the Performance Period or Prior to Implementation
We refer readers to CY 2018, CY 2019, and CY 2021 Quality Payment Program final rules (82 FR 53714 through 53716, 83 FR 59845 through 59847, and 85 FR 84898 through 84901 respectively) and § 414.1380(b)(1)(vii)(A) for our previously establish scoring flexibilities policy.
In the CY 2018 Quality Payment Program Final rule (82 FR 53714 through 53716), we finalized that, beginning with the 2018 MIPS performance period, we will assess performance on measures considered significantly impacted by ICD-10 coding changes during the performance period based only on the first 9 months of the 12-month performance period. We noted that we believe that 9 months of data is sufficient to assess performance when 12 months of data is not available. We finalized that we would publish a list of measures requiring 9 months of data on the CMS website by October 1st of the performance period if technically feasible, but no later than the beginning of the data submission period (for example, January 2, 2021 for the 2020 performance period) (82 FR 53716). We refer readers to § 414.1380(b)(1)(viii) for more on our policy for scoring flexibility for ICD-10 changes.
In the CY 2019 Quality Payment Program final rule (83 FR 59845 through 59847), we finalized policies beginning with the 2019 performance period/2021 MIPS payment year to reduce the total available measure achievement points from the quality performance category by 10 points for MIPS eligible clinicians for each measure submitted that is significantly impacted by clinical guideline changes or other changes when we believe adherence to the guidelines in the existing measures could result in patient harm or otherwise no longer be comparable to a historic benchmark. We wanted the flexibility to respond to instances in which the clinical evidence and guidelines change and approved measures no longer reflect the most up-to-date clinical evidence and could even result in a practice that is harmful to patients. We finalized expanding the list of reasons that a quality measure may be impacted during the performance period in addition to revising when we would allow scoring of the measure with a performance period truncation (to 9 months of data) or the complete suppression of the measure if 9 months of data are not available.
In the CY 2021 Quality Payment Program final rule, we finalized a consolidation of the CY 2018 and CY 2019 scoring flexibilities policy that allowed, beginning with the 2021 performance period/2023 MIPS payment year, truncation of the performance period or suppression of a quality measure if CMS determines that revised clinical guidelines, measure specifications or codes impact clinician's ability to submit information on the measure or may lead to potentially misleading results. Based on the timing of the changes to clinical guidelines, measure specifications or codes, we would assess the measure on 9 months of data, and if 9 consecutive months of data are not available, we would suppress the measure by reducing the total available measure achievement points from the quality performance category by 10 points for each measure submitted that is impacted (85 FR 84898 through 84901).
In previous rules, we noted that we believe that there may be instances when there are changes after the final approval of quality measures including changes to the measure specification, or updates to coding that may lead to misleading results (85 FR 84899). Additionally, we believe that there may be instances in which there is an inadvertent omission of codes, or inclusion of deactivated codes in the measure specifications that do not have the correct status. Typically, codes that are contained within the measure specifications either have a reimbursable status or a non-reimbursable status that still allows processing for purposes of quality reporting programs. We have encountered instances where CMS has been alerted that codes have inadvertently received an inactive status which results in the associated codes not being processed and stored in the National Claims History (NCH) database and therefore not available for quality purposes. As the measure specifications are considered technical documents that include several fields such as, but not limited to: the numerator, denominator, measure description, denominator exclusions, clinical guidance statements, and codes relevant to how the measure should be captured. The measure specifications are finalized and published in coordination with the final rule, and prior to the start of the performance period. An inadvertent omission of codes, or inclusion of inactive codes in the measure specifications, may result in misleading results by affecting clinicians who submit the measure. In these instances, implementation errors could lead to misleading performance rates by failure to reflect accurate numerator and/or denominator values for calculation of the measure. These are not changes that occur during the performance period, but errors that would affect the performance period.
It recently came to our attention through Help Desk inquiries that Medicare Administration Carriers (MACs) were rejecting 2021 Part B Claims submissions for Quality Measure ID (QID) 001: Hemoglobin A1c (HbA1c) Poor Control (>9%) and QID 117 Diabetes: Eye Exam due to an inactive status for certain CPT II codes. The omission of these claims from the total denominator population of the measure could skew scores and lead to misleading results. We believe suppression of the measures is consistent with current § 414.1380(b)(1)(vii)(A). Information on the suppression of the Part B claims collection type of these measures for the 2021 MIPS performance period/2023 MIPS payment year was announced via the QPP listserv on July 1st, 2021. For more information please refer to our website qpp.cms.gov.
We propose to amend § 414.1380(b)(1)(vii)(A) to clarify our intended policy on instances in which we become aware of changes to the active or payable status of the codes and/or implementation errors included in the measure specifications as finalized that would lead to misleading results. Accordingly, we propose to revise § 414.1380(b)(1)(vii)(A) to change "significant changes" to "significant changes or errors" and to include the omission of codes or inclusion of inactive or inaccurate codes to provide that for each measure that is submitted, if applicable, and impacted by significant changes or errors prior to the applicable data submission deadline at § 414.1325(e), performance is based on data for 9 consecutive months of the applicable CY performance period. If such data are not available or CMS determines that they may result in patient harm or misleading results, the measure is excluded from a MIPS eligible clinician's total measure achievement points and total available measure achievement points. For purposes of paragraph (b)(1)(vii)(A), "significant changes or errors" means changes to or errors in a measure that are outside the control of the clinician and its agents and that CMS determines may result in patient harm or misleading results. Significant changes or errors include, but are not limited to, Start Printed Page 39431 changes to codes (such as ICD-10, CPT, or HCPCS codes) or the active status of codes, the inadvertent omission of codes, or inclusion of inactive or inaccurate codes; changes to clinical guidelines; or measure specifications. We will publish on the CMS website a list of all measures scored under paragraph (b)(1)(vii)(A) as soon as technically feasible, but by no later than data submission deadline at § 414.1325(e)(1).
We invite public comments on our proposal to provide scoring flexibilities in response to account for errors included in the finalized measures.
(ii) Quality Measure Benchmarks
We refer readers to the CY 2017, CY 2018, CY 2019, CY 2020, and CY 2021 Quality Payment Program final rules (81 FR 77277 through 77282, 82 FR 53699 through 53718, 83 FR 59841 through 59842, 84 FR 63014 through 63016, and 85 FR 84901 through 84904) for our previously established benchmarking policies.
In the CY 2017 QPP final rule (81 FR 77277 through 77282), we finalized that we would use performance in the baseline period to set benchmarks for the quality performance category, with the exception of new quality measures, quality measures that lack historical data, or where we do not have comparable data from the baseline period, for which we would set the benchmarks using performance in the performance period. We defined the baseline period to be the 12-month CY that is 2 years prior to the performance period for the MIPS payment year. For example, for the CY 2022, the baseline period two performance periods prior would be the CY 2020 performance period (81 FR 77277). Additionally, we further clarified that CMS can establish benchmarks either by the applicable baseline or performance period in the CY 2019 PFS final rule (83 FR 59842), where we finalized the terminology change amending § 414.1380(b)(1)(ii) to remove the mention of each individual benchmark and instead state that benchmarks will be based on collection type, from all available sources, including MIPS eligible clinicians and APMs, to the extent feasible, during the applicable baseline or performance period.
Because of the flexibility provided to MIPS eligible clinicians to allow for no data submission for the 2020 performance period (https://qpp-cm-prod-content.s3.amazonaws.com/uploads/1198/2020%20MIPS%20Automatic%20EUC%20Fact%20Sheet.pdf), we may not have as representative of a sample of data as we would have had without the national PHE for COVID-19. Therefore, we want to revisit our benchmarking policy for the 2022 performance period/2024 MIPS payment year similarly to how we revisited the benchmarking policy for the 2021 performance period/2023 MIPS payment year (85 FR 84901 through 84902). We anticipate that we may have a gap in our data due to receiving fewer submissions for CY 2020, which could skew the benchmarking results. We believe this gap in data could result in different distributions of scores from what we normally see; thus, skewing the benchmarks when using CY 2020 data as the baseline for the CY 2022 performance period/2024 MIPS payment year. Additionally, we anticipate that only those not experiencing a hardship will submit data, thus skewing benchmarks much higher than normal. We ultimately, did not finalize this proposal for CY 2021 because analysis of the submitted data from the CY 2019 performance period/2021 payment period showed that it was suitable for use in calculating benchmarks. However, as we know from the events of 2020, we anticipate that the effects of the national PHE for COVID-19 may be more significant for the CY 2020 performance period.
For this reason, we consider two benchmarking options for the CY 2022 performance period/2024 MIPS payment year. We propose to use performance period benchmarks for the CY 2022 performance period/2024 MIPS payment year in accordance with § 414.1380(b)(1)(ii). As discussed in the CY 2021 PFS final rule (85 FR 84902), this would mean that benchmarks for the CY 2022 performance period/2024 MIPS payment year are based on the actual data submitted during the CY 2022 performance period. Last year, we received comments supportive of our proposal to use performance period benchmarks citing that the performance period benchmarks would capture any changes in care due to the national PHE for COVID-19 and avoid unfairly penalizing practices for variation in performance compared to data from prior to the national PHE for COVID-19 (85 FR 84902 through 84903). We also received comments that opposed the use of performance period benchmarks, as clinicians would not have advance notice of performance targets. As a result, we are also considering and seeking feedback as an alternative to performance period benchmarks, utilizing the historic benchmarks from the 2021 MIPS performance period (which are based on submissions for CY 2019 MIPS performance period/2021 MIPS payment year) for the CY 2022 performance period/2024 MIPS payment year. We believe that this option would allow clinicians to continue to receive advance notice for quality performance category measures so that MIPS eligible clinicians can set a clear performance goal for these measures for the CY 2022 performance period/2024 MIPS payment year. However, we remain concerned that utilizing outdated data could also potentially result in distributions of scores used for benchmarks that no longer reflect the standard of care especially as care changes in response to the public health emergency. Additionally, any new or substantively changed measures in the CY 2022 performance period/2024 MIPS payment year will lack a benchmark, as would measures that were suppressed in the CY 2019 performance period/2021 MIPS payment year alternate baseline period. We will analyze the CY 2020 performance period data and compare the distribution of the CY 2020 performance period data to that of previous years to assess if we can in fact use data from the CY 2020 performance period for benchmarks for the CY 2022 performance period/2024 MIPS payment year and if not, evaluate the suitability of the alternatives.
Additionally, we propose to expand the definition of the baseline period. In instances in which a measure is suppressed 2 performance periods prior in the standard baseline period and cannot be used to calculate a benchmark, we propose to use the data from 3 performance periods prior to calculate benchmarks in the event that a performance period benchmark cannot be calculated. This would mean that for the CY 2022 performance period/2024 MIPS payment year, if a measure was suppressed in the 2020 performance period and a performance period benchmark could not be calculated using the 2022 performance period data because it was not reported by 20 different clinicians or groups that met data completeness and case minimum requirements, we would calculate a benchmark from performance period data from CY 2019. If a measure had undergone a substantive change or was also suppressed in the baseline period 3 performance periods prior, we would not use it to calculate benchmarks and the measure would be subject to our scoring policies for class 2 measures. We would not use benchmarks calculated from performance periods that are older than 3 performance periods prior. We believe it is important to continue using the most up-to-date Start Printed Page 39432 data to drive clinical quality improvement. We believe that this policy will reduce burden to clinicians and allow them to continue to have performance targets in the event that a measure is suppressed.
We invite public comments on our intent to use performance period benchmarks for the CY 2022 performance period and to expand the baseline period to 3 performance periods prior for measures that are suppressed two performance periods prior. Table 47 reflects a summary of the benchmarking hierarchy as a result of this proposal.

(iii) Assigning Quality Measure Achievement Points
We refer readers to § 414.1380(b)(1)(i) for more details on our policies for scoring performance on quality measures (81 FR 77276 through 77307, 82 FR 53694 through 53701, 83 FR 59841 through 59856, 84 FR 63011 through 63019, and 85 FR 84906 through 84907).
(A) Scoring Measures Based on Achievement
We previously established at § 414.1380(b)(1)(i) a global 3-point floor for each scored quality measure, as well as each administrative claims measure. MIPS eligible clinicians receive between 3 and 10 measure achievement points for each submitted measure that can be reliably scored against a benchmark, which requires meeting the case minimum and data completeness requirements. In the CY 2017 Quality Payment Program final rule (81 FR 77282), we established that measures with a benchmark based on the performance period (rather than on the baseline period) would continue to receive between 3 and 10 measure achievement points for performance periods after the first transition year. For measures with benchmarks based on the baseline period, we stated that we would revisit the 3-point floor in future years. For the 2021 performance period/2023 MIPS payment year, we maintained the application of the 3-point floor for each measure that can be reliably scored against a benchmark.
For the 2022 performance period/2024 MIPS payment year, we propose to remove 3-point floor for each measure that can be reliably scored against the benchmark and score the measure from 1 to 10 points. We are scoring from 1-10 for clinicians with a performance rate greater than 0% for non-inverse measures or lesser than 100 percent for inverse measures in accordance with our original intent outlined in the CY 2017 Quality Payment Program final rule (81 FR 77282). As we move towards the MVP framework discussed the CY 2021 PFS final rule (85 FR 84904), we are moving towards a simplified scoring standard in which we can score quality measures from 1 to 10 for measures in MVPs and as such will amend the MIPS program to begin promoting this alignment. As a result, we previously discussed that we would wait until there is further policy development under the MVP framework before proposing to remove the 3-point floor (85 FR 84904). However, with the delay in the implementation of MVPs, we will begin the transition to MVPs by removing policies established on the transition years of MIPS. We have signaled through rulemaking for several years that we would revisit policies established during the transition years of the program from the legacy programs to MIPS. As the legacy programs have been sunsetted for many years now, we believe that it is appropriate to transition the MIPS program to its mature state. Additionally, we believe that transitioning MIPS to its mature state will serve as a transition period to allow clinicians to adjust to the simplified scoring standard that we will be using in MVPs.
Accordingly, we propose to revise § 414.1380(b)(1)(i) to add beginning the 2022 performance period/2024 MIPS payment year, MIPS eligible clinicians will receive between 1 and 10 measure achievement points (including partial points) for each measure required under § 414.1335 on which data is submitted in accordance with § 414.1325 that has a benchmark at paragraph (b)(1)(ii) of this section, meets the case minimum requirement at paragraph (b)(1)(iii) of this section, and meets the data completeness requirement at § 414.1340.
We invite public comment on our proposal to remove the 3-point floor for each measure that can be reliably scored against a benchmark for the 2022 performance period/2024 MIPS payment year. Start Printed Page 39433
(B) Scoring Measures That Do Not Meet Case Minimum, Data Completeness, and Benchmark Requirements
We refer readers to § 414.1380(b)(1)(i)(A) and (B) for more on our scoring policies for a measure that is submitted but is unable to be scored because it does not meet the required case minimum, does not have a benchmark, or does not meet the data completeness requirement (84 FR 63012).
In the 2017 QPP final rule (81 FR 77288) and the 2018 QPP final rule (82 FR 53727), we identified "classes of measures" which were intended to characterize measures for the ease of discussion. Class 1 measures are measures that can be scored based on performance because they have a benchmark, meet the case minimum and data completeness requirements. Class 2 measures are measures that cannot be scored based on performance because they do not have a benchmark or do not meet the case minimum which is generally 20 cases. Class 3 measures are measures that do not meet the data completeness requirement. We also noted that policies for Class 2 and Class 3 measures would not apply to measures submitted with the CMS Web Interface or administrative claims-based measures.
We propose to modify how we score these measures within MIPS, as we consider policies for transitioning to MVPs. For class 2 measures, for the 2022 performance period/2024 MIPS payment year, we propose to remove special scoring policies for measures that meet the data completeness requirement but do not have a benchmark, due to fewer than 20 individual clinicians or groups adequately reporting the measure, or meet the case minimum requirement except in the case of small practices, for which we will maintain the 3-point floor. Practices of other sizes will now receive zero points.
As we move to MVPs, we are seeking to simplify scoring by removing transition policies. To encourage complete and meaningful reporting, we cannot continue to award measure achievement points for measures that cannot be reliably scored against a benchmark. In a quality measurement program such as MIPS, it is imperative that we are able to reliably measure performance on clinical quality measures to achieve the goals of the program and incentivize clinicians that are providing the high-quality care. However, we remain concerned that it may be harder to reach case minimums for smaller practices and as such are maintaining the three-point floor policy for small practices. We believe that measure selection that is better reflective of the care most commonly provided in a practice will increase the value of the program by allowing us to score for achievement in clinical quality and subsequently drive changes in performance on clinical quality measures.
Measure benchmarks are posted in advance of the MIPS performance period and can be used to drive clinician measure selection. While not all measures relevant to a clinician's practice may have a historic benchmark posted, clinicians have the option to make informed decisions about their participation in the MIPS program. Additionally, we believe that while clinicians do not have control over the case volume and mix that their practices treat, under normal circumstances, they can anticipate which measures would be most reflective of the care generally provided by said practices. Scoring 3 points for class 2 measures was established as a policy during the transition years of the program, when the performance threshold was 3 points and scoring 3 points for quality would help clinicians transitioning to the program without receiving scores for poor performance. The intent of the policy was to provide time for clinicians to acclimate to the scoring standard before being held fully accountable for their participation in the program (81 FR 77287). As we believe we have provided sufficient time for clinicians to acclimate to our reporting requirements and scoring practices, we believe that we can hold clinicians accountable for their meaningful participation in the program.
We remain concerned about that case minimums and the availability of a benchmark are in some instances out of the control of the clinician, but believe that clinician choice of measure allows us to move forward with this option. We note that we also have policies in place to account for the calculation of a performance period benchmark if a historical benchmark is unavailable. Simulations of the effects of this policy show minimal effects of removing the 3-point floor as described above, with only a 0.4 point decrease in median quality performance category score and 0.2 point decrease in mean performance quality performance category score when compared to a baseline score that maintains the previously finalized class 2 measure scoring policy (section VII.F.18 of this proposed rule). We considered other approaches to address this issue such as a denominator reduction for class 2 measures; however, we remain concerned over the potential for gaming under this approach, such as where a clinician only selects class 2 measures resulting in their quality performance category score being reweighted.
We do not want this policy to discourage the reporting of new measures in the program because they may lack a benchmark or fail to meet the case minimum requirement. For this reason, we are proposing a 5-point floor for new measures in the program for all collection types for their first 2 years in the program, as well as a new class for these measures. Measures in either their first or second performance period in the program will be classified as class 4 measures. Measures that can be reliably scored against a benchmark because they meet data completeness requirement can have a performance period benchmark calculated, and meet case minimum requirements will be considered class 4a and scored from 5 to 10 measure achievement points. In the event that a measure cannot be reliably scored against a benchmark because it lacks a benchmark or does not meet case minimum, but meets the data completeness requirement, it will be considered class 4b and receive a score of 5. Measures that cannot be scored because they do not meet the data completeness requirement will remain subject to the class 3 measure policy and receive a score of 0 for clinicians other than small practices, while small practices will continue to receive 3 points.
We believe that a 5-point floor for new measures will allow us to introduce new, applicable measures into the program, by reducing the burden to clinicians in selecting them. We believe that this policy is responsive to stakeholder concerns that new measures run the risk of lacking enough data to have a benchmark and clinicians earning only 3 points according to the current policy on measures that cannot be reliably scored against a benchmark. We believe that the possibility of earning 5 points for a measure that lacks a benchmark would not unfairly penalize clinicians who report on new measures. Additionally, clinicians have the possibility of earning up to 10 points with the lowest possible score of 5. The 5-point floor will only be in place for a measure's first 2 years in the program to ease their transition, and will be scored in accordance with policies for standard class 1, 2, and 3 measures thereafter, to maintain a simplified scoring methodology. In the CY 2017 Quality Payment Program final rule, we received comments of support on our proposal to provide a scoring floor for new Start Printed Page 39434 measures citing that a scoring floor for new measures would encourage clinicians to report new measures and prevent gaming. Commenters were also supportive other approaches to reducing the burden of reporting new measures such as reducing the weight of new measures in the performance category score (81 FR 77281).
Accordingly, we propose to revise § 414.1380(b)(1)(i)(A)(1) to provide that except as provided in paragraph (b)(1)(i)(A)(2) and (3) of this section, for the 2017 through 2021 CY performance periods/2019 through 2023 MIPS payment years, MIPS eligible clinicians receive 3 measure achievement points for each submitted measure that meets the data completeness requirement, but does not have a benchmark or meet the case minimum requirement. Beginning within the CY 2022 performance period/2024 MIPS payment year, MIPS eligible clinicians other than small practices receive 0 measure achievement points for each such submitted measures and small practices receive 3 measure achievement points.
We also propose to add the policy for new measures at § 414.1380(b)(1)(i)(C)(1) to provide that beginning with the CY 2022 performance period/2024 MIPS payment year, MIPS eligible clinicians receive between 5 and 10 measure achievement points (including partial points) for each measure required under § 414.1335 on which data is submitted in accordance with § 414.1325 that has a benchmark at paragraph (b)(1)(ii) of this section, meets the case minimum requirement at paragraph (b)(1)(iii) of this section, and meets the data completeness requirement at § 414.1340. We also propose to add a policy at § 414.1380(b)(1)(i)(C)(2) to provide that for purposes of this section "new measures" means a quality measure this is in its first 2 years in the program.
We invite public comments on our proposal to modify the special scoring policies for measures that meet the data completeness requirement but do not have a benchmark or meet the case minimum requirement for the MIPS 2024 payment year and to introduce a 5-point scoring floor for new measures in for their first 2 years in the program. Table 48 reflects a summary of our proposed policies.

Start Printed Page 39435
(iv) Scoring for MIPS Eligible Clinicians That Do Not Meet Quality Performance Category Criteria
In the CY 2018 Quality Payment Program final rule (82 FR 53732), we finalized that, beginning with the 2019 performance period/2021 MIPS payment year, we will validate the availability and applicability of quality measures only with respect to the collection type that a MIPS eligible clinician utilizes for the quality performance category for a performance period, and only if a MIPS eligible clinician collects via claims only, MIPS CQMs only, or a combination of MIPS CQMs and claims collection types. We will not apply the validation process to any data collection type that the MIPS eligible clinician does not utilize for the quality performance category for the performance period. We sought comment on how to modify the validation process for the 2019 performance period/2021 MIPS payment year when clinicians may submit measures collected via multiple collection types.
In the CY 2019 PFS final rule (83 FR 59847), we finalized a proposal to modify our validation process to provide that it only applies to MIPS CQMs and the claims collection type, regardless of the submitter type chosen. For example, this policy would not apply to eCQMs even if they are submitted by a registry. We noted that a MIPS eligible clinician may not have available and applicable quality measures. If we are unable to score the quality performance category, then we may reweight the clinician's score according to the reweighting policies.
In this year's rule, we are proposing to modify our validation process to provide that it only applies to MIPS CQMs or the Part B claims collection type, but it will not apply in instances when a MIPS eligible clinician submits for both collection types. Currently, very few reporters report for a combination of Part B claims and MIPS CQMs. In CY 2020, of submissions with less than 6 measures, only 0.08 percent of individual submissions and 0.3 percent of group submissions included a combination of Part B claims and other submission types. In CY 2019, of submissions with less than 6 measures, only 0.094 percent of individual submissions and 0.07 percent of group submissions included a combination of Part B claims and other submission types. We see that reporting using both collection types is an option very few clinicians have chosen to utilize, and consequently believe that the associated costs for validation of a combination of the both the Part B claims based and MIPS CQM collection types is too great in these instances. To align with this reality that very few clinicians submit data in this manner, we are proposing to update our policy.
We invite public comments on our proposal to modify our validation process to provide that it only applies to MIPS CQMs or the claims collection type and not a combination of the two.
(v) Assigning Measure Achievement Points for Topped Out Measures
We refer readers to § 414.1380(b)(1)(iv) for our previously finalized policies regarding the identification of topped out measures and § 414.1380(b)(1)(iv)(B) for our finalized policies regarding the scoring of topped out measures. Under § 414.1380(b)(1)(iv), we will identify topped out measures in the benchmarks published for each Quality Payment Program year. Under § 414.1380(b)(1)(iv)(B), beginning with the 2021 MIPS payment year, measure benchmarks (except for measures in the CMS Web Interface) that are identified as topped out for 2 or more consecutive years will receive a maximum of 7 measure achievement points beginning in the second year the measure is identified as topped out (82 FR 53726 through 53727).
We noted in the CY 2021 PFS proposed rule (85 FR 50307) that we intended to use performance period benchmarks for the 2021 performance period/2021 MIPS payment year, which would mean we would not be able to publish measures that are topped out prior to the 2021 MIPS performance period. As discussed in that proposed rule (85 FR 50309), this also means we would not be able to identify those that have been topped-out for 2 or more consecutive years for purposes of the topped out scoring of 7 measure achievement points. As we are intending to use performance period benchmarks again for the 2022 performance period/2024 MIPS payment year, these problems would occur again. We believe it is still important to retain a topped-out scoring cap of 7 measure achievement points so that clinicians have incentives to pick alternate measures that are not topped out. We also believe that if a measure is not topped out in the 2022 performance period benchmark, then it should have the ability to achieve up to 10 measure achievement points.
In this year's proposed rule, we are proposing to use performance period benchmarks again and subsequently need to the topped out measure lifecycle to account for this. Therefore, in the event that we finalize the use of performance period benchmarks for the CY 2022 performance period/2024 MIPS payment year, as an exception from the general rule at § 414.1380(b)(1)(iv)(B), we propose at paragraph (b)(1)(iv)(B)(1) to provide that for the CY 2022 MIPS performance period/2024 MIPS payment year, MIPS eligible clinicians receive no more than 7 measure achievement points for each measure (except for measures in the CMS Web Interface) for which the applicable benchmark is identified as topped out for 2 or more consecutive years based on the historical benchmarks published for the CY 2021 MIPS performance period and continues to be identified as topped out based on the performance period benchmarks published for the CY 2022 MIPS performance period/2024 MIPS payment year.
We believe these two criteria collectively would provide clinicians the information to know prior to the CY 2022 performance period/2024 MIPS payment year which measures would have the topped-out scoring applied but would also account for the scenario where a measure is no longer topped out. We would not limit the number of measure achievement points for measures that have not been topped out for at least 2 years as published in the 2021 MIPS performance period/2023 MIPS payment year historical benchmarks.
We invite public comments on our proposal to apply the 7 measures achievement point cap to measures that meet the two criteria.
(vi) Minimum Case Requirements
We refer readers to § 414.1380(b)(1)(iii) for our previous finalized policies regarding case minimum requirements for the quality performance category, which establishes the case minimum requirement threshold at 20 cases for quality measures, with the exception of administrative claims-based measures in the MIPS final list of quality measures described in § 414.1330(a)(1). As more measures are introduced under MIPS, there may be measures, similar to the administrative claims-based measures, that warrant a different case minimum requirement from the other quality measures. In Table Group A of Appendix 1 of this proposed rule, we are proposing to add the following quality measure starting with the 2022 MIPS performance period: Person-Centered Primary Care Measure Patient Reported Outcome Performance Measure (PCPCM PRO-PM), which uses a comprehensive and parsimonious set Start Printed Page 39436 of 11 patient-reported items to assess the broad scope of primary care. In order to achieve the appropriate reliability of the measure—the survey results (adequate number of surveys to provide an accurate representation of the MIPS eligible clinician, group, subgroup, virtual group, and APM Entity), for each MIPS eligible clinician, group, subgroup, virtual group, and APM Entity, a minimum of 30 PCPCM PROM instruments per clinician are needed for submission of this measure. For MIPS eligible groups, subgroups, virtual groups, and APM Entities with 5 or more clinicians, a minimum of 150 PCPCM PROM instruments per TIN for each site/location associated with the clinicians' part of the group, subgroups, virtual groups, and APM Entities are needed for submission of this measure. For TINs with a composition of multiple specialty practices at one site/location, a minimum of 150 PCPCM PROM instruments per specialty practice within a TIN are needed for submission of this measure. If the MIPS eligible group, subgroup, virtual group, and APM Entity with 5 or more clinicians encompasses multiple sites/locations, each site/location would need to meet the PCPCM PROM instruments requirements as noted above. To allow for the reliable implementation of PCPCM PRO-PM under MIPS, such measure requires a case minimum that exceeds 20 cases.
With more measures being introduced under MIPS that warrant differing case minimum requirements (other than administrative claims-based measures), we are proposing to modify our minimum case requirements policy that would broaden the type of measures (to not only include administrative claims-based measures) having an exception for meeting the minimum case requirement threshold of 20 cases and provide for a measure-specific case minimum requirement for measures identified and determined on a case-by-case basis by CMS. For each measure with a measure-specific case minimum, the minimum case requirement is specified in the annual list of MIPS measures. Specifically, we are proposing to amend § 414.1380(b)(1)(iii) to reflect that, except as otherwise specified in the MIPS final list of quality measures described in § 414.1330(a)(1), the minimum case requirement is 20 cases.
We seek public comment on our proposal to modify the minimum case requirement policy that expands the exception to meeting the minimum case requirement threshold of 20 cases to measures with measure-specific case minimum requirements identified and determined by CMS.
We request feedback on our proposal.
(vii) Incentives To Report High-Priority Measures
We refer readers to § 414.1380(b)(1)(v)(A) for our previously finalized policies regarding incentives to report high priority measures. In the CY 2017 Quality Payment Program final rule (81 FR 77293), we established the scoring policies for high priority measure bonus points to encourage the selection of additional high-priority and outcome measures that impact beneficiaries and were closely aligned to our measurement goals. In the CY 2019 PFS final rule (83 FR 59850), we discontinued awarding measure bonus points to CMS Web Interface reporters for reporting high priority measures since CMS Web Interface reporters have no choice in measures.
We stated in the CY 2019 PFS proposed and final rules (83 FR 35950, 59851) that as part of our move towards fully implementing high value measures, we believe that bonus points for high priority measures for all collection types may no longer be needed. We noted in the CY 2019 PFS final rule (83 FR 59851) that measure bonus points were created as transition policies which were not meant to continue through the life of the program. We believe with the finalized framework for transforming MIPS through MVPs (84 FR 62948), we will find ways in the future to emphasize high priority measures without needing to incentivize with bonus points.
In this proposed rule, we propose to end the high-priority measure bonus points. As we move to MVPs we are simplifying our scoring by ending transition policies that were established in the initial years of the program. We have signaled for many years now that this policy was temporary and would be removed from the program. As we transition to a new state, we anticipate that there will no longer be a need to incentivize high-priority measures by usage of bonus points in MIPS and MVPs as we increase the share of high-priority measures in the program.
We propose to end measure points for reporting high priority measures beginning the 2022 performance period/2024 MIPS payment year. Accordingly, we propose to revise § 414.1380(b)(1)(v)(A) to provide that for the 2017 through 2021 performance periods/2019 through 2023 MIPS payment years, MIPS eligible clinicians receive 2 measure bonus points for each outcome and patient experience measure and 1 measure bonus point for each other high priority measure. Beginning with the 2019 performance period/2021 MIPS payment year, MIPS eligible clinicians do not receive such measure bonus points for CMS Web Interface measures. Beginning in the 2022 performance period/2024 MIPS payment year, MIPS eligible clinicians will no longer receive measure bonus points.
We invite public comments on our proposal to end measure points for reporting high priority measures beginning the 2022 performance period/2024 MIPS payment year.
(viii) Incentives To Use CEHRT To Support Quality Performance Category Submissions
Section 1848(q)(5)(B)(ii)(I) of the Act requires the Secretary to encourage MIPS eligible clinicians to report on applicable quality measures through the use of CEHRT and qualified clinical data registries. Section 1848(q)(5)(B)(ii)(II) of the Act requires that the Secretary shall treat such clinicians as satisfying the clinical quality measures reporting requirement described in section 1848(o)(2)(A)(iii) of the Act if they report such measures through the use of such EHR technology for a given performance period. In the CY 2017 Quality Payment Program final rule (81 FR 77297), we established the measure bonus point and bonus cap for using CEHRT for end-to-end electronic reporting. We refer readers to § 414.1380(b)(1)(v)(B) for our previously finalized policies regarding measure bonus points for end-to-end electronic reporting. We believe that with the framework for transforming MIPS through MVPs discussed in the CY 2020 PFS proposed rule (84 FR 40739) and the CY 2021 PFS proposed rule (85 FR 50279 through 50285), we will find ways to encourage the use of CEHRT for reporting quality measures without needing to incentivize the use of CEHRT for end-to-end electronic reporting with bonus points. In the CY 2018 Quality Payment Program final rule (82 FR 53636), we encouraged stakeholders to consider electronically specifying their quality measures as eCQMs, to encourage clinicians and groups to move towards the utilization of electronic reporting. As noted in the CY 2019 PFS final rule (83 FR 59851), bonus points were created as transition policies which were not meant to continue through the life of the program.
In this proposed rule, we propose to end measure bonus points for end-to-end electronic reporting. As we move to MVPs we are simplifying scoring by removing many of the transition policies that we established in the early years of the program. We believe it is imperative Start Printed Page 39437 to remove transition policies in order to develop a stronger MVP program and promote alignment between MIPS and MVPs. As stated in previous rulemaking, we are working to develop ways to encourage the use of CEHRT for electronic reporting without offering measure bonus points. Measure bonus points act to introduce scoring inflation into the program and preclude our ability to accurately compare clinicians' clinical quality. As the program works to focus on the quality of care provided to beneficiaries, we intend to score for performance on measures and not for reporting. Additionally, we believe that we can fulfill the statutory requirement at sections 1848(q)(5)(B)(ii)(I) of the Act to encourage the usage of certified electronic health record technology (CEHRT), through other means. As a program and across the Department of Health and Human Services, we are working towards the goal of all measures within MIPS being digital by 2025 through a movement towards the inclusion of more digital quality measures (dQMs), emphasizing approaches to electronic data sharing that utilize the FHIR standard. As part of these approaches we intend to further leverage the use of electronic reporting from CEHRT for all quality measurement completed in the MIPS program including measurement and reporting facilitated by third party intermediaries such as QCDRs and plan to further address this issue through rulemaking in future years. We are also giving preference to dQMs including eCQMs in our annual call for measures. More information on dQMs and the FHIR standard is available in section IV.A. of this proposed rule, information on the call for measures and CMS measurement needs and priorities is available at https://qpp-cm-prod-content.s3.amazonaws.com/uploads/1313/2021%20Call%20for%20Measures%20and%20Activities%20Toolkit.zip, and https://www.cms.gov/files/document/cms-measurement-priorities-and-needs.pdf, respectively. Furthermore, over the past few years, we have reduced the availability and limited who can submit data for the Medicare Part B claims collection type to only small practices. We note that the Medicare Part B claims collection type is not an electronic means of submission. We invited comments on other methods we can use to encourage the use of CEHRT for electronic reporting.
Accordingly, we proposed to add the policy at § 414.1380(b)(1)(v)(B) to provide that for the 2017 through 2021 performance period/2019 through 2023 MIPS payment years, MIPS eligible clinicians receive 1 measure bonus point for each measure (except claims-based measures) submitted with end-to-end electronic reporting for a quality measure under certain criteria determined by the Secretary. Beginning in the 2022 performance period/2024 MIPS payment year, MIPS eligible clinicians will no longer receive measure bonus points for submitting using end-to-end electronic reporting.
We invite public comments on our proposal to end measure bonus points for end-to-end electronic reporting beginning in the 2022 performance period/2024 MIPS payment year.
(ix) Improvement Scoring for the MIPS Quality Performance Category Score
We refer readers to § 414.1380(b)(1)(vi)(C)(4) for more on our policy stating that for the 2020 through 2023 MIPS payment years, for the purpose of improvement scoring, we will assume a quality performance category achievement percent score of 30 percent in the previous year if a MIPS eligible clinician earned a quality performance category score less than or equal to 30 percent in the previous year.
In this proposed rule, we propose to continue our previously established policy for improvement scoring for the 2022 performance period/2024 MIPS payment year and to revise § 414.1380(b)(1)(vi)(C)(4) to remove the phrase "2020 through 2023 MIPS payment years" and adding in its place the phrase "beginning the 2018 performance period/2020 MIPS payment year" to indicate that for each MIPS payment year after 2020, we will assume a quality performance category achievement percent score of 30 percent in the previous year if a MIPS eligible clinician earned a quality performance category score less than or equal to 30 percent in the previous year. Specifically, for the 2022 performance period/2024 MIPS payment year, we would compare the MIPS eligible clinician's quality performance category achievement percent score for the 2022 performance period/2024 MIPS payment year to an assumed quality performance category achievement percent score of 30 percent if the MIPS eligible clinician earned a quality performance score less than or equal to 30 percent for the 2021 performance period/2023 MIPS payment year.
We invite public comments on our proposal to assume a quality performance category achievement percent score of 30 percent in the previous year if a MIPS eligible clinician earned a quality performance category score less than or equal to 30 percent in the previous year for the 2022 performance period/2024 MIPS payment year.
(d) Cost Performance Category
(i) Scoring Flexibility for Changes That Impact Cost Measures During the Performance Period
We refer readers to § 414.1380(b)(2) for our policies regarding scoring for the cost performance category and to previous rules where these policies were finalized, including the CY 2017 Quality Payment Program final rule (81 FR 77308 through 77311), the CY 2018 Quality Payment Program final rule (82 FR 53748 through 53752), the CY 2019 PFS final rule (83 FR 59856), and the CY 2021 PFS final rule (85 FR 84877 through 84880). In § 414.1380(b)(2)(v), we finalized that a cost performance category score is not calculated if a MIPS eligible clinician or group is not attributed any cost measures for the performance period because the clinician or group has not met the minimum case volume specified by CMS for any of the cost measures or a benchmark has not been created for any of the cost measures that would otherwise be attributed to the clinician or group.
We have identified that there is a need for additional flexibility in calculating the scores for cost measures to account for the impact of changing conditions that are beyond the control of MIPS eligible clinicians and groups. This flexibility would allow us to ensure that clinicians are not impacted negatively when performance is affected not due to the care provided, but due to external factors. Thus, beginning with the 2022 MIPS performance period/2024 MIPS payment year, we propose a policy to provide scoring flexibility in instances where changes during a performance period impede the effective measurement of resource use. We believe that there may be instances when there are rapid or unprecedented changes to the delivery of healthcare services that are reflected in administrative claims data used to calculate cost measures. An example of the type of external factor that could affect cost measurement is the PHE for COVID-19. We refer readers to section IV.A.3.e.(2)(b)(ii)(A) of this proposed rule, where we summarize our assessment of the impact of COVID-19 on the calculation of cost measure scores for the 2020 performance period. In this discussion, we conclude that due to the unique and rapid changes to healthcare in the COVID-19 pandemic, which significantly affected service utilization and the underlying data used Start Printed Page 39438 to calculate cost measures, we cannot reliably calculate scores for the cost measures that adequately capture and reflect clinician performance for 2020. This example demonstrates that the assessment of costs could be impeded by substantial changes to clinical practice and service utilization, and result in misleading or inaccurate results.
We will determine whether such external changes impede the effective measurement of resource use by considering factors including: The extent and duration of the changes, and the conceptual and empirically tested relationship between the changes and each measure's ability to accurately capture clinician cost performance. Empirical testing could include assessing whether there are rapid or unprecedented changes to patient case volume or case mix, and the extent to which this could lead to misleading or inaccurate results. We note that substantial changes in service utilization may not impede our ability to accurately calculate a cost measure score. Consider for example the case of a hypothetical measure that includes the cost of a series of procedures to treat the condition being measured. Due to advances in clinical knowledge, the procedures are declared to no longer be appropriate to treat the condition during a performance period. This would hypothetically lead to rapid and substantial changes in the services captured in the measure in that performance period; however, these changes would not necessarily lead to misleading or inaccurate results as the measure would be appropriately capturing the costs of care for that condition and appropriately reflecting changes in clinician practice. We would assess this empirically for each measure, as we believe that even widespread external changes to service utilization could have a range of different impacts across measures as they focus on diverse types of care. For example, a hypothetical national shortage of blood products may lead to the suspension of elective surgeries. This may not affect the care patterns for a particular outpatient screening or preventative care procedure, meaning that a hypothetical cost measure focused on that outpatient procedure would still accurately reflect clinician cost performance.
While we can monitor the impact of these changes during the performance period, we note that there is an inherent delay between the time we can identify trends in administrative claims data and when we can test for potential impact on the cost measures, due to the claims run-out period necessary to ensure that data included in our analyses reflect final claims for all services in episodes. To ensure sufficient case volume, we expect that we would not be able to properly test the full impact of external changes on the cost measures until after the end of the performance period in which the changes occurred. Thus, we would not be able to determine whether a cost measure should be suppressed due to changes in the performance period until after the completion of the performance period. To allow sufficient time for claims data query and investigatory work to test for potential impacts on cost measure calculations, we expect that we would reach a conclusion as to whether a cost measure should be suppressed and notify clinicians affected by the suppression no later than when performance feedback is issued for the performance period. We believe this would not put undue burden on clinicians since cost measures are calculated by CMS using administrative claims data without any additional data submission by clinicians.
Accordingly, we propose to add § 414.1380(b)(2)(v)(A) to provide that beginning with the 2024 MIPS payment year, if data used to calculate a score for a cost measure are impacted by significant changes during the performance period, such that calculating the cost measure score would lead to misleading or inaccurate results, then the affected cost measure is excluded from the MIPS eligible clinician's or group's cost performance category score. For purposes of this paragraph (b)(2)(v)(A), "significant changes" are changes external to the care provided, and that CMS determines may lead to misleading or inaccurate results. Significant changes include, but are not limited to, rapid or unprecedented changes to service utilization, and will be empirically assessed by CMS to determine the extent to which the changes impact the calculation of a cost measure score that reflects clinician performance.
We seek comments on this proposal.
(e) Promoting Interoperability Performance Category
For our previously established policies regarding scoring the Promoting Interoperability performance category, we refer readers to § 414.1380(b)(4), the CY 2017 Quality Payment Program final rule (81 FR 77216 through 77227), the CY 2018 Quality Payment Program final rule (82 FR 53663 through 53670), the CY 2019 PFS final rule (83 FR 59785 through 59796), the CY 2020 PFS final rule (84 FR 63020) and the CY 2021 PFS final rule (85 FR 84893 through 84894). We also refer readers to § 414.1375 and the CY 2017 Quality Payment Program final rule (81 FR 77199 through 77245), the CY 2018 Quality Payment Program final rule (82 FR 53663 through 53688), the CY 2019 PFS final rule (83 FR 59785 through 59820), and the CY 2021 PFS final rule (85 FR 84886 through 84895 for our previously established policies regarding the Promoting Interoperability (formerly the advancing care information) performance category generally.
In the CY 2019 PFS final rule, we established a new performance-based scoring methodology for the Promoting Interoperability performance category that applies beginning with the 2019 performance period/2021 MIPS payment year (83 FR 59787 through 59796). The scoring methodology is codified at § 414.1380(b)(4)(ii); however, the regulation text refers only to the 2021 and 2022 MIPS payment years, and does not mention subsequent years. We are proposing to revise § 414.1380(b)(4)(ii) for consistency with the previously established policy, by indicating that the methodology applies beginning with the 2019 MIPS performance period/2021 MIPS payment year and continuing to subsequent years. We are proposing to revise § 414.1380(b)(4)(ii) to remove "for the 2021 and 2022 payment years" and add in its place "beginning with the 2019 performance period/2021 MIPS payment year" and remove the word "six" to provide that beginning with the 2019 performance period/2021 MIPS payment year, a MIPS eligible clinician's Promoting Interoperability performance category score equals the sum of the scores for each of the required measures and any applicable bonus scores, not to exceed 100 points.
In addition, for the 2020 performance period/2022 MIPS payment year, each optional measure in the Promoting Interoperability performance category was worth five bonus points (84 FR 63003), and for the 2021 performance period/2023 MIPS payment year, each optional measure was worth ten bonus points (85 FR 84894). In section IV.3.d.(4)(c)1. of this proposed rule, we are proposing that the Query of Prescription Drug Monitoring Program measure would be worth 10 bonus points, and in section IV.3.d.(4)(c)3 of this proposed rule, we are proposing that submission of a "yes" for the Public Health Registry Reporting measure or the Clinical Data Registry Reporting measure or the Syndromic Surveillance Reporting measure would be worth 5 bonus points. To reflect these policies, we are proposing a change to Start Printed Page 39439 § 414.1380(b)(4)(ii)(C) to reflect that the optional measures are worth five or ten bonus points, as specified by CMS.
We invite public comments on our proposal to make these revisions.
(2) Calculating the Final Score
For a description of the statutory basis and our policies for calculating the final score for each MIPS eligible clinician, we refer readers to § 414.1380(c) and the discussion in the CY 2017 and CY 2018 Quality Payment Program final rules, and the CY 2019, CY 2020, and CY 2021 PFS final rules (81 FR 77319 through 77329, 82 FR 53769 through 53785, 83 FR 59868 through 59878, 84 FR 63020 through 63031, 85 FR 84908 through 84917 respectively) on final score calculations, performance category weights, reweighting the performance categories, and the complex patient bonus.
As described in more detail in the following sections, we:
- Propose to continue doubling the complex patient bonus for the CY 2021 MIPS performance period/CY 2023 MIPS payment year, as was previously finalized with a cap of 10 bonus points for the CY 2020 MIPS performance period/CY 2022 MIPS payment year (85 FR 50311).
- Propose to revise the complex patient bonus formula, which currently provides a bonus to all MIPS eligible clinicians, groups, virtual groups, and APM entities who submit data for at least one MIPS performance category, so that beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year the complex patient bonus better target clinicians who treat a higher caseload of more complex and high-risk patients.
- Request comment on potential circumstances where we may not be able to reliably calculate a score for any of the cost measures as described under § 414.1380(c)(2)(i)(A)(2). Additionally, we note that we are reweighting the cost performance category for the CY 2020 MIPS performance period/CY 2022 MIPS payment year because we have concluded there are not sufficient measures and activities applicable and available for us to score any clinicians on performance due to the national PHE for COVID-19.
- Propose to continue performance category weight redistribution policies finalized for the CY 2022 MIPS performance period/CY 2024 MIPS payment year.
- Propose policies for redistributing the weight of the performance categories for small practices.
- Clarify how our application-based and automatic extreme and uncontrollable circumstances policies intersect.
- Propose a new policy to determine the MIPS final score for clinicians and groups who are eligible for facility-based measurement.
(a) Complex Patient Bonus
(i) Background
Section 1848(q)(1)(G) of the Act requires us to consider risk factors in our MIPS scoring methodology. Specifically, it provides that the Secretary, on an ongoing basis, shall, as the Secretary determines appropriate and based on an individual's health status and other risk factors, assess appropriate adjustments to quality measures, cost measures, and other measures used under MIPS; and assess and implement appropriate adjustments to payment adjustments, final scores, scores for performance categories, or scores for measures or activities under MIPS. In doing so, the Secretary is required to take into account the relevant studies conducted under section 2(d) of the Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act) (Pub. L. 113-185, October 6, 2014) and, as appropriate, other information, including information collected before completion of such studies and recommendations. In the CY 2018 Quality Payment Program final rule, under the authority in section 1848(q)(1)(G) of the Act, we established at § 414.1380(c)(3) a complex patient bonus of up to 5 points to be added to the final score for the 2020 MIPS payment year (82 FR 53771 through 53776). In subsequent rulemaking, we continued the complex patient bonus at § 414.1380(c)(3) for the 2021, 2022, and 2023 MIPS payment years (83 FR 59870, 84 FR 63023, and 85 FR 84910, respectively). Additionally, we finalized for the 2022 MIPS payment year at § 414.1380(c)(3)(iv) that the complex patient bonus will be calculated under the existing formulas in paragraphs (c)(3)(i) and (ii), and the resulting numerical value will then be multiplied by 2 (85 FR 84911 through 84913). We refer readers to these final rules for additional details on the background, statutory authority, policy rationale, and previously finalized calculation of the complex patient bonus.
We intended for this bonus to serve as a short-term strategy to address the impact patient complexity may have on MIPS scoring while we continue to work with stakeholders on methods to account for patient risk factors. The overall goal, when considering a bonus for complex patients, is two-fold: (1) To protect access to care for complex patients and provide them with excellent care; and (2) to avoid placing MIPS eligible clinicians who care for complex patients at a potential disadvantage while we review the completed studies and research to address the underlying issues. We used the term "patient complexity" to consider a multitude of factors that describe and have an impact on patient health outcomes; such factors include the health status and medical conditions of patients, and social risk factors. We believe as the number and intensity of these factors increase for a single patient, the patient may require more services, more clinician focus, and more resources to achieve health outcomes similar to those who have fewer factors. In developing the policy for the complex patient bonus, we assessed whether there was a MIPS performance discrepancy by patient complexity using two well-established indicators in the Medicare program: Medical risk as measured through Hierarchical Condition Category (HCC) risk scores, and social risk as measured through the proportion of patients that is dually eligible for Medicare and Medicaid (82 FR 53771 through 53776).
(ii) Complex Patient Bonus for the CY 2021 MIPS Performance Period/CY 2023 MIPS Payment Year
In this section of the proposed rule, we are proposing to modify the complex patient bonus for the CY 2021 MIPS performance period/CY 2023 MIPS payment year in response to the PHE for COVID-19. In the CY 2020 PFS final rule, we finalized a policy to continue the complex patient bonus for the CY 2020 performance period/CY 2022 MIPS payment year (84 FR 63021 through 63023). However, due to the national PHE for COVID-19 during performance period 2020, we noted in the CY 2021 PFS proposed rule that we need to re-evaluate the previously established policy for the complex patient bonus for the CY 2020 MIPS performance period/2022 MIPS payment year (85 FR 50311). We refer readers to the CY 2021 PFS proposed rule (85 FR 50311 through 50313) for further details on how the PHE for COVID-19 impacts care delivery both directly and indirectly. We acknowledged there are direct effects of COVID-19 for those patients who tested positive for SARS-CoV-2 and indirect effects of COVID-19 for other patients whose care was impacted because of the PHE, including increased complexity and barriers such as postponing care, accessing care in a different way (for example, via telecommunications), and disruptions to Start Printed Page 39440 lab results and medications, which are not accounted for in our existing final score calculations using these complexity indicators.
Considering both these direct and indirect effects, we finalized § 414.1380(c)(3)(iv) (85 FR 84913), under which the complex patient bonus is calculated for the CY 2022 MIPS payment year pursuant to the existing formulas in paragraphs (c)(3)(i) and (ii), and the resulting numerical value is then multiplied by 2 but cannot exceed 10.0. The doubled numerical value (subject to the 10-point cap) is added to the final score.
We believe we have made significant progress against COVID-19. As noted by the Centers for Disease Control and Prevention (CDC), the number of COVID-19 cases are predicted to decrease through the week of July 17th, 2021 in the United States and territories.[235] As of June 1, 2021, the number of daily new cases in the United States is at a similar level compared to the number of daily new cases during this time last year.[236] While we are encouraged by these results, we believe that there may be some residual direct and indirect effects from the PHE that could affect the CY 2021 MIPS performance period/CY 2023 MIPS payment year. In particular, for the first quarter, we had high COVID cases in multiple regions.[237] Additionally, we cannot quantify the effect of beneficiaries who delayed care because of the PHE and are now seeking care.
Because of the concerns of the direct and indirect effects of the COVID-19 PHE, we propose to continue doubling the complex patient bonus as described at § 414.1380(c)(3)(iv) for the CY 2023 MIPS payment year, and we propose corresponding revisions to the regulation text at § 414.1380(c)(3)(iv). The doubled numerical value (subject to the 10-point cap) would be added to the final score. Since the COVID-19 cases and deaths have decreased throughout the year, we considered applying a smaller multiplier (like 1.5 for a cap of up to 7.5 points) or to not apply a multiplier at all. However, we believe doubling the complex patient bonus is appropriate given the continuation of the national PHE for COVID-19 into the 2021 MIPS performance period, the potential direct and indirect effects of COVID-19 on care delivery and care postponement, and additional uncertainties. We seek comment on this proposal and whether a different multiplier should be applied as we plan to consider the options based on public comment.
(iii) Complex Patient Bonus Beginning With the CY 2022 MIPS Performance Period/CY 2024 MIPS Payment Year
(A) Complex Patient Bonus Background and Analysis
As discussed in the CY 2021 PFS final rule (85 FR 84908), we intended the complex patient bonus as a short-term solution to address the impact patient complexity may have on MIPS scoring. However, during the development of the CY 2021 PFS final rule, we did not have sufficient information available to develop a long-term solution to account for patient risk factors in MIPS that we could include as a finalized policy for the CY 2021 MIPS performance period/CY 2023 MIPS payment year. In the CY 2020 PFS proposed and final rules, we considered whether newly available data from the Quality Payment Program still supported the complex patient bonus at the final score level (84 FR 40793 through 40795). More specifically, within the data analysis, we did not observe a consistent linear relationship for any reporting type or complexity measure, HCC risk score or dual proportion (84 FR 63021 through 63023). However, we only had a few years of data and believed more recent data may bring different results than the findings we explained in detail in the CY 2020 PFS final rule. We refer readers to the CY 2020 PFS final rule for further details on the methodology and findings (84 FR 63021 through 63023).
Further, section 1848(q)(1)(G) of the Act requires us to consider the relevant studies conducted under section 2(d) of the IMPACT Act and, as appropriate, other information, including information collected before completion of such studies and recommendations. The HHS Assistant Secretary for Planning and Evaluation (ASPE) completed its first report in December 2016, which examined the effect of individuals' socioeconomic status on quality, resource use, and other measures under the Medicare program, and included analyses of the effects of Medicare's current value-based payment programs on clinicians serving socially at-risk beneficiaries and simulations of potential policy options to address these issues. ASPE's second report, Social Risk and Performance in Medicare's Value-Based Purchasing Programs, was -released in June 2020, which builds on the analyses included in the initial report and provides additional insight for addressing risk factors in MIPS and other value-based payment programs.[238] More specifically, the report has a 3-pronged strategy approach to: Measure and report quality; set high, fair quality standards; and reward and support better outcomes for beneficiaries with social risk. As a part of this 3-pronged strategy, the report supports use of the complex patient bonus in MIPS, explaining that it is well supported because this policy gives additional points to clinicians with a higher share of medically and socially complex patients and does not lower the standard of care. Further, the report suggested that we should not include the complex patient bonus within the final score that is publicly reported to ensure that patients can see the true clinician performance. Based on the ASPE report's suggestion, in the future we expect to also publicly report the final score without the complex patient bonus included. We noted in the CY 2021 PFS final rule (85 FR 84909) that as we continue to review the findings from the report, we intend to consider its recommendations, along with any updated data that would become available, for future rulemaking. In the CY 2021 PFS final rule (85 FR 84909), we also noted we plan to continue working with ASPE, the public, and other key stakeholders on this important issue to identify longer term policy solutions that achieve the goals of attaining health equity for all beneficiaries, and minimizing unintended consequences, and would propose modifications to the complex patient bonus in future rulemaking as appropriate.
Under § 414.1380(c)(3), the complex patient bonus is calculated as follows. For MIPS eligible clinicians and groups: [The average HCC risk score assigned to beneficiaries (under the HCC risk adjustment model established by CMS under section 1853(a)(1) of the Act) seen by the MIPS eligible clinician or seen by clinicians in a group] + [the dual eligible ratio × 5]. For APM entities and virtual groups: [The beneficiary weighted average HCC risk score for all MIPS eligible clinicians, and if technically feasible, TINs for models and virtual groups which rely on complete TIN participation within the APM entity or virtual group, respectively] + [the average dual eligible ratio for all MIPS eligible clinicians, and if technically feasible, TINs for models and virtual groups which rely on complete TIN participation, within the Start Printed Page 39441 APM entity or virtual group, respectively, × 5].
For this proposed rule, we continue to explore alternative calculation methodologies to modify the complex patient bonus formula based on several factors including stakeholder feedback, updated data analysis, and implications from ASPE reports to Congress. We also reviewed a series of literature recently published utilizing the 2017 MIPS performance period data. The Johnston KJ, Hockenberry JM et al. article[239] found that the current complex patient bonus formula appears unlikely to mitigate the most regressive effects of MIPS. The article stated this is particularly concerning for future years in MIPS as the performance threshold increases over time. Additionally, the Johnston KJ, Wiemken TL et al. article[240] indicated that lower composite MIPS scores in the higher-risk quintile reflected lower scores in each of the performance categories rather than one specific performance category. Essentially, both articles concluded that in the first year of the Medicare MIPS program, clinicians with the highest proportion of patients dually eligible for Medicare and Medicaid had significantly lower MIPS scores compared with clinicians with the lowest proportion of dually eligible patients. Two other research studies we considered are related to how risk scores, specifically related to HCCs, differ between both rural and urban areas. The Tyler Malone, Denise Kirk et al. study[241] indicated that Medicare beneficiaries in rural counties have lower average CMS-HCC risk scores compared to urban counties despite previous research suggesting that rural populations are sicker than urban populations. The study also emphasized the differences in average CMS-HCC risk scores may be driven partially by differences in the intensity of types of health care interventions received by rural versus urban beneficiaries. However, further research is needed to account for differences in coding patterns and/or resource limitations between urban versus rural counties. The second study[242] found CMS-HCC risk scores provide utility in predicting which patients are expected to be more costly. However, the results also suggested there are systematic differences in the risk adjustment model's predictive capabilities in rural versus urban populations, as rural beneficiaries in the Tyler Malone, Denise Kirk et al. study generally had greater health care utilization even after controlling for risk scores. In conclusion, both studies suggested Medicare provider payment models using CMS-HCC risks scores may underestimate the costs associated with treatment of rural beneficiaries.
While ASPE's reports to Congress support the use of a complex patient bonus at the final score level, we also acknowledge the findings reported in the published literature by identifying ways to make the complex patient bonus more targeted for clinicians caring for high risk and complex patients and to mitigate differences in resources that affect MIPS scores. Once it became available, we reviewed the 2018 MIPS actual data to see if we could replicate the findings and identified some structural issues within the current complex patient bonus formula. We describe the findings below. We also note that the analyses reported in the Johnston KJ, Wiemken TL et al. studies include all MIPS eligible clinicians irrespective of whether they submitted data, while our data analyses with 2018 actual scored MIPS data were restricted to MIPS clinicians who submitted data. We restricted our analyses to MIPS eligible clinicians who submit MIPS data because under current policy as established at § 414.1380(c)(3), the MIPS eligible clinician must engage in, that is submit data for, at least one MIPS performance category for the applicable performance period for the MIPS payment year to receive the complex patient bonus. Our analysis of 2018 actual MIPS data was further limited to MIPS eligible clinicians scored as individuals or groups because the HCC and dual proportion individual data elements were not available for virtual groups and APM Entities at the time of our analysis.
For our updated analysis, we used actual 2018 MIPS scored data to evaluate whether the complex patient bonus sufficiently supports MIPS eligible clinicians scored as a group or individual who submit data and treat high risk and medically complex patients. We grouped the MIPS eligible clinicians into quintiles based on the complex patient bonus points they would receive. Quintiles were created by first ranking the actual complex patient bonus scores from lowest to highest for MIPS eligible clinicians who submitted data and were scored as an individual or group. All clinicians who received the same group score had the same complex patient bonus scores. Then, five groupings were created, each containing 20 percent of the MIPS eligible clinicians in our analysis. We found that MIPS final scores, prior to assigning the complex patient bonus points, were substantially lower for clinicians in the top two quintiles for the complex patient bonus compared to clinicians in the lower quintiles for the complex patient bonus when we refined the analysis by examining the HCC and dual proportion quintiles separately, we observed a similar pattern.
Currently, the complex patient bonus is awarded to all engaged clinicians, meaning those who have submitted data for at least one MIPS performance category or are facility-based, even clinicians serving beneficiaries with below-average dual proportion or HCC scores. Given our updated analysis showed that the MIPS performance of clinicians in the top two quintiles for each risk indicator, HCC and dual proportion, is substantially affected by the complexity of their patients among clinicians who submitted data and were scored as individuals and groups, we compared the respective contributions of the two risk indicators to the total points assigned. We found that approximately 55 percent of the points assigned are for the HCC risk indicator component and 45 percent for the dual proportion risk indicator component. However, we also observed that the additional points attributable to a one standard deviation increase in dual proportion were greater than those attributable to a similar increase in the HCC score.
In summary, our updated analyses using actual 2018 MIPS scored data found that clinicians who have a higher share of complex patients have lower final scores, on average, prior to the assignment of the complex patient bonus than other clinicians. Additionally, our analyses showed that there is little evidence of association between the complex patient risk indicators (HCC and dual proportion) and MIPS final scores among the clinicians with a lower share of complex patients. We also noted, based on the above analysis using actual 2018 MIPS Start Printed Page 39442 scored data for clinicians scored as individuals and groups examined separately, the median raw final scores for engaged clinicians in the calculated top complex patient bonus quintile were more than 10 points less than the median raw final score of those in the bottom complex patient bonus quintile. Specifically, the median raw final scores for engaged clinicians in the top complex patient bonus quintile were 82.33 for groups and 60.76 for individuals compared to median raw scores of 94.36 for groups and 81.28 for individuals in the lowest quintile. Additionally, when we compared the median raw final scores for engaged clinicians in the calculated middle complex patient bonus quintile to the highest quintile, we still observed a difference in median raw final scores of greater than 10 points for both individuals and groups. Table 49 shows 2018 median MIPS raw final scores for the engaged individuals and groups within the lowest, middle and highest complex patient bonus (CPB) quintiles.

Furthermore, we found that, while the two risk indictors, dual proportion and HCC risk score, are correlated substantially, each has a different scale. For example, the dual proportion has a natural upper bound of 1 whereas the HCC risk score does not. The distribution of each risk indicator around its mean and median is also different. Given these findings, we have identified areas within the complex patient bonus that can be improved. Most importantly, the current complex patient bonus formula gives bonus points to clinicians not adversely affected by social risk and medical risk of their patients and does not sufficiently account for clinicians who treat patients who are high-risk and/or medically complex.
(B) Proposed Updates to the Complex Patient Bonus Beginning With the CY 2022 MIPS Performance Period/CY 2024 MIPS Payment Year
Based on the ASPE report and our analyses, we are proposing to revise the complex patient bonus by—(1) limiting the bonus to clinicians who have a median or higher value for at least one of the two risk indicators (HCC and dual proportion); (2) standardizing the distribution of the two risk indicators so that the policy can target clinicians who have a higher share of socially and/or medically complex patients; and (3) providing one overall complex patient bonus cap at 10 bonus points. To accomplish this, we include five separate proposals to update our complex patient bonus for the CY 2022 MIPS performance period/CY 2024 MIPS payment years and future MIPS performance periods/MIPS payment years. We note that in section IV.A.3.e.(2)(a)(iii)(B) of this proposed rule, we use the term "clinician(s)" to generally refer to those individuals and entities that these proposals apply to, including MIPS eligible clinicians, groups, subgroups, APM entities, and virtual groups. First, we propose to add to § 414.1380(c)(3) that, similar to our current policy, beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year, provided that a MIPS eligible clinician, group, subgroup, virtual group or APM entity submits data for at least one MIPS performance category for the applicable performance period for the MIPS payment year, a complex patient bonus will be added to the final score for the MIPS payment year, if applicable, as described in paragraph (c)(3)(v) through (c)(3)(viii). Second, we propose at § 414.1380(c)(3)(v) that beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment years, the complex patient bonus is limited to MIPS eligible clinicians, groups, subgroups, APM entities, and virtual groups with a risk indicator at or above the risk indicator calculated median. Third, we propose the revised formulas at § 414.1380(c)(3)(vi). For MIPS eligible clinicians, groups, and subgroups, the complex patient bonus components are calculated as follows for the specific risk indicators: medical complex patient bonus component = 1.5 + 4*associated HCC standardized score calculated with the average HCC risk score assigned to beneficiaries (pursuant to the HCC risk adjustment model established by CMS pursuant to section 1853(a)(1) of the Act) seen by the MIPS eligible clinician or seen by clinicians in a group or subgroup; social complex patient bonus component =1.5 + 4* associated dual proportion standardized score. The components are added together to calculate one overall complex patient bonus. A standardized score for each risk indicator is determined based on the mean and standard deviation of the raw risk indicator score and provides a standardized measurement of how far each risk score is from the mean: (raw risk indicator score−risk indicator mean)/risk indicator standard deviation. Fourth, we propose the revised formulas at § 414.1380(c)(3)(vii). For APM entities and virtual groups, the complex patient bonus components are calculated as follows for the specific risk indicators: medical complex patient bonus component = 1.5 + 4* the beneficiary weighted average HCC risk standardized score for all MIPS eligible clinicians, and if technically feasible, TINs for models and virtual groups which rely on complete TIN participation within the APM entity or virtual group, respectively; social complex patient bonus component = 1.5 + 4* the average dual proportion standardized score for all MIPS eligible clinicians, and if technically feasible, TINs for models and virtual groups which rely on complete TIN participation, within the APM entity or virtual group, respectively. The components are added together to calculate one overall complex patient bonus. A standardized score for each risk indicator is determined based on the mean and standard deviation of the raw risk indicator score and provides a standardized measurement of how far each risk score is from the mean: (raw risk indicator score−risk indicator mean)/risk indicator standard deviation. Finally, we propose at § 414.1380(c)(3)(viii) that beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment years, Start Printed Page 39443 the complex patient bonus cannot exceed 10.0 and cannot be below 0.0.
(aa) Continuing the Requirement To Submit Data and To Use the Same Risk Indicators
Under the first proposal, beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment years, we are proposing to continue the requirement to submit data for at least one MIPS performance category for the applicable performance period for the MIPS payment year for a MIPS eligible clinician, group, subgroup, virtual group or APM entity to receive a complex patient bonus added to the final score for the MIPS payment year, if applicable, as described in paragraphs (c)(3)(v) through (viii).
As discussed below in section IV.A.3.e.(2)(a)(iii)(B)(cc) of this proposed rule, the proposals at § 414.1380(c)(3)(vi) and (vii) include complex patient bonus components within the formulas proposed beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year to account for social and medical risk, while still using our current established risk indicators of dual proportion and HCC risk scores, respectively. We also continue to believe that applying this bonus at the final score is appropriate because caring for complex and vulnerable patients can affect all aspects of a practice and not just specific performance categories.
(bb) Cutoff at the Median for Complex Patient Bonus Beginning With the CY 2022 MIPS Performance Period/CY 2024 MIPS Payment Year
Beginning with the CY 2018 MIPS performance period/CY 2020 MIPS payment year, a complex patient bonus was added to the final score for the MIPS payment year for all MIPS eligible clinicians, groups, virtual groups and APM entities if they submitted data for at least one MIPS performance category for the applicable performance period for the MIPS payment year (§ 414.1380(c)(3)). We did not include any requirement that clinicians had to attain a certain threshold to receive the complex patient bonus. Under the second proposal, we are proposing at § 414.1380(c)(3)(v), beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment, the complex patient bonus is limited to MIPS eligible clinicians, groups, subgroups, APM entities, and virtual groups with a risk indicator at or above the risk indicator calculated median. This proposal limits the component for social risk to clinicians with a dual proportion value at or above the median dual proportion, and it limits the component for medical risk to clinicians with an HCC score value at or above the median HCC score. Those at or above the median for a risk indicator will get complex bonus points for that risk indicator component. To ensure the points are impactful and provided specifically to clinicians treating higher numbers of medically and socially complex patients, we propose to provide bonus points beginning at the respective calculated medians.
Both medical and social factors within the newly proposed methodology, discussed in section IV.A.3.e.(2)(a)(iii)(B)(cc) of this proposed rule, would have separate medians and distributions calculated to provide more transparent bonuses to directly target those clinicians who treat a higher caseload of high-risk and complex patients. Both the dual proportion and the HCC risk indicators attributed to each clinician will be calculated in the same manner as under our current policy (for more information about the current policy, see the CY 2018 Quality Payment Program final rule (82 FR 30135)).
To determine the median for the respective risk indicator (HCC and dual proportion), we propose to use the risk indicators associated with the final score assigned to a clinician from the prior performance period for all engaged MIPS clinicians, which means those who have submitted data for at least one MIPS performance category or are facility-based. We would also use this same final score distribution to determine the standardized score discussed in section IV.A.3.e.(2)(a)(iii)(B)(cc) of this proposed rule. These statistics would be applied to the applicable performance period values of the risk indicators for each clinician to calculate the social and medical risk component scores for the MIPS performance period. For example, the median from the CY 2021 performance period would be compared to the CY 2022 MIPS performance period risk indicator values to determine who would be eligible for a CY 2022 MIPS performance period/CY 2024 MIPS payment year complex patient bonus. We believe we need a prospective determination of the median, and standardized score from a prior performance period where the final scores are already resolved.
In our proposal, only those clinicians with risk indicator scores at or above the median for either one or both risk indicators would receive complex patient bonus points. Based on this proposed rule's regulatory impact analysis (see section VII.F.17 of this proposed rule), we estimate 64 percent of clinicians would receive a complex patient bonus because there is not 100 percent overlap in the clinicians that are at or above the median for both risk indicators. Approximately 36.3 percent of engaged clinicians would not receive any complex patient bonus points, as they are below the median for both the HCC risk score and the dual proportion. We believe this number is appropriate as we would be targeting the complex patient bonus points to clinicians treating a higher caseload of highly complex patients.
Additionally, the complex patient bonus points would be more impactful as the difference in awarded bonus points between those with high caseloads of complex patients and those with low caseloads, would be greater. We believe establishing a cutoff point at the median, as opposed to the mean, is appropriate given the median is less impacted by outlier cases since the median is simply the middle value. We considered whether to use the mean, instead of the proposed median, but estimated that would result in about 50 percent of clinicians no longer receiving any complex patient bonus points because outliers could increase the mean.
We acknowledge that alternate approaches could be used to target the bonus at clinicians with higher caseloads of complex patients. For example, we could consider using a different distribution of risk indicator scores, such as one from an earlier performance period, so clinicians could know their scores in advance, or through using a distribution that includes individual clinician scores and counts group scores, APM Entity scores, virtual group scores, and, starting in the CY 2023 MIPS performance period/CY 2025 MIPS payment year, subgroup scores only once, so the group, APM Entity, virtual group, or subgroup score is not weighted by the number of clinicians in the group, APM Entity, virtual group, or subgroup. We could also consider building risk benchmarks based on our current quality measure benchmark methodology (81 FR 77282 and 82 FR 53718) to determine the number of complex patient bonus points. Under that methodology, risk indicator scores higher than the decile in which the median risk factor lies would receive complex patient bonus points and all of the clinicians in the top decile would receive 5 points for each risk indicator. We request comments on alternate approaches for calculating the complex patient bonus. Start Printed Page 39444
(cc) Revised Complex Patient Bonus Formulas Beginning With the CY 2022 MIPS Performance Period/CY 2024 MIPS Payment Year
Under the third proposal, we propose at § 414.1380(c)(3)(vi) that beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment, for MIPS eligible clinicians, groups, and subgroups, the complex patient bonus components are calculated as follows for the specific risk indicators: Medical complex patient bonus component = 1.5 + 4* associated HCC standardized score calculated with the average HCC risk score assigned to beneficiaries (pursuant to the HCC risk adjustment model established by CMS under section 1853(a)(1) of the Act) seen by the MIPS eligible clinician or seen by clinicians in a group or subgroup; social complex patient bonus component = 1.5 + 4* associated dual proportion standardized score. The components are added together to calculate one overall complex patient bonus. A standardized score for each risk indicator is determined based on the mean and standard deviation of the raw risk indicator score and provides a standardized measurement of how far each risk score is from the mean: (raw risk indicator score−risk indicator mean)/risk indicator standard deviation. For example, we would use the mean and standard deviation from the CY 2021 MIPS performance period and the indicator assigned in the CY 2022 MIPS performance period to determine the clinician's standard score for the CY 2022 MIPS performance period/CY 2024 MIPS payment year.
Under the fourth proposal, we also propose at § 414.1380(c)(3)(vii), beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment years, for APM entities and virtual groups, the complex patient bonus components are calculated as follows for the specific risk indicators: Medical complex patient bonus component = 1.5 + 4* the beneficiary weighted average HCC risk standardized score for all MIPS eligible clinicians, and if technically feasible, TINs for models and virtual groups which rely on complete TIN participation within the APM entity or virtual group, respectively; social complex patient bonus component = 1.5 + 4* the average dual proportion standardized score for all MIPS eligible clinicians, and if technically feasible, TINs for models and virtual groups which rely on complete TIN participation, within the APM entity or virtual group, respectively. The components are added together to calculate one overall complex patient bonus. A standardized score for each risk indicator is determined based on the mean and standard deviation of the raw risk indicator score and provides a standardized measurement of how far each risk score is from the mean: (raw risk indicator score−risk indicator mean)/risk indicator standard deviation.
The mean and standard deviation statistics will be used along with the performance period values of the risk indicators for each clinician to calculate the standardized score used to calculate the social and medical risk component scores for the MIPS payment year. For example, if the raw dual proportion score calculated for a clinician is 0.25, the dual proportion mean (from the prior performance period) is 0.23, and the dual proportion standard deviation (from the prior performance period) is 0.15, the clinician's standardized score would be (0.25−0.23)/0.15 = 0.13. We believe that the standardized score calculation is appropriate as it is typically used to understand how far the raw risk indicator scores fall from the mean. We note that those with a mean score would have a standardized score of zero, and those with a standardized score of 1 are one standard deviation above the mean. Those below the mean would have a negative standardized score. The proposed formulas would also provide bonus points to clinicians who have a negative risk indicator standardized score and fall between the calculated median and mean for the respective risk indicator and to those who fall at or above the mean and thus have a positive risk indicator standardized score. As noted in section IV.A.3.e.(2)(a)(iii)(B)(cc) of the proposed rule, at § 414.1380(c)(3)(vi) and (vii), the new bonus components would use respective means to calculate standardized scores to then calculate and provide bonus points for clinicians with risk indicators, HCC and dual proportion, at or above the median, to capture medical and social risk, separately. For each of the two component calculations, higher standardized scores above the mean would be associated with more bonus points. For example, a clinician with a standardized score of zero (the mean value) for each risk indicator, HCC and dual proportion, would receive bonus points for each component that is equivalent to the constant, additive factor within the new formula, 1.5. The proposed formulas acknowledge complexity without creating a large cliff between those who are just below the median and those who have a marginally higher caseload of complex patients. The bonus, as proposed, would increase proportionally with the increased number of complex patients a clinician is treating and/or the degree of patient complexity. For example, we would not want to provide those who fall at the mean 1.5 bonus points, and then provide 10 bonus points to those who only have only a slightly higher caseload. Hence, we intend to incorporate a standardized score multiplied by 4 to ensure the score gets proportionally higher as complexity increases.
The additive factor of 1.5 ensures that all clinicians at or above the mean for the social complex patient bonus calculation, based on dual proportion, would receive at least 1.5 complex patient bonus points and all clinicians at or above the mean for the medical complex patient bonus calculation, based on HCC risk score, would receive at least 1.5 complex patient bonus points. Further, the additive factor of 1.5 would allow clinicians between the median and mean to still receive complex patient bonus points. For example, if the dual proportion risk indicator median is 0.2 and the dual proportion risk indicator mean is 0.23, a clinician with an associated standardized score of −0.1 (0.1 standard deviations below the mean, but still above the median), the clinician would receive a complex patient bonus equal to 1.5 + 4 * (−0.1) which equals 1.1 for the dual proportion component. If the dual proportion risk indicator calculated for a clinician falls below the median, they would receive zero complex patient bonus points for the dual proportion component. Based on the standardized score calculation, if the dual proportion risk indicator calculated for a clinician falls above the mean, they would receive complex patient bonus component points greater than the additive factor of 1.5 for the dual proportion component.
We believe this formula avoids a major complex patient bonus points cliff created by providing the clinicians right above the cutoff point significant bonus points compared to the clinicians right below the cutoff point (who do not receive any complex patient bonus points). We acknowledge those who fall below the median will no longer receive any complex patient bonus points, per proposal at § 414.1380(c)(3)(v) that for the CY 2022 MIPS performance period/CY 2024 MIPS payment years and future MIPS performance periods/MIPS payment years, the complex patient bonus is limited to MIPS eligible clinicians, groups, subgroups, APM entities, and virtual groups with a risk Start Printed Page 39445 indicator at or above the risk indicator calculated median.
(dd) Cap for the Complex Patient Bonus Beginning With the CY 2022 MIPS Performance Period/CY 2024 MIPS Payment Year
When we originally established the complex patient bonus policy in the CY 2018 Quality Payment Program final rule (82 FR 53774), we finalized at § 414.1380(c)(3)(iii) that the complex patient bonus cannot exceed 5.0. At that time, our data analysis estimated a decrease in simulated scores of 5.4 points (for individuals who report 6 or more quality measures) and 4.5 points (for groups) from the top quartile to the bottom quartile for the average patient HCC risk scores and 4.8 points (for individuals who report 6 or more quality measures) and 4.5 points (for groups) from the top quartile to the bottom quartile for the dual proportion. Therefore, we believed a cap for the complex patient bonus of 5 points was supported by the data and was sufficient to compensate for the estimated differences in performance based on HCC risk scores and dual proportion (82 FR 53773). In CY 2019, CY 2020, and CY 2021 PFS final rules, we continued to believe that the 5-point cap was appropriate given that we had not updated our prior analyses (83 FR 58769 through 59870, 84 FR 63021 through 63023, 85 FR 84908 through 84913, respectively). However, based on our updated analyses using actual 2018 MIPS scored data, described above, about 5 percent of clinicians scored as group or individual had a complex patient bonus that would have been more than 5-points had the cap not been in place. The complex patient bonus cap of 5-points (82 FR 53773 through 53776) affects clinicians who are most likely carrying higher caseloads for patient complexity, which is inconsistent with the intent of the policy, and we no longer believe we should maintain the overall cap of 5-points. As discussed in section IV.A.3.e.(2)(a)(iii)(A), our updated analyses using actual 2018 MIPS scored data also found that clinicians with a higher caseload of complex patients have lower final scores, by more than 10 points on average, prior to the assignment of the complex patient bonus than clinicians with lower caseloads of complex patients. We believe, with a cap of 10 bonus points, we are targeting the bonus to address the differences in final scores these clinicians face more appropriately without capping at a larger number, such as 15 or 20 bonus points, which could result in inappropriate score inflation and masking of poor performance. A cap of fewer than 10 bonus points would not address the differences in the observed raw final scores and would not adequately address the need to provide more equity within our scoring system for those clinicians treating higher caseloads of socially and/or medically complex patients. We did consider lower caps, such as 5 or 7 bonus points, or higher caps, such as 20 bonus points, but, for the reasons described above, have proposed the cap of 10 bonus points. However, if future analyses and more recent data show differences in raw final scores that differ from our previous findings, we would consider proposing in future rulemaking the use of a cap that aligns with new findings.
As discussed in section IV.A.3.e.(2)(a)(iii)(B)(cc) of this proposed rule, we are proposing the formulas as: risk indicator complex patient bonus component = 1.5 + 4* associated risk indicator standardized score. The formula, as proposed, has a multiplier of 4. We also considered a multiplier of 2 with a cap of 5 bonus points which results in a lower final score mean and median but does not change the number of clinicians receiving a complex patient bonus when compared to the proposed policy. We do not believe that this alternate approach would produce a bonus impactful enough to overcome the differences seen at the final score level when comparing complex patient bonus quintiles. We request feedback on this alternate approach and additional alternate approaches we should consider.
Under the fifth proposal, we are proposing at § 414.1380(c)(3)(viii) that beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year, the complex patient bonus cannot exceed 10.0 and cannot be below 0.0. Based on the proposed formulas at § 414.1380(c)(3)(vi) and (vii), the social complex patient bonus component (using dual proportion standardized score) and the medical complex patient bonus component (using HCC risk score standardized score) will be added together to calculate one overall complex patient bonus. Then, based on the proposal at § 414.1380(c)(3)(viii) the complex patient bonus would then be capped at the 10 points overall. The benefit would be targeted toward those clinicians treating medically and socially complex patients.
For example, if a clinician's dual proportion standardized score is 1.5, their complex patient bonus social risk component is 1.5 + 4 * 1.25 = 6.5. If their HCC risk score standardized score is 0.25, their complex patient bonus medical risk component is 1.5 + 4 * 0.25 = 2.5. The two complex patient bonus components are then added together to total 9 complex patient bonus points for this clinician. Had the total been above 10 bonus points, the 10-point cap would have applied and the clinician would have received 10 complex patient bonus points added to their final MIPS score.
We seek feedback on whether we should use the multiplier of 4 and cap of 10 bonus points as proposed, a multiplier of 2 and cap of 5 bonus points as discussed as an alternative, or if we should consider additional options and rationale for the complex patient bonus formula and bonus cap, such as a cap of 7 or 20 bonus points.
In summary, we propose the five separate proposals for the CY 2022 MIPS performance period/CY 2024 MIPS payment year and future MIPS performance periods/MIPS payment years as follows:
- To revise § 414.1380(c)(3), by adding, beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year, provided that a MIPS eligible clinician, group, subgroup, virtual group or APM entity submits data for at least one MIPS performance category for the applicable performance period for the MIPS payment year, a complex patient bonus will be added to the final score for the MIPS payment year, if applicable, as described in paragraphs (c)(3)(v) through (viii).
- At § 414.1380(c)(3)(v), beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year, we propose the complex patient bonus is limited to MIPS eligible clinicians, groups, subgroups, APM entities, and virtual groups with a risk indicator at or above the risk indicator calculated median.
- At § 414.1380(c)(3)(vi), beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year, we proposed for MIPS eligible clinicians, groups, and subgroups, the complex patient bonus components are calculated as follows for the specific risk indicators: Medical complex patient bonus component = 1.5 + 4 * associated HCC standardized score calculated with the average HCC risk score assigned to beneficiaries (under the HCC risk adjustment model established by CMS pursuant to section 1853(a)(1) of the Act) seen by the MIPS eligible clinician or seen by clinicians in a group or subgroups; social complex patient bonus component = 1.5 + 4 * associated dual proportion standardized score. The components are added together to calculate one overall complex patient bonus. A standardized score for each Start Printed Page 39446 risk indicator is determined based on the mean and standard deviation of the raw risk indicator score and provides a standardized measurement of how far each risk score is from the mean: (raw risk indicator score−risk indicator mean)/risk indicator standard deviation.
- At § 414.1380(c)(3)(vii), we propose that beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year, for APM entities and virtual groups, the complex patient bonus components are calculated as follows for the specific risk indicators: Medical complex patient bonus component = 1.5 + 4 * the beneficiary weighted average HCC risk standardized score for all MIPS eligible clinicians, and if technically feasible, TINs for models and virtual groups which rely on complete TIN participation within the APM entity or virtual group, respectively; social complex patient bonus component = 1.5 + 4 * the average dual proportion standardized score for all MIPS eligible clinicians, and if technically feasible, TINs for models and virtual groups which rely on complete TIN participation, within the APM entity or virtual group, respectively. The components are added together to calculate one overall complex patient bonus. A standardized score for each risk indicator is determined based on the mean and standard deviation of the raw risk indicator score and provides a standardized measurement of how far each risk score is from the mean: (raw risk indicator score−risk indicator mean)/risk indicator standard deviation.
- At § 414.1380(c)(3)(viii), beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year, we propose the complex patient bonus cannot exceed 10.0 and cannot be below 0.0.
We seek comments on these proposals including the different aspects of the updated complex patient bonus formulas, such as the additive and multiplicative components, the cutoff point (the median), and the 10-point overall cap. For all of the reasons described above in sections IV.A.3.e.(2)(a)(iii), IV.A.3.e.(2)(a)(iii)(A) and (B), and IV.A.3.e.(2)(a)(iii)(B)(aa) through (dd) of this rule, we have proposed such policies. However, we did also consider the alternative of continuing the use of the complex patient bonus formulas, as previously finalized at § 414.1380(c)(3)(i) and (ii), along with the 5.0 point cap as previously finalized at § 414.1380(c)(3)(iii) for the CY 2022 MIPS performance period/CY 2024 MIPS payment year. We also seek comment on that alternative.
(b) Final Score Performance Category Weights
(i) General Weights
Section 1848(q)(5)(E)(i) of the Act specifies weights for the performance categories included in the MIPS final score: In general, 30 percent for the quality performance category; 30 percent for the cost performance category; 25 percent for the Promoting Interoperability performance category; and 15 percent for the improvement activities performance category. For more of the statutory background and descriptions of our current policies, we refer readers to the CY 2017 through CY 2018 Quality Payment Program final rules, and CY 2019 through CY 2021 PFS final rules (81 FR 77320 through 77329, 82 FR 53779 through 53785, 83 FR 59870 through 59878, and 84 FR 62950 through 62959, 85 FR 84898 through 84908, respectively). Table 50 summarizes the weights established in prior rulemaking (85 FR 84913) for each performance category for the CY 2022 MIPS performance period/CY 2024 MIPS payment year and each subsequent MIPS payment year.

(ii) Flexibility for Weighting Performance Categories
Under section 1848(q)(5)(F) of the Act, if there are not sufficient measures and activities applicable and available to each type of MIPS eligible clinician involved, the Secretary shall assign different scoring weights (including a weight of zero) for each performance category based on the extent to which the category is applicable to the type of MIPS eligible clinician involved and for each measure and activity with respect to each performance category based on the extent to which the measure or activity is applicable and available to the type of MIPS eligible clinician involved.
Under section 1848(q)(5)(B)(i) of the Act, in the case of a MIPS eligible clinician who fails to report on an applicable measure or activity that is required to be reported by the clinician, the clinician must be treated as achieving the lowest potential score applicable to such measure or activity. In this scenario of failing to report, the MIPS eligible clinician generally would receive a score of zero for the measure or activity, which would contribute to the final score for that MIPS eligible clinician. Under certain circumstances, however, a MIPS eligible clinician who fails to report could be eligible for an assigned scoring weight of zero percent and a redistribution of the performance category weights. For a description of our existing policies for reweighting performance categories, please refer to § 414.1380(c)(2) and the CY 2021 PFS final rule (85 FR 84914 through 84916).
(A) Reweighting the Cost Performance Category
Under § 414.1380(c)(2)(i)(A), we will assign a different weight to a performance category and redistribute its prescribed weight to another performance category or categories in certain circumstances where we determine there are not sufficient measures and activities applicable and available under section 1848(q)(5)(F) of the Act. For the cost performance category, this includes circumstances where we cannot reliably calculate a score for the cost measures which adequately captures and reflects the performance of a MIPS eligible clinician (see § 414.1380(c)(2)(i)(A)(2)). In the CY 2018 Quality Payment Program final rule (82 FR 53780), for the cost performance category, we noted in the Start Printed Page 39447 proposed rule we had stated that we continue to believe having sufficient measures applicable and available means that we can reliably calculate a score for the cost measures which adequately captures and reflects the performance of a MIPS eligible clinician, and that MIPS eligible clinicians who are not attributed enough cases to be reliably measured should not be scored for the cost performance category. We noted (82 FR 53780) we had established a policy in the CY 2017 Quality Payment Program final rule that if a MIPS eligible clinician is not attributed enough cases for a measure (in other words, has not met the required case minimum for the measure), or if a measure does not have a benchmark, then the measure will not be scored for that clinician (81 FR 77323). We stated (82 FR 53780) if we do not score any cost measures for a MIPS eligible clinician in accordance with this policy, then the clinician would not receive a cost performance category percent score. In this section of the proposed rule, we are requesting comments on potential circumstances where we may not be able to reliably calculate a score for any of the cost measures which adequately captures and reflects the performance of a MIPS eligible clinician, and may assign a different weight to the cost performance category and redistribute its prescribed weight to another performance category or categories. We are interested in comments on circumstances that could affect all MIPS eligible clinicians, as well as those that could affect a subset of MIPS eligible clinicians or individual MIPS eligible clinicians. The cost performance category reweighting provision at § 414.1380(c)(2)(i)(A)(2) is distinct from the measure suppression policy proposed in section IV.A.3.e.(1)(d)(i) of this proposed rule, as it applies to the performance category as a whole and not on a measure-by-measure basis.
In the CY 2021 PFS final rule (85 FR 84880), we responded to commenters' concerns regarding the potential for COVID-19 to impact cost measures, noting various features of the cost measures could allow us to calculate cost measures without penalizing practices in COVID-19 hotspots, and policies were in place to account for circumstances when a cost measure cannot be reliably calculated to adequately capture and reflect clinician performance. First, we noted service assignment allows the episode-based measures to capture the cost of services clinically related to the triggering event for the episode, reducing the likelihood of the measures to capture costs resulting from high volumes of COVID-19 treatment services. Then, we noted the measures are adjusted for clinical risk to account for different levels of care beneficiaries may require due to comorbidities, disability, age, and other risk factors (including clinical characteristics based on the beneficiary's recent medical history) to ensure attributed clinicians are not penalized for factors beyond their influence. Finally, we noted the cost measures use standardized claims to account for differences in Medicare payments for the same services across health care providers, removing the effect of regional differences in health care costs and the payment standardization process removes the 20 percent increase in the IPPS relative weight under the CARES Act for individuals diagnosed with COVID-19.
After the publication of the CY 2021 PFS final rule, we continued to monitor the cost measures to determine whether we could reliably calculate the cost measures for the 2020 performance period. Due to the claims run-out period, there is an inherent delay between the time at which we can observe trends in claims and when we can test for potential impact on cost measures, to ensure that the data included in our analyses reflect final claims for all services in episodes. In addition, a drop-in service utilization early in 2020 would only affect the amount of information available to risk adjust for patient clinical factors for episodes beginning in the second half of the year as the episode-based measures use a 120-day look back period during which we look for HCC indicators. When considering these factors, along with the length of the episode windows for the measures and the need to include as many episodes in our study as possible, we had to complete our monitoring and assessment after the end of the CY 2020 MIPS performance period.
Based on our analyses using the full year of data from 2020, we considered many factors and concluded we cannot reliably calculate scores for the cost measures that adequately capture and reflect clinician performance due to the unique changes to healthcare in the COVID-19 pandemic. Our analyses showed substantial decreases in service utilization in 2020 compared to 2019 across all care settings. This can affect risk adjustment and the ability to adequately reflect clinician performance on cost measures. Since we look for diagnosis information in a beneficiary's claims history during a lookback period before the start of the episode to capture HCC indicators and other clinical risk factors used in risk adjustment, a decrease in service utilization means that there are fewer claims from which we can identify risk adjustors. There was a systematic decrease in the ratio of the number of HCCs identified for risk adjustment in 2020 compared to 2019 in the second half of the year across all the measures. We note this finding coincides with when we observed the steepest drops in service utilization due to the length of the lookback period. There was also a systematic decrease in the ratio of the number of episodes in 2020 compared to 2019. This indicates the overall decrease in service utilization is indeed reflected in fewer episodes being triggered and demonstrates the wide-ranging impact of COVID-19 across the range of care that the cost measures capture. Separately from the decrease in service utilization, our analyses demonstrate that episodes with COVID-19 diagnoses generally have higher observed, payment-standardized costs than episodes without COVID-19 diagnoses. These differences are also present when comparing risk-adjusted costs for episodes with and without COVID-19 diagnoses. This shows despite service assignment rules for episode-based measures, beneficiaries diagnosed with COVID-19 had higher payment-standardized costs of care which was not sufficiently accounted for through risk adjustment or other features of cost measures.
Because we determined we cannot reliably calculate scores for the cost measures that adequately capture and reflect the performance of MIPS eligible clinicians, we announced via email communication (subject: CMS Reweighting 2020 MIPS Cost Performance Category) on May 20, 2021, in accordance with § 414.1380(c)(2)(i)(A)(2), we will assign a weight of 0 percent to the cost performance category for the CY 2020 MIPS performance period/CY 2022 payment year and redistribute the prescribed weight of 15 percent to another performance category or categories, as established at § 414.1380(c)(2)(ii)(D). We note as with all measures, we continue to monitor the evolving impact of COVID-19.
We recognize there may be additional circumstances where we may not be able to reliably calculate a score for any of the cost measures within the cost performance category that adequately captures and reflects the performance of a MIPS eligible clinician, which could include external factors beyond the control of clinicians. Similar to the Measure Suppression Factors discussed Start Printed Page 39448 in the FY 2022 IPPS/LTCH PPS proposed rule (86 FR 25473), such external factors may include significant national shortages or rapid or unprecedented changes in: (1) Healthcare personnel; (2) medical supplies, equipment, or diagnostic tools or materials; or (3) patient case volumes or patient case mix. We request comment on whether these external factors should inform our future decision-making on whether to reweight the cost performance category under § 414.1380(c)(2)(i)(A)(2). We also request comment on whether there are other external factors we should consider, or other circumstances in general that could affect our ability to reliably calculate a score for the cost performance category as described under § 414.1380(c)(2)(i)(A)(2).
(iii) Redistributing Performance Category Weights
In the CY 2017 through CY 2018 Quality Payment Program final rules, and CY 2019 through CY 2021 PFS final rules (81 FR 77325 through 77329, 82 FR 53783 through 53785, 83 FR 59876 through 59878, 84 FR 63027 through 63031, and 85 FR 84914 through 84916), and at § 414.1380(c)(2)(ii), we established policies for redistributing the weights of the performance categories in the event that a scoring weight different from the generally applicable weight is assigned to a category or categories.
In CY 2021 PFS final rule, we finalized a policy for redistributing the performance category weights for the CY 2022 MIPS performance period/CY 2024 MIPS payment year and noted that we planned to revisit our redistribution policies in future rulemaking and may consider redistributing more weight to the cost performance category after clinicians have more experience with cost being weighted at 30 percent (85 FR 84914). While we still intend to redistribute more weight to the cost performance category in future years, we believe it would be beneficial to establish the redistribution policies for the CY 2023 MIPS performance period/CY 2025 MIPS payment year and future years to provide as much notice as possible to clinicians and other stakeholders. Hence, we propose to apply the redistribution policy finalized for the 2022 MIPS performance period/2024 MIPS payment year at § 414.1380(c)(2)(ii)(F) to the 2025 MIPS payment year and each subsequent MIPS payment year, and we propose corresponding revisions to § 414.1380(c)(2)(ii)(F). We still plan to revisit our redistribution policies in future rulemaking after clinicians have more experience with cost being weighted at 30 percent and gain more experience with our newly proposed MVPs. Our proposed redistribution policies for the 2025 MIPS payment year and each subsequent MIPS payment year are included in Table 51. We note that not all the redistribution scenarios described in Table 51 would apply to MIPS eligible clinicians in small practices, so we also propose at § 414.1380(c)(2)(ii)(F) an exception for a new paragraph (G) which applies to small practices. Please refer to Table 52 for the proposed redistribution policy for small practices.
We request public comment on this proposal.

(A) Redistributing Performance Category Weight for Small Practices
Clinicians and groups who work in small practices are a crucial part of the health care system. The Quality Payment Program provides options designed to make it easier for these clinicians and groups to report on performance and quality and participate in advanced alternative payment models for incentives. We have heard directly from clinicians in small practices that they face unique challenges related to financial and other resources, environmental factors, and access to health information technology. We heard from many commenters that the Quality Payment Program gives an advantage to large organizations because such organizations have more resources invested in the infrastructure required to track and report measures to MIPS (82 FR 53776). In response to the feedback on the potential burden on small practices, we have established special policies available for small practices including the small practice bonus and special scoring policies. For example, in the CY 2018 QPP final rule (82 FR 53682 through 53683), we established a significant hardship exception for small practices for the Start Printed Page 39449 Promoting Interoperability performance category.
In this section of the proposed rule, we discuss how we would redistribute the Promoting Interoperability performance category weight for small practices. Within the reweighting policy for the CY 2022 MIPS performance period/CY 2024 MIPS payment year, the Promoting Interoperability performance category weight is redistributed fully to the quality performance category, unless the quality performance category is weighted at zero percent. In general, our reweighting policies have emphasized the quality performance category over the improvement activities performance category. We have noted it is important to prioritize performance on measures that show a variation in performance such as quality measures, rather than the activities under the improvement activities performance category, which are based on attestation of completion (85 FR 84914). We believe this helps reduce incentives to not report measures for the quality performance category in circumstances when a clinician may be able to report but chooses not to do so. However, given stakeholder input and recently published literature, we believe there could be other reasons why a small practice would not report quality measures. One recent article[243] stated that physicians in larger group practices, multispecialty practices, or participating through alternative payment models had higher MIPS scores, possibly reflecting such practices' greater infrastructure and resources to collect, analyze, and report measures to CMS. We have also heard directly from clinicians in small practices that they face unique challenges related to financial and other resources and access to health information technology. Many commenters have shared their belief that the Quality Payment Program gives an advantage to large organizations because such organizations have more resources invested in the infrastructure required to track and report measures to MIPS (82 FR 53776). Indeed, 85 percent of clinicians who are not engaged with MIPS (who do not submit data) are clinicians in small practices (85 FR 85018), which we believe may be due to their limited resources. Given infrastructure and resource limitations within small practices, we believe it is appropriate to place more emphasis on a performance category that poses a reduced reporting burden such as the improvement activities performance category. We propose that for small practices, as defined at § 414.1305, when the Promoting Interoperability performance category is reweighted, the quality performance category will be weighted at 40 percent, the cost performance category will be weighted at 30 percent, and the improvement activities performance category will be weighted at 30 percent. When both the cost performance category and the Promoting Interoperability performance category are reweighted, the quality performance category will be weighted at 50 percent and the improvement activities performance category will be weighted at 50 percent. We plan to revisit this redistribution policy in future rulemaking and may consider redistributing more weight to the cost performance category after clinicians have more experience with cost being weighted at 30 percent.
We anticipate that our proposal noted in the two rows in Table 52 will greatly assist small practices by providing further flexibilities to help with our goal of increasing engagement across the MIPS program and to be able to meet the MIPS requirements. Beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year, we propose at § 414.1380(c)(2)(ii)(G) redistribution policies for small practices, as shown in Table 52.
We request public comment on this proposal.

Start Printed Page 39450
(iv) MIPS Reweighting Based on Extreme and Uncontrollable Circumstances
(A) MIPS Applications for Reweighting for the CY 2021 MIPS Performance Period/CY 2023 MIPS Payment Year Based on Extreme and Uncontrollable Circumstances
We anticipate that the national PHE for COVID-19 will continue through CY 2021. Therefore, we remind clinicians that the application-based extreme and uncontrollable circumstances policy, as described in § 414.1380(c)(2)(i)(A)(6) and (c)(2)(i)(C)(2), will be available for the CY 2021 performance period/CY 2023 MIPS payment year (85 FR 84916 through 84917). Please refer to https://qpp.cms.gov/about/covid19?py=2021 for details. The application allows clinicians, groups, and virtual groups significantly impacted by the PHE for COVID-19 to request reweighting for any or all MIPS performance categories. Under this policy, if a clinician, group, or virtual group submits a reweighting application and also submits data for a performance category for which an application was submitted, the data submission will override the application, and the clinician, group, or virtual group will be scored on the data submitted.
Additionally, if an application is submitted for one performance category only, and data is submitted for the other 2 performance categories, only the performance category for which the application was submitted will be reweighted and the other performance categories will be scored. We believe this approach maintains a balance of encouraging participation in the Quality Payment Program while still providing for flexibility in weighting the performance categories for those who have been affected by the national PHE for COVID-19. Please refer to https://qpp.cms.gov/about/covid19?py=2021 for more information.
(B) MIPS Reweighting Based on Extreme and Uncontrollable Circumstances; Automatic and Application-Based Policies Clarification
Under the application-based extreme and uncontrollable circumstances policy codified at § 414.1380(c)(2)(i)(A)(6) for the quality, cost, and improvement activities performance categories and at § 414.1380(c)(2)(i)(C)(2) for the promoting interoperability performance category, clinicians who are subject to extreme and uncontrollable circumstances may submit an application to CMS to request reweighting of a performance category or categories. We also established an automatic extreme and uncontrollable circumstances policy at § 414.1380(c)(2)(i)(A)(8) for the quality, cost, and improvement activities performance categories and at § 414.1380(c)(2)(i)(C)(3) for the promoting interoperability performance category, under which we automatically reweight the performance categories for clinicians who are located in an area affected by extreme and uncontrollable circumstances as identified by us.
Based on stakeholder inquiries, we recognize not all stakeholders understand how individual MIPS eligible clinicians who are eligible for reweighting under the automatic extreme and uncontrollable circumstances policy and who also submit an application for reweighting based on extreme and uncontrollable circumstances are affected by the intersection of these policies. Currently, under both the application-based and automatic extreme and uncontrollable circumstances policies, if a MIPS eligible clinician who is located in an area affected by extreme and uncontrollable circumstances as identified by CMS submits data for any of the MIPS performance categories by the applicable submission deadline for the MIPS performance period, they will be scored on each performance category for which they submit data, and the performance category will not be reweighted to zero percent in the final score. Under the automatic extreme and uncontrollable circumstances policy, the other performance categories for which data was not submitted will remain reweighted to zero percent (82 FR 53898, 83 FR 59874). Additionally, as described in the CY 2019 PFS final rule (83 FR 59874), under the automatic extreme and uncontrollable circumstances policy, a MIPS eligible clinician who is located in an area affected by extreme and uncontrollable circumstances as identified by CMS will not be scored on the cost performance category. As we stated in the CY 2019 PFS final rule (83 FR 59874), if a MIPS eligible clinician is located in an affected area, we would assume the clinician does not have sufficient cost measures applicable to him or her and assign a weight of zero percent to that category in the final score, even if we receive administrative claims data that would enable us to calculate the cost measures for that clinician.
The following example is intended to illustrate the intersection of the automatic and application-based extreme and uncontrollable circumstances policies. A MIPS eligible clinician who is located in an area affected by extreme and uncontrollable circumstances as identified by CMS and eligible for the automatic extreme and uncontrollable circumstances policy submits an application for reweighting based on extreme and uncontrollable circumstances. The application requests reweighting for the Promoting Interoperability performance category, and the clinician submits data for the quality and improvement activities performance categories. The clinician will be scored on the quality and improvement activities performance categories because they submitted data for those categories; the cost performance category is reweighted to zero percent under the automatic extreme and uncontrollable circumstances policy, as discussed above; and the Promoting Interoperability performance category is also reweighted to zero percent under the automatic extreme and uncontrollable circumstances policy. The application for reweighting was not needed in this example to reweight the Promoting Interoperability performance category.
Please refer to https://qpp.cms.gov/about/covid19?py=2021 for more information.
(v) Redistributing Performance Category Weights for Facility-Based Measurement
(A) Background
In the CY 2018 Quality Payment Program final rule, we established facility-based measurement under section 1848(q)(2)(C)(ii) of the Act which provides that the Secretary may use measures used for payment systems other than for physicians, such as measures for inpatient hospitals, for purposes of the quality and cost performance categories (82 FR 53752 through 53767). Scoring under facility-based measurement was available for clinicians beginning with the CY 2019 MIPS performance period/CY 2021 MIPS payment year. We established facility-based measurement to better align incentives between facilities and the MIPS eligible clinicians who provide services there (82 FR 53753). For more background on facility-based measurement, we refer readers to both the CY 2018 Quality Payment Program final rule (82 FR 53752 through 53767) and the CY 2019 PFS final rule (83 FR 59856 through 59867).
(B) Redistribution of Performance Category Weights Under Facility-Based Measurement
In the CY 2019 PFS final rule, we established that clinicians and groups would not need to elect or opt-in to Start Printed Page 39451 facility-based measurement, but instead we would automatically apply facility-based measurement to MIPS eligible clinicians and groups who are eligible for facility-based measurement and who would benefit by having a higher combined quality and cost performance category score (83 FR 59863). In this same final rule, we finalized policies for redistributing weight among the performance categories for the 2019 MIPS performance period/2021 MIPS payment year under § 414.1380(c)(2)(ii)(C). Under those redistribution policies, if the cost performance category is reweighted to zero percent of the final score, its weight is redistributed entirely to the quality performance category, unless the quality performance category is reweighted to zero percent, in which case the quality and cost performance category weights would be redistributed to the improvement activities and Promoting Interoperability performance categories. A clinician or group could have the weight of the cost performance category redistributed because they did not meet the case minimum for any of the measures in the cost performance category. Because facility-based measurement always includes both the quality and cost performance categories, it is possible a clinician or group would be scored on the cost performance category under facility-based measurement but not outside of facility-based measurement. There are two common scenarios for a facility-based clinician or group which could occur in the 2019 MIPS performance period/2021 MIPS payment year. In the first scenario, a facility-based clinician or group meets the case minimum for at least one cost performance category measure and receives a cost performance category percent score as defined at § 414.1380(b)(2). The respective quality and cost scores would be multiplied by the available points in the quality performance category (45 points) and the available points in the cost performance category (15 points) to determine the combined contribution of the quality performance category and the cost performance category to the final score out of the available 60 points. In the second scenario, a facility-based clinician or group does not meet the case minimum for any cost performance category measure and the cost performance category weight is redistributed to the quality performance category so the quality performance category score alone determines the score out of the available 60 points. Table 53 shows these two scenarios.

In the CY 2020 PFS final rule, we established a redistribution policy for the CY 2020 MIPS performance period/CY 2022 MIPS payment year at § 414.1380(c)(2)(ii)(D), for scenarios when the cost performance category weight is redistributed to the Promoting Interoperability performance category, as well as to the quality performance category (84 FR 63028). Under this policy, the weights of the combined quality and cost performance categories could be different for a clinician or group under facility-based measurement and outside of facility-based measurement in circumstances in which the clinician or group was not scored on the cost performance category outside of facility-based measurement but was scored on all other performance categories. Table 54 shows the scenario in which the combined weights of the quality and cost performance categories differ if cost is included, which occurs when the cost performance category is redistributed and all other categories are scored.

We established similar redistribution policies for CY 2021 MIPS performance period/CY 2023 MIPS payment year and CY 2022 MIPS performance period/CY 2024 MIPS payment year at § 414.1380(c)(2)(ii)(E) and (F) in that same rule (84 FR 63029 through 63031), which also described situations where the combined weight of the cost and quality performance categories was not always consistent. For more on the background and proposed policies related to redistribution of performance categories, please see section IV.A.3.e.(2)(b)(iii) of this proposed rule.
Based on inquires we received from clinicians who were eligible for facility-based measurement, we believe our policy for determining the combined quality and cost performance category scores via facility-based measurement and outside of facility-based measurement is not ideal because it could result in a facility-based clinician or group receiving a lower final score Start Printed Page 39452 than they would otherwise receive outside of facility-based measurement. We considered whether this more complex consideration of the scores and the weights in the performance categories necessitated a reconsideration of an opt-in requirement for facility-based measurement. However, we believe that establishing such a requirement would create administrative burden for clinicians and groups.
Instead of adding an opt-in requirement, we propose a new policy to determine the MIPS final score for clinicians and groups who are eligible for facility-based measurement. We propose at § 414.1380(e)(6)(vi)(B) that beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year, the MIPS quality and cost performance category scores will be based on the facility-based measurement scoring methodology unless a clinician or group receives a higher MIPS final score through another MIPS submission. Under this proposed policy, we would calculate two final scores for clinicians and groups who are facility-based. One score would be based on the clinician or group's performance and the weights of the performance categories if facility-based measurement did not apply, and the other would be based on the application of facility-based measurement. The example below shows how this proposed policy would apply for a facility-based group that did not meet the case minimum for any of the cost measures but was scored on all other performance categories.

As a result of this proposed policy, the group in this example would receive a final score on the basis of their performance outside of facility-based measurement because they have obtained a higher final score through the combination of their submitted quality measures, submitted improvement activities and submitted promoting interoperability measures.
We request comments on this proposal.
f. MIPS Payment Adjustments
(1) Background
For our previously established policies regarding the final score used to determine MIPS payment adjustments, we refer readers to the CY 2021 PFS final rule (85 FR 84917 through 84926), CY 2020 PFS final rule (84 FR 63031 through 63045), CY 2019 PFS final rule (83 FR 59878 through 59894), CY 2018 Quality Payment Program final rule (82 FR 53785 through 53799) and CY 2017 Quality Payment Program final rule (81 FR 77329 through 77343). In this proposed rule, we are proposing: (1) To select the mean as our methodology for calculating the performance threshold; (2) to establish the performance threshold for the 2024 MIPS payment year using 2019 MIPS payment year data; (3) to establish the additional performance threshold for exceptional performance for the 2024 MIPS payment year; and (4) to update the scoring hierarchy to include subgroups. In addition, we are including information about our expected timeframe for providing MIPS performance feedback to clinicians for the performance period in 2020.
(2) Establishing the Performance Threshold
Under section 1848(q)(6)(D)(i) of the Act, for each year of MIPS, the Secretary shall compute a performance threshold with respect to which the final scores of MIPS eligible clinicians are compared for purposes of determining the MIPS payment adjustment factors under section 1848(q)(6)(A) of the Act for a year. The performance threshold for a year must be either the mean or median (as selected by the Secretary, and which may be reassessed every 3 years) of the final scores for all MIPS eligible clinicians for a prior period specified by the Secretary.
Section 1848(q)(6)(D)(iii) of the Act included a special rule for the initial 2 years of MIPS, which requires the Secretary, prior to the performance period for such years, to establish a performance threshold for purposes of determining the MIPS payment adjustment factors under section 1848(q)(6)(A) of the Act and an additional performance threshold for purposes of determining the additional MIPS payment adjustment factors under section 1848(q)(6)(C) of the Act, each of which shall be based on a period prior to the performance period and take into account data available for performance on measures and activities that may be used under the performance categories and other factors determined appropriate by the Secretary. Section 51003(a)(1)(D) of the Bipartisan Budget Act of 2018 (Pub. L. 115-123, February 9, 2018) amended section 1848(q)(6)(D)(iii) of the Act to extend the special rule to apply for the initial 5 years of MIPS instead of only the initial 2 years of MIPS.
In addition, section 51003(a)(1)(D) of the Bipartisan Budget Act of 2018 added a new clause (iv) to section 1848(q)(6)(D) of the Act, which includes an additional special rule for the third, fourth, and fifth years of MIPS (the 2021 through 2023 MIPS payment years). This additional special rule provides, for purposes of determining the MIPS payment adjustment factors under section 1848(q)(6)(A) of the Act, in addition to the requirements specified in section 1848(q)(6)(D)(iii) of the Act, the Secretary shall increase the performance threshold for each of the third, fourth, and fifth years to ensure a gradual and incremental transition to the performance threshold described in section 1848(q)(6)(D)(i) of the Act (as estimated by the Secretary) with respect to the sixth year (the 2024 MIPS payment year) to which the MIPS applies.
We have applied these special rules for the past 5 years to provide for a gradual and incremental transition to the year 6 performance threshold. For further information on established Start Printed Page 39453 performance threshold policies we refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77333 through 77338), CY 2018 Quality Payment Program (82 FR 53787 through 53794), CY 2019 PFS final rule (83 FR 59880 through 59883), the CY 2020 PFS final rule (84 FR 63031 through 63037), and the CY 2021 PFS final rule (85 FR 84919 through 84923). We codified the performance thresholds for each of the first 5 years of MIPS at § 414.1405(b)(4), (5), (6), (7), and (8) as presented in Table 56.

In the CY 2020 PFS final rule (84 FR 63031 through 63037) at § 414.1405(b)(7) and (8), we finalized the performance thresholds for the 2022 and 2023 MIPS payment years at 45 and 60 points, respectively, an increase of 15 points each year until the 2024 MIPS payment year, for which we estimated that the performance threshold would be 74.01 points. We believe that this approach effectively provided for a gradual and incremental transition to the performance threshold we had estimated for the 2024 MIPS payment year, as required by the statute.
Beginning with the 2024 MIPS payment year, section 1848(q)(6)(D)(i) of the Act requires the performance threshold to be the mean or median (as selected by the Secretary) of the final scores for all MIPS eligible clinicians with respect to a prior period specified by the Secretary. That section also provides that the Secretary may reassess the selection of the mean or median every three years. Thus, we considered whether to use the mean or median as the methodology for determining the performance threshold. We would use this methodology to determine a performance threshold for each of the following three years: The 2024 MIPS payment year, 2025 MIPS payment year, and 2026 MIPS payment year. We would then reassess and establish the methodology (mean or median) that we would use for each of the next 3 years (2027 MIPS payment year, 2028 MIPS payment year, and 2029 MIPS payment year). At the time of publication of this proposed rule, we have final score data from the 2017 MIPS performance period/2019 MIPS payment year through the 2019 MIPS performance period/2021 MIPS payment year to use in our assessment of whether to use the mean or median as our methodology for the next 3 years.
From our review of the available data, we have identified the mean and median final scores for each of the 2019 through 2021 MIPS payment years, as shown in Table 57. These six values represent all available prior year mean and median final scores that could be utilized for the 2024 MIPS payment year performance threshold.

As shown in Table 57, using the median final score gives a possible range of performance thresholds from 89.71 points to 99.63 points. Given our performance threshold of 60 points in year 5, these values would result in an increase of 29.71 points to 39.63 points for year 6. Selecting the median of final scores as our methodology would, at a minimum, nearly double the annual increase in the performance threshold of 15 points that we had from year 2 to year 5 of the program. Section 1848(q)(6)(D)(iv) of the Act required that we increase the performance threshold for each of the third, fourth, and fifth years of MIPS to ensure a gradual and incremental transition to the performance threshold we estimated with respect to the sixth year of MIPS. In prior rules we estimated the year six performance threshold to be 74.01 points and used this estimate to determine how to gradually raise the performance threshold (83 FR 59881, 84 FR 63032, 84 FR 40802). Although section 1848(q)(6)(D)(iv) of the Act does not require this approach for the sixth year and subsequent years of MIPS, we believe that it is appropriate to set the performance threshold at a level that is in line with our previous estimates for year 6. We believe that continuing the gradual and incremental increase into year 6 would provide consistency to our stakeholders. After evaluating the possible values shown in Table 57, we believe that using the mean as our methodology would continue this approach.
Using the mean final score as the methodology would yield a possible range of performance thresholds from 74.65 points to 85.61 points (rounded to 75 points and 86 points respectively). Given our performance threshold of 60 points in year 5, these values would result in an increase of 15 points to 26 Start Printed Page 39454 points for year 6. Given these values and our annual performance threshold increases of 15 points for years 2 to 5 of the program, 75 is the value that is most consistent with the gradual and incremental approach that we have elected to continue. Therefore, we are proposing at § 414.1405(g) that for each of the 2024, 2025, and 2026 MIPS payment years, the performance threshold is the mean of the final scores for all MIPS eligible clinicians from a prior period as specified under § 414.1405(b). This methodology will be used for MIPS payment years 2024 through 2026 of the program after which we will reassess the methodology for MIPS payment years 2027 through 2029.
In addition to selecting the methodology (mean or median), section 1848(q)(6)(D)(i) of the Act also requires us to specify a prior period from which we would use the final scores for all MIPS eligible clinicians to calculate the mean or median. As shown in Table 57, the mean final scores are 74.65, 87, and 85.61 points for MIPS payment years 2019 through 2021 respectively. In previous rules (83 FR 59881, 84 FR 63032), we used the MIPS payment year 2019 mean final score to estimate a performance threshold of 74.01 points for year 6 of the program. Our data have been updated to reflect completed targeted reviews since the time we made this estimate, and the mean final score for the 2019 MIPS payment year is now 74.65 points (see Table 57). This value would be an increase of almost exactly15 points from the MIPS payment year 2023 performance threshold of 60 points, which is identical to the increases of the previous three years and consistent with our intention to continue the gradual and incremental approach that has been utilized in prior years. After reviewing the available final score data, we are proposing at § 414.1405(b)(9) to use the MIPS payment year 2019 as the prior period and the rounded mean final score of 75 points as the year 6 performance threshold. When we establish the performance threshold for future MIPS payment years in future rulemaking, we will reassess using the mean final score for MIPS payment year 2019 as mean final scores for subsequent years become available.
We request comments on these proposals, as well as the alternative methodology of the median that we considered but did not propose. Additionally, we request comments on calculating the performance threshold using an alternative year's final scores that we considered but did not propose.
(3) Additional Performance Threshold for Exceptional Performance
Section 1848(q)(6)(D)(ii) of the Act requires the Secretary to compute, for each year of the MIPS (beginning with the 2019 MIPS payment year and ending with the 2024 MIPS payment year), an additional performance threshold for purposes of determining the additional MIPS payment adjustment factors for exceptional performance under section 1848(q)(6)(C) of the Act. For each such year, the Secretary shall apply either of the following methods for computing the additional performance threshold: (1) The threshold shall be the score that is equal to the 25th percentile of the range of possible final scores above the performance threshold determined under section 1848(q)(6)(D)(i) of the Act; or (2) the threshold shall be the score that is equal to the 25th percentile of the actual final scores for MIPS eligible clinicians with final scores at or above the performance threshold with respect to the prior period described in section 1848(q)(6)(D)(i) of the Act. Under section 1848(q)(6)(C) of the Act, a MIPS eligible clinician with a final score at or above the additional performance threshold will receive an additional MIPS payment adjustment factor and may share in the $500 million of funding available for the year under section 1848(q)(6)(F)(iv) of the Act. We note that under section 1848(q)(6)(F)(iv) of the Act, funding is available for additional MIPS payment adjustment factors under section 1848(q)(6)(C) of the Act only through the 2024 MIPS payment year, which is the sixth year of the MIPS program.
In the CY 2020 PFS final rule (84 FR 63037 through 63040), we used the special rule under section 1848(q)(6)(D)(iii) of the Act to set the additional performance threshold at 85 points for the 2022 and 2023 MIPS payment years. We note that the special rule under section 1848(q)(6)(D)(iii) of the Act applies only to the initial 5 years of MIPS, so we cannot use that rule to establish the additional performance threshold for the 2024 MIPS payment year. As noted above, under section 1848(q)(6)(D)(ii) of the Act, we may set the additional performance threshold at either: (1) The 25th percentile of the range of possible final scores above the performance threshold, or (2) the 25th percentile of the actual final scores for MIPS eligible clinicians with final scores at or above the performance threshold with respect to the prior period described in section 1848(q)(6)(D)(i) of the Act.

For illustrative purposes, the possible additional performance thresholds are shown in Table 58. Note that mean or median refers to the methodology for calculation of the performance threshold. As can be seen in Table 58, the potential values for the additional performance threshold range from a low of 81.26 to a high of 100. However, to remain consistent with our gradual and incremental approach, we have proposed to use the mean as our methodology for setting the performance threshold during the next 3 years and we have proposed to use the final score data from MIPS payment year 2019. The selection of the mean for the methodology and final score data from the 2019 MIPS payment year leaves us with the options in the first column of Table 58 for where we can set the additional performance threshold.
As stated above, with a proposed performance threshold of 75 points for the 2024 MIPS payment year based on final scores for the 2019 MIPS payment year, the calculation methods in section 1848(q)(6)(D)(ii) of the Act give us two possible options for where we can set the additional performance threshold for MIPS payment year 2024. The first calculation method (described in section 1848(q)(6)(D)(ii)(I) of the Act), Start Printed Page 39455 using the range of possible final scores above the proposed performance threshold for the 2024 MIPS payment year, yields a value of 81.26 points (the 25th percentile of the range of 75.01 to 100). The calculation is as follows: 75.01 + [(100-75.01) * 0.25] = 81.26. The second calculation method (described in section 1848(q)(6)(D)(ii)(II) of the Act), the 25th percentile of the actual final scores for the 2019 MIPS payment year at or above the proposed performance threshold for the 2024 MIPS payment year, yields a value of 88.94. For the second calculation method, we would apply the 25th percentile calculation of (n+1)p/100 to the 2019 MIPS payment year final score data that are at or above 75.
We considered using each of these methods, but we do not believe that it would be appropriate to lower the additional performance threshold to 81.26 points from its present value of 85 points. Maintaining or increasing the additional performance threshold likely would serve as a greater incentive to clinicians to continue to improve their performance on the MIPS measures and activities and to achieve exceptional performance. We believe that an additional performance threshold of 88.94 points rounded to 89 points is appropriate. This is an increase of 4 points from the prior year, which we believe would be a gradual increase. Therefore, using the second calculation method described above, we are proposing at § 414.1405(d)(7) to set the additional performance threshold for the 2024 MIPS payment year at 89 points.
We request comments on these proposals as well as the alternative additional performance thresholds listed that we considered but did not propose.
(4) Example of Adjustment Factors
Figure A provides an illustrative example of how various final scores would be converted to a MIPS payment adjustment factor and potentially an additional MIPS payment adjustment factor, using the statutory formula and based on our proposed policies for the 2024 MIPS payment year. In Figure A, the performance threshold is set at 75 points. The applicable percentage is 9 percent for the 2024 MIPS payment year. The MIPS payment adjustment factor is determined on a linear sliding scale from zero to 100, with zero being the lowest possible score which receives the negative applicable percentage (negative 9 percent for the 2024 MIPS payment year) and resulting in the lowest payment adjustment, and 100 being the highest possible score which receives the highest positive applicable percentage and resulting in the highest payment adjustment. However, there are two modifications to this linear sliding scale. First there is an exception for a final score between zero and one-fourth of the performance threshold (zero and 18.75 points based on the proposed performance threshold of 75 points for the 2024 MIPS payment year). All MIPS eligible clinicians with a final score in this range would receive the lowest negative applicable percentage (negative 9 percent for the 2024 MIPS payment year). Second, the linear sliding scale line for the positive MIPS payment adjustment factor is adjusted by the scaling factor, which cannot be higher than 3.0.
If the scaling factor is greater than zero and less than or equal to 1.0, then the MIPS payment adjustment factor for a final score of 100 would be less than or equal to 9 percent. If the scaling factor is above 1.0 but is less than or equal to 3.0, then the MIPS payment adjustment factor for a final score of 100 would be greater than 9 percent.
Only those MIPS eligible clinicians with a final score equal to 75 points (which is the proposed performance threshold) would receive a neutral MIPS payment adjustment. Because the performance threshold is 75 points, we anticipate that more clinicians will receive a positive adjustment than a negative adjustment and that the scaling factor would be less than 1 and the MIPS payment adjustment factor for each MIPS eligible clinician with a final score of 100 points would be less than 9 percent.
Start Printed Page 39456

Table 59 illustrates the changes in payment adjustment based on the final policies from the CY 2021 PFS final rule (85 FR 84923 through 84925) for the 2023 MIPS payment year and the proposed policies for the 2024 MIPS payment year, as well as the applicable percent required by section 1848(q)(6)(B) of the Act.
Start Printed Page 39457

(5) Final Score Hierarchy Used in Payment Adjustment Calculation
In the CY 2021 PFS final rule (85 FR 84917 through 84919), we modified the final score hierarchy that applies when more than one final score is associated with a TIN/NPI, as displayed in Table 60. Beginning with the 2021 performance period/2023 MIPS payment year, if a TIN/NPI has a virtual group final score associated with it, we use the virtual group final score to determine the MIPS payment adjustment. If a TIN/NPI does not have a virtual group final score associated with it, we use the highest available final score associated with the TIN/NPI to determine the MIPS payment adjustment.

In section IV.A.3.b.(3) of this proposed rule, we are proposing policies applicable to subgroups, including a definition of a subgroup at § 414.1305 as a subset of a group which contains at least one MIPS eligible Start Printed Page 39458 clinician and is identified by a combination of the group TIN, subgroup identifier, and each eligible clinician's NPI. Each clinician in a subgroup would be identifiable by a unique TIN/NPI combination just as in any MIPS group or APM Entity. In addition, a clinician, group, subgroup, or APM Entity could choose more than one MIPS reporting option for a performance period. A clinician, group, subgroup, or APM Entity could choose to report through MVPs, traditional MIPS, and/or the APP (assuming they are eligible for each of these reporting options) for a performance period. As a result, there could be more than one final score for a clinician, group, subgroup, or APM Entity for a performance period from MVPs, traditional MIPS, and/or the APP. Therefore, we propose to update the scoring hierarchy to include subgroups and to specify that the scoring hierarchy would apply with respect to any available final score that is associated with a TIN/NPI from MVPs, traditional MIPS, and/or the APP. The proposed updated scoring hierarchy can be seen in Table 61. We are seeking comment on this proposal.

g. Review and Correction of MIPS Final Score
(1) Feedback and Information To Improve Performance
Under section 1848(q)(12)(A)(i) of the Act, we are at a minimum required to provide MIPS eligible clinicians with timely (such as quarterly) confidential feedback on their performance under the quality and cost performance categories beginning July 1, 2017, and we have discretion to provide such feedback regarding the improvement activities and Promoting Interoperability performance categories. In the CY 2018 Quality Payment Program final rule (82 FR 53799 through 53801), we finalized that on an annual basis, beginning July 1, 2018, performance feedback will be provided to MIPS eligible clinicians and groups for the quality and cost performance categories, and if technically feasible, for the improvement activities and advancing care information (now called the Promoting Interoperability) performance categories.
On July 1, 2018, we provided the first performance feedback for the Quality Payment Program. The second performance feedback was provided on July 1, 2019. In the CY 2021 PFS proposed rule (85 FR 50321), we noted that we aim to provide performance feedback on or around July 1 of each year, but due to the Public Health Emergency (PHE) and COVID-19, we estimated that we would provide performance feedback for the performance period in 2019 in late July or early August of 2020. The third performance feedback (for the 2019 performance period) was provided on August 5, 2020. We note that similar to the 2019 performance period, due to the PHE for COVID-19, we may provide performance feedback for the 2020 performance period after July 1, 2021. Although we aim to provide performance feedback on or around July 1 of each year, it is possible that the release date could be later than July 1 depending on the circumstances. We estimate that we will provide performance feedback for the performance period in 2020 in early August of 2021, although this timeframe could be subject to change. We direct readers to qpp.cms.gov for more information.
h. Third Party Intermediaries
We refer readers to §§ 414.1305 and 414.1400, the CY 2017 Quality Payment Program final rule (81 FR 77362 through 77390), the CY 2018 Quality Payment Program final rule (82 FR 53806 through 53819), the CY 2019 PFS final rule (83 FR 59894 through 59910), the CY 2020 PFS final rule (84 FR 63049 through 63080), the May 8th COVID-19 IFC (85 FR 27594 through 27595), and the CY 2021 PFS final rule (85 FR 84926 through 84947) for our previously established policies regarding third party intermediaries.
In the CY 2022 PFS proposed rule, we propose to make several changes: (1) Reorganization and consolidation of § 414.1400 generally; (2) new third party intermediaries general requirements; (3) new requirements specific to both QCDR and qualified registries; (4) new requirements specific to only QCDRs; and (5) remedial action and termination of third parties.
(1) Proposed Reorganization and Consolidation of § 414.1400 Generally
We recognize that many of our policies for third party intermediaries are similar or verbatim, and yet in prior rules, we have described them separately. To minimize the lengthiness and burden of reading our policies, we are proposing to consolidate our regulatory text under § 414.1400. To be clear, our proposed updates would not change previously finalized requirements for third party intermediaries, but would bring more clarity and simplicity to the regulatory text. These proposed changes are discussed by topic in more detail below. We also note that in several places at § 414.1400 the regulation text was only updated to reflect both the applicable MIPS performance period/MIPS payment year.
(a) Proposed Reorganization for Requirements Related to MIPS Performance Categories That Must be Supported by Third Party Intermediaries
We previously established in the CY 2017 Quality Payment Program final rule (81 FR 77363 through 77364), and further revised in the Quality Payment Program provisions in the CY 2019 and Start Printed Page 39459 CY 2020 PFS final rules ((83 FR 60088 and 84 FR 63049 through 63052, respectively), and further clarified our requirements of QCDRs, qualified registries, and health IT vendors with regards to submitting data for purposes of the MIPS program in the Quality Payment Program provisions in the CY 2021 PFS final rule, at § 414.1400(a)(2), for our current policy regarding the types of MIPS data that third party intermediaries may submit. Our current requirements at § 414.1400(a)(2) state, beginning with the CY 2021 MIPS performance period/2023 MIPS payment year, QCDRs and qualified registries must be able to submit data for all of the following MIPS performance categories, and Health IT vendors must be able to submit data for at least one of the following MIPS performance categories: Except as provided under (a)(2)(ii), QCDRs, qualified registries, and health IT vendors must be able to submit data for all of the following MIPS performance categories:
- Quality, except:
++ The CAHPS for MIPS survey; and
++ For qualified registries and Health IT vendors, QCDR measures;
- Improvement activities; and
- Promoting Interoperability, if the eligible clinician, group, or virtual group is using CEHRT; however, a third party intermediary may be excepted from this requirement if its MIPS eligible clinicians, groups or virtual groups fall under the reweighting policies at § 414.1380(c)(2)(i)(A)(4) or (5) or (c)(2)(i)(C)(1) through (7) or (c)(2)(i)(C)(9)).
++ Health IT vendors that do not support MIPS Value Pathways must be able to submit data for at least one of the MIPS performance categories described in paragraphs (a)(2)(i)(A) through (C) of this section.
++ Promoting Interoperability, if the eligible clinician, group, or virtual group is using CEHRT; however, a third party intermediary may be excepted from this requirement if its MIPS eligible clinicians, groups or virtual groups fall under the reweighting policies at § 414.1380(c)(2)(i)(A)(4) or (5) or § 414.1380(c)(2)(i)(C)(1) through (7) or § 414.1380(c)(2)(i)(C)(9)).
In an effort to simplify, we propose reorganizing existing language at § 414.1400(a)(2). Specifically, we propose providing updates to separately identify and provide clarity to data submission requirements since data requirements vary based on third party intermediary type and to provide clarification to exceptions to Promoting Interoperability for virtual groups and subgroups. We propose the following updates:
- To revise and redesignate existing paragraph at § 414.1400(a)(2) through (a)(2)(i) to proposed paragraphs § 414.1400(b)(1)(i) and (c)(1) through (c)(1)(i) to state the following:
- To state at proposed § 414.1400(b)(1)(i), beginning with the CY 2021 MIPS performance period/2023 MIPS payment year, QCDRs and qualified registries must be able to submit data for all of the following MIPS performance categories:
- Quality, except:
++ The CAHPS for MIPS survey; and
++ For qualified registries, QCDR measures.
- Improvement activities; and
- Promoting Interoperability, if the eligible clinician, group, virtual group, or subgroup is using CEHRT, unless:
++The third party intermediary's MIPS eligible clinicians, groups, virtual groups, or subgroups fall under the reweighting policies at § 414.1380(c)(2)(i)(A)(4)(i) through (iii) or (c)(2)(i)(C)(1) through (7) or (c)(2)(i)(C)(9)).
- To state at proposed § 414.1400(c)(1), beginning with the CY 2021 MIPS performance period/2023 MIPS payment year, health IT vendors must be able to submit data for the MIPS performance categories as follows:
- To state at proposed § 414.1400(c)(1)(i) through (c)(1)(i)(B), health IT vendors that support MVPs must be able to submit data for all of the MIPS performance categories:
- Quality, except:
++ The CAHPS for MIPS survey; and
++ QCDR measures;
- Improvement activities; and
- To revise and redesignate existing paragraph at § 414.1400(a)(2)(iii) to proposed paragraph § 414.1400(c)(1)(i)(C) state, Promoting Interoperability, if the eligible clinician, group, virtual group, or subgroup is using CEHRT, unless:
++ The third party intermediary's MIPS eligible clinicians, groups, virtual groups, or subgroups fall under the reweighting policies at § 414.1380(c)(2)(i)(A)(4)(i) through (iii) or (c)(2)(i)(C)(1) through (7) or (c)(2)(i)(C)(9).
- To revise and redesignate existing paragraph at § 414.1400(a)(2)(ii) to proposed paragraph § 414.1400(c)(1)(ii) to state, health IT vendors that do not support MVPs must be able to submit data for at least one of the MIPS performance categories described in paragraphs (c)(1)(i) through (iii) of this section.
- We propose to create a new requirement at § 414.1400(c)(1)(iii) for health IT vendors to support MVPs. For more information on this proposal, please refer to section "proposed new requirement for third party intermediaries to support MVPs and the APP" below at IV.A.3.h.(2)(b)(i).
- To move the current Health IT vendor requirements from paragraphs § 414.1400 (a)(2)(ii) through (iii) and § 414.1400 (d) to a proposed new section applicable to Health IT vendor requirements at § 414.1400(c). This will separately identify and provide clarity to data submission requirements specific to Health IT vendors.
- To move the current CMS-approved survey vendor requirements from paragraphs (a)(3) and (e) to a proposed new section applicable to CMS-approved survey vendor requirements at § 414.1400(d). We propose to the redesignate paragraph (a)(3) current requirements to paragraph (d)(1) CMS-approved survey vendors may submit data on the CAHPS for MIPS survey for the MIPS quality performance category. For the current requirements at paragraph (e), we propose to move up those requirements to paragraph (d)(2).
- To redesignate paragraph (a)(4) as paragraph (a)(2).
- To redesignate paragraph (a)(5) as paragraph (a)(3).
We request public comment on our proposals.
(b) Proposed Reorganization for Requirements Related QCDR and Qualified Registries Self-Nomination
We are proposing to consolidate and redesignate the existing language at § 414.1400(b)(1) and (c)(1) to proposed § 414.1400(b)(2) to reference both QCDR and qualified registries. We are proposing this consolidation to provide clarity and alignment with the aforementioned proposals and consolidate the duplicative criteria of QCDRs and qualified registries. As discussed below, we are also proposing to consolidate and redesignate the performance feedback requirements previously at existing § 414.1400(b)(1) and (c)(1) to § 414.1400(b)(3)(iii). We propose to state at § 414.1400 (b)(2), Self-nomination. For the 2018 and 2019 performance periods/2020 and 2021 MIPS payment years, entities seeking to qualify as a QCDR or qualified registry must self-nominate September 1 until November 1 of the CY preceding the applicable performance period. For the 2020 MIPS performance period/2022 MIPS payment year and future years, entities seeking to qualify as a QCDR or qualified registry must self-nominate during a 60-day period during the CY preceding the applicable performance period (beginning no earlier than July 1 and ending no later than September 1). Entities seeking to qualify as a QCDR or Start Printed Page 39460 qualified registry for a performance period must provide all information required by CMS at the time of self-nomination and must provide any additional information requested by CMS during the review process. For the 2019 MIPS performance period/2021 MIPS payment year and future years, existing QCDRs and qualified registries that are in good standing may attest that certain aspects of their previous year's approved self-nomination have not changed and will be used for the applicable performance period.
We also propose removing the last two sentences of existing § 414.1400(b)(1), which are duplicative with existing § 414.1400(b)(3)(iii). We propose consolidating this language with existing paragraph (b)(3)(iii). We request public comment on our proposals.
(c) Proposed Reorganization for Requirements Related to QCDR and Qualified Registries Conditions for Approval
We refer readers to existing § 414.1400(b)(2) for QCDR conditions for approval and existing § 414.1400(c)(2) for qualified registries conditions for approval. In this proposed rule, we are proposing the following in order to better organize, consolidate the duplicative criteria of QCDRs and qualified registries, and refer to both "QCDR and qualified registry" instead of one or the other:
- We propose to redesignate existing paragraph (b)(2) to proposed paragraph (b)(3) and revising the paragraph heading as, Conditions for approval.
- We also propose updating the reference to both QCDR and qualified registry in proposed paragraph (b)(3).
- We propose to revise to include both QCDR and qualified registry and redesignate existing paragraph (b)(2)(i) to proposed paragraph (b)(3)(i).
- We propose to revise and redesignate existing paragraph (b)(2)(ii) to proposed paragraph (b)(3)(ii). We also propose to extend our policy for collaboration. For more information on this proposal, please refer to section "collaboration of entities to become a QCDR and proposal to extend policy for collaboration of entities to become a qualified registry" below at section IV.A.3.h.(3)(a)(ii) of this proposed rule.
- As discussed above, we are proposing to consolidate and redesignate the performance feedback requirements previously at existing § 414.1400(b)(1) and (c)(1) to § 414.1400(b)(3)(iii). Furthermore, to consolidate similar performance feedback requirements, we also propose to revise and redesignate existing paragraph (b)(2)(iii) to proposed paragraph (b)(3)(iii) to state, beginning with the 2021 MIPS performance period/2023 MIPS payment year, require the QCDR or qualified registry must to provide performance feedback to their clinicians and groups at least 4 times a year, and provide specific feedback to their clinicians and groups on how they compare to other clinicians who have submitted data on a given measure within the QCDR or qualified registry. Exceptions to this requirement may occur if the QCDR or qualified registry submits notification to CMS within the reporting period promptly within the month of realization of the impending deficiency and provides sufficient rationale as to why they do not believe they would be able to meet this requirement (for example, if the QCDR does not receive the data from their clinician until the end of the performance period).
- We propose to consolidate and redesignate paragraphs (b)(2)(iv) and (c)(2)(iii) in their entirety, into a new paragraph (b)(3)(v), and to correct a typographical error in which the word "MIPS" was omitted in the first sentence.
- We propose to consolidate and redesignate paragraphs (b)(2)(v) and (c)(3)(iv), in their entirety, into a new paragraph (b)(3)(vi).
We invite public comment on our proposals.
(d) Proposed Reorganization for Requirements Related to QCDR Measures
(i) Proposed Reorganization for Requirements Related to QCDR Measures for the Quality Performance Category
We refer readers to existing language at § 414.1400(b)(3) for QCDR measures for the quality performance category. We currently define "QCDR measure" at existing § 414.1400(b)(3). We recognize that the QCDR measure definition is referred to throughout our policies and that it is not specific to § 414.1400(b)(3) or third party intermediaries. Therefore, to provide further clarity and to better align with the current policy, we propose moving the QCDR measure definition to the definitions section at § 414.1305. We are also proposing the following revisions to better organize regulation text at § 414.1400(b)(4) and to update cross-references to correspond to the new section numbers as reflected in this proposed rule:
- We propose to redesignate paragraphs (b)(3)(i), (b)(3)(i)(A), and (b)(3)(i)(B) to definitions at § 414.1305.
- We propose to revise and redesignate existing paragraphs at (b)(3)(ii) to proposed paragraphs (b)(4)(i) to state, for the 2018 MIPS performance period/2020 MIPS payment year and future years, at the time of self-nomination an entity seeking to become a QCDR must submit the following information for any measure it intends to submit for the payment year:
++ For MIPS quality measures, the entity must submit specifications including the MIPS measure IDs and specialty-specific measure sets, as applicable.
+ For QCDR measures, the entity must submit for CMS-approval measure specifications including: Name/title of measures, NQF number (if NQF-endorsed), descriptions of the denominator, numerator, and when applicable, denominator exceptions, denominator exclusions, risk adjustment variables, and risk adjustment algorithms. In addition, no later than 15 calendar days following CMS approval of any QCDR measure specifications, the entity must publicly post the measure specifications for that QCDR measure (including the CMS-assigned QCDR measure ID) and provide CMS with a link to where this information is posted.
- We also propose adding a header to state, "QCDR measure self-nomination requirements". We believe adding a heading will help readers clearly distinguish QCDR measure self-nomination requirements.
- We propose moving existing paragraph at (b)(3)(iii) in its entirety to proposed paragraph (b)(4)(ii) and adding a header to state, "QCDR measure submission requirements". We believe adding a heading will help readers clearly distinguish QCDR measure submission requirements.
- We propose moving existing paragraphs at (b)(3)(v) through (v)(C)(1) in its entirety, to proposed paragraph (b)(4)(iii) through (b)(4)(iii)(A)(3).
- We propose to revise and redesignate existing paragraph at (b)(3)(v)(C)(2) to proposed paragraph (b)(4)(iii)(A)(3)(i) to state, to be included in an MVP for the 2022 MIPS performance period/2024 MIPS payment year and future years, a QCDR measure must be fully tested.
- We propose moving existing paragraph at (b)(3)(vii) in its entirety, to proposed paragraph (b)(4)(iv).
(ii) Proposed Reorganization for Requirements Related to QCDR Measure Approval Criteria
We refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77374 through 77375) and the Quality Payment Program provisions in the CY 2020 PFS final rule (84 FR Start Printed Page 39461 63059), where we finalized existing § 414.1400(b)(3)(v).
We propose to reorganize and make minor updates to the existing requirements at § 414.1400(b)(3)(v) to proposed § 414.1400(b)(4)(iii). We propose to reorganize the existing requirements so that QCDR measure approval at § 414.1400(b)(3)(v) is discussed before QCDR measure considerations at § 414.1400(b)(3)(iv). Therefore, we propose the following revisions:
- To revise and redesignate existing paragraph (b)(3)(v) "QCDR measure requirement for approval include" to proposed paragraph (b)(4)(iii) and add a heading to state, "QCDR measure approval criteria". We believe adding a heading will help readers clearly distinguish QCDR measure approval criteria. We also propose to include the following updates:
++ Move existing (b)(3)(v) to proposed revised paragraph (b)(4)(iii)(A) to state, QCDR measure requirements for approval are.
++ Move existing paragraph (b)(3)(v)(A) in its entirety to proposed paragraph (b)(4)(iii)(A)(1).
++ Move existing paragraph (b)(3)(v)(B) in its entirety to proposed paragraph (b)(4)(iii)(A)(2).
++ Revise existing paragraphs (b)(3)(v)(C) and (b)(3)(v)(C)(1) to proposed paragraph (b)(4)(iii)(A)(3) to state, beginning with the 2022 MIPS performance period/2024 MIPS payment year, all QCDR measures must meet face validity. To be approved for the 2023 MIPS performance period/2025 MIPS payment year, all QCDR measures must be must meet face validity for the initial MIPS payment year for which it is approved. For subsequent years, all QCDR measures must be fully developed and tested, with complete testing results at the clinician level, prior to submitting the QCDR measure at the time of self-nomination.
++ Move existing paragraph (b)(3)(v)(C)(2) in its entirety to proposed paragraph (b)(4)(iii)(A)(3)(i).
++ Move existing paragraph (b)(3)(v)(D) in its entirety to proposed paragraph (b)(4)(iii)(A)(4).
++ Move existing paragraph (b)(3)(v)(E) in its entirety to proposed paragraph (b)(4)(iii)(A)(5).
We invite public comment on our proposals.
(iii) Proposed Reorganization for Requirements Related to QCDR Measure Considerations for Approval
We refer readers to the Quality Payment Program provisions in the CY 2019 PFS final rule (84 FR 63198 through 63199), where we finalized existing § 414.1400(b)(3)(iv) "QCDR measure considerations for approval". We propose to reorganize and make minor updates to the language at existing § 414.1400(b)(3)(iv) to proposed § 414.1400(b)(4)(iii)(B). We propose to reorganize the existing requirements so that QCDR measure approval at § 414.1400(b)(3)(v) is discussed before QCDR measure considerations at § 414.1400(b)(3)(iv).
We also propose to redesignate existing § 414.1400(b)(3)(vi) to proposed § 414.1400(b)(4)(iii)(C). Specifically, we propose the following revisions:
- To revise and redesignate existing paragraph (b)(3)(iv) "QCDR measure considerations for approval include" to proposed paragraph (b)(4)(iii)(B) "QCDR measure considerations for approval include, but are not limited to".
- Move existing paragraph (b)(3)(iv)(A) in its entirety to proposed paragraph (b)(4)(iii)(B)(1).
- Move existing paragraph (b)(3)(iv)(B) in its entirety to proposed paragraph (b)(4)(iii)(B)(2).
- Move existing paragraph (b)(3)(iv)(C) in its entirety to proposed paragraph (b)(4)(iii)(B)(3).
- Move existing paragraph (b)(3)(iv)(D) in its entirety to proposed paragraph (b)(4)(iii)(B)(4).
- Move existing paragraph (b)(3)(iv)(E) in its entirety to proposed paragraph (b)(4)(iii)(B)(5).
- Move existing paragraph (b)(3)(iv)(F) in its entirety to proposed paragraph (b)(4)(iii)(B)(6).
- Move existing paragraph (b)(3)(iv)(G) in its entirety to proposed paragraph (b)(4)(iii)(B)(7).
- Revise and consolidate existing paragraph (b)(3)(iv)(G)(1) to proposed paragraph (b)(4)(iii)(B)(7)(i) to state that QCDR link their QCDR measures as feasible to at least one cost measure, improvement activity, or an MVP at the time of self-nomination.
- Move existing paragraph (b)(3)(iv)(G)(2) in its entirety to proposed paragraph (b)(4)(iii)(B)(7)(ii).
- Move existing paragraph (b)(3)(iv)(H) in its entirety to proposed paragraph (b)(4)(iii)(B)(8).
- Move existing paragraph (b)(3)(iv)(I) in its entirety to proposed paragraph (b)(4)(iii)(B)(9).
- Move existing paragraph (b)(3)(iv)(J) in its entirety to proposed paragraph (b)(4)(iii)(B)(10).
- Move existing paragraph (b)(3)(iv)(J)(2) to proposed paragraph Reserve (b)(4)(iii)(B)(10)(ii).
- Move existing paragraph (b)(3)(vi) in its entirety to proposed paragraph (b)(4)(iii)(C).
We invite public comment on our proposals.
(iv) QCDR Measure Rejection Criteria
We refer readers to the existing requirements at § 414.1400(b)(3)(vii). We propose reorganizing existing requirements at § 414.1400(b)(3)(vii) to proposed § 414.1400(b)(4)(iv). Therefore, we propose the following revisions:
- To revise and redesignate existing paragraph (b)(3)(vii) "QCDR measure rejection criteria" to proposed paragraph (b)(4)(iv) and add a heading to state, QCDR measure rejection criteria. We believe adding a heading will help readers clearly distinguish measure rejection criteria.
We also propose to include the following updates:
- To move existing paragraph (b)(3)(vii) to proposed paragraph (b)(4)(iv) to state, QCDR measure rejection criteria. Beginning with the 2020 MIPS performance period/2022 MIPS payment year, QCDR measure rejection considerations include, but are not limited to.
- Move existing paragraphs (b)(3)(vii)(A) through (L) in their entirety to proposed (b)(4)(iv)(A) through (L).
We invite public comment on our proposals.
(e) Proposed Reorganization for Requirements Related to Remedial Action and Termination of Third Party Intermediaries
We refer readers to § 414.1400(f), the CY 2017 Quality Payment Program final rule (81 FR 77548), CY 2019 PFS final rule (83 FR 59908 through 59910), the CY 2020 PFS final rule (84 FR 63077 through 63080), and the CY 2021 PFS final rule (85 FR 84930 through 84937) for previously finalized policies for remedial action and termination of third party intermediaries. With the proposed updates being made at § 414.1400, we propose to redesignate the following sections:
- We propose to redesignate current paragraph (f) as paragraph (e) and to update cross-references to correspond to the new section numbers as reflected in this proposed rule.
- We also propose to redesignate current paragraph (g) as paragraph (f) and to update cross-references to correspond to the new section numbers as reflected in this proposed rule.
We invite public comment on our proposals.
(2) Third Party Intermediaries General Requirements
We refer readers to previously established § 414.1400(a) and the CY 2017 Quality Payment Program final Start Printed Page 39462 rule (81 FR 77363 through 77364), and as further revised in the CY 2019 PFS final rule (83 FR 60088), CY 2020 PFS final rule (84 FR 63049 through 63052), CY 2021 PFS final rule (85 FR 84926 through 84947) for our established policy regarding third party intermediaries general requirements.
In this proposed rule, we propose a couple changes for third party intermediaries: (1) Third party intermediary submissions for APM Entities; and (2) MIPS performance categories that must be supported by third party intermediaries. We are also requesting comment on third party intermediaries that derive data from CEHRT. These proposals and request for comment are discussed in more detail below.
(a) Third Party Intermediary Submissions for APM Entities
As finalized in the Quality Payment Program provisions in the CY 2021 PFS final rule (85 FR 84895), APM Entities now have the option of reporting to MIPS on behalf of the MIPS eligible clinicians participating in their APM Entity. They have the option of reporting to traditional MIPS or via the APP (85 FR 84859). APM Entities have historically used Third Party Intermediaries for submitting their quality measures to their APMs, rather than to MIPS, however, these third party intermediaries now have the opportunity to submit these data for purposes of MIPS.
In this proposed rule, we are proposing to add APM Entities to § 414.1400(a)(1), expanding the general participation requirements of third party intermediaries to third party intermediaries reporting to MIPS on behalf of APM Entities in order to align reporting requirements for all participants in MIPS.
We note that the Promoting Interoperability performance category is scored for APM Entities based on data submitted by the participant MIPS eligible clinicians and groups as described at § 414.1317(b)(1), and therefore would not be required to be submitted by the third party intermediary on behalf of the APM Entity.
We request comment on this proposal.
(b) MIPS Performance Categories That Must Be Supported by Third Party Intermediaries
We refer readers to previously established § 414.1400(a)(2) and the CY 2017 Quality Payment Program final rule (81 FR 77363 through 77364), and as further revised in the CY 2019 PFS final rule (83 FR 60088), CY 2020 PFS final rule (84 FR 63049 through 63052), CY 2021 PFS final rule (85 FR 84926 through 84947) for our established policy regarding the types of MIPS data that third party intermediaries may submit.
In this proposed rule, we are proposing new requirements in alignment with our proposals in section IV.A.3.b. of this proposed rule to adopt MVPs and subgroups.
(i) Proposed New Requirement for Third Party Intermediaries To Support MVPs and the APP
As described in the Quality Payment Program provisions finalized in the CY 2021 PFS final rule (85 FR 84849), MVPs should include measures and activities from the quality, cost, improvement activities, and Promoting Interoperability performance categories. As described in section IV.A.3.b. of this proposed rule, we discuss our proposals related to furthering our transition to MIPS Value Pathways (MVPs). As MVPs are implemented, proposed beginning with the 2023 MIPS performance period/2025 MIPS payment year, we believe it is important to also propose the methods in which an MVP participant may report on an MVP or the APP. Since QCDRs, qualified registries, and health IT vendors are required under existing § 414.1400(a)(1) to submit data for quality, improvement activities, and promoting interoperability, we believe they would have the experience needed to support MVP and APP reporting.
Therefore, we propose to create a new requirement at § 414.1400(b)(1)(ii) to state that, beginning with the 2023 MIPS performance period/2025 MIPS payment year, QCDRs and qualified registries must support MVPs that are applicable to the MVP participants on whose behalf they submit MIPS data. QCDRs and qualified registries may also support the APP. Additionally, we propose to create a new requirement at paragraph § 414.1400(c)(1)(iii) to state that beginning with the 2023 MIPS performance period/2025 MIPS payment year, Health IT vendors must support MVPs that are applicable to the MVP participants on whose behalf they submit MIPS data. Health IT vendors may also support the APP.
Based off historical participation, we are aware that some third party intermediaries (QCDRs and qualified registries) support a single specialty or subspecialty, while others support multiple specialties. Therefore, we believe that it is not appropriate to expect that all third party intermediaries are able to support all MVPs that are implemented in the program. Rather, the third party intermediaries should identify and support MVPs that are relevant to the clinicians and groups they support. We do not believe that CMS-approved survey vendors will be able to support MVP reporting, because they are historically limited, in that they only support the CAHPS for MIPS Survey Measure.
As proposed in section IV.A.3.b of this proposed rule, MVPs would start with the 2023 MIPS performance period/2025 MIPS payment year. We believe this delay in implementation would allow third party intermediaries sufficient time for programming and system preparation for MVP reporting success. We request comment on our proposals.
(ii) Proposed Requirements for All Third Party Intermediaries To Support Subgroup Reporting
As proposed in section IV.A.3.b.(3) of this proposed rule, subgroup reporting would allow clinicians in multispecialty practices to participate in MIPS more meaningfully. Since subgroups would be implemented concurrently with MVPs, it is important that third party intermediaries have the capability to support subgroup reporting of MVPs. As described above, we believe QCDRs, qualified registries, and health IT vendors would have the capacity to support MVP and APP reporting.
In this proposed rule, we propose to require QCDRs, qualified registries, health IT vendors, and CAHPS for MIPS survey vendors to support subgroup reporting, beginning with the 2023 MIPS performance period/2025 MIPS payment year. Therefore, we propose to revise § 414.1400(a)(1) to state that MIPS data may be submitted on behalf of a MIPS eligible clinician, group, virtual group, subgroup or APM Entity by any of the following third party intermediaries: QCDR; qualified registry; health IT vendor; or CMS-approved survey vendor. We believe it is imperative for all third party intermediaries to be able to support subgroup reporting as we envision that to be the future of the program.
While the CAHPS for MIPS survey vendors cannot support MVPs or the APP, we believe they can support the reporting of the CAHPS for MIPS measure within an MVP and the APP, if a subgroup decides to report on that measure. Due to the limited experience, CAHPS for MIPS survey vendors have in quality reporting, we do not believe it is feasible for them to support MVP reporting since MVP reporting would require experience with reporting across the performance categories and the use of several collection types for quality Start Printed Page 39463 reporting. However, there may be instances where the CAHPS for MIPS survey measure may be included in an MVP. For example, in the Optimizing Chronic Conditions Management MVP, as described in Appendix 3: MVP Inventory, of this proposed rule. In such instances, if groups or subgroups would like to report this measure, they should be able to utilize a CAHPS for MIPS survey vendor to do so. We believe it is important that all third party intermediaries support subgroup reporting in order to support meaningful quality reporting. We understand that there may be a level of burden to third party intermediaries that are required in supporting subgroup reporting by requiring them to support another clinician type. However, we believe that requiring third party intermediaries to support subgroup reporting will allow for clinicians to participate in a manner that is more meaningful. We note that as proposed in section IV.A.3.b.(4)(f) of this proposed rule, subgroups would have to register through the MVP participant registration process. Third party intermediaries would need to be able to track the subgroup identifiers and support the data submission process accordingly.
We request comments on our proposal.
(c) Request for Comment on Third Party Intermediaries That Derive Data From CEHRT
For third party intermediaries that will be submitting quality measure data on behalf of MIPS eligible clinicians, we believe that EHR systems will be able to provide measure results for a set of providers that are part of a subgroup where required for subgroup reporting. We note that the existing CEHRT definition for eligible clinicians at § 414.1305 includes the 45 CFR 170.315(c)(4) "Clinical quality measures—filter" as an optional element. This criterion requires health IT to be able to filter CQM results at both patient and aggregate levels. Moreover, a Health IT Module must be able to filter by a single proposed data element (for example, provider type) or a combination of any of the data elements). Historically, the "Clinical quality measures—filter" at 45 CFR 170.315(c)(4)" (CQM-filter) criterion has been applicable for certified health IT modules supporting quality measurement for participants in certain APMs.
We believe technology certified to this optional criterion could support subgroup reporting via third party intermediaries that derive data from CEHRT by ensuring that an EHR can produce CQM results filtered for a specific group of provider NPIs that are part of a subgroup. These filtered CQM results could then be shared with a third party intermediary, which provides this data for reporting to CMS. However, we also believe health IT developers are offering non-certified functionality that can effectively support reporting of measure results for a subgroup. As a result, we are not proposing any changes at this time to the language in the CEHRT definition for eligible clinicians regarding the "optional" status of technology certified to the CQM-filter criterion.
We are interested in general feedback from stakeholders on the current capabilities of third party intermediaries that derive data from CEHRT to successfully receive and transmit data to CMS for CQMs based on subgroups; capabilities of EHR systems to support subgroup reporting, including reporting facilitated by third party intermediaries, and whether requiring the adoption of technology certified to the CQM-filter criterion would help to support subgroup reporting; and challenges which entities may face in meeting requirements to report on subgroups when deriving data from CEHRT. We request feedback on this topic.
(3) New Requirements for Both Qualified Clinical Data Registries (QCDRs) and Qualified Registries
(a) Background
We refer readers to §§ 414.1305 and 414.1400, the CY 2017 Quality Payment Program final rule (81 FR 77362 through 77390), the CY 2018 Quality Payment Program final rule (82 FR 53806 through 53819), the CY 2019 PFS final rule (83 FR 59894 through 59910), the CY 2020 PFS final rule (84 FR 63049 through 63080), the May 8th COVID-19 IFC (85 FR 27594 through 27595), and the CY 2021 PFS final rule (85 FR 84926 through 84947) for our previously established policies regarding QCDRs and qualified registries.
In this proposed rule, we propose several changes for both QCDRs and qualified registries: (1) New requirement for approved QCDRs and qualified registries that have not submitted performance data; (2) collaboration of entities to become a QCDR and qualified registry; and (3) data validation audit and targeted audit requirements. These proposals are discussed in more detail below.
(i) Proposed New Requirement for Approved QCDRs and Qualified Registries That Have Not Submitted Performance Data
We require that both QCDRs and qualified registries must have a minimum of 25 participants signed up by the prior performance period at existing § 414.1400(b)(2) and (c)(2). We refer readers to CY 2017 Quality Payment Program final rule (81 FR 77362 through 77390), the CY 2018 Quality Payment Program final rule (82 FR 53806 through 53819), the CY 2019 PFS final rule (83 FR 59894 through 59910), the CY 2020 PFS final rule (84 FR 63049 through 63080), the May 8th COVID-19 IFC (85 FR 27594 through 27595), and the CY 2021 PFS final rule (85 FR 84926 through 84947). We identified a number of QCDRs and qualified registries that have continued to self-nominate to become a third party intermediary for the MIPS program, but have not submitted clinician, group or virtual group data to CMS. As the MIPS program continues to mature, we wish to reduce the number of vendors that self-nominate to become a qualified vendor, but do not actively participate in the MIPS program. We believe that maintaining these vendors who do not actively participate does not provide a benefit to the MIPS program, rather it creates stakeholder confusion by including these vendors in our qualified postings.
We are proposing a two-tiered approach to solve this issue. First, we propose to create a new requirement at § 414.1400(b)(3)(vii) to require QCDRs and qualified registries that have never submitted data since the inception of MIPS (2017 MIPS performance period/2019 MIPS payment year) through the 2020 MIPS performance period/2022 MIPS payment year, to submit a participation plan as part of their self-nomination for CY 2023. Exceptions to this requirement may occur if data is received for the 2021 MIPS performance period/2023 MIPS payment year. Under this scenario, QCDRs and qualified registries would not need to submit a participation plan for CY 2023 of the self-nomination period. If they do not submit data, their participation plan must be submitted as part of self-nomination for CY 2023 and must be accepted by CMS to continue to be an approved QCDR or qualified registry.
Secondly, we propose to codify a new requirement at paragraph (b)(3)(viii) to state, beginning with the 2024 MIPS performance period/2026 MIPS payment year, a QCDR or qualified registry that was approved but did not submit any MIPS data for either of the Start Printed Page 39464 2 years preceding the applicable self-nomination period must submit a participation plan for CMS's approval. For example, for the 2024 MIPS performance period/2026 MIPS payment year, vendors will be required to have submitted performance data for the 2021 and 2022 MIPS performance periods/2023 and 2024 MIPS payment years. Under this proposal, the participation plan must explain the QCDR's or qualified registry's detailed plans about how the vendor intends to encourage clinicians to submit MIPS data to CMS through the third party intermediary on behalf of clinicians or groups. The vendor must also explain why they should still be allowed to participate as a qualified vendor. We note that this proposed participation plan was modeled off of the current requirement for QCDR measure participation at existing § 414.1400(b)(3)(iv)(J)(1) (redesignated to proposed § 414.1400(b)(4)(iii)(B)(10)(i)). We request comments on this proposal.
(ii) Collaboration of Entities To Become a QCDR and Proposal To Extend Policy for Collaboration of Entities To Become a Qualified Registry
(A) Background
In the CY 2017 Quality Payment Program final rule (81 FR 77377), we finalized to allow collaboration of entities to become a QCDR based on our experience with the qualifying entities wishing to become QCDRs for performance periods. We stated that we believed our previously finalized policy supporting entity collaboration should be continued under MIPS. Therefore, we discussed that an entity that may not meet the criteria of a QCDR solely on its own, but could do so in conjunction with another entity and would be eligible for qualification through collaboration with another entity. Additionally, we finalized at § 414.1400(b)(2)(ii), specifically for QCDRs, that if the entity uses an external organization for purposes of data collection, calculation, or transmission, it must have a signed, written agreement with the external organization that specifically details the responsibilities of the entity and the external organization. The written agreement must be effective as of September 1 of the year preceding the applicable performance period.
For example, an entity, such as a specialty society, that needs technical support may partner with an outside entity such as a health IT vendor to qualify as a QCDR. While entities, such as QCDRs, Health IT vendors, and qualified registries, can collaborate with external organizations, those entities could only do so to meet requirements to be a QCDR. We did not explicitly create a policy for entities to collaborate to meet the requirements to be a qualified registry.
(B) Proposal To Extend to Qualified Registries
We believe we should extend the previously finalized policy to apply to entities who wish to collaborate to become a qualified registry, as well because extending this policy to qualified registries would also help smaller societies that may not have the resources on their own to become a qualified registry. This will allow those societies to be able to partner with other entities to meet the definition of a qualified registry. Therefore, in this proposed rule, we propose to revise and redesignate existing paragraph (b)(2)(ii) to new paragraph (b)(3)(ii) to state, if the entity seeking to qualify as a QCDR or qualified registry uses an external organization for purposes of data collection, calculation, or transmission, it must have a signed, written agreement with the external organization that specifically details the responsibilities of the entity and the external organization. The written agreement must be effective as of September 1 of the year preceding the applicable performance period. For example, an entity, such as a specialty society, that needs technical support may partner with an outside entity such as a health IT vendor to qualify as a qualified registry. We request comments on this proposal.
(iii) Data Validation Audit and Targeted Audit Requirements
(A) Information Required at the Time of Self-Nomination
In the CY 2017 Quality Payment Program final rule (81 FR 77366 through 77367; 81 FR 77383 through 77384) we discussed our expectation for QCDRs and qualified registries to conduct validation on the data they intend to submit for the MIPS performance period. We also discussed that the full self-nomination process would require the following: A submission of basic information, a description of the process the QCDR and qualified registry will use for completion of a targeted audit of a subset of data prior to submission, the provision of a data validation plan along with the results of the executed data validation plan by May 31 of the year following the performance period. Additionally, in the Quality Payment Program provisions in the CY 2021 PFS final rule (85 FR 84930 through 84937; 85 FR 84944 through 84947) at existing § 414.1400(b)(2)(iv) and (v) and § 414.1400(c)(2)(iii) and (iv), we finalized the data validation audit requirements as condition for approval. While we did finalize the requirements for the data validation audits as condition for approval, we did not codify the requirements for QCDR and qualified registries to submit data validation plan during self-nomination along with the results of the executed data validation plan by May 31 of the year following the performance period.
In order to provide clarification and to better align with the previously finalized policy (81 FR 77366 through 77367; 81 FR 77383 through 77384), we propose to codify the following revisions. As stated in previous polices (81 FR 77366 through 77367;81 FR 77383 through 77384), QCDRs and qualified registries are required to submit the results of their data validation plan to CMS by May 31 of the year following the performance period. Therefore, we propose to codify at § 414.1400(b)(3)(v)(G)(1) to state that QCDRs and qualified registries must conduct validation on the data they intend to submit for the applicable MIPS performance period, and provide the results of the executed data validation plan by May 31st of the year following the performance period.
Furthermore, QCDRs and qualified registries are required to submit their data validation plan explaining their process of data validation submission annually during self-nomination, and it must be approved by CMS for before use. To provide further clarity and to better align with the existing policy (81 FR 77366 through 77367; 81 FR 77383 through 77384), we also propose to codify a new requirement at § 414.1400(b)(3)(iv) to state that, beginning with the CY 2023 MIPS performance period/2025 MIPS payment year, the QCDR or qualified registry must submit a data validation plan annually, at the time of self-nomination, for CMS' approval, and may not change the plan once approved, without the prior approval of the agency.
As discussed above we propose to codify at § 414.1400(b)(3)(iv) to provide further clarity to better align with previous policies. Therefore, we propose to reorganize at § 414.1400(b)(2)(iv) though (viii) to better align with the above changes. We propose with the following revisions:
- We propose to revise and redesignate existing paragraph (b)(2)(iv) to proposed paragraph (b)(3)(v) to state, that beginning with the 2021 MIPS Start Printed Page 39465 performance period/2023 MIPS payment year, the QCDR or qualified registry must conduct annual data validation audits in accordance with this paragraph (b)(3)(v).
- We propose to revise and redesignate existing paragraph (b)(2)(iv)(A) to proposed paragraph (b)(3)(vi)(A) to state that, if a data validation audit under paragraph (b)(3)(v) identifies one or more deficiency or data error, the QCDR or qualified registry must conduct a targeted audit into the impact and root cause of each such deficiency or data error for that MIPS payment year.
- We propose to revise and redesignate existing paragraph (b)(2)(v) to proposed paragraph (b)(3)(vi) to state that beginning with the 2021 MIPS performance period/2023 MIPS payment year, the QCDR or qualified registry must conduct targeted audits in accordance with this paragraph (b)(3)(vi).
- We propose to revise and redesignate paragraph (b)(2)(vi) to proposed paragraph (b)(3)(vii), to state for the 2023 MIPS performance period/2025 MIPS payment year, a QCDR or qualified registry that was approved but did not submit any MIPS data for any of the 2017 through 2021 MIPS performance periods/2019 through 2023 MIPS payment years must submit a participation plan for CMS's approval. This participation plan must include the QCDR's detailed plans and changes to encourage eligible clinicians and groups to submit data on the low-reported QCDR measure for purposes of the MIPS program.
- We propose to revise and redesignate existing paragraph (b)(2)(vii) to proposed paragraph (b)(4)(viii) to state that beginning with the 2024 MIPS performance period/2026 MIPS payment year, a QCDR or qualified registry that was approved but did not submit any MIPS data for either of the 2 years preceding the applicable self-nomination period must submit a participation plan for CMS's approval.
(4) New Requirements Specific to QCDRs
(a) Background
We refer readers to § 414.1400(b), the CY 2017 Quality Payment Program final rule (81 FR 77374 through 77375), the CY 2018 Quality Payment Program final rule (82 FR 53813 through 53814), the CY 2019 PFS final rule (83 FR 59900 through 59906), the CY 2020 PFS final rule (84 FR 63058 through 63074), the May 8th COVID-19 IFC (85 FR 27594 through 27595), and the CY 2021 PFS final rule (84937 through 84944) for where we previously finalized standards and criteria for QCDRs, specifically QCDR measure requirements. In this section, we propose to update policies related to QCDR measure rejections.
(b) QCDR Measures
(i) QCDR Measure Rejections
(A) Proposed New QCDR Measure Rejection Criteria
We refer readers to the Quality Payment Program provisions in the CY 2020 PFS final rule (84 FR 63070 through 63073) at § 414.1400(b)(3)(vii) where we have previously adopted QCDR measure rejection criteria. In this proposed rule, we are proposing to add two new criteria: (1) QCDR does not have permission to use a QCDR measure; and (2) QCDR not approved or not in good standing. These are discussed in more detail below.
(aa) QCDR Does Not Have Permission To Use a QCDR Measure
In the CY 2018 Quality Payment Program final rule (82 FR 53813 through 53814), we discussed that beginning with the 2018 performance period and for future program years, QCDR vendors may seek permission from another QCDR to use an existing measure that is owned by the other QCDR. We noted that the QCDR measure owner (QCDR vendor) would still own and maintain the QCDR measure, but would allow other QCDRs to utilize their measure with proper notification. We intended for this policy to help reduce the number of QCDR measures that are similar in concept or clinical topic, or duplicative of other QCDR measures that are being approved. Additionally, in the Quality Payment Program provisions in the CY 2020 PFS final rule (84 FR 63070 through 63073) at § 414.1400(b)(3)(vii), we finalized the QCDR measure rejection criteria considerations. We noted that these considerations would help to ensure that QCDR measures are meaningful and measurable. Although we finalized the QCDR measure rejection criteria, we did not codify that QCDRs may seek permission from another QCDR to use an existing measure that is owned by another QCDR. In order to provide further clarity to the existing policies (82 FR 53813 through 53814; 84 FR 63070 through 63073), we propose to codify a new requirement and add a rejection criterion at § 414.1400(b)(4)(iv)(M) to state, a QCDR does not have permission to use a QCDR measure owned by another QCDR for the applicable performance period. We request comment on this proposal.
(bb) QCDR Not Approved or Not in Good Standing
Additionally, if a QCDR measure owner is not approved or is not in good standing, any QCDR measures associated with that QCDR would also not be approved. We believe it is important to have an approved QCDR measure owner for all approved QCDR measures. This would ensure that there is active involvement by the QCDR measure owner so that any potential measure issues can be mitigated during the specified MIPS performance period. For example, any mid-year guideline changes or measure questions would need to be immediately clarified to avoid negative impacts to clinicians such as the inability to construct a benchmark due to an error in the measure specifications. Therefore, we propose to codify another rejection criterion at § 414.1400(b)(4)(iv)(N) to state that, if a QCDR measure owner is not approved during a given self-nomination period, any associated QCDR measures with that QCDR would also not be approved. We request comment on this proposal.
We have received inquiries from stakeholders on what can be done in circumstances when an active QCDR wishes to use an inactive QCDR's measure. We are interested in feedback from stakeholders on what should be done in such circumstances. For example, what should happen if "QCDR A" is using "QCDR B's" measures in a given performance period and "QCDR B" is terminated mid performance period? Alternatively, what if "QCDR A" is using a measure from "QCDR B" and "QCDR B" decides not to self-nominate for the subsequent performance period? While "QCDR A" could partner with "QCDR B" as described at § 414.1400(b)(3)(ii), are there other policy options we should consider to minimize impact to the MIPS eligible clinician who has selected the QCDR measure for reporting?
We seek comment on the above circumstances.
(5) Remedial Action and Termination of Third Party Intermediaries
We refer readers to § 414.1400(f), the CY 2017 Quality Payment Program final rule (81 FR 77548), CY 2019 PFS final rule (83 FR 59908 through 59910), the CY 2020 PFS final rule (84 FR 63077 through 63080), and the CY 2021 PFS final rule (85 FR 84930 through 84937) for previously finalized policies for remedial action and termination of third party intermediaries.
In the Quality Payment Program provisions in the CY 2019 PFS final rule Start Printed Page 39466 (83 FR 59908 through 59910), we discussed that the threshold for "inaccurate, unusable or otherwise compromised" may be met if the submitted data includes TIN/NPI mismatches, formatting issues, calculation errors, or data audit discrepancies that affect more 3 percent of the total number of MIPS eligible clinicians or groups for which data was submitted by the third party intermediary. We are proposing to update the existing language at § 414.1400(f)(3)(ii) to broadly explain that it is up CMS's discretion on whether third party intermediaries' inaccuracies may lead to possible remedial action or termination. As discussed earlier, we propose consolidating and redesignating the existing language at § 414.1400(f) as § 414.1400(e) and § 414.1400(g) as § 414.1400(f) to provide clarity and alignment with the aforementioned proposals to consolidate the duplicative criteria of QCDRs and qualified registries. Therefore, we propose to revise and redesignate existing language at § 414.1400(f)(3)(ii) to proposed § 414.1400(e)(3) to state, contains data inaccuracies affecting the third party intermediary's total clinicians may lead to remedial action/termination of the third party intermediary for future program year(s) based on CMS discretion.
i. Public Reporting on the Compare Tools Hosted by the U.S. Department of Health & Human Services (HHS)
In this section of the proposed rule, we propose to amend § 414.1395(c) to add a 1-year delay of publicly reporting new improvement activities and Promoting Interoperability measures and attestations reported via MVP. We also propose a one-time, 1-year delay to subgroup-level public reporting, such that subgroup-level public reporting will begin with CY 2024 performance information available in 2025, and each year thereafter, on the Compare Tools hosted by the U.S. Department of Health and Human Services (HHS), referred to as "compare tool" throughout this proposed rule, available at https://www.medicare.gov/care-compare/ and data.medicare.gov, as technically feasible. We are proposing to add facility affiliations, beyond the hospital affiliations currently displayed on individual profile pages. Additional facility affiliations would include: Inpatient rehabilitation facilities (IRFs); long-term care hospitals (LTCHs); skilled nursing facilities (SNFs); inpatient psychiatric facilities (IPFs); home health agencies (HHAs); hospices; and dialysis facilities. Finally, we seek comment on publicly reporting utilization data on clinician and group profile pages.
For previous discussions on public reporting, we refer readers to the CY 2016 PFS final rule (80 FR 71116 through 71123), the CY 2017 Quality Payment Program final rule (81 FR 77390 through 77399), the CY 2018 Quality Payment Program final rule (82 FR 53819 through 53832), the CY 2019 PFS final rule (83 FR 59910 through 59915), the CY 2020 PFS final rule (84 FR 63080 through 63083), the CY 2021 PFS final rule (85 FR 84947 through 85 FR 84948) and the Care Compare: Doctors and Clinicians Initiative Page at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Compare-DAC.
(1) MVP and Subgroup Public Reporting
The introduction of MVPs and subgroup reporting provides for new types of performance information that are available for public reporting, provided they meet the established public reporting standards at § 414.1395(b). In consideration of our MVP and subgroup performance information public reporting proposals, we wish to remind readers that all submitted MIPS performance information is available for public reporting (81 FR 77395 through 77397). Additionally, we previously finalized at § 414.1395(c) that, for each program year, CMS does not publicly report any first year measures for the first 2 years, meaning any measure in its first 2 years of use in the quality and cost performance categories. We also note that MIPS performance category and composite final scores for MIPS eligible clinicians participating in MVPs will continue to be publicly reported as required under section 1848(q)(A)(i)(I) of the Act and finalized at § 414.1395(a)(1)(i).
We believe delaying public reporting of certain MVP and subgroup performance information provides a catalyst to encourage clinician participation in MVPs and subgroups while they familiarize themselves with these options. For this reason, we propose, for individuals, groups, and subgroups reporting via MVP, to add a 1-year delay for new improvement activities and Promoting Interoperability measures and attestations, as technically feasible. This means that new improvement activities and Promoting Interoperability measures and attestations would be available for public reporting at their inception in traditional MIPS, but we would delay public reporting of new improvement activities and Promoting Interoperability measures and attestations by 1 year after inception for those reporting via MVP. We note that improvement activities and Promoting Interoperability measures and attestations that have already been in MIPS for more than 1 year and become newly available as part of an MVP would be available for public reporting in the first year the MVP is in the program. That is, non-first year improvement activities and Promoting Interoperability measures and attestations that are newly part of an MVP would be available for public reporting in the first year the MVP is in the program. Table 62 further clarifies when this 1-year delay would apply.

We recognize that under this proposal, we would be further delaying the release of performance information for improvement activities and Promoting Interoperability measures and attestations reported via MVP. Because of this, as a potential incentive, we also considered whether to delay public reporting of quality and cost Start Printed Page 39467 measure information reported via MVP by 1 additional year, for a total of 3 years. We request comments on our proposal to delay public reporting of new improvement activities and Promoting Interoperability measures and attestations reported via MVP by 1 year, as well as any feedback on alternate approaches we should consider spurring clinicians to report performance data on MVPs while making performance data available for patients on the compare tool. We propose to amend this MVP public reporting policy at § 414.1395(c)(2) to state CMS does not publicly report any MVP data on new improvement activities or Promoting Interoperability measure, objective, or activity included in an MVP for the first year in which it is included in the MVP. We also propose to amend § 414.1395(c)(1) to state that CMS does not publicly report any data on new quality or cost measure for the first 2 years in which it is in the program, after which CMS evaluates the measure to determine whether it is suitable for public reporting under § 414.1395(b). Currently, § 414.1395(c) refers to these quality and cost measures as "first year measures". We are proposing to change "first year measures" to "new measures".
The introduction of MVPs and subgroup reporting in MIPS, provides for new types of performance information that are available for public reporting, provided they meet the public reporting standards. Currently, we display information on profile pages at the individual clinician and group level, since this is the level of information we provide for and at which patients and caregivers search for on the compare tool. To ensure that patients and caregivers have access to subgroup performance information, we propose creating a separate workflow from the established ones for individuals and groups, since we only display information at the level at which it was publicly reported. That is, we only publicly report individual-level performance information on individual clinician profile pages and group-level performance information on group profile pages. We do not publicly report group-level performance information on individual profile pages or individual-level information on group profile pages, as doing so would not be truly representative of either the group's or individual's own performance, and we do not want to mislead website users. Instead, we would link from the individual or group profile page to the corresponding subgroup performance information. That is, we propose to create a subgroup public reporting workflow, in which we would indicate with plain language on an individual profile page that the clinician reports performance information as part of a subgroup or on a group profile page that the group has subgroups for purposes of performance information and then link to that subgroup's performance information. Future user testing would determine how to best display and put in plain language subgroup performance information. Subgroup performance information would also be available on http://data.medicare.gov/.
Subgroups represent a new type of reporting for MIPS, that is available for clinicians reporting on MVPs or via the APP. For this reason, we also propose to delay all subgroup-level public reporting for 1 year, including measures, activities and attestations across the quality, cost, improvement activities, and Promoting Interoperability performance categories in order to encourage clinician participation in subgroups without the risk of displaying subgroup performance information as clinicians familiarize themselves with the option of subgroup reporting. This would only be a one-time delay in public reporting of subgroup-level information. That is, we would not publicly report any CY 2023 subgroup-level measure, attestation, or activity performance information; this information would be available for public reporting beginning with CY 2024 performance period. We would publicly report PY 2024 subgroup performance information and for each performance year thereafter if the information meets our established public reporting standards. Since we are moving toward more granular level performance information, we believe delaying subgroup public reporting by 1 year provides an incentive for subgroup participation and experience. As an alternative, we also considered a 1-year public reporting delay of performance information for all new subgroups each performance year, as technically feasible. For example, subgroups that begin in CY 2023 are not eligible for public reporting until CY 2024, subgroups that begin in CY 2024 are not eligible for public reporting until CY 2025, and so on for each subsequent year. Another alternative we considered was to publicly report all subgroup performance information without delay and provide new subgroups the opportunity to opt-out, during the preview period, of having their performance information publicly reported for their first year. Some subgroups may want to have their performance information publicly reported and having an overall 1-year delay may be a disincentive to subgroup participation. We seek comment on these considerations. We note that MIPS performance category and composite final scores for MIPS eligible clinicians participating in MVPs will continue to be publicly reported for those participating in subgroups, as required under section 1848(q)(A)(i)(I) of the Act and finalized at § 414.1395(a)(1)(i), and will not be delayed by 1 year for public reporting.
We also seek comment on additional factors that we should consider as we look to expand the availability of MVP and subgroup data on the compare tools. For example, should there be a certain threshold of MVPs available, or clinicians participating in MVPs prior to public reporting? For public reporting of subgroups, are there factors we should consider to make this information usable to the patient but reflective of the subgroups characteristics and composition? Should we test an indicator of MVP participation for compare tool profile pages to see if this is useful information for patients making healthcare decisions? We seek comment on this proposal and additional ways public reporting may encourage MVP participation.
(2) Publicly Reporting APM Performance Pathway Information
In the CY 2021 Quality Payment Program final rule, we finalized to establish an APM performance pathway (APP) beginning in the 2021 MIPS performance year. This is an optional MIPS reporting and scoring pathway for MIPS eligible clinicians who participate in MIPS APMs. We also note that since APP participants are MIPS eligible clinicians, their MIPS performance category and composite final scores will be publicly reported as required under section 1848(q)(A)(i)(I) of the Act and finalized at § 414.1395(a)(1)(i).
In the CY 2017 Quality Payment Program final rule, we finalized, as technically feasible, to use ACO profile pages as a guide to publicly reporting more APM data (81 FR 77398). Currently, groups who participate in an ACO have an indicator showing their participation as well as a link to the ACO profile page with available performance information. User testing has shown that website users find the ACO information meaningful and displayed in a user-friendly way. For this reason, we plan to continue this approach for APM performance information, including that which comes in via the APP, as technically feasible. We also seek comment on Start Printed Page 39468 alternative ways to publicly report performance information reporting via APPs and additional considerations to publicly reporting this information.
(3) Facility Affiliations
Compare tool profile pages for clinicians currently provide demographic information, including names, addresses, phone numbers, medical specialties, APM affiliations, Medicare assignment status, board certifications, education and residency, gender, and group and hospital affiliations. User testing consistently shows that Medicare patients and caregivers find value in these types of information. For hospital affiliations, website users have consistently noted the importance of understanding up front the relationships clinicians may have with facilities where they perform services when searching for a clinician. Specifically, patients and caregivers have noted during user testing that hospital affiliation is important to them, since they may be looking for a clinician to perform a procedure at a hospital or want to know the hospitals a clinician could potentially admit them if needed. Linking from the clinician profile page to their affiliated hospital page has provided a seamless experience for patients and caregivers, as they do not need to separately search for clinicians and hospitals; rather, they can navigate to a hospital profile page directly from the clinician's profile page.
With these user testing findings in mind, and because the Compare Tools include information on a number of other types of facilities beyond hospitals, we believe it would benefit patients and caregivers to also be able to navigate from clinician profile pages to profile pages for other types of facilities such as: IRFs; LTCHs; SNFs; IPFs; HHAs; hospices; and dialysis facilities.
Expanding the types of clinician-facility affiliations, beyond hospital affiliation, publicly reported would allow us to provide additional information about clinicians with or without any hospital affiliation but who are affiliated with other types of facilities. User testing with patients and caregivers has shown that facility affiliations not only for hospitals but also for IRFs, LTCHs, SNFs, IPFs, HHAs, hospices, and dialysis facilities would be helpful to their healthcare decision-making. Specifically, we propose adding affiliations to clinician profile pages for each of the following types of facilities, pending the results of user testing, as applicable and technically feasible: IRFs; LTCHs; SNFs; IPFs; HHAs; hospices; and dialysis facilities. User testing will determine how to best display these affiliations on compare tool clinician profile pages. To determine clinician affiliations to these facilities, we would use claims data the same way we do to display the hospital affiliations currently available on clinician profile pages (77 FR 69165). We build the clinician-hospital affiliations based on observing a clinician practicing at a given hospital caring for at least three different Medicare patients on three different dates of service in the preceding 6 months, as documented in Medicare claims. We would use similar criteria for determining additional facility affiliations. Clinicians can email the Quality Payment Program Service Center at QPP@cms.hhs.gov if they believe their facility affiliations are displayed incorrectly. We seek comment on the proposal to add affiliations to clinician profile pages for each of the following types of facilities and link to the specific facility's page on the compare tool: IRFs; LTCHs; SNFs; IPFs; HHAs; hospices; and dialysis facilities. Further, we also seek comment on whether there should be a limit on the number of procedures done or conditions treated at a given facility to determine clinician-facility affiliations.
(4) Utilization Data Request for Information
Under section 104(e) of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), beginning with 2016, the Secretary is required to integrate utilization data information on Physician Compare.[244] To satisfy section 104(e) of the MACRA, we previously implemented a policy to begin to include utilization data in a downloadable format in late 2017 using the most currently available data, and previously finalized that the specific codes to be included would be determined via data analysis and reported at the eligible clinician level (80 FR 71130). We finalized to continue to include utilization data in the downloadable database (81 FR 77398). This information continues to be available today on www.data.cms.gov/provider-data.
To date, we have gathered utilization data for procedures from physician/supplier Medicare Part B non-institutional claims on certain services and procedures and published it in the public use file (PUF) file entitled "Physician and Other Supplier Data." These data are useful to the healthcare industry, healthcare researchers, and other stakeholders who can accurately interpret these data and use them in meaningful analyses. However, this information is presented in a technical way that is not easily accessible or usable by patients, who do not frequently visit data.cms.gov or understand medical procedure coding. This information also does not provide detail on the specific conditions clinicians treat, though in select cases it may be inferred by the clinicians and researchers reviewing this information.
Section 10331(b)(3) of the Affordable Care Act requires that for public reporting, to the extent practicable, to include processes to assure that the data made available provides a robust and accurate portrayal of a clinician's performance. In our efforts to continue to provide patients and caregivers with meaningful information to make informed healthcare decisions, we believe utilization data may also have a place on clinician and group profile pages, if presented in a consumer-friendly way. We envision utilization data on patient-facing profile pages providing two main areas of benefit. The first is allowing for more granular clinician searches, so that patients not only find specific types of clinicians but also those clinicians experienced in performing specific types of procedures and/or treating specific conditions. The second is providing categories of utilization data in a more plain language display that is usable to patients and their caregivers. In summary, utilization data could provide information to Medicare patients and their caregivers on the specific diagnoses clinicians treat and the frequency with which certain services or procedures are performed by a clinician or group and/or which types of clinicians do not provide certain services.
For example, someone with severe arthritis of the knee may want to search for an orthopedic surgeon who specifically does knee replacements. The way the clinician search works currently would only show results for "orthopedic surgeons" generally. That is, the patient would not see which of these clinicians specialize in this procedure, and likely would need to spend time calling clinicians to ascertain more detail. This could similarly be the case for finding a clinician who focuses on treatment of a certain condition. We believe indicating which clinicians focus on certain procedures or conditions would relieve some of this patient burden, as it would yield more specific search results. There are a number of factors that could influence how procedure- and condition-specific information is Start Printed Page 39469 determined, which is why we are seeking comment on this topic in several areas.
For display purposes, we may wish to apply a minimum experience level, such as the number of times a clinician performed a procedure or treated a condition, before a clinician profile is annotated to indicate experience with the condition or procedure. Regarding the methods in which we would identify clinician volume of procedures conducted or treat specific conditions, we would need to set a threshold for making these assertions. We have considered several options. The threshold could be based on the number of times a clinician has performed a procedure or treated a condition within a certain time-period, or the proportion of the clinician's practice that is represented by the procedure or condition. Alternatively, thresholds may be devised based on ranking clinicians compared to their peers (specialty and geography may be considered when defining peers) in volume of procedures performed or frequency with which they treat each condition.
We note too that these approaches utilize Medicare claims data only. That is, these data would not include procedures performed or conditions treated for patients who have other types of insurance, since this information is not available. We also acknowledge that this utilization data only represents the care provided to Medicare beneficiaries and clinicians offer care to those with other forms of insurance. This disclaimer could be added to any data that may be publicly reported. We seek comment on these approaches and whether there are any additional ones we should consider.
Additionally, because the Compare Tools utilize a location-based search, national or local thresholds may be appropriate. For example, clinicians in urban centers may specialize in a small number of procedures that they perform on a weekly basis, while a clinician in a rural area might be the most experienced at a given procedure, but not have comparable volume to the urban clinician who practices a very narrow scope. We seek comment on these considerations as well as if there are others.
We also seek comment on the potential types of utilization data that, if publicly reported, could help Medicare patients and their caregivers make informed healthcare decisions, as well as on technical considerations for presenting a specific affiliation between clinicians and diagnoses and/or procedures. Specifically, we seek comment on:
- The types of conditions and procedures that would most benefit patients' clinician searches;
- Important features and considerations for clinician searches by conditions or procedures;
- The lookback period for Medicare claims in order to identify a clinician's volume of procedures balancing frequency with recent experience (for example, 6 months, 1 year, 2 years);
- Clinician specialties or conditions with special considerations (for example, non-patient facing clinicians);
- The maximum number of conditions treated or procedures performed to display on a given clinicians profile page; and
- Methods to set a threshold of treatment volume to display that a clinician commonly performs a procedure or treats a condition. For example, the threshold could be: (1) The number of times a clinician treated a condition or performed a procedure; (2) the total scope that a condition or treatment represents in a clinician's practice; or (3) the clinician's rank—either overall among all clinicians or among a subset of clinicians—in the number of times that clinician treated a condition or performed a procedure.
- Any other factors or considerations not listed above.
4. Overview of the APM Incentive
(a) Overview
Under the Quality Payment Program, eligible clinicians who are Qualifying APM Participants (QPs) for a year are eligible to receive an APM Incentive Payment in the corresponding payment year for payment years 2019 through 2024. In the CY 2017 Quality Payment Program final rule (81 FR 77480 through 77489), we finalized at § 414.1450(d) that this payment is made based on the clinician's QP status in the QP Performance Period that is 2 years prior (for example, the 2021 payment will correspond to the 2019 performance year), and at § 414.1450(b)(1) that the payment is equal to 5 percent of the estimated aggregate payments for covered professional services in the base period (the year between the QP performance and payment years).
We also finalized at § 414.1450(c)(1) (82 FR 31729) that the APM Incentive Payment would go to the TIN associated with the Advanced APM Entity through which an eligible clinician becomes a QP during the QP Performance Period. In 2019, our first year of making APM Incentive Payments, we learned that the amount of time between the QP Performance Period (during which QP status is attained) and the QP payment year (during which APM Incentive Payments are issued) creates challenges to disbursing the payment for some QPs in a routine and efficient manner. Consistent with section 1833(z) of the Act, QP status is determined for, and connected to, an eligible clinician (identified by their NPI) for the QP payment year based on their Advanced APM participation during the QP Performance Period. We do not believe that changes in a QP's practice or TIN in the interim year between the QP determination and the QP payment year should affect a QP's ability to receive the APM Incentive Payment. To address some of the unanticipated challenges we encountered in disbursing the APM Incentive Payments, in the CY 2021 PFS final rule, we finalized a hierarchy, codified at § 414.1450, that, based on our experience and lessons learned in making payments in 2019, would provide more ways to identify an appropriate TIN to which we can make the APM Incentive Payment when a QP has experienced changes in their practice or TIN since the performance year in which they attained QP status.
(c) APM Incentive Payment Recipient
In the 2021 PFS final rule (85 FR 84472), we revised our approach to identifying the TIN or TINs to which we make the APM Incentive Payment, and established a process that enables QPs to provide CMS with updated enrollment information that could be used to complete the payment in the event our approach does not yield an appropriate TIN or TINs. The process for those QPs to update their information, as well as a preliminary list of NPIs to whom it may be applicable, is included in a Public Notice published annually in the Federal Register. We explained in the CY 2021 PFS final rule that the revised approach would involve looking at a QP's relationship with TINs at different, specified periods in time, as well as considering the relationships such TINs have with certain APM Entities and Advanced APMs. We stated that we believe this revised approach enables us to more appropriately identify TINs with which QPs currently have relationships to receive other Medicare payments, and through which the QPs likely would anticipate receiving their APM Incentive Payments. We noted that, when the QP is no longer affiliated with the TIN through which they achieved QP status, this approach would prioritize identifying an alternate TIN with which the QP is affiliated at the time the APM Incentive Payment is made, and to which it would be appropriate to make the payment. The approach we adopted Start Printed Page 39470 also serves to reduce uncertainty for QPs as they anticipate their APM Incentive Payments, as well as potential delays in our ability to make their payments.
To improve and expand the ways we identify the TIN(s) to which we make the APM Incentive Payment for a QP in a timely and efficient manner, we finalized a policy to sequentially apply a decision hierarchy and codified the hierarchy in § 414.1450(c). We apply the hierarchy by beginning at the first step, and if we are unable to identify one or more TINs with which the QP has a current affiliation at this step, we move to the next and successive steps of the hierarchy until we do identify one or more TINs with which the QP is affiliated.
As discussed in the CY 2021 PFS final rule, if we identify more than one TIN at the applicable step in the hierarchy, we divide the APM Incentive Payment proportionally between the QP's TINs based on the relative paid amount for Part B covered professional services that are billed through each of the TINs. We propose to clarify that, when we divide the APM incentive payment between two or more TINs, we apportion the APM incentive payment among TINs based on the share of total payments for covered professional services made to each TIN in the same base year that we use to calculate the APM incentive payment for the year. To calculate the APM incentive payment, we sum the total estimated aggregate payments for covered professional services for a QP for the base year, which is based on claims submitted for covered professional services, as codified at § 414.1450(b)(1) through (3). We propose to codify this policy at § 414.1450(c).
In the course of making APM Incentive Payments during CY 2020 PFS final rule, we explored the possibility of expanding our search at each step of the hierarchy at § 414.450(c) to identify potential payee TINs that are associated with the QP during the QP payment year. Based on our findings, we believe expanding our search in this way would enable us to make payments earlier in the calendar year and reduce the number of QP NPIs for whom we cannot identify a payee TIN using our hierarchy, and thus, rely on our Public Notice to request additional information. Therefore, we now propose to revise the hierarchy at § 414.1450(c) so that, using the criterion described in each step of our current regulation, we would first seek to identify a TIN associated with the QP during the base year, and if no such TIN is identified in the base year, we would then seek to identify a TIN associated with the QP during the payment year. We have found in many instances that there are changes in enrollment information in PECOS for a QP over the span of 2 years between the QP performance period and payment year. By using enrollment information for the QP during the payment year, we are more likely to identify an appropriate TIN to which to make the APM incentive payment hierarchy. Under the proposal, applying the steps in the APM incentive payment hierarchy, we would make the APM Incentive Payment to one or more solvent TINs associated with the QP, identified by paid Medicare Part B claims for covered professional services and associated PECOS enrollment information during the base period, and if no such TIN is identified, we would make the payment to such TINs associated with QP during the payment year, according to this section. If no such TIN or TINs can be identified at a particular step, we would move to the next and successive steps listed in § 414.1450(c)(1) through (8) until we identify one or more solvent TIN or TINs with which the QP is associated, and then would make the APM Incentive Payment to any such TIN(s). If more than one TIN is identified at a step based on paid claims during the applicable year, either the base year or payment year, as we explain earlier and propose to codify in the regulation under § 414.1450(c), we divide the APM Incentive Payment proportionately among such TINs according to the relative total paid amounts for Part B covered professional services to each TIN in same the base year we use to calculate the APM incentive payment.
We propose, for each step in the APM incentive payment decision hierarchy, we would first search for a payment TIN or TINs associated with the QP during the base period., If no such TIN is found during the base year, we would search for any TIN or TINs that are similarly situated with respect to the criterion at that step in the hierarchy and associated with the QP during the payment year. If such a TIN or TINs are found, we would make the APM incentive payment to such TIN or TINs. We would continue at each step in the hierarchy to first attempt to identify the relevant base year TIN or TINs associated with the QP because we believe such TINs are more likely to be associated with the APM Entity through which the QP attained their QP status during the QP performance period. However, if no such TIN is found in the base year, we would proceed at that step to search for a TIN or TINs with which the QP is associated in the payment year.
We believe this approach creates the greatest opportunity to identify and pay an appropriate TIN as efficiently and early as possible during the payment year. The proposed change would maintain the current hierarchy while adding a sub-step at each level in which we would conduct our search based on more current enrollment information. The proposed change would allow for the identification of an appropriate TIN or TINs at each step by first checking the base year, and then checking the payment year before moving on to the next step in the process. We believe that by maintaining the current hierarchy we would continue to incent Advanced APM participation by prioritizing making payments to TINs affiliated with Advanced APMs, even if they are not in the same Advanced APM Entity through which QP status originally was achieved. For example, we anticipate that many eligible clinicians who earned QP status in 2020 through a practice participating in the CPC+ model would join the new Primary Care First (PCF) model in 2022.
In the event the eligible clinician's CPC+ participant TIN is no longer active, our proposed modification to the hierarchy would enable us to pay the APM Incentive Payment to a TIN participating in the PCF model in 2022. We continue to believe it would be appropriate to first identify the relevant base year TIN or TINs at each step in of the hierarchy because we believe those TINs are more likely to be associated with the APM Entity through which the QP attained their QP status during the QPs performance period. However, if no TIN is found in the base year, we would proceed to identify any TINs associated with the QP in the payment year; and then use the same process for the subsequent steps in the hierarchy until we identify one or more TINs associated with the QP at a particular step for a particular year (base year or payment year). We believe this approach will be a more efficient and expeditious way to identify a TIN or TINs to which to make the APM incentive payment for QPs.
We seek comment on this proposal to amend our APM Incentive Payment decision hierarchy to include an additional attempt to identify and pay, at each step, one or more solvent TINs associated with the QP during the payment year when no such TIN is identified for the QP in the base year. Start Printed Page 39471
c. Advanced APMs
1. Qualifying APM Participant Determination
a. General Overview:
In the CY 2017 Quality Payment Program final rule (81 FR 77439 through 77445), we finalized our policy at § 414.1425(b) for Qualifying APM Participant (QP) determinations. For the purposes of making QP determinations, an eligible clinician must be present on the Participation List of an APM Entity in an Advanced APM on one of the "snapshot dates" (March 31, June 30, or August 31) for the QP Performance Period. An eligible clinician included on a Participation List on any one of such dates is included in the APM Entity group even if that eligible clinician is not included on that Participation List at one of the prior- or later-listed dates. We perform QP determinations for the eligible clinicians in an APM entity group three times during the QP Performance Period using claims data for services furnished from January 1 through each of the respective QP snapshot dates. An eligible clinician can be determined to be a QP only if the eligible clinician appears on the Participation List on a snapshot date that we use to determine the APM Entity group and to make QP determinations at the APM Entity group level based on participation in the Advanced APM. For eligible clinicians who appear on a Participation List in more than one APM Entity, but do not to achieve QP status based on any APM Entity level determinations, we make QP determinations at the individual level as described in § 414.1425(c)(4). Likewise, for eligible clinicians on an Affiliated Practitioner list for an Advanced APM we make QP determinations at the individual level three times during the QP Performance Period using claims data for services furnished from January 1 through each of the respective QP determination snapshot dates as described in § 414.1425(b)(2).
b. QP thresholds and Partial QP thresholds
Section 1833(z)(2)(B) of the Act describes the thresholds for the level of participation in Advanced APMs required for an eligible clinician to become a QP for a year. The Medicare Option, based on Part B payments for covered professional services or counts of patients furnished covered professional services under Part B, is applicable beginning in the payment year 2019. The All-Payer Combination Option, which uses the Medicare Option, as well as an eligible clinician's participation in Other Payer Advanced APMs, is applicable beginning in the payment year 2021. In the CY 2017 Quality Payment Program final rule (81 FR 77433 through 77439) we finalized our policy for the Medicare Option as codified at § 414.1430(a) and the All-Payer Option at § 414.1430(b).
Section 114 of division CC of the CCA amended section 1833(z)(2)(B) of the Act with regard to payment years 2023 and 2024 (which correspond respectively to performance years 2021 and 2022), specifically by freezing for such years the applicable payment amount and patient count thresholds used to make determine an eligible clinician's QP determinations status. Specifically, the CAA amended section 1833(z)(2)(B) of the Act to continue the QP payment amount thresholds that apply in payment years 2021 and 2022 to payment years 2023 and 2024. Additionally, the CAA, 2021 amended section 1833(z)(2)(D) of the Act to require that, for payment years 2023 and 2024, the Secretary use the same percentage criteria for the QP patient count threshold that are applied in payment year 2022. As such, the Medicare Option QP thresholds payment years 2023 and 2024 (for performance years 2021 and 2022) will remain at 50 percent for the payment amount method and 35 percent for the patient count method. The CAA also amended section 1848(q)(1)(C)(iii) of the Act to extend the Partial QP thresholds that are established for payment years 2021 and 2022 through payment year 2024. Therefore, the Partial QP thresholds for payment years 2023 and 2024 (performance years 2021 and 2022) will remain at 40 percent for the payment amount method and 25 percent for the patient count method. For performance years beginning with 2023 (corresponding to payment years beginning with 2025) the statute prescribes the QP thresholds for the payment amount method, and the QP thresholds we established for the patient count method at § 414.1430 will take effect. Specifically, for performance years beginning with 2023, the Medicare Option QP thresholds will be 75 percent for the payment amount method and 50 percent for the patient count method. The Partial QP thresholds under the Medicare Option will be 50 percent for the payment amount method and 35 percent for the patient count method.
Under the All-Payer Combination Option, the QP thresholds for performance years 2021 and 2022 (corresponding to payment years 2023 and 2024) will be 50 percent for the payment amount method and 35 percent for the patient count method. The Partial QP thresholds for performance years 2021 and 2022 will be 40 percent for the payment amount method and 25 percent for the patient count method. In order to become a QP through the All-Payer Combination Option, eligible clinicians must first meet certain threshold percentages under the Medicare Option. For performance years 2021 and later (corresponding to payment year 2023 and later), the minimum Medicare Option threshold an eligible clinician must meet for the All-Payer Combination Option is 25 percent for the payment amount method or 20 percent under the patient count method.
Start Printed Page 39472

V. Collection of Information Requirements
Under the Paperwork Reduction Act of 1995 (PRA) (44 U.S.C. 3501 et seq.), we are required to publish a 60-day notice in the Federal Register and solicit public comment before a "collection of information" requirement is submitted to the Office of Management and Budget (OMB) for review and approval. For the purposes of the PRA and this section of the preamble, collection of information is defined under 5 CFR 1320.3(c) of OMB's implementing regulations. To fairly evaluate whether an information collection should be approved by OMB, PRA section 3506(c)(2)(A) requires that we solicit comment on the following issues:
- The need for the information collection and its usefulness in carrying out the proper functions of our agency.
- The accuracy of our burden estimates.
- The quality, utility, and clarity of the information to be collected.
- Our effort to minimize the information collection burden on the affected public, including the use of automated collection techniques.
We are soliciting public comment on each of the required issues under section 3506(c)(2)(A) of the PRA for the following information collection requirements.
A. Wage Estimates
To derive average costs, we used data from the U.S. Bureau of Labor Statistics' May 2020 National Occupational Employment and Wage Estimates for all salary estimates (http://www.bls.gov/oes/current/oes_nat.htm). In this regard, Table 64 presents the mean hourly wage, the cost of fringe benefits and overhead (calculated at 100 percent of salary), and the adjusted hourly wage.
Start Printed Page 39473

For the CY 2019 and CY 2020 PFS final rules, we used the BLS wage for "Physicians and Surgeons" (occupation code 29-1060) to estimate the cost for Physicians. In BLS' most recent set of occupational wage rates (dated May 2020) they have discontinued this occupation in their wage data. As a result, in order to estimate the burden for Physicians, we are using a rate of $217.32/hr which is the average of the following BLS occupations and adjusted wage estimates.

As indicated, we BLS' hourly wage estimates by a factor of 100 percent. This is necessarily a rough adjustment, both because fringe benefits and overhead costs vary significantly from employer to employer, and because methods of estimating these costs vary widely from study to study. Nonetheless, we believe that doubling the hourly wage to estimate total cost is a reasonably accurate estimation method. Start Printed Page 39474
B. Proposed Information Collection Requirements (ICRs)
1. ICRs Requiring Certain Manufacturers To Report Drug Pricing Information for Part B (§§ 414.802 and 414.806)
Pending our finalization of the following provisions, the proposed changes will be subject to the standard PRA process under OMB control number 0938-0921 (CMS-10110). The standard PRA process includes the publication of 60- and 30-day Federal Register notices that will provide the public with opportunities for public review and comment. We expect to publish the 60-day notice shortly after the publication of the CMS-1751-F final rule.
The new provisions at §§ 414.802 and 414.806 would implement new statutory requirements under sections 1847A and 1927 of the Act, as amended by section 401 of Division CC, Title IV of the CAA, 2021 (for the purposes of this section of this proposed rule, hereinafter is referred to as "section 401"), which requires manufacturers without a Medicaid drug rebate agreement to report ASP information to CMS for calendar quarters beginning on January 1, 2022, for drugs or biologicals payable under Medicare Part B and described in sections 1842(o)(1)(C), (E), or (G) or 1881(b)(14)(B) of the Act, including items, services, supplies, and products that are payable under Part B as a drug or biological. Specifically, to implement the new reporting requirements for manufacturers without Medicaid drug rebate agreements, we are proposing to modify: (1) The definition of drug at § 414.802; and (2) the regulations describing civil money penalties at § 414.806. The new requirements will improve the accuracy of reported payment limits and limit the use of WAC-based pricing.
For the purposes of section 401's new reporting requirements, for manufacturers without Medicaid drug rebate agreements, confidentiality requirements appear in section 1847A(f)(2)(D) of the Act which states that the ASP data are confidential and shall not be disclosed by the Secretary in a form which discloses the identity of a specific manufacturer or wholesaler or prices charged for drugs or biologicals by such manufacturer or wholesaler, except—as the Secretary determines to be necessary to carry out this section (including the determination and implementation of the payment amount), or to carry out section 1847B of the Act; to permit the Comptroller General of the United States to review the information provided; to permit the Director of the Congressional Budget Office to review the information provided; to permit the Medicare Payment Advisory Commission to review the information provided; and to permit the Medicaid and CHIP Payment and Access Commission to review the information provided.
For manufacturers with Medicaid drug rebate agreements, confidentiality requirements appear in section 1927(b)(3)(D) of the Act which states that the ASP data are confidential and shall not be disclosed by the Secretary in a form which discloses the identity of a specific manufacturer or wholesaler, prices charged for drugs by such manufacturer or wholesaler, except—as the Secretary determines to be necessary to carry out this section, to permit the Comptroller General to review the information provided, and to permit the Director of the Congressional Budget Office to review the information provided.
The burden associated with these requirements is the time and effort required by manufacturers of drugs and biologicals payable under Medicare Part B to prepare and submit the required ASP data to CMS. We have previously estimated the burden associated with ASP reporting requirements for manufacturers with Medicaid drug rebate agreements. Because section 401 extends the ASP reporting requirements to manufacturers without Medicaid drug rebate agreements, we are updating our burden estimates to account for the additional manufacturers who will now be required to report ASP data to us.
As described in section III.D.1. of this proposed rule, in considering whether to exclude repackagers from the reporting requirements at section 1847A(f)(2) of the Act, we conducted analyses to estimate: (1) The proportion of repackaged products in our existing ASP data; (2) the number of new ASP submissions we can expect as a result of the new reporting requirements under section 401; and (3) the proportion of those (new) submissions that involve repackaged products.
Based on our existing ASP data, 547 manufacturers (respondents) report ASP data to us. Of these, 331 respondents have products for which they are required to submit ASP data, and 216 respondents have products for which they currently submit ASP data voluntarily, but will now be required to do so under section 1847A(f)(2) of the Act. (331 + 216 = 547)
We also estimate that under the new reporting requirements of section 401, a total of 568 respondents have products for which they will now be required to report ASP data to us. The 568 includes the 216 respondents (above) and 361 respondents who have products (identified by us) for which they will now be required to submit ASP data under section 1847A(f)(2) of the Act and did not previously voluntarily submit these data to us. There were 9 respondents who voluntarily submitted ASP data for some, but not all, of their products identified in our analysis. (216 + 361−9 overlap = 568)
We estimate a total of 740 respondents will report ASP data to us. This includes the 547 respondents who currently submit ASP data to us (voluntarily, or as currently required), and the 361 respondents who have products (identified by us) for which they will now be required to submit ASP data under section 1847A(f)(2) of the Act and did not previously voluntarily submit these data to us. However, there were 168 respondents who currently are required to submit ASP data to us, or who voluntarily submit ASP data to us, for whom we identified additional products that they did not previously submit ASP data, and will now be required to submit ASP data for these additional products under the new reporting requirements of section 401. (547 + 361−168 overlap = 740)
These respondents submit ASP data four times per year for a total of 2,960 submissions (740 respondents × 4 submissions/year).
Based on our experience with ASP data reporting, we continue to estimate that the time associated with reporting, record keeping, and third-party disclosure for ASP data reporting is 13 hours: 10 hours to review instructions and search existing data resources and 3 hours to gather the data, compile the data, submit via electronic media and upload to the automated system. This estimate includes labor costs for respondents to extract data from their information systems and to compile and submit the ASP data, including signature, to CMS via the internet-based automated system and electronic media. This estimate also includes the cost of the compact disc (CD) and overnight mail service used to report the data, time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection.
Based on these analyses and assumptions, we estimate an annual burden of 38,480 hours (2,960 submissions/yr × 13 hours per response) at a cost of $1,495,332.80 (38,480 hr × $38.86/hr), rounding to $1,495,333.
Start Printed Page 39475

We welcome comment on the likely costs or savings manufacturers from this provision.
2. ICRs Regarding the Medicare Shared Savings Program (Sections VII.F.8.a. and b.)
Section 1899(e) of the Act provides that chapter 35 of title 44 U.S.C., which includes such provisions as the PRA, shall not apply to the Shared Savings Program. Accordingly, we are not setting out burden under the authority of the PRA. Please refer to sections VII.F.8.a. and b. of this proposed rule for a discussion of the impacts associated with this rule's proposed changes to the Shared Savings Program's quality reporting requirements, beneficiary assignment methodology, repayment mechanism requirements, requirements for disclosure of prior participation in the Shared Savings Program by the ACO, ACO participants, and ACO providers/suppliers, requirements for ACOs to submit sample ACO participant agreements and executed ACO participant agreements to CMS, and beneficiary notification requirements.
3. ICRs Regarding the Medicare Ground Ambulance Data Collection System (§ 414.626)
Section 1834(l)(17) of the Act requires that the Secretary develop a ground ambulance data collection system that collects cost, revenue, utilization, and other information determined appropriate by the Secretary with respect to providers of services and suppliers of ground ambulance services (ground ambulance organizations). Section 1834(l)(17)(I) of the Act states that the PRA does not apply to the collection of information required under section 1834(l)(17) of the Act. Accordingly, this collection of information section does not set out any burden for the proposed provisions. Please see section VII. of this preamble for a discussion of the estimated impacts.
4. ICRs Regarding the Medicare Diabetes Prevention Program (MDPP) Expanded Model (§§ 410.79, 414.84, 424.205, and 424.502)
In section III.L. of this proposed rule, we propose policies necessary to shorten the Medicare Diabetes Prevention Program (MDPP) services period to one (1) year on a prospective basis, amend and update the amount of the performance payments for the Core Sessions and Core Maintenance Sessions, and make changes to eliminate the ongoing maintenance phase for MDPP beneficiaries who start MDPP set of services on or after January 1, 2022. In addition, we propose to add a provision to waive the provider enrollment Medicare application fee for all organizations enrolling in Medicare as MDPP suppliers during the MDPP expanded model. We expect the proposed policies will increase the number of eligible organizations willing to enroll as MDPP suppliers. We also anticipate that the shortened service period will make MDPP more marketable to beneficiaries in that their time commitment is reduced and less intimidating with a 12-month vs 24-month service period. We anticipate the shortened MDPP services period will reduce the recordkeeping burden for suppliers. Section 1115A(d)(3) of the Act exempts Innovation Center model tests and expansions, which include the MDPP expanded model, from the provisions of the PRA. Accordingly, this collection of information section does not set out any burden for the proposed provisions. Please see section VII. of this preamble for a discussion of the estimated impacts.
5. ICRs for Prepayment and Post-payment Definitions, Documentation Request Timeframes, and Payment Denials for Noncompliance with Documentation Requests (§§ 405.902, 405.903, 405.929, and 405.930)
In section III.N.2. of this proposed rule, we are proposing to: (1) Define key terms including "additional documentation," "additional documentation request," "post-payment medical review," and "prepayment medical review;" (2) codify contractors' authority to request additional documentation for prepayment and post-payment review within established timeframes; (3) codify timeframes for response to requests for documentation; (4) codify result of a failure to comply with prepayment or post-payment documentation request(s) by a provider or supplier, specifically denial of payment.
The codification of contractor authority to request additional documentation for post-payment reviews, associated timeframes, and resulting denials for failure to comply with these requests is not subject to the PRA per 5 CFR 1320.3(h)(9). The request for additional documentation would be on a case-by-case basis using non-standardized follow-up questions.
With regard to the (1) definitions for "additional documentation" and "additional documentation request," "post-payment medical review," and "prepayment medical review;" (2) the codification of contractor authority to request additional documentation for pre-payment reviews; (3) the associated provider and supplier timeframes for providing additional documentation from the pre-payment reviews; and (4) possible denials for failure to comply with these requests, we do not expect that these proposals, if finalized, would affect our information collection burden estimates because these policies do not require providers or suppliers to submit any more documentation to CMS than what is already approved by OMB under control number 0938-0969 (CMS-10417). The proposed regulations simply codify certain requirements by clarifying definitions, timeframes, and results for noncompliance.
6. ICRs Regarding the Requirement for Electronic Prescribing for Controlled Substances for a Covered Part D Drug Under a Prescription Drug Plan or an MA-PD Plan (§ 423.160(a))
Pending our finalization of the following provisions, the proposed changes will be subject to the standard PRA process under OMB control number 0938-1396 (CMS-10755) to give Start Printed Page 39476 stakeholders optimal opportunity to comment on our proposed burden for this provision, given how dynamic the burden for EPCS is. The standard PRA process includes the publication of 60- and 30-day Federal Register notices that will provide the public with opportunities for public review and comment. We expect to publish the 60-day notice shortly after the publication of the final rule.
The purpose of this proposal is to continue to implement section 2003 of the SUPPORT for Patients and Communities Act, which requires that the prescribing of a Schedule II, III, IV, or V controlled substance under Medicare Part D be done electronically in accordance with an electronic prescription drug program beginning January 1, 2021, subject to any exceptions, which HHS may specify. We refer readers to the CY 2021 PFS final rule (85 FR 84472) for our previously finalized requirements and burden for the first phase of implementing this statutory mandate, which required prescribers to use the NCPDP SCRIPT 2017071 standard for Electronic Prescription for Controlled Substances (EPCS) prescription transmissions. The purpose of this proposed rule is to propose delaying the date for CMS to begin taking compliance actions, proposing certain exceptions to the mandate, and proposing a compliance threshold.
In the CY 2021 PFS final rule, we estimated that the one-time burden to implement this provision would be 828,750 hours (165,750 prescribers * 6 hr) at a cost of $36,418,590 (994,500 hr * $36.62/hr). We arrived at the estimate of 165,750 prescribers having to implement EPCS based on taking the 425,000 Part D prescriber practices, and decreasing that amount by 60 percent to account for the 60 percent of prescriber practices that likely already had EPCS in place by January 1, 2021. Based on our current PDE data, we estimate that 70 percent of Part D prescribers already conduct EPCS,[245] which would leave 30 percent of Part D prescribers that would have to implement EPCS, if we did not propose any exceptions to this mandate. We also propose that long term care facilities will have an extension until January 1, 2025 along with the following exceptions to the EPCS mandate: (1) For prescriptions issued when the prescriber and dispensing pharmacy are the same entity; (2) cases where prescribers issue only a small number of Part D; (3) cases where a prescriber's NCPDP database address is in a geographic service area of an emergency or disaster declared by a federal, state or local government entity; and (4) cases where a prescriber has received a CMS-approved waiver. These exceptions will result in fewer prescribers being required to conduct EPCS.
Based on our PDE data, we believe that these exceptions will substantially decrease the number of prescribers having to implement EPCS as a result of this regulation. We have listed the exceptions and the estimated number of prescribers falling under each exception in the Table 67.[246] We do not anticipate that our proposal to include a compliance threshold of 70 percent will have any material impact on the impact of this provision. The reason for this is that based on our PDE data and conversations with prescribers, we believe that the 30 percent or less of the time that prescribers are not e-prescribing is because they are unable to e-prescribe, so they would have applied for a waiver. Although there are sometimes scenarios where beneficiaries may request that their prescriptions not be transmitted electronically, it appears as though those circumstances are not enough to make a material impact, since beneficiaries often change their views when they are given countervailing reasons that the prescriptions should be transmitted via EPCS.

Table 67 gives our estimate of the number of prescribers affected by our proposed exceptions broken down by the type of exception. As shown in Table 67, we estimate that our exceptions will exempt approximately 582,664 prescribers from the EPCS requirement, which consistutes approximately 38 percent of prescribers, since there are an estimated 1,548,221 Part D prescribers[247] (582,664/1,548,221). Since the number of exempted prescribers from this mandate far exceeds the number of prescribers who currently do not e-prescribe controlled substances in Part D, we do not expect that the total number of Part D prescribers who electronically prescribe controlled substances will increase following our implementation of this mandate. As a result, we do not believe there will be a measurable impact to the prescriber community as a whole, should this provision be finalized, as proposed.
However, for individual prescribers who have to implement this mandate, Start Printed Page 39477 we expect that the implementation costs will be the same amounts that we finalized in the CY 2021 PFS final rule. Based on the modeling that we have seen, we have found that EHR companies provide the initial set-up of e-prescribing software free of charge, provided the prescribers pay the per transaction cost of $1.88 mentioned in the CY 2021 PFS final rule. Based on the comments received on our CY2021 PFS proposed rule, we understand that implementing EPCS can lead to technological glitches, and then fixing those issues. We understand that the EHR companies remedy the issues free of charge. However, we also understand that such fixes take time away from the medical office staff. We estimate that such fixes would take the staff approximately 1 extra hour from the estimate given in our CY 2020 PFS proposed rule, when averaged across all prescribers. As a result, we have changed our one-time burden estimate of e-prescribing set-up from 5 hours to 6 hours per provider, which means a total of 994,500 hours (165,750 prescribers * 6 hr) at a cost of $36,617,490 (994,500 hr * $36.82/hr), since we anticipate that this work would be completed by an Administrative Support Worker. In this regard, the impact of this rule is plus 1 hour per response, plus 165,750 hours (165,750 prescribers × 1 hr/response), and $6,102,915 (165,750 hr * $36.82/hr).
We have proposed that prescribers have the ability to apply for a waiver from the EPCS requirement, should they be facing circumstances beyond their control that prevent them from e-prescribing, and these circumstances are not the result of a natural disaster or public health emergency. Due to the high prevalence of EPCS, the miniscule compliance actions that we are proposing for non-compliance, and the number of prescribers that we expect to exempt from the mandate, we only expect to receive about 100 attestations per year. Although we proposed certain fields be in this attestation, these were minimal, and there was no accompanying documentation required. (Note, as outlined in section II.Q. of this final rule, to meet the standard for a waiver, prescribers must provide documentation showing the existence of a circumstance beyond their control and that such a circumstance prevents them from conducting EPCS.) We expect that each attestation would take 10 minutes (0.1667 hr) for a prescriber at $210.44/hr to complete. In aggregate, CMS estimates an annual burden for filling out attestations of 16.67 hours (100 attestations × 0.1667 hr) at a cost of $3,508.04 (16.67 hr × $210.44/hr). In addition, we solicit comment on any other potential information collection implications.

7. ICRs Regarding Open Payments Proposals Included in the CY 2022 PFS (42 CFR Part 403)
The following requirement and burden changes will be submitted to OMB for approval under control number 0938-1237 (CMS-10495). The following estimates burden changes to the Open Payments final rule at §§ 403.900 through 403.914 as proposed by this proposed rule.
a. Payment Context Field for Teaching Hospitals
The proposed mandatory context field is a new requirement for reporting entities submitting and attesting to records that are attributed to teaching hospitals only. The field will be freeform text entry. We estimate that for each applicable manufacturer and applicable group purchasing organization (GPO), the inclusion of this field for collection and reporting activities will average an additional 6 total hours. The applicable instrument for these activities in the current PRA package is the "General-Research-Ownership Submission Data Elements". At the support staff cost per FTE of $42.40/hr, this would increase costs by $254.40 (6 hr × $42.40/hr) per applicable manufacturer or applicable GPO submitting teaching hospital records. However, because we anticipate fewer disputes due to this proposed field, we believe it will decrease dispute resolution by 2 total hours for support staff at $42.40/hr respectively, reducing costs by $84.80 (2 hr × $42.40/hr) per applicable manufacturer and applicable GPO. This results in a net increase in burden for each applicable manufacturer and applicable GPO submitting teaching hospital records of $169.60 ($254.40−$84.80). In Program Year (PY) 2019, 794 applicable manufacturers and applicable GPOs submitted at least one teaching hospital record, meaning the increase in burden will be a total of 3,176 hours (4 hours × 794 reporting entities) at a cost of $42.40/hr or a total of $134,662.40 (3,176 × $42.40).
In addition, we estimate this proposal would reduce teaching hospital dispute resolution estimates by 2 hours per support staff FTE at $37.82/hr or $75.64 (2 hr × $37.82/hr) per teaching hospital with records attributed to them. In PY 2019, 1,202 hospitals had record attributed to them, so for teaching hospitals we estimate a total burden reduction of 2,404 hours at a cost of $90,919.28 (2,404 × $75.64).
In aggregate, we estimate an annual burden of 772 hours (3,176−2,404) at a cost of $43,743.12 ($134,662.40−$90,919.28).
b. Optional Annual Recertification
The annual recertification is voluntary for applicable manufacturers or applicable group purchasing organizations. We approximate that 15 percent of applicable manufacturers and group purchasing organizations, or 240 reporting entities (0.15 [1,595 applicable manufacturers and applicable GPOs]) will complete and submit the proposed optional annual recertification. We anticipate that it will be a simple check box form to be included in the AM (Attestation) and GPO (Attestation) Annual IC Requirement and the "Attestation and Assumptions Screen Shots" Instrument in the existing PRA Start Printed Page 39478 package. We estimate that it would take 0.5 hours at $42.40/hr for support staff to complete and submit the recertification. In aggregate, we estimate an added annual burden of 120 hours (240 entities × 0.5 hr/response) at a cost of $5,088 (120 hr × $42.40/hr).
c. Defining a Physician-Owned Distributorship (42 CFR 403.902)
The proposed new definition is not subject to the PRA since it would not revise, add, or remove any collection of information requirements or burden.
d. Disallowing Record Deletion Without Reason (§ 403.904(a)(3))
This proposal clarifies that entities are not permitted to delete records without reason once their timeliness, completeness, and accuracy has been attested to. In order to ensure compliance with this requirement, a freeform text dialogue box will be added to the system when records are deleted that asks the applicable manufacturer or GPO to input a reason for the deletion. This would be included in the AM (Data collection and submission) and Applicable GPO (Data Collection and Submission) IC requirements and the "Open Payments User Guide" Instrument in the existing PRA package. We anticipate that this would take an average of 2 hours at $42.40/hr to input a reason for the deletion. In aggregate, we estimate an added annual burden of 80 hours (40 applicable manufacturers or GPOs deleting records annually × 2 hr/response) at a cost of $3,392 (80 hr * $42.40/hr).
e. Disallow Publication Delays of General Payments
A very small number of general payments are delayed from publication by reporting entities every year, and these records will simply either be reported as research records instead, or not delayed at all. Therefore, we anticipate a negligible burden for this proposal.
f. Short Term Loans (§ 403.902)
This proposal is merely a clarification of an existing requirement in regulation text. The purpose of this language is to clarify that the exemption for short-term loans from reporting requirements only applies for loans of less than 91 cumulative days per calendar year. In other words, multiple short-term loans in a calendar year would still meet reporting requirements if they add up to 91 days or greater. We do not believe this proposal will change reporting behavior, and therefore do not anticipate an increase in burden.
g. Remove General Ownership Records
Currently the Open Payments system allows for a reporting entity to submit either a general record with a nature of payment category of ownership, or an ownership and investment interest record. For Program Years 2015-2019, approximately 92 applicable manufacturers and GPOs reported records with the nature of payment category of ownership. Since reporting these general records as ownership records will require the addition of two additional pieces of information, we anticipate that it will take these 92 entities an additional 3 hours at $42.40/hr to report the two extra fields. In aggregate, we estimate an added annual burden of 276 hours (92 entities × 3 hr/response) at a cost of $11,702 (276 hr × $42.40/hr). This would be included in the AM (Data collection and submission) and Applicable GPO (Data Collection and Submission) IC requirements and the "Open Payments User Guide" Instrument in the existing PRA package.
h. Updated Contact Information (§ 403.908(c)(3))
This proposal creates a requirement for reporting entities to keep their contact information up to date with CMS. The ability to communicate with a reporting entity is important because CMS may need to contact the entity in the case of perceived issues with the records. Applicable manufacturers and applicable GPOs will only be required to update their contact information if the two contacts provided become obsolete due to a change in the organization. This will also only apply to entities that do not have records to report for 2 years after a program year in which they reported. Therefore, we anticipate that it will only affect approximately 30 applicable manufacturers and applicable group purchasing organizations. We estimate that it would take 0.5 hours at $42.40/hr to update the contact information. In aggregate, we estimate an added annual burden of 15 hours (30 entities × 0.5 hr/response) at a cost of $636 (15 hr × $42.40/hr). This would be included in the AM (Data collection and submission) and Applicable GPO (Data Collection and Submission) IC requirements and the "Open Payments User Guide" Instrument in the existing PRA package.
i. Summary

Start Printed Page 39479
8. The Quality Payment Program (QPP) (42 CFR Part 414 and Section IV. of This Proposed Rule)
The following QPP-specific ICRs reflect this rule's proposed policy changes as well as adjustments to the policies that have been finalized in the CY 2017 and 2018 Quality Payment Program final rules (81 FR 77008 and 82 FR 53568, respectively), the CY 2019, CY 2020 and CY 2021 PFS final rules (83 FR 59452, 84 FR 62568 and 85 FR 84472, respectively).
a. Background
(1) ICRs Associated With MIPS and Advanced APMs
There is a series of ICRs associated with the Quality Payment Program, including for MIPS and Advanced APMs. The MIPS ICRs consist of: Registration for virtual groups (see section V.B.8.b of this proposed rule); QCDR self-nomination applications and other requirements (see section V.B.8.c.(2) of this proposed rule); qualified registry self-nomination applications and other requirements (see section V.B.8.c.(3) of this proposed rule); CAHPS survey vendor applications (see section V.B.8.c.(4) of this proposed rule); Health IT vendors (see section V.B.8.c.(5) of this proposed rule); Open Authorization credentialing and token request process (see section V.B.8.d of this proposed rule); Quality Payment Program Identity Management Application Process (see section V.B.8.e.(3) of this proposed rule); quality performance category data submission by Medicare Part B claims collection type (see section V.B.8.e.(4) of this proposed rule), QCDR and MIPS CQM collection type (see section V.B.8.e.(5) of this proposed rule), eCQM collection type (see section V.B.8.e.(6) of this proposed rule), MVP Quality submission (see section V.B.8.e.(7)(a)(iii) of this proposed rule), and CMS Web Interface collection type (see section V.B.8.e.(8) of this proposed rule); CAHPS for MIPS survey beneficiary participation (see section V.B.8.e.(9) of this proposed rule); group registration for CMS Web Interface (see section V.B.8.e.(10) of this proposed rule); group registration for CAHPS for MIPS survey (see section V.B.8.e.(11) of this proposed rule); MVP registration (see section V.B.8.e.(7)(a)(i) of this proposed rule); subgroups registration (see section V.B.8.e.(7)(a)(ii) of this proposed rule); and call for quality measures (see section V.B.8.f of this proposed rule); reweighting applications for Promoting Interoperability and other performance categories (see section V.B.8.g.(2) of this proposed rule); Promoting Interoperability performance category data submission (see section V.B.8.g.(3) of this proposed rule); call for Promoting Interoperability measures (see section V.B.8.h of this proposed rule); improvement activities performance category data submission (see section V.B.8.i of this proposed rule); nomination of improvement activities (see section V.B.8.j of this proposed rule); nomination of MVPs (see section V.B.8.k of this proposed rule); and opt-out of Physician Compare for voluntary participants (see section V.B.8.o of this proposed rule).
The ICRs for Advanced APMs consist of: Partial Qualifying APM Participant (QP) election (section V.B.8.m of this proposed rule); Other Payer Advanced APM identification: Payer Initiated and Eligible Clinician Initiated Processes (sections V.B.8.n.(1) and V.B.8.n.(2) of this proposed rule); and submission of data for QP determinations under the All-Payer Combination Option (section V.B.8.n.(3) of this proposed rule).
(2) Summary of Quality Payment Program Changes: MIPS
The following 10 MIPS ICRs show changes in burden due to the proposed policies in this rule: (1) QCDR self-nomination applications; (2) Qualified Registry self-nomination applications; (3) Quality performance category data submission by Medicare Part B Claims collection type; (4) Quality performance category data submission by QCDR and MIPS CQM collection type; (5) Quality performance category data submission by eCQM collection type; (6) Group registration for CMS Web Interface; (7) CMS Web Interface submission burden; (8) Reweighting applications for Promoting Interoperability and other performance categories; (9) Promoting Interoperability performance category data submission; and (10) Nomination of improvement activities. In aggregate, we estimate the proposed policies will result in a net decrease in burden of 40,010 hours and $4,053,151 for the CY 2022 MIPS performance period/2024 MIPS payment year and 84,803 hours and $8,577,728 for the CY 2023 MIPS performance period/2025 MIPS payment year. The remaining changes to our currently approved burden estimates are adjustments due to the revised assumptions based on the data available at the time of publication of this proposed rule.
We have also added 3 new ICRs (MVP Registration, MVP Quality Submissions, and Subgroups Registration) for the associated burden related to the proposals for implementation of MVPs and subgroups beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. The MVP and subgroup registration ICRs reflect the burden associated with the proposed MVP and subgroup registration requirements described in section IV.A.3.b(4)(f) of this rule. The MVP quality submission ICR reflects the change in burden associated with the proposed requirements for the quality performance category of MVPs described in section IV.A.3.b(4)(d)(ii) of this rule.
We are not making any changes or adjustments to the following ICRs: Registration for virtual groups; CAHPS survey vendor applications; Quality Payment Program Identity Management Application Process; group registration for CAHPS for MIPS survey; CAHPS for MIPS survey beneficiary participation; Open Authorization (OAuth) Credentialing and Token Request Process; nomination of MVPs; call for Promoting Interoperability measures; and improvement activities performance category data submission. See section V.B.8. of this proposed rule for a summary of the ICRs, the overall burden estimates, and a summary of the assumption and data changes affecting each ICR.
The accuracy of our proposed estimates of the total burden for data submission under the quality, Promoting Interoperability, and improvement activities performance categories may be impacted due to two primary reasons. First, we are unable to predict with absolute certainty who will be a QP for the CY 2022 MIPS performance period/2024 MIPS payment year. New eligible clinician participants in Advanced APMs who become QPs would be excluded from MIPS reporting requirements and payment adjustments, and as such, unlikely to report under MIPS; while some current Advanced APM participants may end participation such that the APM Entity's eligible clinicians would not be QPs for a year based on § 414.1425(c)(5), and thus be required to report under MIPS. Second, it is difficult to predict what Partial QPs, who can elect whether to report to MIPS, will do in the CY 2022 MIPS performance period/2024 MIPS payment year compared to the CY 2019 MIPS performance period/2021 MIPS payment year, and therefore, the actual number of Advanced APM participants and how they elect to submit data may be different than our estimates. However, we believe our estimates are the most appropriate given the available data. Start Printed Page 39480
We acknowledge a recent JAMA article (Khullar, et al., 2021)[248] which includes new data on the burden involved in submitting data for the Quality Payment Program. We have chosen not to include this data in our estimates because of the small sample size included (30 TINs, half of which are APM participants, which we do not include in our estimates). In addition, the article does not indicate the time spent per activity involved in submissions for MIPS, so we are unable to determine if the totals in the article represent only the activities relevant for regulatory burden or separate the totals for the individual ICRs. We seek comment on our assumptions for estimating the burden for clinicians submitting data for the Quality Payment Program.
(3) Summary of Quality Payment Program Changes: Advanced APMs
For these ICRs (identified above under, "ICRs Associated with MIPS and Advanced APMs"), the changes to currently approved burden estimates are adjustments based on updated projections for the CY 2022 MIPS performance period/2024 MIPS payment year. We are not proposing any changes to the Other Payer Advanced APM identification: Eligible Clinician Initiated Process and submission of Data for QP determinations under the All-Payer Combination Option ICRs.
(4) Framework for Understanding the Burden of MIPS Data Submission
Because of the wide range of information collection requirements under MIPS, Table 70 presents a framework for understanding how the organizations permitted or required to submit data on behalf of clinicians vary across the types of data, and whether the clinician is a MIPS eligible clinician or other eligible clinician voluntarily submitting data, MIPS APM participant, or an Advanced APM participant. As shown in the first row of Table 70, MIPS eligible clinicians and other clinicians voluntarily submitting data will submit data either as individuals, groups, or virtual groups for the quality, Promoting Interoperability, and improvement activities performance categories. Note that virtual groups are subject to the same data submission requirements as groups, and therefore, we will refer only to groups for the remainder of this section unless otherwise noted. We want to note that we have included subgroups to Table 70 due to the proposed introduction of subgroups for clinicians choosing to report MVPs or the APP in the CY 2023 MIPS performance period/2025 MIPS payment year described in section IV.A.3.b.(2)(d)(ii) of this rule. Because MIPS eligible clinicians are not required to submit any additional information for assessment under the cost performance category, the administrative claims data used for the cost performance category is not represented in Table 70.
For MIPS eligible clinicians participating in MIPS APMs, the organizations submitting data on behalf of MIPS eligible clinicians will vary between performance categories and, in some instances, between MIPS APMs. As discussed in section IV.A.3.c. of this proposed rule, for clinicians in APM Entities, the APM Performance Pathway is available for both ACO and non-ACOs to submit quality data. Due to data limitations and our inability to determine who would use the APM Performance Pathway versus the traditional MIPS submission mechanism for the CY 2022 MIPS performance period/2024 MIPS payment year, we assume ACO APM Entities will submit data through the APM Performance Pathway, using the CMS Web Interface option, and non-ACO APM Entities would participate through traditional MIPS, thereby submitting as an individual or group rather than as an entity.
For the Promoting Interoperability performance category, group TINs may submit data on behalf of eligible clinicians in MIPS APMs, or eligible clinicians in MIPS APMs may submit data individually. For the improvement activities performance category, we will assume no reporting burden for MIPS APM participants. In the CY 2017 PFS final rule, we described that for MIPS APMs, we compare the requirements of the specific MIPS APM with the list of activities in the improvement activities Inventory and score those activities in the same manner that they are otherwise scored for MIPS eligible clinicians (81 FR 77185). Although the policy allows for the submission of additional improvement activities if a MIPS APM receives less than the maximum improvement activities performance category score, to date all MIPS APM have qualified for the maximum improvement activities score. Therefore, we assume that no additional submission will be needed.
Eligible clinicians who attain Partial QP status may incur additional burden if they elect to participate in MIPS, which is discussed in more detail in the CY 2018 PFS final rule (82 FR 53841 through 53844).
Start Printed Page 39481

The policies finalized in the CY 2017 and CY 2018 Quality Payment Program final rules, the CY 2019, CY 2020 and CY 2021 PFS final rules, and continued in this proposed rule create some additional data collection requirements not listed in Table 70. These additional data collections, some of which are currently approved by OMB under the control numbers 0938-1314 (Quality Payment Program, CMS-10621) and 0938-1222 (CAHPS for MIPS, CMS-10450), are as follows: Start Printed Page 39482
Additional ICRs Related to MIPS Third-Party Intermediaries (See Section V.B.8.c).
- Self-nomination of new and returning QCDRs (81 FR 77507 through 77508, 82 FR 53906 through 53908, and 83 FR 59998 through 60000) (OMB 0938-1314).
- Self-nomination of new and returning registries (81 FR 77507 through 77508, 82 FR 53906 through 53908, and 83 FR 59997 through 59998) (OMB 0938-1314).
- Approval process for new and returning CAHPS for MIPS survey vendors (82 FR 53908) (OMB 0938-1222).
- Open Authorization Credentialing and Token Request Process (New) (OMB 0938-1314) (see section V.B.8.d).
Additional ICRs Related to the Data Submission and the Quality Performance Category (See Section V.B.8.e).
- CAHPS for MIPS survey completion by beneficiaries (81 FR 77509, 82 FR 53916 through 53917, and 83 FR 60008 through 60009) (OMB 0938-1222).
- Quality Payment Program Identity Management Application Process (82 FR 53914 and 83 FR 60003 through 60004) (OMB 0938-1314).
Additional ICRs Related to the Promoting Interoperability Performance Category (See Section V.B.8.g).
- Reweighting Applications for Promoting Interoperability and other performance categories (82 FR 53918 and 83 FR 60011 through 60012) (OMB 0938-1314).
Additional ICRs Related To Call for New MIPS Measures and Activities (See Sections V.B.8.f, V.B.8.h, V.B.8.j. and V.B.8.k).
- Nomination of improvement activities (82 FR 53922 and 83 FR 60017 through 60018) (OMB 0938-1314).
- Call for new Promoting Interoperability measures (83 FR 60014 through 60015) (OMB 0938-1314).
- Call for MIPS quality measures (83 FR 60010 through 60011) (OMB 0938-1314).
- Nomination of MVPs (OMB 0938-1314).
Additional ICRs Related to MIPS (See Section V.B.8.o).
- Opt out of performance data display on Physician Compare for voluntary reporters under MIPS (82 FR 53924 through 53925 and 83 FR 60022) (OMB 0938-1314).
Additional ICRs Related to APMs (See Sections V.B.8.m and V.B.8.n).
- Partial QP Election (81 FR 77512 through 77513, 82 FR 53922 through 53923, and 83 FR 60018 through 60019) (OMB 0938-1314).
- Other Payer Advanced APM determinations: Payer Initiated Process (82 FR 53923 through 53924 and 83 FR 60019 through 60020) (OMB 0938-1314).
- Other Payer Advanced APM determinations: Eligible Clinician Initiated Process (82 FR 53924 and 83 FR 60020) (OMB 0938-1314).
- Submission of Data for All-Payer QP Determinations (83 FR 60021) (OMB 0938-1314).
b. ICRs Regarding the Virtual Group Election (§ 414.1315)
This rule is not proposing any new or revised collection of information requirements or burden related to the virtual group election. The virtual group election requirements and burden are currently approved by OMB under control number 0938-1343 (CMS-10652). Consequently, we are not proposing any changes under that control number.
c. ICRs Regarding Third-Party Intermediaries (§ 414.1400)
In section IV.A.3.h. of this rule, we propose multiple changes to the third-party intermediary regulations at § 414.1400. Specifically, we are proposing: (1) Requirement for third-party intermediaries to submit MIPS data for APM Entities; (2) requirement for QCDRs and qualified registries to support MVPs and the APP; (3) requirement for all QCDRs and qualified registries to support subgroup reporting; (4) requirements for approved QCDRs and qualified registries that have not submitted performance data; and (5) new QCDR measure rejection criteria. The proposed burden associated with each of these topics are discussed separately below for qualified registries, QCDRs, and survey vendors.
(1) Background
Under MIPS, the quality, Promoting Interoperability, and improvement activities performance category data may be submitted via relevant third-party intermediaries, such as qualified registries, QCDRs, and health IT vendors. Data on the CAHPS for MIPS survey, which counts as either one quality performance category measure, or towards an improvement activity, can be submitted via CMS-approved survey vendors. Entities seeking approval to submit data on behalf of clinicians as a qualified registry, QCDR, or survey vendor must complete a self-nomination process annually.[249] The processes for self-nomination for entities seeking approval as qualified registries and QCDRs are similar with the exception that QCDRs have the option to nominate QCDR measures for approval for the reporting of quality performance category data. Therefore, differences between QCDRs and qualified registry self-nomination are associated with the preparation of QCDR measures for approval.
(2) QCDR Self-Nomination Applications
The proposed requirements and burden associated with this rule's data submission changes related to QCDRs will be submitted to OMB for approval under control number 0938-1314 (CMS-10621). As explained below, in this rule we propose to: Adjust the number of self-nomination applications based on current data (from 82 to 90), change the number of QCDR measures submitted for consideration by each QCDR at the time of self-nomination (from 2 to 12), and adjust the average time required to submit information for each QCDR measure (from 2.5 hours to 0.75 hours).
(a) Self-Nomination Process and Other Requirements
In section IV.A.3.h.(1) of this rule, we are proposing reorganization and consolidation of § 414.1400 generally. We assume that this proposal does not change the existing requirements for third-party intermediaries during the self-nomination process. Therefore, we are not revising our burden estimates related to these proposals. We refer readers to § 414.1400 which states that QCDRs interested in submitting MIPS data to us on behalf of a MIPS eligible clinician, group, or virtual group will need to complete a self-nomination process to be considered for approval to do so. We also refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77507 through 77508), CY 2018 Quality Payment Program final rule (82 FR 53906 through 53908), CY 2019 PFS final rule (83 FR 59998 through 60000), the CY 2020 PFS final rule (84 FR 63116 through 63121) and the CY 2021 PFS final rule (85 FR 84964 through 84969) for our previously finalized requirements and burden for self-nomination of QCDRs and nomination of QCDR measures.
In section IV.A.3.h.(2)(a) of this rule, we propose to: Add APM Entities to § 414.1400(a)(1), and expand the general Start Printed Page 39483 participation requirements of third-party intermediaries, to third party intermediaries reporting to MIPS on behalf of APM Entities reporting to MIPS in order to align reporting requirements for all participants in MIPS. We are also proposing that beginning with the CY 2023 MIPS performance period/2025 MIPS payment year, QCDRs and qualified registries must support the APP, and MVPs that are applicable to the MVP participants on whose behalf they submit MIPS data. As finalized in the CY 2017 PFS final rule, third-party intermediaries currently support MIPS data submission on behalf of eligible clinicians (81 FR 77016). APM Entities have historically used third party intermediaries for submitting their quality measures to their APMs. Additionally, QCDRs, qualified registries and health IT vendors are required under existing § 414.1400(a)(1) to submit data for the quality, improvement activities, and Promoting Interoperability performance categories in MIPS. Therefore, we anticipate no additional steps being added to the self-nomination process as a result of this proposal for third-party intermediaries to submit MIPS data on behalf of APM Entities, and to support measures and activities in MVPs that are applicable to the MVP participants on whose behalf they submit MIPS data. For this proposed rule, we assume that there will be no impact on the time required for QCDRs to complete either the simplified or full self-nomination process because of the above proposals. Additionally, we are proposing to require QCDRs, qualified registries, health IT vendors, and CAHPS for MIPS survey vendors to support subgroup reporting, beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. We anticipate that at the time of self-nomination, QCDRs would be using a checkbox to indicate their compliance for the proposed requirement to support data submission for subgroups beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. We assume that this would not impact the overall time estimated for QCDRs to submit their information at the time of self-nomination. Therefore, we are not proposing to make any adjustments in the time required for QCDRs during the simplified or full self-nomination process because of this proposal. However, we anticipate that third-party intermediaries would need to make administrative changes to their existing workflows for submission of MVPs and APP data for clinicians participating as subgroups beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. We refer readers to section VII.C.17.f.(2)(f) of this proposed rule where we discuss our impact analysis.
In section IV.A.3.h.(3)(a)(iii) of this rule, to provide further clarity and to better align with the existing policy (81 FR 77366 through 77367; 81 FR 77383 through 77384), we are proposing to codify QCDRs, and qualified registries must conduct validation on the data they intend to submit for the applicable MIPS performance period and provide the results of the executed data validation plan by May 31st of the year following the performance period. Additionally, we are proposing to codify a new requirement at § 414.1400(b)(3)(iv) to state that, beginning with the CY 2023 MIPS performance period/2025 MIPS payment year, the QCDR or qualified registry must submit a data validation plan annually, at the time of self-nomination, for CMS' approval, and may not change the plan once approved, without the prior approval of the agency. We anticipate that this proposal does not make any changes to the existing data validation requirements for QCDRs and qualified registries. Through this proposal, CMS is codifying the finalized policies related to data validation for QCDRs and qualified registries in previous rules. We are not revising our burden estimates as a result of the above proposal and the associated burden was captured in the CY 2017 PFS final rule (81 FR 77383 through 77384) and the CY 2019 PFS final rule (83 FR 59998 through 59999) and submitted to OMB for approval under control number 0938-1314 (CMS-10621).
In section IV.A.3.h(3)(a)(i) of this proposed rule, we are proposing new requirements for approved QCDRs and qualified registries that have not submitted performance data. First, we are proposing to create a new requirement at § 414.1400(b)(3)(vii) to require QCDRs and qualified registries that have never submitted data since the inception of MIPS (CY 2017 MIPS performance period/2019 MIPS payment year) through the 2020 MIPS performance period/2022 MIPS payment year, to submit a participation plan as part of their self-nomination for CY 2023. If the QCDRs and qualified registries did not submit data, their participation plan must be submitted as part of self-nomination for the 2023 self-nomination period and must be accepted by CMS to continue to be an approved QCDR or qualified registry. We are also proposing to codify a new requirement at paragraph (b)(3)(viii) to state that, beginning with the CY 2024 MIPS performance period/2026 MIPS payment year, a QCDR or qualified registry that was approved but did not submit any MIPS data for either of the 2 years preceding the applicable self-nomination period must submit a participation plan for CMS's approval. Under this proposal, the participation plan must explain the QCDR and/or qualified registry's detailed plans about how the vendor intends to encourage clinicians to submit MIPS data to CMS through the third-party intermediary on behalf of clinicians or groups. The vendor must also explain why they should still be allowed to participate as a qualified vendor.
Based on our review of the existing list of approved QCDRs that did not submit performance data since the inception of MIPS (CY 2017 MIPS performance period/2019 MIPS payment year), we estimate that approximately 10 QCDRs will submit participation plans for the CY 2022 and the CY 2023 self-nomination periods. Similar to our assumptions for submission of a Corrective Action Plan (CAP) in the CY 2021 PFS final rule (85 FR 84968), we anticipate that the effort involved in developing a participation plan including the proposed policies specified in this rule and submitting it to CMS is likely to be no more than 3 hours for a computer systems analyst at a rate of $95.22/hr. For the CY 2022 MIPS performance period/2024 MIPS payment year, we estimate an annual burden of 30 hours (3 hr × 10 participation plans) at a cost of $2,857 (30 hr × $95.22/hr) for QCDRs that would need to develop and submit a participation plan.
In section IV.A.3.h.(4) of this rule, we are proposing to codify new requirements that if a QCDR measure owner is not an approved active QCDR for a given self-nomination period, that QCDR measure will not be available for use. Additionally, we propose to codify a new requirement in section IV.A.3.h.(1)(d)(iv) and add a rejection criterion at § 414.1400(b)(4)(iv)(M) to state, a QCDR does not have permission to use a QCDR measure owned by another QCDR for the applicable performance period. It was finalized in the CY 2018 PFS final rule (82 FR 53813) that beginning with the CY 2018 MIPS performance period/2020 MIPS payment year, QCDR vendors may seek permission from another QCDR to use an existing measure that is owned by the other QCDR. Additionally, in the CY 2020 PFS final rule (84 FR 63070 through 63073), we finalized the QCDR measure rejection criteria Start Printed Page 39484 considerations. Specifically, we stated that all previously approved QCDR measures and new QCDR measures would be reviewed on an annual basis (as a part of the QCDR measure review process that occurs after the self-nomination period closes on September 1st) to determine whether they are appropriate for the program. In the CY 2020 PFS final rule, we indicated to stakeholders that as information becomes available in future years, we will revisit our assumptions to better reflect the impact of these requirements on QCDRs and the quantity of measures annually (84 FR 63118 through 63119). As discussed in the CY 2019 PFS final rule (83 FR 60000) and CY 2020 PFS final rule (84 FR 63118), we are not accounting for QCDR measure licensing costs as part of our burden estimate.
Based on the number of QCDR measures submitted at the time of self-nomination for the CY 2021 MIPS performance period/2023 MIPS payment year, we assume that 82 QCDRs will submit 984 measures for consideration in the CY 2022 MIPS performance period/2024 MIPS payment year, approximately 12 measures per QCDR, on average. We anticipate that out of the 984 measures, 820 measures would be existing or borrowed measures, approximately 10 measures submitted per QCDR self-nomination application. The remaining 104 measures would be new measures, approximately 2 measures on average per QCDR.
Using the above assumption that each QCDR submitting measures for approval during the self-nomination process will submit approximately 12 measures (10 existing or borrowed measures + 2 new measures), we estimate an increase of 10 measures from the currently approved estimate of 2 measures per QCDR. The estimated increase in the total number of measures submitted by a QCDR at the time of self-nomination is due to the inclusion of the existing or borrowed QCDR measures in our assumptions. Additionally, we anticipate that less information is needed for a QCDR to submit an existing or borrowed measure for approval, therefore, we estimate that the time needed for a QCDR to submit an existing or borrowed measure is 0.5 hours, independent of the selection of the simplified or full self-nomination process. Consistent with our assumption in the CY 2020 PFS final rule (84 FR 63119), we continue to estimate that each QCDR will require 2 hours to submit a new QCDR measures for approval, independent of the selection of the simplified or full self-nomination process. To account for the difference in the time for submission of new vs existing QCDR measures for approval, we are using the weighted average to estimate the time required for QCDR measure submission at the time of self-nomination. Therefore, we assume that the weighted average of the time required for each QCDR to submit a new or existing or borrowed measure for approval during the self-nomination process is 0.75 hours [((2 new measures × 2 hours) + (10 existing or borrowed measures × 0.5 hours))/total # of measures (12)]. Based on the above assumptions, we are proposing to revise our estimates in the amount of time required for a QCDR to submit measures during the self-nomination process from a total of 2 hours to approximately 0.75 hours, a decrease of 1.75 hours from the currently approved estimated burden per QCDR measure submission.
In the CY 2019 PFS final rule, we estimated that it would take 0.5 hours and 3 hours for a QCDR to submit all the required information during the simplified and full self-nomination process, respectively (83 FR 59999). Based on our experience with the amount of time needed for QCDRs during the 2020 self-nomination period, we assume that the estimated time of 3 hours per QCDR for a full self-nomination process is an overestimate and are proposing to revise our estimated time required for the QCDR full-self-nomination process to 2.5 hours, a decrease of 0.5 hours. We are not making any adjustments in the amount of time needed for simplified self-nomination process.
Based on the trends noticed in the number of QCDRs that submit applications for self-nominations and consistent with our assumptions in the CY 2021 PFS final rule (85 FR 84965), we estimate that 90 QCDRs will submit applications for consideration during the self-nomination process for the CY 2022 MIPS performance period/2024 MIPS payment year, an increase of 8 applications from the currently approved estimate of 82. For QCDRs that submit measures as part of their self-nomination process, while simultaneously accounting for the estimated increase in the number of existing or borrowed QCDR measures submitted with the self-nomination application and the decrease in the estimated time for the QCDR full-nomination process, we are proposing to revise our estimated time for the QCDR self-nomination process to a minimum of 9.5 hours [0.5 hours for the simplified self-nomination process + (12 measures × 0.75 hr/measure for QCDR measure submission)] and a maximum of 11.5 hours [2.5 hours for the full self-nomination process + (12 measures × 0.75 hr/measure for QCDR measure submission)], an increase of 4 hours at a cost of $380.88 (4 hr × $95.22/hr) and 3.5 hours at a cost of $333.27 (3.5 hr × $95.22/hr) from the currently approved burden per respondent estimate in the CY 2021 PFS final rule (85 FR 84965).
Consistent with our assumptions in the CY 2021 PFS final rule (85 FR 84967), based on the number of targeted audits received for the 2020 self-nomination period, we estimate that 20 QCDRs will submit targeted audits for the CY 2022 MIPS performance period/2024 MIPS payment year, an increase of 3 from the currently approved estimate of 17 QCDRs submitting targeted audits in the CY 2021 PFS final rule. Using the currently approved unchanged burden per respondent estimate, the proposed estimated burden associated with QCDRs completing targeted audits will range from 100 hours (20 audits × 5 hr/audit) at a cost of $9,522 (20 audits × $476.10/audit) for the simplified self-nomination process to 200 hours (20 audits × 10 hr/audit) at a cost of $19,044 (20 audits × $952.20/audit) for the full self-nomination process (see Table 68 for the cost per audit). We assume that this would adjust our burden estimates for targeted audits by +15 hours (+3 respondents × 5 hr/audit) at a cost of $1,428.30 (15 hrs × $95.22/hr) and +30 hours (+3 respondents × 10 hr/audit) at a cost of $2,856.60 (30 hrs × $95.22/hr) for the simplified and full self-nomination process, respectively. Based on the assumptions discussed in this section, we provide an estimate of the total annual burden associated with a QCDR self-nominating to be considered "qualified" to submit quality measures results and numerator and denominator data on behalf of MIPS eligible clinicians.
As shown in Table 71, we assume that the staff involved in the QCDR self-nomination process will continue to be computer systems analysts or their equivalent, who have an average labor rate of $95.22/hr.
Using the change in the number of respondents and the estimated time per respondent for QCDRs that submit measures for approval during the self-nomination process, the proposed annual burden for the simplified and full-self nomination process will range from 855 hours (90 QCDRs × 9.5 hr) to 1,035 hours (90 QCDRs × 11.5 hr) at a cost ranging from $81,413 (855 hr × $95.22/hr) and $98,553 (1,035 hr × $95.22/hr), respectively.
As shown in Table 71, combined with our proposed adjusted estimate of annual burden for targeted audits and the proposed burden for submission of participation plans, we are proposing to Start Printed Page 39485 revise our estimated burden for the QCDR self-nomination process, ranging from 985 hours [855 hr (90 QCDRs × 9.5 hr) + 100 hr (20 audits × 5 hr) + 30 hr (10 participation plans × 3 hr)] at a cost of $93,792 [$81,414 (855 hr × $95.22/hr) + $9,522 (20 audits × $476.10/audit) + $2,857 (30 hr × $95.22/hr)] for a simplified self-nomination process to 1,265 hours [1,035 hr (90 QCDRs × 11.5 hr) + 200 hr (20 audits × 10 hr) + 30 hr (10 participation plans × 3 hr)] at a cost of $120,454 [$98,553 (1,035 hr × $95.22/hr) + $19,044 (20 audits × $952.20/audit) + $2,857 (30 hr × $95.22/hr)] for the full self-nomination process.

As shown in Table 72, for the CY 2022 MIPS performance period/2024 MIPS payment year, independent of the proposed change to our per response time estimate, the estimated increase in 8 respondents from the currently approved 82 respondents to 90 results in an increase of between +76 hours (+8 respondents × 9.5 hrs/respondent for the simplified self-nomination process) and +92 hours (+8 respondents × 11.5 hrs/respondent for the full self-nomination process) at a cost of between +$7,237 (+8 respondents × $904.60/respondent for the simplified self-nomination process) and +$8,760 (+8 respondents × $1,095.03/respondent for the full self-nomination process) (see Table 71 for the cost per QCDR).
Accounting for the proposed change in time required for the QCDR self-nomination process results in an adjustment of between +328 hours (82 respondents × +4 hr for the simplified self-nomination process or also referred to as minimum burden) at a cost + $31,232 [82 respondents × $380.88 (+4 hr × $95.22/hr)/respondent) and +287 hours (82 respondents × 3.5 hr for the full self-nomination process or also referred to as maximum burden) at a cost of and +$27,328 (82 respondents × $333.27 (+3.5 hr × $95.22/hr)/respondent). The reason for the increase in minimum burden compared to the maximum burden is due to an increase in the change in the number of hours required for the simplified self-nomination process compared to the increase in the number of hours for the full self-nomination process.
In aggregate, as shown in Table 72, when these impacts are combined with the proposed estimate for targeted audits and participation plans discussed above, the net impact ranges between +449 hours [76 hr (+8 respondents × 9.5 hrs/respondent) + 15 hr (+3 targeted audits × 5 hrs/audit) + 30 hr (10 participation plans × 3 hr/plan) + 328 hr (82 respondents × 4 hr)] at a cost of $42,754 ($7,237 + $1,428 + $2,857 + $31,232) for the simplified self-nomination process (also referred to as minimum burden) and +439 hours [92 hr (+8 respondents × 11.5 hrs/respondent) + 30 hr (+3 targeted audits × 10 hrs/audit) + 30 hr (10 participation plans × 3 hr/plan) + 287 hr (+82 respondents × 3.5 hr)] at a cost of $41,802 [$8,760 (+8 respondents × $1,095.03/respondent + $2,857 (30 hr × $95.22/hr) + $2,857 (30 hr × $95.22/hr) + $27,328 (82 respondents × $333.27/respondent)] for the full self-nomination process (also referred to as maximum burden) for the CY 2022 MIPS performance period/2024 MIPS payment year.
Start Printed Page 39486

(b) QCDR Measure Requirements
In the CY 2018 Quality Payment Program final rule (82 FR 53813 through 53814), we discussed that beginning with the 2018 performance period and for future program years, QCDR vendors may seek permission from another QCDR to use an existing measure that is owned by the other QCDR. Additionally, in the CY 2020 Quality Payment Program rule (84 FR 63070 through 63073) we finalized the QCDR measure rejection criteria considerations.
In section IV.A.3.h.(4)(a)(i)(A)(aa) of this rule, we are proposing to codify a new requirement and add a rejection criterion that a QCDR does not have permission to use a QCDR measure owned by another QCDR for the applicable performance period. Additionally, we are proposing to codify new requirements that if a QCDR measure owner is not an approved active QCDR for a given self-nomination period, that QCDR measure will not be available for use. The inactive QCDR measure owner has the option to transfer ownership of the QCDR measure to an active QCDR or agree upon terms set forth with the active QCDR allowing co-ownership of the QCDR measure. We refer readers to section IV.A.3.h.(4)(a)(i)(A) of this rule for additional details on the proposed policies for transfer of ownership of QCDR measures. This proposal is to codify the existing requirements for the QCDR self-nomination process. We are not proposing to revise our burden estimates as result of this proposal because we assume that this does not change the requirements, or the time required for a QCDR to submit information for a QCDR measure at the time of self-nomination.
Additionally, we are proposing to codify another rejection criterion at § 414.1400(b)(4)(iv)(N) to state that, if a QCDR measure owner is not approved during a given self-nomination period, any associated QCDR measures with that QCDR would also not be approved. We are not revising our burden estimates as a result of the above proposal because we assume that there would not be additional requirements for QCDRs to submit at the time of self-nomination. This is part of the measure specification requirements for QCDRs which submit measures for approval during the self-nomination process.
(3) Qualified Registry Self-Nomination Process and Other Requirements
The requirements and burden associated with this rule's data submission changes related to qualified registries will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
We refer readers to § 414.1400 which states that qualified registries interested in submitting MIPS data to us on behalf of MIPS eligible clinicians, groups, or virtual groups need to complete a self-nomination process to be considered for approval to do so. We also refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77507 through 77508), CY 2018 Quality Payment Program final rule (82 FR 53906 through 53908), CY 2019 PFS final rule (83 FR 59997 through 59998), CY 2020 PFS final rule (84 FR 63114 through 63116) and the CY 2021 PFS final rule (85 FR 84967 through 85 FR 84969) for our previously finalized requirements and burden for self-nomination of qualified registries.
In section IV.A.3.h.(1) of this rule, we are proposing reorganization and consolidation of § 414.1400 generally. We assume that this proposal does not change the existing requirements for third-party intermediaries during the self-nomination process. Therefore, we are not revising our burden estimates related to these proposals.
In section IV.A.3.h.(2)(a) of this rule, we are proposing to add APM Entities to § 414.1400(a)(1), expanding the general participation requirements of third-party intermediaries, to third party intermediaries reporting to MIPS on behalf of APM Entities reporting to MIPS to align reporting requirements for all participants in MIPS. We are also proposing that beginning with the CY 2023 MIPS performance period/2025 MIPS payment year, QCDRs and qualified registries must support APP, and MVPs that are applicable to the MVP participants on whose behalf they submit MIPS data. As finalized in the CY 2017 PFS final rule, third-party intermediaries currently support MIPS data submission on behalf of eligible clinicians (81 FR 77016). APM Entities have historically used third party intermediaries for submitting their quality measures to their APMs. Additionally, QCDRs, qualified registries and health IT vendors are required under existing § 414.1400(a)(1) to submit data for the quality, improvement activities, and promoting interoperability performance categories in MIPS. Like our discussion for QCDRs above, we anticipate no additional steps being added to the qualified registry self-nomination process as a result of this proposal for third party intermediaries to submit MIPS data on behalf of APM Entities, and to support measures and activities in MVPs that are applicable to the MVP participants on whose behalf they submit MIPS data. For this proposed rule, we assume that there will be no impact on the time required for qualified registries to complete either the simplified or full self-nomination process because of the above proposals. Additionally, we are proposing to require QCDRs, qualified registries, health IT vendors, and CAHPS for MIPS survey vendors to support subgroup reporting, beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. We anticipate that at the time of self-nomination, qualified registries would be using a checkbox to indicate their compliance for the proposed requirement to support data submission Start Printed Page 39487 for subgroups beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. We assume that this would not impact the overall time estimated for qualified registries to submit their information at the time of self-nomination. Therefore, we are not proposing to make any adjustments in the time required for qualified registries during the simplified or full self-nomination process because of this proposal. However, we anticipate that third-party intermediaries would need to make administrative changes to their existing workflows for submission of MVPs and APP data for clinicians participating as subgroups beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. We refer readers to section VII.C.17.f.(2)(f) of this rule where we discuss our impact analysis.
Based on previous trends for the number of self-nominations for qualified registries received during the 2020 self-nomination period for the CY 2021 MIPS performance period/2023 MIPS payment year and consistent with our assumptions in the CY 2021 PFS final rule (85 FR 84967), we anticipate an increase in the number of submissions from qualified registries for the CY 2022 MIPS performance period/2024 MIPS payment year. Therefore, we estimate that we will receive 210 nomination applications from qualified registries for the CY 2022 MIPS performance period/2024 MIPS payment year, an increase of 27 from the currently approved estimate of 183. Based on our estimates in the CY 2021 PFS final rule (85 FR 84967), we are proposing to adjust the estimated number of qualified registries that will submit targeted audits for the CY 2022 MIPS performance period/2024 MIPS payment year. Similar to our assumptions in the CY 2021 PFS final rule (85 FR 84967) and based on the number of targeted audits received from qualified registries for the CY 2019 MIPS performance period/2021 payment year, we estimate 63 qualified registries would be required to conduct targeted audits, an increase of 7 from the currently approved estimate of 56. Therefore, we estimate the total impact associated with qualified registries completing targeted audits will range from 315 hours (63 registries ×5 hours/audit) at a cost of $29,994 (63 registries × $476.10/audit) to 630 hours (63 registries × 10 hours/audit) at a cost of $59,989 (63 registries × $952.20/audit) for the simplified and full self-nomination process, respectively (see Table 71 for the cost per audit). We assume that this would adjust our burden estimates for targeted audits by +35 hours (+7 respondents × 5 hr/audit) at a cost of $3,332.70 (35 hrs × $95.22/hr) and +70 hours (+7 respondents × 10 hr/audit) at a cost of $6,665.40 (70 hrs × $95.22/hr) for the simplified and full self-nomination process, respectively.
Using our currently approved time per response estimate of 3 hours, the resulting adjustment in burden for QCDRs and qualified registries to submit CAPs is 30 hours (10 respondents × 3 hrs/respondent) at a cost of $2,857 (30 hours × $95.22/hr).
In section IV.A.3.h.(3)(a)(1) of this proposed rule, we are proposing new requirements for approved QCDRs and qualified registries that have not submitted performance data. First, we are proposing to create a new requirement at paragraph § 414.1400(b)(3)(vii) to require QCDRs and qualified registries that have never submitted data since the inception of MIPS (CY 2017 MIPS performance period/2019 MIPS payment year) through the CY 2020 MIPS performance period/2022 MIPS payment year, to submit a participation plan as part of their self-nomination for CY 2023. Exceptions to this requirement may occur if data is received for the CY 2021 MIPS performance period/2023 MIPS payment year. Under this scenario, QCDRs and qualified registries would not need to submit a participation plan for the 2023 self-nomination process. If the QCDRs and qualified registries did not submit data, their participation plan must be submitted as part of self-nomination for 2023 MIPS self-nomination period and must be accepted by CMS to continue to be an approved QCDR or qualified registry. We are also proposing to codify a new requirement that, beginning with the CY 2024 MIPS performance period/2026 MIPS payment year, a QCDR or qualified registry that was approved but did not submit any MIPS data for either of the 2 years preceding the applicable self-nomination period must submit a participation plan for CMS' approval. Under this proposal, the participation plan must explain the QCDR and/or qualified registry's detailed plans about how the vendor intends to encourage clinicians to submit MIPS data to CMS through the third-party intermediary on behalf of clinicians or groups. The vendor must also explain why they should still be allowed to participate as a qualified vendor.
Based on our review of the existing list of approved qualified registries that did not submit performance data since the inception of MIPS (CY 2017 MIPS performance period/2019 MIPS payment year), we estimate that 19 qualified registries will submit participation plans for the CY 2023 MIPS self-nomination period. Similar to our assumptions used for submission of a CAP in the CY 2021 PFS final rule (85 FR 84968), we anticipate that the effort involved in developing a participation plan including the proposed policies specified in this rule and submitting it to CMS is likely to be no more than 3 hours for a computer systems analyst at a rate of $95.22/hr. For the CY 2023 MIPS performance period/2025 MIPS payment year, we estimate an annual burden of 57 hours (3 hr × 19 participation plans) at a cost of $5,428 (57 hr × $95.22/hr) for qualified registries to develop and submit a participation plan.
As stated above, based on the number of self-nominations received for the CY 2021 MIPS performance period/2023 MIPS payment year, we are proposing to adjust the estimated number of qualified registries that would self-nominate for the CY 2023 performance period to 210, an increase of 27 from the currently approved estimate of 183 in the CY 2021 PFS final rule (85 FR 84969). In the CY 2019 PFS final rule, we estimated that it would take 3 hours for a qualified registry to submit all the required information during the full self-nomination process (83 FR 59998). Based on our experience with the self-nomination process, we believe that the number of fields needed to be submitted for a qualified registry are fewer than those needed for a QCDR. We assume that our previous assumption of 3 hours is an overestimate. Therefore, we propose to revise our estimated time required for a qualified registry submitting a full-self-nomination process to 2 hours, a decrease of 1 hour.
We assume that the staff involved in the qualified registry self-nomination process will continue to be computer systems analysts or their equivalent, who have an average labor rate of $95.22/hr. Using the change in estimated burden per respondent time, associated with the self-nomination process range from a minimum of 0.5 hours to a maximum of 2 hours, we estimate that the annual burden will range from 105 hours (210 qualified registries × 0.5 hr) to 420 hours (210 qualified registries × 2 hr) at a cost ranging from $9,998 (105 hr × $95.22/hr) and $39,992 (420 hr × $95.22/hr), respectively (see Table 73).
Combined with our estimates of burden associated with completing targeted audits and developing and submitting participation plans and corrective action plans, our total burden estimate ranges from 507 hours [105 hr (210 qualified registries × 0.5 hr) + 57 hr (+19 participation plans × 3hr/plan) Start Printed Page 39488 + 315 hr (63 targeted audits × 5 hours/audit) + 30 hr (10 CAPs × 3 hr) at a cost of $48,277 [$9,998 (105 hr × $95.22/hr) + $5,428 (57 hr × $95.22/hr) + $29,994 (63 registries × $476.10/audit) + $2,857 (30 hours × $95.22/hr)] to 1,137 hours [420 hr (210 qualified registries × 2 hr) + 57 hr (+19 participation plans × 3hr/plan) + 630 hr (63 targeted audits × 10 hours/audit) + 30 hr (10 CAPs × 3 hr)] at a cost of $108,266 [$39,992 (420 hr × $95.22/hr) + $5,428 (57 hr × $95.22/hr) + $59,989 (63 registries × $952.20/audit) + $2,857 (30 hours × $95.22/hr) for the simple self-nomination process (see minimum burden in Table 73 below) and full self-nomination process (see maximum burden in Table 73) respectively.
Based on the assumptions discussed in this section, we provide a proposed estimate of the total annual burden associated with a qualified registry self-nominating to be considered "qualified" to submit quality measures results and numerator and denominator data on MIPS eligible clinicians.

As shown in Table 74, for the CY 2022 MIPS performance period/2024 MIPS payment year, independent of the proposed change to our per response time estimate, the estimated increase in 27 respondents from the currently approved 183 respondents to 210 results in an increase of between +13.5 hours (+27 respondents × 0.5 hrs/respondent) at a cost of +$1,285 (13.5 hours × $95.22/hr) and +54 hours (+27 respondents × 2 hrs/respondent) at a cost of +$5,142 (54 hours × $95.22/hr). Accounting for the proposed change in time required for the qualified registry self-nomination process results in an adjustment of 0 hours for the simplified self-nomination process and −183 hours (183 respondents × −1 hours) at a cost of −$17,425 (−183 hours × $95.22/hr) for the full self-nomination process.
When the above impacts are combined with the proposed estimates for targeted audits, participation plans and corrective action plans discussed above, the net impact ranges between +106 hours [13.5 hr (+27 respondents × 0.5 hrs/respondent) + 0 hr + 35 hr (+7 audits × 5 hr/audit) + 57 hr (+19 participation plans × 3hr/plan) + 0 hr)] at $10,046 [($1,286 (13.5 hours × $95.22/hr) + $0 + $3,333 (35 hrs × $95.22/hr) + $5,428 (+57 hr × $95.22/hr) + $0)] for the simplified self-nomination process and −2 hours [(54 hr (+27 respondents × 2 hrs/respondent) −183 hr (183 respondents × −1 hours) + 70 hr (+7 audits × 10hr/audit) + 57 hr (+19 participation plans × 3hr) + 0 hr)] at a cost of −$190 [($5,142 (54 hours × $95.22/hr)−$17,425 (−183 hours × $95.22/hr) + $6,665 (70 hrs × $95.22/hr) + $5,428 (+57 hr × $95.22/hr) + $0)] for the full self-nomination process for the CY 2022 MIPS performance period/2024 MIPS payment year.
Start Printed Page 39489

(4) Survey Vendor Requirements
In section IV.A.3.h(2)(b)(ii) of this rule, we are proposing to require CAHPS for MIPS survey vendors to support subgroup reporting, beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. Because of this proposal, we anticipate no additional steps being added to the requirements for CAHPS for MIPS survey vendors to submit a participation form and assume there will be no impact on the time required for the survey vendors. Therefore, we are not proposing to make any adjustments in the time required for CAHPS survey vendors to submit the because of this proposal. The requirements and burden for CAHPS survey vendors to submit data for eligible clinicians are currently approved by OMB under control number 0938-1222 (CMS-10450). Consequently, we are not proposing any changes under that control number.
(5) Health IT Vendors
In section IV.A.3.h.(2)(b) of this rule, we propose to create a new requirement at paragraph § 414.1400(c)(1)(iii) to state that, beginning with the CY 2023 MIPS performance period/2025 MIPS payment year, health IT vendors must support MVPs that are applicable to the MVP participants on whose behalf they submit MIPS data. Health IT vendors may also support the APP. Additionally, we propose to require health IT vendors to support subgroup reporting beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. We do not anticipate any requirement/burden changes as it relates to the support of reporting data.
d. ICR Regarding Open Authorization (OAuth) Credentialing and Token Request Process
This rule is not proposing any new or revised collection of information requirements or burden related to the identity management application process. The requirements and burden are currently approved by OMB under control number 0938-1314 (CMS-10621). Consequently, we are not proposing any changes under that control number.
e. ICRs Regarding Quality Data Submission (§§ 414.1318, 414.1325, 414.1335, and 414.1365)
(1) Background
We refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77502 through 77503), CY 2018 Quality Payment Program final rule (82 FR 53908 through 53912), CY 2019 PFS final rule (83 FR 60000 through 60003), CY 2020 PFS final rule (84 FR 63121 through 63124), and the CY 2021 PFS final rule (85 FR 84970 through 84974) for our previously finalized requirements for data submission for the quality performance category.
Under our current policies, two groups of clinicians must submit quality data under MIPS: Those who submit as MIPS eligible clinicians and those who submit data voluntarily but are not subject to MIPS payment adjustments. Clinicians are ineligible for MIPS payment adjustments if they are newly enrolled to Medicare; are QPs; are partial QPs who elect to not participate in MIPS; are not one of the clinician types included in the definition for MIPS eligible clinician; or do not exceed the low-volume threshold as an individual or as a group.
(2) Changes and Adjustments to Quality Performance Category Respondents
To determine which QPs should be excluded from MIPS, we used the Advanced APM payment and patient percentages from the APM Participant List for the final snapshot date for the 2019 QP performance period. From this data, we calculated the QP determinations as described in the Qualifying APM Participant (QP) definition at § 414.1305 for the CY 2022 MIPS performance period/2024 MIPS payment year. Due to data limitations, we could not identify specific clinicians who have not yet enrolled in APMs, but who may become QPs in the future CY 2022 MIPS performance period/2024 MIPS payment year (and therefore will no longer need to submit data to MIPS); hence, our model may underestimate or overestimate the number of respondents.
In the CY 2019 PFS final rule, we finalized limiting the Medicare Part B claims collection type to small practices beginning with the CY 2019 MIPS performance period/2021 MIPS payment year and allowing clinicians in small practices to report Medicare Part B claims as a group or as individuals (83 FR 59752). As in the CY 2021 PFS final rule, we continue to use CY 2019 MIPS performance period/2021 MIPS payment year data to estimate the number of respondents in the CY 2022 PFS proposed rule.
There may be an undercount in submissions due to the PHE for COVID-19, because of the automatic extreme and uncontrollable circumstances policy, and application-based policy that allowed clinicians to elect not to submit during the submission period for the CY 2019 MIPS performance period/2021 MIPS payment year that we are using to inform our burden estimates. Despite this limitation, we believe the data from the CY 2019 MIPS performance period/2021 MIPS payment year is still the best data source available as it most accurately reflects the impacts of policies finalized in previous rules and trends toward increased group reporting.
In section IV.A.3.d.(1)(d) of this rule, we are proposing to continue the CMS Web Interface measures as a collection type for the CY 2022 MIPS performance period/2024 MIPS payment year. Additionally, we are proposing to sunset the CMS Web Interface measures as a collection type/submission type starting with the CY 2023 MIPS performance period/2025 MIPS payment year. In the CY 2021 PFS final rule (85 FR 84981), we finalized the sunset of CMS Web Interface as a collection type for the CY 2022 MIPS Start Printed Page 39490 performance period/2024 MIPS payment year. We refer readers to the CY 2021 PFS final rule for discussion on our assumptions for the CY 2022 MIPS performance period/2024 MIPS payment year, where we estimated a burden of zero due to our assumption that all Web Interface respondents will alternately utilize either the MIPS CQM and QCDR or eCQM collection types. Based on the number of groups that submitted quality performance data via the CMS Web Interface in the CY 2019 MIPS performance period/2021 MIPS payment year, we are not able to ascertain what alternative collection type(s) the groups would elect. In order to estimate the number of groups that will select each of these collection types, we first clustered the number of groups which submitted data via the CMS Web Interface collection type during the CY 2019 MIPS performance period/2021 MIPS payment year by practice size (between 25 and 49 clinicians, between 50 and 99 clinicians, etc.). Then, for each cluster, we allocated these groups to each of the MIPS CQM and QCDR and eCQM collection types based on the percent of TINs that submitted MIPS data via these two collection types. For example, of the 1,629 TINs with a practice size of 25 to 49 clinicians which submitted data for the CY 2019 MIPS performance period/2021 MIPS payment year, 1,066 (65 percent) submitted data via the MIPS CQM and QCDR collection type and 563 (35 percent) submitted data via the eCQM collection type. We applied these percentages to the 7 TINs with a practice size of 25 to 49 clinicians which submitted data via the CMS Web Interface collection type for the CY 2019 MIPS performance period/2021 MIPS payment year to estimate that 4 (7 TINs × 0.56) would elect to submit data via the MIPS CQM and QCDR collection type and the remaining 3 (7 TINs × 0.44) would elect to submit data via the eCQM collection type. In total, beginning with the CY 2023 MIPS performance period/2025 MIPS payment year, we estimate that 64 of the 114 groups that submitted data via the CMS Web Interface collection type for the CY 2019 MIPS performance period/2021 MIPS payment year will submit quality data via the MIPS CQM and QCDR collection type and 50 groups will now submit quality data via the eCQM collection type. Note that the 114 groups is an increase of 114 from our currently approved estimate of 0 groups in the CY 2022 performance period/2024 payment year. We also performed this analysis to determine the number of clinicians that would be affected and would need to submit quality data via an alternate collection type beginning with the CY 2023 performance period/2025 payment year. In total, of the estimated 45,599 individual clinicians affected by this provision, we estimate that 11,432 would submit quality data as part of a group via the MIPS CQM and QCDR collection type and 34,167 would submit quality data as part of a group via the eCQM collection type. These estimates are reflected in Tables 78 and 80 and the associated changes in burden are reflected in Tables 79 and 81. In aggregate, as discussed in section V.B.8.p of this proposed rule, we estimate the provision to sunset the CMS Web Interface measures as a collection type/submission type will result in a net decrease in quality performance data reporting burden while acknowledging the additional financial impacts on clinicians as discussed in section VII.F.17.f.(2)(a) of the Regulatory Impact Analysis. We assume that 100 percent of ACO APM Entities will submit quality data to CMS as required under their models. While we do not believe there is additional reporting for ACO APM entities, consistent with assumptions used in the CY 2020 and CY 2021 PFS final rules (84 FR 63122 and 85 FR 84972), we include all quality data voluntarily submitted by MIPS APM participants made at the individual or TIN-level in our respondent estimates. As stated in section V.B.8.e.(2) of this proposed rule, we assume non-ACO APM Entities will participate through traditional MIPS and submit as an individual or group rather than as an entity. To estimate who will be a MIPS APM participant in the CY 2022 MIPS performance period/2024 MIPS payment year, we used the Advanced APM payment and patient percentages from the APM Participant List for the final snapshot date for the 2019 QP performance period. We elected to use this data source because the overlap with the data submissions for the CY 2019 MIPS performance period/2021 MIPS payment year enabled the exclusion of Partial QPs that elected to not participate in MIPS and required fewer assumptions as to who is a QP or not. Based on this information, if we determine that a MIPS eligible clinician will not be scored as a MIPS APM, then their reporting assumption is based on their reporting as a group or individual for the CY 2019 MIPS performance period/2021 MIPS payment year.
Our burden estimates for the quality performance category do not include the burden for the quality data that APM Entities submit to fulfill the requirements of their APMs. The burden is excluded from this collection of information section but is discussed in the regulatory impact analysis section of this proposed rule because sections 1899(e) and 1115A(d)(3) of the Act (42 U.S.C. 1395jjj(e) and 1315a(d)(3), respectively) state that the Shared Savings Program and the testing, evaluation, and expansion of Innovation Center models tested under section 1115A of the Act (or section 3021 of the Affordable Care Act) are not subject to the PRA.[250] Tables 72, 73, and 74 explain our revised estimates of the number of organizations (including groups, virtual groups, and individual MIPS eligible clinicians) submitting data on behalf of clinicians segregated by collection type.
Table 75 provides our estimated counts of clinicians that will submit quality performance category data as MIPS individual clinicians or groups in the CY 2022 and 2023 MIPS performance periods/2024 and 2025 MIPS payment years based on data from the CY 2019 MIPS performance period/2021 MIPS payment year.
For the CY 2022 MIPS performance period/2024 MIPS payment year, respondents will have the option to submit quality performance category data via Medicare Part B claims, direct, and log in and upload submission types, and Web Interface. For the CY 2023 MIPS performance period/2025 MIPS payment year, respondents would no longer have the option to submit quality performance category data via the Web Interface. We estimate the burden for collecting data via collection type: Medicare Part B claims, QCDR and MIPS CQMs, eCQMs, and the CMS Web Interface. We believe that, while estimating burden by submission type may be better aligned with the way clinicians participate with the Quality Payment Program, it is more important to reduce confusion and enable greater transparency by maintain consistency with previous rulemaking.
For the CY 2023 MIPS performance period/2025 MIPS payment year, we propose in section IV.A.3.b.(2)(d) of this rule that clinicians in MIPS would have the option to submit measures and activities in MVPs. We refer readers to section IV.A.3.b.(4) of this rule for additional details on the proposed reporting requirements for MVPs. For the quality performance category of MVPs, we assume that MVP Participants would choose to report via the Medicare Start Printed Page 39491 Part B claims, QCDR, MIPS CQMs, and eCQMs collection type. Table 87 of this rule includes the estimated burden for collecting data for the quality performance category of MVPs.
As shown in Table 75, using participation data from the CY 2019 MIPS performance period/2021 MIPS payment year, combined with the estimate of QPs for the CY 2022 MIPS performance period/2024 MIPS payment year, we estimate a total of 625,703 clinicians will submit quality data as individuals or groups in each of the CY 2022 and 2023 MIPS performance periods/2024 and 2026 MIPS payment years, a decrease of 25,811 clinicians when compared to our estimate of 651,514 clinicians in the CY 2021 PFS final rule (85 FR 84972). For the CY 2022 performance period/2024 payment year, we estimate 28,252 clinicians will submit data as individuals for the Medicare Part B claims collection type; 279,247 clinicians will submit data as individuals or as part of groups for the MIPS CQM and QCDR collection type; 273,819 clinicians will submit data as individuals or as part of groups via eCQM collection types; and 44,385 clinicians will submit as part of groups via the CMS Web Interface. Compared to the CY 2022 MIPS performance period/2024 MIPS payment year burden estimated in the CY 2021 PFS final rule (85 FR 84972), these are decreases from the estimates of 29,273, 295,941, and 326,300 for Medicare Part B claims, MIPS CQM and QCDR, eCQM, and an increase of 44,385 for the CMS Web Interface collection types, respectively. These adjustments are due to the availability of updated data from the CY 2019 MIPS performance period/2021 MIPS payment year and the delay in sunsetting the CMS Web Interface from the CY 2022 MIPS performance period/2024 MIPS payment year to the CY 2023 MIPS performance period/2025 MIPS payment year. For the CY 2023 performance period/2025 payment year, we estimate 25,427 clinicians will submit data as individuals for the Medicare Part B claims collection type; 288,637 clinicians will submit data as individuals or as part of groups for the MIPS CQM and QCDR collection type; 311,326 clinicians will submit data as individuals or as part of groups via the eCQM collection type.
Table 75 provides estimates of the number of clinicians to collect quality measures data via each collection type, regardless of whether they decide to submit as individual clinicians or as part of groups. Because our burden estimates for quality data submission assume that burden is reduced when clinicians elect to submit as part of a group, we also separately estimate the expected number of clinicians to submit as individuals or part of groups.

Because MIPS eligible clinicians may submit data for multiple collection types for a single performance category, the estimated numbers of individual clinicians and groups to collect via the various collection types are not mutually exclusive and reflect the occurrence of individual clinicians or groups that collected data via multiple collection types during the 2019 MIPS performance period/2021 MIPS payment year. We captured the burden of any eligible clinician that may have historically collected via multiple collection types, as we assume they will continue to collect via multiple collection types and that our MIPS scoring methodology will take the highest score where the same measure is submitted via multiple collection types.
Table 76 uses methods similar to those described to estimate the number of clinicians that will submit data as individual clinicians via each collection type in the CY 2022 and CY 2023 MIPS performance periods/2024 and 2025 MIPS payment years. For the CY 2022 MIPS performance period/2024 MIPS payment year, we estimate that approximately 28,252 clinicians will submit data as individuals using the Medicare Part B claims collection type; approximately 40,507 clinicians will submit data as individuals using MIPS CQM and QCDR collection type; and approximately 40,446 clinicians will submit data as individuals using eCQMs collection type. Based on performance data from the CY 2019 MIPS performance period/2021 MIPS payment year, these are decreases of −1,021, −833, and −1,809 respondents from the currently approved estimates of 29,273, 41,340, and 42,255 for the Medicare Part B claims, MIPS CQM and Start Printed Page 39492 QCDR, and eCQM collection types, respectively.
As shown in Table 76, for the CY 2023 MIPS performance period/2025 MIPS payment year, we estimate that approximately 25,427 clinicians will submit data as individuals using the Medicare Part B claims collection type; approximately 36,456 clinicians will submit data as individuals using MIPS CQM and QCDR collection type; and approximately 36,401 clinicians will submit data as individuals using eCQMs collection type. Based on performance data from the CY 2019 MIPS performance period/2021 MIPS payment year, these are decreases of −3,846, −4,884, and −5,854 respondents from the currently approved estimates of 29,273, 41,340, and 42,255 for the Medicare Part B claims, MIPS CQM and QCDR, and eCQM collection types, respectively.

Consistent with the policy finalized in the CY 2018 Quality Payment Program final rule that for MIPS eligible clinicians who collect measures via Medicare Part B claims, MIPS CQM, eCQM, or QCDR collection types and submit more than the required number of measures (82 FR 53735 through 54736), we will score the clinician on the required measures with the highest assigned measure achievement points and thus, the same clinician may be counted as a respondent for more than one collection type. Therefore, our columns in Table 76 are not mutually exclusive.
Table 77 provides our estimated counts of groups or virtual groups that will submit quality data on behalf of clinicians for each collection type in the CY 2022 and 2023 MIPS performance period/2024 and 2025 MIPS payment years. We assume that clinicians that submitted quality data as groups in the CY 2019 MIPS performance period/2021 MIPS payment year will continue to submit quality data either as groups or virtual groups for the same collection types as they did as a group or TIN within a virtual group for the CY 2022 and 2023 MIPS performance periods/2024 and 2025 MIPS payment years. Specifically, for the CY 2022 MIPS performance period/2024 MIPS payment year we estimate that 11,529 groups and virtual groups will submit data for the MIPS CQM and QCDR collection type on behalf of 243,169 clinicians; 8,127 groups and virtual groups will submit for eCQM collection types on behalf of 249,878 eligible clinicians; and 114 groups will submit data via the CMS Web Interface on behalf of 44,385 clinicians. These are decreases of −75 and −93 respondents from the currently approved estimates of 11,604, and 8,220 groups and virtual groups for the MIPS CQM and QCDR and eCQM collection types, and an increase of +114 groups from the currently approved estimates of 0 groups for the CMS Web Interface collection types, respectively.
As shown in Table 77, for the CY 2023 MIPS performance period/2025 MIPS payment year we estimate that 10,434 groups and virtual groups will submit data for the MIPS CQM and QCDR collection type on behalf of 313,038 clinicians and 7,359 groups and virtual groups will submit for eCQM collection types on behalf of 339,109 eligible clinicians. These are decreases of −1,170 and −861 respondents from the currently approved estimates of 11,604, and 8,220 groups and virtual groups for the MIPS CQM and QCDR and eCQM collection types, respectively. The reason for the difference in estimated number of respondents from the estimates for the CY 2022 MIPS performance period/2024 MIPS payment year describe above, is due to the sunset of the CMS Web Interface as a collection type and the implementation of MVPs beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. As the data does not exist for APM performance pathway or MIPS quality measures for non-ACO APM entities, we assume non-ACO APM Entities would participate through traditional MIPS and base our estimates on submissions received in the CY 2019 MIPS performance period/2021 MIPS payment year.
Start Printed Page 39493

The burden associated with the submission of quality performance category data have some limitations. We believe it is difficult to quantify the burden accurately because clinicians and groups may have different processes for integrating quality data submission into their practices' workflows. Moreover, the time needed for a clinician to review quality measures and other information, select measures applicable to their patients and the services they furnish, and incorporate the use of quality measures into the practice workflows is expected to vary along with the number of measures that are potentially applicable to a given clinician's practice and by the collection type. For example, clinicians submitting data via the Medicare Part B claims collection type need to integrate the capture of quality data codes for each encounter whereas clinicians submitting via the eCQM collection types may have quality measures automated as part of their EHR implementation.
We believe the burden associated with submitting quality measures data will vary depending on the collection type selected by the clinician, group, or third-party. As such, we separately estimated the burden for clinicians, groups, and third parties to submit quality measures data by the collection type used. For the purposes of our burden estimates for the Medicare Part B claims, MIPS CQM and QCDR, and eCQM collection types, we also assume that, on average, each clinician or group will submit 6 quality measures. For the CY 2023 MIPS performance period/2025 MIPS payment year we refer readers to section IV.A.3.b.(4) of the rule for the changes related to MVP and subgroup reporting requirements. In terms of the quality measures available for clinicians and groups to report for the CY 2022 MIPS performance period/2024 MIPS payment year, we are proposing that the total number of quality measures will be 195. The new MIPS quality measures proposed for inclusion in MIPS for the CY 2022 MIPS performance period/2024 MIPS payment year and future years are found in Table Group A of Appendix 1; MIPS quality measures with proposed substantive changes can be found in Table Group D of Appendix 1; and MIPS quality measures proposed for removal can be found in Table Group C of Appendix 1. These proposed measures are stratified by collection type in Table 78, as well as counts of new, removed, and substantively changed measures.
Start Printed Page 39494

For the CY 2022 MIPS performance period/2024 MIPS payment year, we are proposing a net reduction of 15 quality measures across all collection types compared to the 209 measures finalized for the CY 2021 MIPS performance period/2023 MIPS payment year (85 FR 84974). Specifically, as discussed in section IV.A.3.c.(1)(d), we are proposing to add 2 new administrative claims outcome measures, remove 19 quality measures, and make substantive updates to 84 quality measures. We do not anticipate that our proposal to remove these measures will increase or decrease the reporting burden on clinicians and groups as respondents generally are still required to submit quality data for 6 measures. For the change in associated burden related to the proposals introducing MVP and subgroup reporting beginning in the CY 2023 MIPS performance period/2025 MIPS payment year, we refer readers to Table 87 of this section.
(3) Quality Payment Program Identity Management Application Process
This rule is not proposing any new or revised collection of information requirements or burden related to the identity management application process. The requirements and burden are currently approved by OMB under control number 0938-1314 (CMS-10621). Consequently, we are not proposing any changes under that control number.
(4) Quality Data Submission by Clinicians: Medicare Part B Claims-Based Collection Type
This rule is not proposing any new or revised collection of information requirements related to the submission of Medicare Part B claims data for the quality performance category. However, we are adjusting our currently approved burden estimates based on more recent data. For the change in associated burden related to the proposals introducing MVP and subgroup reporting beginning in the CY 2023 MIPS performance period/2025 MIPS payment year, we refer readers to Table 87 of this section.
The following proposed burden will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
We refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77501 through 77504), CY 2018 Quality Payment Program final rule (82 FR 53912), CY 2019 PFS final rule (83 FR 60004 through 60005), CY 2020 PFS final rule (84 FR 63124 through 63126) and the CY 2021 PFS final rule (85 FR 84975 through 84976) for our previously finalized requirements and burden for quality data submission via the Medicare Part B claims collection type.
As noted in Table 76, based on data from the CY 2019 MIPS performance period/2021 MIPS payment year, we assume that 28,252 individual clinicians will collect and submit quality data via the Medicare Part B claims collection type. This rule is proposing to adjust the number of Medicare Part B claims respondents from the currently approved estimate of 29,273 to 28,252 (a decrease of 1,021) based on more recent data and our methodology of accounting only for clinicians in small practices who submitted such claims data in the CY 2019 MIPS performance period/2021 MIPS payment year rather than all clinicians who submitted quality data codes to us for the Medicare Part B claims collection type.
As shown in Table 79, consistent with our currently approved per response time figures, we estimate that the burden of quality data submission using Medicare Part B claims will range from 0.15 hours (9 minutes) for a computer systems analyst at a cost of $14.28 (0.15 hr × $95.22/hr) to 7.2 hours for a computer systems analyst at a cost of $685.58 (7.2 hr × $95.22/hr). The burden will involve becoming familiar with MIPS quality measure specifications.
Consistent with our currently approved per response time figures, we believe that the start-up cost for a clinician's practice to review measure specifications is 7 hours, consisting of 3 hours at $114.24/hr for a medical and health services manager, 1 hour at $217.32/hr for a physician, 1 hour at $48.16/hr for an LPN, 1 hour at $95.22/hr for a computer systems analyst, and 1 hour at $40.02/hr for a billing and posting clerk. We are not revising our currently approved per response time estimates.
As shown in Table 79, considering both data submission and start-up requirements for our adjusted number of clinicians, the estimated time (per clinician) ranges from a minimum of 7.15 hours (0.15 hr + 7 hr) to a maximum of 14.2 hours (7.2 hr + 7 hr). In this regard the total annual time for the CY 2022 MIPS performance period/2024 MIPS payment year ranges from 202,002 hours (7.15 hr × 28,252 clinicians) to 401,178 hours (14.2 hr × 28,252 clinicians). The estimated annual cost (per clinician) ranges from $758 [(0.15 hr × $95.22/hr) + (3 hr × $114.24/hr) + (1 hr × $95.22/hr) + (1 hr × $48.16/hr) + (1 hr × $40.02/hr) + (1 hr × $217.32/hr)] to a maximum of $1,429 [(7.2 hr × $95.22/hr) + (3 hr × $114.24/hr) + (1 hr × $95.22/hr) + (1 hr × $48.16/hr) + (1 hr × $40.02/hr) + (1 hr × $217.32/hr)]. The total annual cost for the CY 2022 MIPS performance period/2024 MIPS payment year ranges from a minimum of $21,407,105 (28,252 clinicians × $758) to a maximum of Start Printed Page 39495 $40,372,673 (28,252 clinicians × $1,429).
As shown in Table 79, for purposes of calculating total burden associated with the Claims collection type for the CY 2023 MIPS performance period/2025 MIPS payment year only the maximum burden is used. The decrease in the number of annual respondents results in an estimated total annual time of 361,061 hours (14.2 hr × 25,427 clinicians) for the CY 2023 MIPS performance period/2025 MIPS payment year. Using the currently approved unchanged estimate for cost per respondent, the total annual cost for the CY 2023 MIPS performance period/2025 MIPS payment year is $36,335,692 (25,427 clinicians × $1,429 per respondent).
Table 79 summarizes our proposed estimated range of total annual burden associated with clinicians submitting quality data via Medicare Part B claims for both the CY 2022 and 2023 MIPS performance periods/2024 and 2025 MIPS payment years.

As shown in Table 76, using the unchanged currently approved hours per respondent, we estimate that the burden per respondent for quality data submission using the Medicare Part B Claims collection type would range from $758 to $1,429. The decrease in number of respondents from 29,273 to 28,252 results in a total adjustment of between −7,300 hours (−1,021 respondents × 7.15 hr/respondent) at a cost of −$167,945 (−1,021 respondents × $758/respondent) and −14,499 hours (−1,021 respondents × 14.2 hr/respondent) at a cost of −$1,257,748 (−1,021 respondents × $1,429/respondent). For purposes of calculating total burden associated with the proposed rule as shown in Tables 113, 114, 115, and 116, only the maximum burden is used.
As shown in Table 80, for purposes of calculating total burden associated the CY 2023 MIPS performance period/2025 MIPS payment year only the maximum burden is used. Using the unchanged currently approved hours per respondent, we estimate that the burden per respondent for quality data submission using the Medicare Part B Claims collection type would be $1,429. The decrease in number of respondents from 29,273 to 25,427 results in a total adjustment of −54,616 hours (−25,427 respondents × 14.2 hr/respondent) at a cost of −$5,294,729 (−25,427 respondents × $1,429/respondent).
Start Printed Page 39496

(5) Quality Data Submission by Individuals and Groups Using MIPS CQM and QCDR Collection Types
The following requirement and burden will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
We refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77504 through 77505), CY 2018 Quality Payment Program final rule (82 FR 53912 through 53914), CY 2019 PFS final rule (83 FR 60005 through 60006), CY 2020 PFS final rule (84 FR 63127 through 63128), CY 2021 PFS final rule (85 FR 84977 through 84979) for our previously finalized requirements and burden for quality data submission via the MIPS CQM and QCDR collection types. For the change in associated burden for quality data submission related to the proposals introducing MVP and subgroup reporting beginning in the CY 2023 MIPS performance period/2025 MIPS payment year, we refer readers to Table 87.
As noted in Tables 72, 73, and 74, and based on data from the CY 2019 MIPS performance period/2021 MIPS payment year, for the CY 2022 performance period/2024 MIPS payment year, we assume that 279,247 clinicians will submit quality data as individuals or groups using MIPS CQM or QCDR collection types; 52,036 clinicians will submit as individuals and the remaining 279,223 clinicians will submit as members of 11,527 groups and virtual groups. This is an increase of 10,696 individuals and a decrease of 32 groups from the estimates of 41,340 individuals and the 11,559 groups provided in the CY 2021 PFS final rule (85 FR 84977). Given that the number of measures required for clinicians and groups is the same, we expect the burden to be the same for each respondent collecting data via MIPS CQM or QCDR, whether the clinician is participating in MIPS as an individual or group.
Under the MIPS CQM and QCDR collection types, the individual clinician or group may either submit the quality measures data directly to us, log in and upload a file, or utilize a third-party intermediary to submit the data to us on the clinician's or group's behalf.
We estimate that the burden associated with the QCDR collection type is similar to the burden associated with the MIPS CQM collection type; therefore, we discuss the burden for both together below. For MIPS CQM and QCDR collection types, we estimate an additional time for respondents (individual clinicians and groups) to become familiar with MIPS quality measure specifications and, in some cases, specialty measure sets and QCDR measures. Therefore, we believe that the burden for an individual clinician or group to review measure specifications and submit quality data is total of 9 hours at a cost of $922.76 per response. This consists of 3 hours at $95.22/hr for a computer systems analyst (or their equivalent) to submit quality data along with 2 hours at $114.24/hr for a medical and health services manager, 1 hour at $95.22/hr for a computer systems analyst, 1 hour at $48.16/hr for a LPN, 1 hour at $40.02/hr for a billing clerk, and 1 hour at $217.32/hr for a physician to review measure specifications. Additionally, clinicians and groups who do not submit data directly will need to authorize or instruct the qualified registry or QCDR to submit quality measures' results and numerator and denominator data on quality measures to us on their behalf. We estimate that the time and effort associated with authorizing or instructing the quality registry or QCDR to submit this data will be approximately 5 minutes (0.083 hours) at $95.22/hr for a computer systems analyst at a cost of $7.90 (0.083 hr × $95.22/hr). Overall, we estimate 9.083 hr/response (3 hr + 2 hr + 1 hr + 1 hr + 1 hr + 1 hr + 0.083 hr) at a cost of $922.76/response [(3 hr × $95.22/hr) + (2 hr × $114.24/hr) + (1 hr × $217.32/hr) + (1 hr × $95.22/hr) + (1 hr × $48.16/hr) + (1 hr × $40.02/hr) + (0.083 hr × $95.22/hr)].
For the CY 2022 MIPS performance period/2024 MIPS payment year, in aggregate, we estimate a burden of 472,643 hours [9.083 hr/response × (40,507 clinicians submitting as individuals + 11,527 groups submitting via QCDR or MIPS CQM on behalf of individual clinicians or 52,036 responses)] at a cost of $ $48,016,739 (52,036 responses × $922.76/response).
For the CY 2023 MIPS performance period/2025 MIPS payment year, in aggregate, we estimate a burden of 425,787 hours [9.083 hr/response × (36,456 clinicians submitting as individuals + 10,432 groups submitting via QCDR or MIPS CQM on behalf of individual clinicians or 46,877 responses)] at a cost of $43,256,221 (46,877 responses × $922.76/response). Based on these assumptions, we have estimated in Table 81 the burden for these submissions.
Start Printed Page 39497

As shown in Table 82, using the unchanged currently approved hours per respondent burden estimate, the decrease of 913 respondents from 52,949 to 52,036 for the CY 2022 MIPS performance period/2024 MIPS payment year results in a decrease of −8,293 hours (−913 respondents × 9.083 hr/respondent) and −$842,483 (913 respondents × $922.76/respondent).
For the CY 2023 MIPS performance period/2025 MIPS payment year, using the unchanged currently approved hours per respondent burden estimate, the decrease of 6,072 respondents from 52,949 to 46,877 results in a decrease of −55,149 hours (−6,072 respondents × 9.083 hr/respondent) and −$5,602,649 (−6,072 respondents × $922.76/respondent).
Start Printed Page 39498

(6) Quality Data Submission by Clinicians and Groups: eCQM Collection Type
The following requirement and burden will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
We refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77505 through 77506), CY 2018 Quality Payment Program final rule (82 FR 53914 through 53915), CY 2019 PFS final rule (83 FR 60006 through 60007), CY 2020 PFS final rule (84 FR 63128 through 63130) and the CY 2021 PFS final rule (85 FR 84979 through 84980) for our previously finalized requirements and burden for quality data submission via the eCQM collection types. For the change in associated burden for quality data submission related to the proposals introducing MVP and subgroup reporting beginning in the CY 2023 MIPS performance period/2025 MIPS payment year, we refer readers to Table 87 of this section.
Based on CY 2019 MIPS performance period/2021 MIPS payment year data, for the CY 2022 MIPS performance period/2024 MIPS payment year, we assume that 322,392 clinicians will elect to use the eCQM collection type; 40,446 clinicians are expected to submit eCQMs as individuals; and 8,127 groups and virtual groups are expected to submit eCQMs on behalf of the remaining 273,819 clinicians. This is a decrease of 2,109 individuals and 27 groups from the estimates of 42,555 individuals and 8,154 groups provided in the CY 2021 PFS final rule (85 FR 84979). We expect the burden to be the same for each respondent using the eCQM collection type, whether the clinician is participating in MIPS as an individual or group.
Under the eCQM collection type, the individual clinician or group may either submit the quality measures data directly to us from their eCQM, log in and upload a file, or utilize a third-party intermediary to derive data from their CEHRT and submit it to us on the clinician's or group's behalf.
To prepare for the eCQM collection type, the clinician or group must review the quality measures on which we will be accepting MIPS data extracted from eCQMs, select the appropriate quality measures, extract the necessary clinical data from their CEHRT, and submit the necessary data to a QCDR/qualified registry or use a health IT vendor to submit the data on behalf of the clinician or group. We assume the burden for collecting quality measures data via eCQM is similar for clinicians and groups who submit their data directly to us from their CEHRT and clinicians and groups who use a health IT vendor to submit the data on their behalf. This includes extracting the necessary clinical data from their CEHRT and submitting the necessary data to a QCDR/qualified registry.
We estimate that it will take no more than 2 hours at $95.22/hr for a computer systems analyst to submit the actual data file. The burden will also involve becoming familiar with MIPS quality measure specifications. In this regard, we estimate it will take 6 hours for a clinician or group to review measure specifications. Of that time, we estimate 2 hours at $114.24/hr for a medical and health services manager, 1 hour at $217.32/hr for a physician, 1 hour at $95.22/hr for a computer systems analyst, 1 hour at $48.16/hr for an LPN, and 1 hour at $40.02/hr for a billing clerk. Overall, we estimate a cost of $812.76/response [(2 hr × $95.22/hr) + (2 hr × $114.24/hr) + (1 hr × $217.32/hr) + (1 hr × $95.22/hr) + (1 hr × $48.16/hr) + (1 hr × $40.02/hr)].
For the CY 2022 MIPS performance period/2024 MIPS payment year, in aggregate, we estimate a burden of 388,584 hours [8 hr × 48.573 (40,446 clinicians + 8,127 groups and virtual groups)] at a cost of $39,812,374 (48,573 responses × $819.64/response). For the CY 2023 MIPS performance period/2025 MIPS payment year, in aggregate, we estimate a burden of 350,186 hours [8 hr × 43,773 (36,401 clinicians + 7,372 groups and virtual groups)] at a cost of $35,878,102 (43,773 responses × $819.64/response). Based on these assumptions, we have estimated in Table 83 the burden for these submissions.
Start Printed Page 39499

As shown in Table 84, using the unchanged currently approved hours per respondent burden estimate, the decrease of 1,897 respondents from 50,470 to 48,573 for the CY 2022 MIPS performance period/2024 MIPS payment year results in a total difference of −15,176 hours at a cost of −$1,544,857. For CY 2023 MIPS performance period/2025 MIPS payment year, using the unchanged currently approved hours per respondent burden estimate, the decrease of 6,697 respondents from 50,470 to 43,773 results in a total difference of −53,574 hours at a cost of −$5,488,883.
Start Printed Page 39500

(7) ICRs Regarding Burden for MVP Reporting
Section IV.A.3.b.(2)(d) of this rule describes proposals related to implementing MVPs beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. The MVPs would include the Promoting Interoperability performance category as a foundational element and incorporate population health claims-based measures, as feasible, along with the relevant measures and activities in the quality, cost and improvement activities performance categories. For the CY 2023 MIPS performance period/2025 MIPS payment year, CMS is proposing an inventory of seven MVPs included in Appendix 3: MVP Inventory of this rule to assess performance across MVPs for the quality, cost, improvement activities, and Promoting Interoperability performance categories. Additionally, in section IV.A.3.b.(2)(c)(i) of this rule, we propose to use the term "MVP Participant" to refer to clinicians who would choose to participate in MIPS for reporting MVPs.
The following new ICRs reflect the burden associated with the first year of data collection related to the proposed implementation of MVPs and subgroup reporting in the CY 2023 MIPS performance period/2025 MIPS payment year as described in section IV.A.3.b.(2)(d) of this rule. The proposed requirements and burden associated with the implementation of MVPs and subgroups will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
While MVP respondents report on all performance categories, we believe that for purposes of data submission, the burden for clinicians submitting information for the Promoting Interoperability and improvement activities performance categories of MVPs will be consistent with the currently approved estimated burden per respondent for clinicians submitting data for these performance categories in traditional MIPS. We acknowledge that clinicians participating through MVPs will have fewer requirements to meet for the improvement activity performance category as discussed in section IV.A.3.b.(4)(d)(iv) of this proposed rule. We assume that these requirement changes will not significantly lower the burden for clinicians reporting MVPs. Therefore, we will not add additional ICRs to capture the burden for the Promoting Interoperability and Improvement Activity performance categories. For this rule, we are proposing to create a separate ICR for estimating the burden associated with data submission for the Quality performance category of MVPs. We considered whether we should have a separate ICR to estimate burden for submission of measures and activities in the Promoting Interoperability performance category of MVPs. Based on our assumption above that the burden for clinicians submitting information for these performance categories of MVPs will be consistent with the currently approved estimated burden per respondent for clinicians submitting data in traditional MIPS, we anticipate that the separate ICRs would not be of value to clinicians.
We seek comment on our proposal to distinctly estimate burden only for data submission in the Quality performance category of MVPs and whether we should revise the MVP submission ICR to include all the four MIPS performance categories and whether our assumptions on Promoting Interoperability and Improvement Activities should be modified for MVPs.
(a) Burden for MVP Quality Submission
In section IV.A.3.b.(2)(d)(i) of this rule, we propose to implement voluntary MVP reporting beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. If this proposal is finalized, clinicians participating in MIPS would have the option to voluntarily submit data using MVPs starting with the CY 2023 MIPS performance period/2025 MIPS payment year. While we recognize the implementation of MVPs in MIPS will result in a burden for registration, we also assume that MVP reporting will result in a decline in burden for MVP participants due to proposed changes in the MVP reporting requirements described in section IV.A.3.b.(4)(d) of this rule. We anticipate that the clinicians choosing to participate in MIPS for reporting MVPs would need to select from a reduced inventory of measures and activities for the quality and improvement activities performance categories. This reduction in burden is described in the quality, improvement activities and Promoting Interoperability performance categories sections below.
For the ICRs related to MVP participants, we used the MIPS submission data from the CY 2021 MIPS performance period/2023 MIPS payment year. Based on our review of the proposed inventory of 7 MVPs in Appendix 3: MVP Inventory of this rule and the existing submission trends in MIPS for the measures and activities included in these MVPs, we anticipate that 10 percent of the clinicians who participate in traditional MIPS in the CY 2022 MIPS performance period/2024 MIPS payment year will report MVPs in the CY 2023 MIPS performance period/2025 MIPS payment year. Given that MVPs are new, voluntary, and represent a reduction in burden per response, we believe that we should be conservative Start Printed Page 39501 in estimating the number of clinicians submitting through MVPs during the initial year. Given that MVPs are a new mechanism available for clinicians, we believe that initial participation numbers will be relatively low. In an effort to be conservative in our estimate of burden reduction due to MVP reporting and reflect the anticipate low uptake by clinicians in the first year of MVP availability, we assume that a total of 10 percent of MIPS submitters will become MVP participants in the CY 2023 MIPS performance period/2025 MIPS payment year.
As described in section IV.A.3.b.(2)(d)(ii) of this rule, beginning with the CY 2023 MIPS performance period/2025 MIPS payment year, we are proposing voluntary subgroup reporting within MIPS limited to clinicians reporting the MVP or the APP. We recognize the implementation of subgroups for clinicians to participate in MVP and APP reporting in MIPS will result in additional burden. We believe that subgroup participation option would allow clinicians in certain specialties and subspecialties to report on measures and activities meaningful to the scope of care provided. We anticipate that public reporting of subgroup performance information would allow patients to identify clinicians in multispecialty groups that are representative of the care specific to their clinical condition. Clinician participation in subgroups is new to MIPS and we do not have any historical participation data to estimate the submission burden for clinicians who would choose to participate as subgroups for reporting the MVP or the APP. We refer readers to section IV.A.3.b.(3) of this proposed rule for details on the proposals related to subgroup composition.
We anticipate that the subgroup reporting option would increase reporting and allow clinicians in specialties to report on measures and activities meaningful to their practice. Due to the delay in implementation of subgroup reporting in the CY 2023 MIPS performance period/2025 MIPS payment year, we anticipate that there is an adequate amount of time for clinicians that historically participate in MIPS to determine if they would be able to participate as subgroups for reporting on the measures and activities in an MVP. However, due to the limited number of MVPs available for clinicians to choose, the additional burden involved in reporting, and also given the voluntary option to participate as subgroups for reporting the MVPs or the APP, we anticipate that a relatively small number of clinicians would choose to participate as subgroups in the CY 2023 MIPS performance period/2025 MIPS payment year. Therefore, we assume there will be 20 subgroups reporters in the CY 2023 MIPS performance period/2025 MIPS payment year. We assume that more clinicians will choose to participate as subgroups in future years. We seek comment on our MVP and subgroup reporting assumptions for the CY 2023 MIPS performance period/2025 MIPS payment year.
(i) Burden for MVP Registration: Individuals, Groups and APM Entities
Beginning with the CY 2023 MIPS performance period/2025 MIPS payment year, we propose that clinicians interested in participating in MIPS through MVP reporting would be required to complete an annual registration process described in section IV.A.3.b.(4)(f) of this rule. At the time of registration, MVP participants would need to select a specific MVP, a population health measure and if administrative claims measures are included in the selected MVP, the MVP participants would also need to choose an applicable administrative claims measure in the MVP. We refer readers to section IV.A.3.b.(4)(f) of this rule for additional details on MVP registration requirements.
Due to the delay in implementation of MVPs in the CY 2023 MIPS performance period/2025 MIPS payment year, we anticipate that there is an adequate amount of time for clinicians that historically participate in MIPS to determine if the measures and activities in an MVP are applicable to the scope of care provided. In Table 85, we estimate that the registration process for clinicians choosing to submit MIPS data for the measures and the activities in an MVP would require 0.25 hours of a computer systems analyst's time, similar to the currently approved burden of group registration process for CMS Web Interface finalized in the CY 2021 PFS final rule (85 FR 84983) for the CY 2023 MIPS performance period/2025 MIPS payment year. We assume that the staff involved in the MVP registration process will mainly be computer systems analysts or their equivalent, who have an average labor cost of $95.22/hour.
As discussed above, based on data from the CY 2019 MIPS performance period/2021 MIPS payment year, we assume that approximately 10 percent of the clinicians that currently participate in MIPS will submit data for the measures and activities in an MVP. Note that we apply this 10 percent calculation after adding the clinicians who begin submitting though the CQM and eCQM collection types due to the proposed sunset of Web Interface in the CY 2023 MIPS performance period/2025 MIPS payment year. For the CY 2023 MIPS performance period/2025 MIPS payment year, we assume that a total of 25,798 submissions would be received for the measures and activities included in MVPs. This total includes our estimate of 20 subgroup reporters that will also be reporting MVPs in addition to MVP reporters who currently participate in MIPS. Therefore, we assume that the total number of individual clinicians, groups, subgroups and APM Entities to complete the MVP registration process is 12,918. We estimate that the total cost to clinicians participating as individuals and groups associated with the MVP registration process will be approximately $307,513. Table 85 includes our burden assumptions related to the MVP registration process for clinicians participating in MIPS for reporting MVPs as individuals, groups, subgroups, and APM Entities.
Start Printed Page 39502

(ii) Burden for Subgroup Registration
We propose to add a separate ICR to estimate the burden associated with subgroup registration to capture the proposed subgroup registration requirements in section IV.A.3.b.(4)(f)(ii)(D) of this rule. In section IV.A.3.b.(3)(c)(ii) of this rule, we propose to define a subgroup at § 414.1305 as a subset of a group, as identified by a combination of the group TIN, the subgroup identifier, and each eligible clinician's NPI. In addition to the burden for MVP registration process described above, clinicians who choose to form subgroups for reporting the MVPs or the APP would need to submit a list of each TIN/NPI associated with the subgroup and a plain language name for the subgroup in a manner specified by CMS, described in section IV.A.3.b.(4)(f)(ii)(D) of this rule.
As discussed above, we estimate that clinicians would choose to form 20 subgroups for reporting the measures and activities in MVPs. Additionally, we estimate that clinicians who choose to participate as subgroups for reporting MVPs would require a minimum of 0.5 hours per subgroup respondent to submit the proposed requirements for subgroup registration. We assume that the staff involved in the subgroup registration process will mainly be computer systems analysts or their equivalent, who have an average labor cost of $95.22/hr.
As all subgroups will report MVPs, the burden associated with subgroup quality reporting will be included with the MVP quality reporting ICR. Burden associated with subgroup submissions for Promoting Interoperability and improvement activities will be included with those ICRs.

(iii) Burden for MVP Quality Performance Category Submission
In the CY 2017 PFS final rule (81 FR 77100 through 77114), we established the submission criteria for quality measures (excluding the CMS Web Interface measures and the CAHPS for MIPS survey measure) at § 414.1335, which requires a MIPS eligible clinician, group, or virtual group that is reporting on Qualified Clinical Data Registry (QCDR) measures, MIPS clinical quality measures (MIPS CQMs), electronic CQMs (eCQMs), or Medicare Part B claims measures to submit data on at least six measures, including at least one outcome measure. As discussed in section IV.A.3.b.(4)(d)(ii) of this proposed rule, we propose that except as provided in paragraph Start Printed Page 39503 § 414.1365(c)(1)(i), an MVP Participant must select and report 4 quality measures, including 1 outcome measure (or, if an outcome measure is not available, 1 high priority measure, included in the MVP. The decrease in the number of required measures in the quality performance category from 6 to 4 is a two-thirds reduction in the number of measures needed for eligible clinicians to submit data for the quality performance category in MVPs described in Appendix 3: MVP Inventory of this proposed rule. Therefore, we estimate that the time for submitting the measures in the MVP quality performance category will, on average, take two-thirds of the currently approved burden per respondent for the quality performance category as it does to complete a MIPS quality submission through the CQM, eCQM, and Claims submission types.
As described above in this section of the proposed rule, we estimate that 10 percent of the clinicians who participated in MIPS for the CY 2019 MIPS performance period/2021 MIPS payment year would submit data for the quality performance category of MVPs beginning with the CY 2023 MIPS performance period/2025 MIPS payment year. We anticipate that there will be 20 subgroups reporters in the CY 2023 MIPS performance period/2025 MIPS payment year. As shown in Table 87, we estimate that approximately 2,825 clinicians would submit data for the MVP quality performance category using the Medicare Part B claims collection type; approximately 5,210 clinicians and 10 subgroups will submit data using MIPS CQM and QCDR collection type; and approximately 4,862 clinicians and 10 subgroups will submit data using eCQMs collection type. We want to note that we used the same methodologies used in sections V.B.8.e.(4), V.B.8.e.(5) and V.B.8.e.(6) to estimate the quality submission burden for each collection type. As shown in Table 87, for the clinicians and subgroups submitting data for the MVP quality performance category, we estimate a burden of 26,670 hours (9.44 hr × 2,825 clinicians) at a cost of $2,691,329 (2,825 respondents × 952.68/respondent) for the Medicare Part B claims collection type, 31,163 hours [5.97 hr × 5,220 (5,210 + 10)] at a cost of $3,211,216 (5,220 × 615.18/respondent) for the MIPS CQM and QCDR collection type, and 25,822 hours [5.3 hr × 4,872 (4,862 + 10) respondents] at a cost of $2,662,191 (4,872 × 546.43/respondent) for the eCQM collection types.
Start Printed Page 39504

(8) Quality Data Submission via CMS Web Interface
The proposed requirements and burden will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
Background
In the CY 2021 PFS final rule, we finalized our policy to sunset the CMS Web Interface measures as a collection type/submission type starting with the CY 2022 MIPS performance period/2024 MIPS payment year. As a result of this provision, for the CY 2022 MIPS performance period/2024 MIPS payment year, we estimated a burden of zero due to our assumption that all Web Interface respondents will alternately utilize either the MIPS CQM and QCDR or eCQM collection types (85 FR 84981).
In section IV.A.3.d.(1)(d) of this rule, we are proposing to continue the CMS Web Interface measures as a collection type for the CY 2022 MIPS performance period/2024 MIPS payment year. Additionally, we are proposing to sunset the CMS Web Interface measures as a collection type for the CY 2023 MIPS performance period/2025 MIPS payment year. For this proposed rule, we are providing a burden estimate for the CY 2022 and CY 2023 MIPS performance periods/2024 and 2025 MIPS payment years.
For the CY 2022 MIPS performance period/2024 MIPS payment year, we assume that 114 groups will submit quality data via the CMS Web Interface based on the number of groups who completed 100 percent of reporting quality data via the Web Interface in the CY 2019 MIPS performance period/2021 MIPS payment year. This is an increase of 114 groups from the currently approved number of 0 groups provided in the CY 2021 PFS final rule (85 FR 84981 due to the proposal to continue with the CMS Web Interface as a collection type for the CY 2022 MIPS performance period/2024 MIPS payment year. We estimate that 44,385 clinicians will submit as part of groups via this method, an increase of 44,385 from our currently approved estimate of 0 clinicians.
The proposed estimated burden associated with the group submission requirements is the time and effort associated with submitting data on a sample of the organization's Start Printed Page 39505 beneficiaries that is prepopulated in the CMS Web Interface. Our proposed burden estimate for submission includes the time (61 hours and 40 minutes or 61.67 hours) needed for each group to populate data fields in the web interface with information on approximately 248 eligible assigned Medicare beneficiaries and submit the data (we will partially pre-populate the CMS Web Interface with claims data from their Medicare Part A and B beneficiaries). The patient data either can be manually entered, uploaded into the CMS Web Interface via a standard file format, which can be populated by CEHRT, or submitted directly. Each group must provide data on 248 eligible assigned Medicare beneficiaries (or all eligible assigned Medicare beneficiaries if the pool of eligible assigned beneficiaries is less than 248) for each measure. In aggregate, we estimate a burden for the CY 2022 MIPS performance period/2024 MIPS payment year of 7,030 hours (114 groups × 61.67 hr) at a cost of $669,432 (114 groups × $5,872.21/group). For the CY 2023 MIPS performance period/2025 MIPS payment year, we propose to revise our estimated burden to zero due to our assumption that with the proposed policy to sunset the CMS Web Interface as a collection type, all Web Interface respondents will alternately utilize either the MIPS CQM and QCDR or eCQM collection types. Based on the assumptions discussed in this section, Table 88 summarizes the proposed estimated burden for groups submitting to MIPS via the CMS Web Interface.


(9) Beneficiary Responses to CAHPS for MIPS Survey
This rule does not propose any new or revised collection of information requirements or burden related to the CAHPS for MIPS survey. The CAHPS for MIPS survey requirements and burden are currently approved by OMB under control number 0938-1222 (CMS-10450). Consequently, we are not proposing any changes under that control number.
(10) Group Registration for CMS Web Interface
The proposed requirements and burden will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
In the CY 2021 PFS final rule, we finalized to sunset the CMS Web Interface measures as a collection type/submission type starting with the CY 2022 MIPS performance period/2024 MIPS payment year. As a result, we estimated that there would be zero hours and $0 burden for group registration for the CMS Web Interface for the CY 2022 MIPS performance period/2024 MIPS payment year (85 FR 84984). As discussed in section IV.A.3.d.(1)(d) of this proposed rule, we are proposing to continue the CMS Web Interface measures as a collection type for the CY 2022 MIPS performance period/2024 MIPS payment year. We are also proposing to sunset the CMS Web Interface as a collection type starting with the CY 2023 MIPS performance period/2025 MIPS payment year.
Groups interested in participating in MIPS using the CMS Web Interface for the first time must complete an online registration process. After first time registration, groups will only need to opt out if they are not going to continue to submit via the CMS Web Interface. In Table 90, we estimate that the registration process for groups under MIPS involves approximately 0.25 hours at $95.22/hr for a computer Start Printed Page 39506 systems analyst (or their equivalent) to register the group.
Because we are finalizing to sunset the CMS Web Interface beginning with the CY 2023 MIPS performance period/2025 MIPS payment year, it is possible that fewer groups will elect to register to submit quality data for the first time in the performance year prior to the collection type/submission type no longer being available; however, we currently have no data with which to estimate what the associated reduction may be. Consistent with our assumptions in the CY 2021 PFS final rule (85 FR 84983), we continue to assume that approximately 90 groups will elect to use the CMS Web Interface for the first time during the CY 2022 MIPS performance period/2025 MIPS payment year based on the estimated number of new registrations during the CY 2021 MIPS performance period/2023 MIPS payment year. As shown in Table 90, we estimate a burden of 22.5 hours (90 new registrations × 0.25 hr/registration) at a cost of $2,142 (22.5 hr × $95.22/hr).

As shown in Table 91, the proposed estimated increase in the number of groups registering for the CMS Web Interface collection type to submit the MIPS data and the estimated increase in burden per respondent results in adjustment to the total time burden of +22.5 hours (+90 respondents × 0.25 hr/respondent) at a cost of $2,142 for the CY 2022 MIPS performance period/2024 MIPS payment year. For the CY 2023 MIPS performance period/2025 MIPS payment year, our proposed burden estimate is $0.

(11) Group Registration for CAHPS for MIPS Survey
This rule is not proposing any new or revised collection of information requirements or burden related to the group registration for the CAHPS for MIPS Survey. The CAHPS for MIPS survey requirements and burden are currently approved by OMB under control number 0938-1222 (CMS-10450). Consequently, we are not proposing any changes under that control number.
f. ICRs Regarding the Call for MIPS Quality Measures
This rule is not proposing any new or revised collection of information requirements or burden related to the call for MIPS quality measures. However, outside of the rulemaking process we are replacing the existing tool for stakeholders beginning with the 2021 Annual Call for Measures. To account for the updated tool (MERIT), we are proposing to revise our currently approved burden estimates. The updated tool and revised burden will be submitted to OMB under control number 0938-1314 (CMS-10621).
Beginning with the 2021 Annual Call for Measures, we replaced the customary Office of the National Coordinator (ONC) Issue Tracking System Jira platform that stakeholders used to submit candidate quality measure specifications and all supporting data files for CMS review with the MUC Entry/Review Information Tool (MERIT). For the ONC Issue Tracking System Jira platform used by stakeholders, the approved estimated time for a practice Start Printed Page 39507 administrator to identify, propose, and link to a quality measure is 0.9 hours and for a clinician to identify, propose, link to quality measure, and complete the Peer Review Journal Article form is 4.6 hours (0.6 hours to identify, propose, and link to quality measure (84 FR 63132) and 4 hours to complete the Peer Review Journal Article Form (84 FR 63133), with a total estimated time of 5.5 hours per quality measure submission. Based on the stakeholder experience with the updated tool and additional information collected at the time of submission, we estimate that it would add approximately 1.5 hours for the practice administrator at $114.24/hr and 0.5 hours at $217.32/hr for a clinician to identify, propose, and link the quality measure, and reduce approximately 2 hours at $217.32/hr for a clinician to complete the Peer Review Journal Article Form, resulting in a new estimated time of 2.4 hours for a practice administrator and 3.1 hours for a clinician, and an unchanged total estimated time of 5.5 hours per quality measure submission. In order to account for the implementation of the MERIT tool starting with the 2021 Annual Call for Measures, we propose to revise the estimated time required for a practice administrator to identify, propose, and link to a quality measure to 2.4 hours (from 0.9 hr) and a clinician to identify, propose, link to quality measure, and complete the Peer Review Journal Article Form to 3.1 hours (from 4.6 hr), resulting in a total estimated time of 5.5 hours per quality measure submission. Based on the number of submissions received during the CY 2020 Call for Quality Measures process, we anticipate receiving the same number of 28 submissions during the CY 2021 Call for Quality Measures process (84 FR 63132).
Although the total estimated time of 5.5 hours for completing a quality measure submission using the MERIT tool (see Table 92) is the same estimated time as the ONC Issue Tracking System Jira platform, we need to account for the changes to the individual components of the estimated time required by a practice administrator and clinician using the MERIT tool. Consistent with our assumptions in the CY 2021 PFS final rule (85 FR 84984), we estimate an annual burden of 154 hours (28 submissions × 5.5 hr/measure). Thus, we are proposing to adjust our estimated annual burden from $30,197 (28 submissions × [(0.9 hr × $110.74/hr) + (4.6 hr × $212.78/hr)) to $26,541 (28 measures × [(2.4 hr × $114.24/hr) + (3.1 hr × $217.32/hr)]) a difference of −$3,656.

g. ICRs Regarding Promoting Interoperability Data (§§ 414.1375 and 414.1380)
(1) Background
For the CY 2022 MIPS performance period/2024 MIPS payment year, clinicians and groups can submit Promoting Interoperability data through direct, log in and upload, or log in and attest submission types. With the exception of submitters who elect to use the log in and attest submission type for the Promoting Interoperability performance category, which is not available for the quality performance category, we anticipate that individuals and groups will use the same data submission type for the both of these performance categories and that the clinicians, practice managers, and computer systems analysts involved in supporting the quality data submission will also support the Promoting Interoperability data submission process. The following burden estimates show only incremental hours required above and beyond the time already accounted for in the quality data submission process. Although this analysis assesses burden by performance category and submission type, we emphasize that MIPS is a consolidated program and submission analysis, and decisions are expected to be made for the program as a whole.
(2) Reweighting Applications for Promoting Interoperability and Other Performance Categories
The requirements and burden associated with this rule's data submission will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
We refer readers to the CY 2018 Quality Payment Program final rule (82 FR 53918 through 53919), CY 2019 PFS final rule (83 FR 60011 through 60012), CY 2020 PFS final rule (84 FR 63134 through 63135), and the CY 2021 PFS final rule (85 FR 84984 through 84985) for our previously finalized requirements and burden for reweighting applications for Promoting Start Printed Page 39508 Interoperability and other performance categories.
As established in the CY 2017 and CY 2018 Quality Payment Program final rules, MIPS eligible clinicians who meet the criteria for a significant hardship or other type of exception may submit an application requesting a zero percent weighting for the Promoting Interoperability, quality, cost, and/or improvement activities performance categories under specific circumstances (81 FR 77240 through 77243, 82 FR 53680 through 53686, and 82 FR 53783 through 53785). Respondents who apply for a reweighting for the quality, cost, and/or improvement activities performance categories have the option of applying for reweighting for the Promoting Interoperability performance category on the same online form. We assume that respondents applying for a reweighting of the Promoting Interoperability performance category due to extreme and uncontrollable circumstances will also request a reweighting of at least one of the other performance categories simultaneously and not submit multiple reweighting applications.
Table 93 summarizes the burden for clinicians to apply for reweighting the Promoting Interoperability performance category to zero percent due to a significant hardship exception or as a result of a decertification of an EHR. Based on the number of reweighting applications received by March, 2021 for the CY 2020 MIPS performance period/2022 MIPS payment year, we assume 20,192 respondents (eligible clinicians or groups) will submit a request to reweight the Promoting Interoperability performance category to zero percent due to a significant hardship or EHR decertification and an additional 22,635 respondents will submit a request to reweight one or more of the quality, cost, Promoting Interoperability, or improvement activities performance categories due to an extreme or uncontrollable circumstance. For the CY 2022 MIPS performance period/2024 MIPS payment year, we estimate that a total of 42,797 reweighting applications would be submitted. This is a decrease of 9,302 respondents compared to our currently approved estimate of 52,099 respondents (85 FR 84984). This decrease is likely due to the proposal in section IV.A.3.e.(2)(b)(iii)(A) of this rule to automatically reweight the Promoting Interoperability performance category for small practices who previously had to apply for reweighting. For the CY 2020 MIPS performance period/2024 MIPS payment year, 13,894 respondents requested reweighting due to significant hardship for small practices. Similar to the data used to estimate the number of respondents in the CY 2021 PFS final rule, our respondent estimate includes a significant number of applications submitted as a result of a data issue CMS was made aware of and is specific to a single third-party intermediary. While we do not anticipate similar data issues to occur in each performance period, we do believe future similar incidents may occur and are electing to use this data without adjustment to reflect this belief. We assume that, out of our total respondent count of 42,797 above, we estimate that 22,605 respondents (eligible clinicians or groups) will submit a request for reweighting the Promoting Interoperability performance category to zero percent due to extreme and uncontrollable circumstances, insufficient internet connectivity, lack of control over the availability of CEHRT, or as a result of a decertification of an EHR.
In the CY 2021 PFS final rule (85 FR 84984) we discussed that, beginning with the CY 2019 MIPS performance period/2021 MIPS payment year, APM Entities may submit an extreme and uncontrollable circumstances exception application for all four performance categories and applicable to all MIPS eligible clinicians in the APM Entity group. As discussed above in this section of this proposed rule, due to data limitations and our inability to determine who would use the APP versus the traditional MIPS submission mechanism for the 2022 MIPS performance period/2024 MIPS payment year, we assume ACO APM Entities will submit data through the APP and non-ACO APM Entities would participate through traditional MIPS, thereby submitting as an individual or group rather than as an entity. Therefore, we limited our analysis to ACOs that were eligible for an exception due to extreme and uncontrollable circumstances during the 2020 MIPS performance period/2022 MIPS payment year and elected not to report quality data. Based on this data, we estimate that 30 APM Entities will submit an extreme and uncontrollable circumstances exception application for the CY 2022 MIPS performance period/2024 MIPS payment year. Combined with our aforementioned estimate of 42,797 eligible clinicians and groups, the total estimated number of respondents for the CY 2022 MIPS performance period/2024 MIPS payment year is 42,827.
Consistent with our assumptions in the CY 2021 PFS final rule (85 FR 84984-84985), we continue to estimate it will take 0.25 hours for a computer system analyst to complete and submit the application. As shown in Table 93, we estimate an annual burden of 10,707 hours (42,827 applications × 0.25 hr/application) and $1,019,521 (10,707 hr × $95.22/hr).

Start Printed Page 39509
As shown in Table 94, using our currently approved burden estimates, the proposed decrease in the estimate number of respondents (from 52,099 to 42,827 respondents) results in an adjustment of minus 2,318 hours (9,272 respondents x 0.25 hr/respondent) and minus $184,747.

(3) Submitting Promoting Interoperability Data
The requirements and burden associated with this rule's data submission will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
We refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77509 through 77511), CY 2018 Quality Payment Program final rule (82 FR 53919 through 53920), CY 2019 PFS final rule (83 FR 60013 through 60014), CY 2020 PFS final rule (84 FR 63135 through 63137), and the CY 2021 PFS final rule (85 FR 84985 through 84987) for our previously finalized requirements and burden for submission of data for the Promoting Interoperability performance category.
We did not propose any changes to our current criteria for automatic reweighting of the Promoting Interoperability performance category for certain MIPS eligible clinicians or MIPS eligible clinicians who have experienced a significant hardship or decertification of an EHR. In section IV.A.3.d.(4)(d)(ii) of this proposed rule, we proposed the additional requirement that MIPS eligible clinicians must attest to conducting an annual assessment of the High Priority Guides of the SAFER Guides beginning with the 2022 performance period. Clinicians will complete this attestation by checking a box when they submit their promoting interoperability performance category data. We estimate that this requirement will add an additional minute to the time it takes to complete the submission of promoting interoperability data. We also proposed to modify the Provide Patients Electronic Access to Their Health Information measure to require MIPS eligible clinicians to ensure that patient health information remains available to the patient (or patient-authorized representative) to access indefinitely. The proposed requirement would apply beginning with the performance period in 2022, and would include all patient health information from encounters on or after January 1, 2016. We do not believe this proposal will impact the burden of Promoting Interoperability data submission. Therefore, we are not revising the currently approved burden per respondent estimate.
As shown in Table 95, based on data from the CY 2019 MIPS performance period/2021 MIPS payment year, we estimate that a total of 51,647 respondents consisting of 40,172 individual MIPS eligible clinicians and 11,475 groups and virtual groups will submit Promoting Interoperability data. Since our CY 2021 PFS final rule estimated 53,636 respondents, this represents a decrease of 1,989 respondents (51,647 respondents−53,636 active respondents).
We assume that MIPS eligible clinicians previously scored under the APM scoring standard, as described in the CY 2020 PFS final rule, will continue to submit Promoting Interoperability data (84 FR 63006) in a similar way through the APP. As a result, we do not anticipate any change in burden. Each MIPS eligible clinician in an APM Entity reports data for the Promoting Interoperability performance category through either their group TIN or individual reporting. Sections 1899 and 1115A of the Act (42 U.S.C. 1395jjj and 42 U.S.C. 1315a, respectively) state that the Shared Savings Program and the testing, evaluation, and expansion of Innovation Center models are not subject to the PRA. However, in the CY 2019 PFS final rule, we established that MIPS eligible clinicians who participate in the Shared Savings Program are no longer limited to reporting for the Promoting Interoperability performance category through their ACO participant TIN (83 FR 59822 through 59823). Burden estimates for this proposed rule assume group TIN-level reporting as we believe this is the most reasonable assumption for the Shared Savings Program, which requires that ACOs include full TINs as ACO participants. As we receive updated information which reflects the actual number of Promoting Interoperability data submissions submitted by Shared Savings Program ACO participants, we will update our burden estimates accordingly.
Start Printed Page 39510

As discussed in section IV.A.3.b.(2)(d)(ii) of this proposed rule, we will be introducing subgroup reporting in CY 2023 MIPS performance period/2025 MIPS payment year. As we discussed above in this section of the proposed rule, we estimate that there will be 20 subgroup submissions in CY 2023 MIPS performance period/2025 MIPS payment year, each of which will have burden related to the submission of Promoting Interoperability data. We have included this burden in Table 96.

With the inclusion of the additional minute (0.02 hr) to attest to conducting an annual assessment of the High Priority Guides of the SAFER Guides, we are proposing to update our estimate of the time required for an individual or group to submit Promoting Interoperability data from 2.67 hours to 2.69 hours (2.67 hr + 0.02 hr). As shown in Table 97, the total burden estimate for submitting data on the specified Promoting Interoperability objectives and measures is estimated to be 138,930 hours (51,647 respondents × 2.69 incremental hours for a computer analyst's time above and beyond the physician, medical and health services manager, and computer system's analyst time required to submit quality data) and $13,228,915 (138,930 hr × $95.22/hr)).

As shown in Table 98, with the introduction of subgroup reporting in CY 2023 MIPS performance period/2025 MIPS payment year, the total proposed burden estimate for submitting data on the specified Promoting Interoperability objectives and measures is estimated to be 138,984 hours (51,667 respondents × 2.69 incremental hours for a computer analyst's time above and beyond the physician, medical and health services manager, and computer system's analyst time required to submit quality data) and $13,234,078 (138,984 hr × $95.22/hr)).
Start Printed Page 39511

Table 99, using our updated per respondent burden estimate (+0.02 hr/response), the decrease in number of respondents and proposed SAFER guide attestation requirement results in a total adjustment of −4,099 hours at a cost of −$390,338 for the CY 2022 MIPS performance period/2024 MIPS payment year and −4,045 hours at a cost of −$385,175 for the CY 2023 MIPS performance period/2025 MIPS payment year.

h. ICRs Regarding the Nomination of Promoting Interoperability (PI) Measures
This rule is not proposing any new or revised collection of information requirements or burden related to the nomination of Promoting Interoperability measures. The requirements and burden are currently approved by OMB under control number 0938-1314 (CMS-10621). Consequently, we are not proposing any changes under that control number.
i. ICR Regarding Improvement Activities Submission (§§ 414.1305, 414.1355, 414.1360, and 414.1365)
The following proposed requirements and burden will be submitted to OMB for approval under control number 0938-1314 (CMS-10621). We refer readers to the CY 2017 Quality Payment Program final rule (81 FR 77511 through 77512), CY 2018 Quality Payment Program final rule (82 FR 53920 through 53922), CY 2019 PFS final rule (83 FR 60015 through 60017), CY 2020 PFS final rule (84 FR 63138 through 63140) and the CY 2021 PFS final rule (85 FR 84987 through 84989) for our previously finalized requirements and burden for submission of data for the improvement activities performance category.
In section IV.A.3.d.(3) of this rule, we are proposing to: (1) Revise group reporting requirements for the 50 percent threshold to address subgroups; (2) add 7 new improvement activities, modify 15 existing improvement activities, and remove 6 previously adopted improvement activities for the CY 2022 MIPS performance period/2024 MIPS payment year and future years; (3) revise the "Drug Cost Transparency to include requirements for use of real-time benefit tools" improvement activity; and (4) add the COVID-19 "Clinical Data Reporting with or without Clinical Trial" improvement activity for CY 2022 MIPS performance period/2024 MIPS payment year and future years. Additionally, we are proposing to adjust our currently approved burden estimates based on more recent data.
Specifically, we are proposing to revise § 414.1360(a)(2) to state that, beginning with the CY 2023 MIPS performance period/2025 MIPS payment year, each improvement activity for which groups and virtual groups submit a yes response in accordance with paragraph (a)(1) of this section must be performed by at least 50 percent of the NPIs that are billing under the group's TIN or virtual group's TINs or that are part of the subgroup, as applicable; and the NPIs must perform the same activity during any continuous 90-day period within the same performance year. In section Start Printed Page 39512 IV.A.3.d.(3)(b) of this rule, we discussed stakeholder requests through the Quality Payment Program help desk to apply the 50 percent threshold to a portion of clinicians in a group. We anticipate that clinicians would find applicable and meaningful activities specific to practice size, specialty, or practice setting. Therefore, we assume that the proposal to apply the 50 percent minimum threshold to clinicians who submit for the improvement activity performance category as part of groups, virtual groups, or choose to participate as subgroups beginning with the CY 2023 MIPS performance period/2025 MIPS payment year would not present additional complexity or burden.
We do not believe the proposed changes to the improvement activities inventory will impact time or financial burden on stakeholders because MIPS eligible clinicians are still required to submit the same number of activities and the per response time for each activity is uniform. Therefore, we are not proposing to revise the estimated time of 5 minutes (per response) currently approved for improvement activities submission.
As represented in Table 100, based on data from the CY 2019 MIPS performance period/2021 MIPS payment year, we estimate that a total of 81,562 respondents consisting of 63,845 individual clinicians and 17,717 groups will submit improvement activities during the 2022 MIPS performance period/2024 MIPS payment year. Since our currently approved burden sets out 79,927 respondents, this represents an increase of 1,635 respondents (81,562 respondents−79,927 active respondents). This is an increase of 1,242 individuals and 393 groups from the estimates of 62,603 individuals and 17,324 groups provided in the CY 2021 PFS final rule due to availability of updated data (85 FR 50362).
As discussed in sections V.B.8.e. and V.B.8.g.(3) of this proposed rule regarding our estimate of clinicians and groups submitting data for the quality and Promoting Interoperability performance categories, we are proposing to update our estimates for the number of clinicians and groups that will submit improvement activities data based on projections of the number of eligible clinicians that were not QPs or participating in an ACO in the CY 2019 MIPS performance period/2021 MIPS payment year but will be QPs in the CY 2022 MIPS performance period/2024 MIPS payment year, and will therefore not be required to submit improvement activities data.

As discussed in section IV.A.3.b.(2)(d)(ii) of this proposed rule, we are proposing subgroup reporting in the CY 2023 MIPS performance period/2025 MIPS payment year. As we discussed in section V.B.8.e.(7)(a) of this proposed rule, we estimate that there will be 20 subgroup reporters in the CY 2023 MIPS performance period/2025 MIPS payment year, each of which will have burden related to the submission of improvement activities. We have included this burden in Table 101.
Start Printed Page 39513

Consistent with the CY 2021 PFS final rule, we continue to estimate that the per response time required per individual or group is 5 minutes for a computer system analyst to submit by logging in and manually attesting that certain activities were performed in the form and manner specified by CMS with a set of authenticated credentials (84 FR 63140).
As shown in Table 102, we estimate an annual burden of 6,797 hours (81,562 responses × 5 minutes/60) and $647,210 (6,797 hr × $95.22/hr)) in CY 2022 MIPS performance period/2024 MIPS payment year.

As shown in Table 103, with the introduction of subgroup reporting in the CY 2023 MIPS performance period/2025 MIPS payment year, we estimate an annual burden of 6,799 hours (81,582 responses × 5 minutes/60) and $647,401 (6,799 hr × $95.22/hr)).
Start Printed Page 39514

As shown in Table 104, using our unchanged currently approved per respondent burden estimate, the increase of 1,635 in the number of respondents results in an adjustment of 136 hours (1,635 responses × 5 minutes/60) at a cost of $12,973 (136 hr × $95.22/hr).

j. ICRs Regarding the Nomination of Improvement Activities (§ 414.1360)
The proposed requirements and burden associated with this rule's data submission will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
We refer readers to the CY 2018 Quality Payment Program final rule (82 FR 53922), CY 2019 PFS final rule (83 FR 60017 through 60018), CY 2020 PFS final rule (84 FR 63141) and the CY 2021 PFS final rule (85 FR 84989 through 85 FR 84990) for our previously finalized requirements and information collection burden for the nomination of improvement activities.
In section IV.A.3.d.(3)(c)(i)(B) of this rule, we are proposing: (1) To revise the required criteria for improvement activity nominations received through the Annual Call for Activities; (2) changes to the timeline for improvement activities nomination during a public health emergency (PHE); and (3) to suspend activities that become obsolete or impacted by clinical practice guideline changes from the program when this occurrence happens outside of the rulemaking process.
In section IV.A.3.d.(3)(c)(i)(B)(cc) of this rule, we are proposing 2 new criteria that beginning with the CY 2022 Annual Call for Activities MIPS improvement activities: (1) Should not duplicate other improvement activities in the Inventory; and (2) should drive improvements that go beyond purely common clinical practices.
Additionally, we are proposing to increase the number of criteria stakeholders are required to meet when submitting an activity proposal from a minimum of 1 to all 8 criteria, which includes the two new proposed criteria. We believe that this proposal would provide clearer guidance to stakeholders when submitting a nomination for an improvement activity. In the CY 2021 PFS final rule, we estimated that it would require 0.6 hours for a medical and health services manager or equivalent and 0.4 hours for a physician to link the nominated improvement activity to existing and related cost and quality measures (85 FR 84989). Given that our current approved estimated time per respondent to nominate an improvement activity is 3 hours (1.8 hours for a medical and health services manager or equivalent and 1.2 hours for a physician), we assume that the proposed new requirement to meet all 8 criteria would require approximately 1 hour at $114.24/hr for a medical and health services manager to identify and submit an activity and 0.4 hours at a rate of $217.32/hr for a clinician to review each activity. Combined with our currently approved burden estimate, we propose to revise our estimate to 2.8 hours at $114.24/hr for a medical and health services manager or equivalent and 1.6 hours at $217.32/hr for a physician to nominate an improvement activity. This represents a change of +1 hours (2.8 hr−1.8 hr) for a medical and health services manager or equivalent and +0.4 hours (2 hr−1.6 hr) for a physician and an overall increase of 1.4 hours. We considered whether we should double our estimates for nomination of an improvement activity to 6 hours. Since only 2 of the required Start Printed Page 39515 8 criteria are new, we assume that stakeholders are familiar with the existing criteria and would not need additional time to review but would need the additional time to verify and confirm if the considered activity meets all the 8 criteria. We seek comment on our proposed estimate to revise the time for nomination of an improvement activity to 4.4 hours and if there are additional burden implications that we should consider for above proposals to revise the criteria.
In the CY 2021 PFS final rule, we finalized an exception stating that during public health emergencies (PHE) stakeholders can nominate improvement activities outside of the established Annual Call for Activities timeframe (85 FR 84989). Instead of only accepting nominations and modifications submitted February 1st through July 1 each year, we would accept nominations for the duration of the PHE as long as the improvement activity is still relevant. No other aspects of the Annual Call for Activities process would be affected (for example, criteria for nominating improvement activities, considerations for selection of improvement activities, or weighting policies would all still apply). In section IV.A.3.d.(3)(c)(i)(B)(aa)of this rule, we are proposing to clarify that in order to implement a new improvement activity for a PHE during the same year as the nomination, the nomination would need to be received no later than January 5th of the nomination year to be included in a rule for notice-and-comment rulemaking during that fiscal or calendar year, a necessary precursor to implementation if it were to be finalized, as described above.
We believe this proposal will not affect our currently approved burden estimates since we assume that the number of nominations will not change, but it would make an activity available for reporting to clinicians in the same performance year it was intended to be implemented. Similar to our assumptions in the CY 2021 PFS final rule (85 FR 84989), we expect additional nominations may be received as a result of this change. However, we do not have any data with which to estimate what the additional number may be. As a result, we are not making any proposed revisions to our currently approved burden estimate.
In section IV.A.3.d.(3)(c)(i)(C)(aa) of this rule, we are proposing that beginning with the CY 2022 MIPS performance period/2024 MIPS payment year we are proposing that for each improvement activity that is in the Inventory, if applicable, and impacted by significant changes or errors prior to the applicable data submission deadline, it will be removed from the program as soon as possible. In the CY 2020 PFS final rule (84 FR 62988 through 62990), we finalized the factors for consideration in removing improvement activities. Following the publication of the CY 2021 PFS proposed rule, the improvement activities team became aware that clinicians could no longer complete the activity from April 1 through December 31, 2020, because one of the improvement activities in the Inventory had expired on March 31, 2020. We do not anticipate any burden for stakeholders because of the above proposal as described, the proposed policy does not change requirements for the nomination of improvement activities. This proposal would help avoid stakeholder confusion and ensure the accuracy of the available activities in the Inventory. Therefore, we are not proposing to revise our estimated burden due to the above proposed policy.
Additionally, consistent with our assumptions in the CY 2021 PFS final rule (85 FR 84990) we continue to use our currently approved assumption that we will receive 31 nominations of new or modified activities which will be evaluated for the Improvement Activities Under Consideration (IAUC) list for possible inclusion in the CY 2023 Improvement Activities Inventory. The 2021 Annual Call for Activities ends on July 1, 2021; assuming updated information is available, we will update our estimate in the final rule.
As shown in Table 105, accounting for the change in burden per respondent estimate due to the provision to require all the 8 criteria for nomination of an improvement activity as described above in this section, we propose to revise our estimated annual information collection burden to 136 hours (31 nominations × 4.4 hr/nomination) at a cost of $20,695 (31 × [(2.8 hr × $114.24/hr) + (1.6 hr × $217.32/hr)]).

As shown in Table 106, using our unchanged estimate of the number of activities nominated, the increase in the burden per nomination results in a change of 43 hours (31 nominations × 1.4 hr/nomination) at a cost of $6,492 (31 activities × [(1 hr × $114.24/hr) + (0.4 hr × $217.32/hr)]).
Start Printed Page 39516

k. Nomination of MVPs
This rule does not propose any new or revised collection of information requirements or burden related to the nomination of MVPs for inclusion in the Quality Payment Program. The requirements and burden are currently approved by OMB under control number 0938-1314 (CMS-10621). Consequently, we are not proposing any changes under that control number.
l. ICRs Regarding the Cost Performance Category (§ 414.1350)
The cost performance category relies on administrative claims data. The Medicare Parts A and B claims submission process (OMB control number 0938-1197; CMS-1500 and CMS-1490S) is used to collect data on cost measures from MIPS eligible clinicians. MIPS eligible clinicians are not required to provide any documentation by CD or hardcopy. Moreover, the proposed policies in this rule do not result in the need to add or revise or delete any claims data fields. Consequently, we are not proposing any changes under that control number.
m. ICRs Regarding Partial QP Elections (§§ 414.1310(b) and 414.1430)
This rule does not propose any new or revised collection of information requirements related to the Partial QP Elections to participate in MIPS as a MIPS eligible clinician. However, we are proposing to adjust our currently approved burden estimates based on updated projections for the CY 2022 MIPS performance period/2024 MIPS payment year. The proposed adjusted burden will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
As shown in Table 107, based on our predictive QP analysis for the 2022 QP performance period/2024 payment year, which accounts for historical response rates in the CY 2020 MIPS performance period/2022 MIPS payment year, we propose to revise our estimate that 150 APM Entities and 100 eligible clinicians (representing approximately 9,000 Partial QPs) will make the election to participate as a Partial QP in MIPS, a total of 250 elections which is a decrease of 50 from the 300 elections that are currently approved by OMB under the aforementioned control number. We continue to estimate it will take the APM Entity representative or eligible clinician 15 minutes (0.25 hr) to make this election. In aggregate, we propose to revise our estimated annual burden to 63 hours (250 respondents × 0.25 hr/election) and $5,999 (63 hr × $95.22/hr).

As shown in Table 108, using our unchanged currently approved per respondent burden estimate, the proposed decrease in the number of Partial QP elections results in an adjustment of 12.5 hours (−50 elections × 0.25 hr) at a cost of −$1,191 (−12.5 hr × $95.22/hr) (85 FR 84991).
Start Printed Page 39517

n. ICRs Regarding Other Payer Advanced APM Determinations: Payer-Initiated Process (§ 414.1445) and Eligible Clinician Initiated Process (§ 414.1445)
The following burden will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
(1) Payer Initiated Process (§ 414.1445)
This rule is not proposing any new or revised collection of information requirements related to the Payer-Initiated Process. However, we are proposing to adjust our currently approved burden estimates based on updated projections for the CY 2022 MIPS performance period/2024 MIPS payment year.
As shown in Table 109, based on the actual number of requests received in the 2020 QP performance period, we propose to revise our estimate that for the 2023 QP performance period, 15 payer-initiated requests for Other Payer Advanced APM determinations will be submitted (6 Medicaid payers, 6 Medicare Advantage Organizations, and 3 remaining other payers), a decrease of 65 from the 80 total requests currently approved by OMB under the aforementioned control number. We continue to estimate it will take 10 hours for a computer system analyst per arrangement submission. We propose to revise our estimated annual burden to 150 hours (15 submissions × 10 hr/submission) and $14,283 (150 hr × $95.22/hr).

As shown in Table 110, using our unchanged currently approved per respondent burden estimate, the proposed decrease in the number of payer-initiated requests from 800 to 150 results in an adjustment of −650 hours (−65 requests × 10 hr) at a cost of −$61,893 (−650 hr × $95.22/hr) (85 FR 84992).
Start Printed Page 39518

(2) Eligible Clinician Initiated Process (§ 414.1445)
This rule does not propose any new or revised collection of information requirements or burden related to the Eligible-Clinician Initiated Process. However, we are proposing to adjust our currently approved burden estimates based on updated projections for the CY 2022 MIPS performance period/2024 MIPS payment year. As mentioned above, the new and adjusted burden will be submitted to OMB for approval.
As shown in Table 111, based on the actual number of requests received in the 2020 QP performance period, we estimate that in CY 2022 for the 2023 QP performance period, 15 Eligible-Clinician Initiated request for Other Payer Advanced APM determinations will be submitted, a decrease of 135 from the 150 total requests currently approved by OMB under the aforementioned control number. We continue to estimate it will take 10 hours for a computer system analyst per arrangement submission. We propose to revise our estimated annual burden to 150 hours (15 submissions × 10 hr/submission) and $14,283 (150 hr × $95.22/hr).

As shown in Table 112, using our unchanged currently approved per respondent burden estimate, the proposed decrease in the number of eligible clinician-initiated requests from 150 to 15 results in an adjustment of −1,350 hours (−135 requests × 10 hr) at a cost of −$128,547 (−1,350 hr × $95.22/hr) (85 FR 84993).
Start Printed Page 39519

(3) Submission of Data for QP Determinations Under the All-Payer Combination Option (§ 414.1440)
This rule does not propose any new or revised collection of information requirements related to the Submission of Data for QP Determinations under the All-Payer Combination Option. The requirements and burden are currently approved by OMB under control number 0938-1314 (CMS-10621). Consequently, we are not proposing any changes under that control number.
o. ICRs Regarding Voluntary Participants Election To Opt-Out of Performance Data Display on Physician Compare (§ 414.1395)
This rule does not propose any new or revised collection of information requirements related to the election by voluntary participants to opt-out of public reporting on Physician Compare. However, we are proposing adjustments to our currently approved burden estimates based on data from the CY 2019 MIPS performance period/2021 MIPS payment year. The proposed adjusted burden will be submitted to OMB for approval under control number 0938-1314 (CMS-10621).
We refer readers to the CY 2018 Quality Payment Program final rule (82 FR 53924 through 53925), CY 2019 PFS final rule (83 FR 60022), CY 2020 PFS final rule (84 FR 63145 through 63146) and the CY 2021 PFS final rule (85 FR 84993) for our previously finalized requirements and burden for voluntary participants to opt-out of public reporting on Physician Compare.
In the CY 2021 PFS final rule (85 FR 84993), we estimated that 10 percent of the clinicians and groups who voluntarily participate in MIPS would opt out of public reporting. Based on the number of opt-out eligible clinicians that chose to opt-out of public reporting in the CY 2019 MIPS performance period/2021 MIPS payment year, we propose to revise our estimates. We anticipate that 0.1 percent of the total clinicians and groups who will voluntarily participate in the CY 2022 MIPS performance period/2024 MIPS payment year will also elect not to participate in public reporting. This results in a total of 38 (0.001 × 37,934 voluntary MIPS participants) clinicians and groups, a decrease of 3,448 from the currently approved estimate of 3,486. Voluntary MIPS participants are clinicians that are not QPs and are expected to be excluded from MIPS after applying the eligibility requirements set out in the CY 2019 PFS final rule but have elected to submit data to MIPS. As discussed in the RIA section of the CY 2019 PFS final rule, we continue to estimate that 33 percent of clinicians that exceed one (1) of the low-volume criteria, but not all three (3), will elect to opt-in to MIPS, become MIPS eligible, and no longer be considered a voluntary reporter (83 FR 60050).
Table 113 shows that for these voluntary participants, we continue to estimate it will take 0.25 hours for a computer system analyst to submit a request to opt-out. In aggregate, we estimate an annual burden of 9.5 hours (38 requests × 0.25 hr/request) and $904 (9.5 hr × $95.22/hr).

As shown in Table 108, using our unchanged currently approved per respondent burden estimate, the decrease of 3,448 opt outs by voluntary participants results in an adjustment of −862 hours (−3,448 requests × 0.25 hr) at a cost of $−82,079 (−862 hr × $95.22/hr).
Start Printed Page 39520

p. Summary of Annual Quality Payment Program Burden Estimates
Table 115 summarizes this proposed rule's total burden estimates for the Quality Payment Program for both the CY 2022 and CY 2023 MIPS performance periods/2024 and 2025 MIPS payment years. In the CY 2021 PFS final rule, the total estimated burden for the CY 2022 MIPS performance period/2024 MIPS payment year was 1,473,741 hours at a cost of $144,034,968 (85 FR 84994). Accounting for updated wage rates and the subset of all Quality Payment Program ICRs discussed in this rule compared to the CY 2021 PFS final rule, the total estimated annual burden of continuing policies and information set forth in the CY 2021 PFS final rule into the CY 2022 MIPS performance period/2024 MIPS payment year is 1,468,547 hours at a cost of $148,093,881. These represent a decrease of 5,194 hours and an increase of $4,058,913. To understand the burden implications of the proposed policies in this rule, we provide an estimate of the total burden associated with continuing the policies and information collections set forth in the CY 2021 PFS final rule into the CY 2022 MIPS performance periods/2024 MIPS payment year. This burden estimate of 1,424,726 hours at a cost of $143,682,174 reflects the availability of more accurate data to account for all potential respondents and submissions across all the performance categories and more accurately reflect the exclusion of QPs from all MIPS performance categories, a decrease of 43,821 hours and $4,411,707. This burden estimate is lower than the burden approved for information collection related to the CY 2021 PFS final rule due to updated data and assumptions. Our total burden estimate for the CY 2022 MIPS performance period/2024 MIPS payment year is 1,428,537 hours and $144,040,730, which represents a decrease of 40,010 hours and $4,053,151 from the CY 2021 PFS final rule. The difference of +3,811 hours (43,821 hours−40,010 hours) and +$358,556 ($4,385,824−$4,026,590) between this estimate and the total burden shown in Table 115 is the reduction in burden associated with impacts of the policies proposed for the CY 2022 MIPS performance period/2024 MIPS payment year, which includes the re-introduction of the CMS Web Interface measures as a collection type/submission type.
Table 115 also offers a comparison between the total currently approved estimated burden from the CY 2021 PFS final rule and our proposed estimated burden for the CY 2023 MIPS performance period/2025 MIPS payment year. Our total burden estimate for the CY 2023 MIPS performance period/2025 MIPS payment year is 1,383,744 hours and $139,516,153, which represents a decrease of 84,803 hours and $8,577,728 from the CY 2021 PFS final rule. The difference of −40,982 hours (43,821 hours−84,803 hours) and −$4,166,021 ($4,411,707−$8,577,728) between this estimate and the total burden shown in Table 115 is the reduction in burden associated with impacts of the policies proposed for the CY 2023 MIPS performance period/2025 MIPS payment year. These policy changes include the proposals to sunset the CMS Web Interface measures as a collection type/submission type and to implement a new information collection for MVPs and subgroups.
We have included Table 115 to assist in understanding these differences. Note that the difference between the burden estimates for the CY 2022 and 2023 MIPS performance periods/2024 and 2025 MIPS payment years is entirely due to the proposed policies to introduce MVP and subgroup reporting and sunset the CMS Web Interface measures as a collection type/submission type beginning in the CY 2023 MIPS performance period/2025 MIPS payment year.
Start Printed Page 39521

Start Printed Page 39522

Start Printed Page 39523

Start Printed Page 39524

The following table represents averages for the estimated changes in burden for the CY 2022 and CY 2023 MIPS performance periods/2024 and 2025 MIPS payment years.

Table 119 provides the reasons for changes in the estimated burden for information collections in the Quality Payment Program segment of this proposed rule. We have divided the reasons for our change in burden into those related to new policies and those related to adjustments in burden from continued Quality Payment Program Year 5 policies that reflect updated data and revised methods.
Start Printed Page 39525

Start Printed Page 39526

Start Printed Page 39527

C. Summary of Annual Burden Estimates for Proposed Changes
Start Printed Page 39528

D. Submission of Comments
We have submitted a copy of this rule to OMB for its review of the rule's proposed information collection requirements and burden. The requirements are not effective until they have been approved by OMB.
To obtain copies of the supporting statement and any related forms for the proposed collections previously discussed, please visit CMS's website at https://www.cms.gov/RegulationsandGuidance/Legislation/PaperworkReductionActof1995/PRAListing.html, or call the Reports Clearance Office at (410) 786-1326.
We invite public comments on the proposed information collection requirements and burden. If you wish to comment, please submit your comments electronically as specified in the DATES and ADDRESSES sections of this proposed rule and identify the rule (CMS-1751-P) and where applicable the ICR's CFR citation, CMS ID number, and OMB control number.
VI. Response to Comments
Because of the large number of public comments we normally receive on Federal Register documents, we are not able to acknowledge or respond to them individually. We will consider all comments we receive by the date and time specified in the DATES section of this preamble, and, when we proceed with a subsequent document, we will respond to the comments in the preamble to that document.
VII. Regulatory Impact Analysis
A. Statement of Need
In this proposed rule, we are proposing to make payment and policy changes under the Medicare PFS and required statutory changes under the Consolidated Appropriations Act, 2016 and sections 2003 and 2005 of the SUPPORT for Patients and Communities Act of 2018. We are also proposing changes to payment policy and other related policies for Medicare Part B. In addition, if finalized, this proposed rule would make modest revisions to certain Medicare provider and supplier enrollment regulatory provisions and add already existing provider and supplier requirements pertaining to prepayment and post-payment review activities.
This proposed rule is necessary to make policy changes under Medicare fee-for-service and to address various provider and supplier enrollment issues. Therefore, we include a detailed Regulatory Impact Analysis (RIA) to assess all costs and benefits of available regulatory alternatives and explain the selection of these regulatory approaches that we believe adhere to statutory requirements and, to the extent feasible, maximize net benefits.
B. Overall Impact
We examined the impact of this rule as required by Executive Order 12866 on Regulatory Planning and Review (September 30, 1993), Executive Order 13563 on Improving Regulation and Regulatory Review (February 2, 2013), the Regulatory Flexibility Act (RFA) (September 19, 1980, Pub. L. 96-354), section 1102(b) of the Social Security Act, section 202 of the Unfunded Mandates Reform Act of 1995 (March 22, 1995; Pub. L. 104-4), Executive Order 13132 on Federalism (August 4, 1999), and the Congressional Review Act (5 U.S.C. 804(2)).
Executive Orders 12866 and 13563 direct agencies to assess all costs and benefits of available regulatory alternatives and, if regulation is necessary, to select regulatory approaches that maximize net benefits (including potential economic, environmental, public health and safety effects, distributive impacts, and equity). An RIA must be prepared for major rules with economically significant effects ($100 million or more in any 1 year). We estimated, as discussed in this section, that the PFS provisions included in this proposed Start Printed Page 39529 rule would redistribute more than $100 million in 1 year. Therefore, we estimate that this rulemaking is "economically significant" as measured by the $100 million threshold, and hence also a major rule under the Congressional Review Act. Accordingly, we prepared an RIA that, to the best of our ability, presents the costs and benefits of the rulemaking. The RFA requires agencies to analyze options for regulatory relief of small entities. For purposes of the RFA, small entities include small businesses, nonprofit organizations, and small governmental jurisdictions. Most hospitals, practitioners and most other providers and suppliers are small entities, either by nonprofit status or by having annual revenues that qualify for small business status under the Small Business Administration standards. (For details, see the SBA's website at http://www.sba.gov/content/table-small-business-size-standards (refer to the 620000 series)). Individuals and states are not included in the definition of a small entity.
The RFA requires that we analyze regulatory options for small businesses and other entities. We prepare a regulatory flexibility analysis unless we certify that a rule would not have a significant economic impact on a substantial number of small entities. The analysis must include a justification concerning the reason action is being taken, the kinds and number of small entities the rule affects, and an explanation of any meaningful options that achieve the objectives with less significant adverse economic impact on the small entities.
Approximately 95 percent of practitioners, other providers, and suppliers are considered to be small entities, based upon the SBA standards. There are over 1 million physicians, other practitioners, and medical suppliers that receive Medicare payment under the PFS. Because many of the affected entities are small entities, the analysis and discussion provided in this section, as well as elsewhere in this proposed rule is intended to comply with the RFA requirements regarding significant impact on a substantial number of small entities.
In addition, section 1102(b) of the Act requires us to prepare an RIA if a rule may have a significant impact on the operations of a substantial number of small rural hospitals. This analysis must conform to the provisions of section 603 of the RFA. For purposes of section 1102(b) of the Act, we define a small rural hospital as a hospital that is located outside of a Metropolitan Statistical Area for Medicare payment regulations and has fewer than 100 beds. Medicare does not pay rural hospitals for their services under the PFS; rather, the PFS pays for physicians' services, which can be furnished by physicians and NPPs in a variety of settings, including rural hospitals. We did not prepare an analysis for section 1102(b) of the Act because we determined, and the Secretary certified, that this proposed rule will not have a significant impact on the operations of a substantial number of small rural hospitals.
Section 202 of the Unfunded Mandates Reform Act of 1995 also requires that agencies assess anticipated costs and benefits on state, local, or tribal governments or on the private sector before issuing any rule whose mandates require spending in any 1 year of $100 million in 1995 dollars, updated annually for inflation. In 2021, that threshold is approximately $158 million. This proposed rule will impose no mandates on state, local, or tribal governments or on the private sector.
Executive Order 13132 establishes certain requirements that an agency must meet when it issues a proposed rule (and subsequent final rule) that imposes substantial direct requirement costs on state and local governments, preempts state law, or otherwise has federalism implications. Since this proposed rule does not impose any costs on state or local governments, the requirements of Executive Order 13132 are not applicable.
We prepared the following analysis, which together with the information provided in the rest of this preamble, meets all assessment requirements. The analysis explains the rationale for and purposes of this proposed rule; details the costs and benefits of the rule; analyzes alternatives; and presents the measures we would use to minimize the burden on small entities. As indicated elsewhere in this proposed rule, we discussed a variety of changes to our regulations, payments, or payment policies to ensure that our payment systems reflect changes in medical practice and the relative value of services, and implementing statutory provisions. We provide information for each of the policy changes in the relevant sections of this proposed rule. We are unaware of any relevant federal rules that duplicate, overlap, or conflict with this proposed rule. The relevant sections of this proposed rule contain a description of significant alternatives if applicable.
C. Changes in Relative Value Unit (RVU) Impacts
1. Resource-Based Work, PE, and MP RVUs
Section 1848(c)(2)(B)(ii)(II) of the Act requires that increases or decreases in RVUs may not cause the amount of expenditures for the year to differ by more than $20 million from what expenditures would have been in the absence of these changes. If this threshold is exceeded, we make adjustments to preserve budget neutrality.
Our estimates of changes in Medicare expenditures for PFS services compared payment rates for CY 2021 with payment rates for CY 2022 using CY 2020 Medicare utilization. The payment impacts described in this proposed rule reflect averages by specialty based on Medicare utilization. The payment impact for an individual practitioner could vary from the average and would depend on the mix of services he or she furnishes. The average percentage change in total revenues will be less than the impact displayed here because practitioners and other entities generally furnish services to both Medicare and non-Medicare patients. In addition, practitioners and other entities may receive substantial Medicare revenues for services under other Medicare payment systems. For instance, independent laboratories receive approximately 83 percent of their Medicare revenues from clinical laboratory services that are paid under the Clinical Laboratory Fee Schedule (CLFS).
The PFS update adjustment factor for CY 2022, as required by section 1848(d)(19) of the Act, is 0.00 percent before applying other adjustments. In addition, section 101 of Division N of the CAA provided a 3.75 percent increase in PFS payment amounts for services furnished on or after January 1, 2021, and before January 1, 2022 and required that the increase shall not be taken into account in determining PFS payment rates for subsequent years. The expiration of this 3.75 percent increase in payment amounts will result in the CY 2022 conversion factor being calculated as though the 3.75 percent increase for the CY 2021 conversion factor had never been applied.
To calculate the CY 2022 PFS conversion factor (CF), we took the CY 2021 conversion factor without the 1-year 3.75 percent increase provided by the CAA and multiplied it by the budget neutrality adjustment required as described in the preceding paragraphs. We estimate the CY 2022 PFS CF to be 33.5848 which reflects the budget Start Printed Page 39530 neutrality adjustment under section 1848(c)(2)(B)(ii)(II) of the Act, the 0.00 percent update adjustment factor specified under section 1848(d)(19) of the Act, and the expiration of the 3.75 percent increase for services furnished in CY 2021, as provided in the CAA. We estimate the CY 2022 anesthesia CF to be 21.0442 which reflects the same overall PFS adjustments with the addition of anesthesia-specific PE and MP adjustments.

Table 123 shows the payment impact of the policies contained in this proposed rule on PFS services, should CMS finalize this rule as proposed. To the extent that there are year-to-year changes in the volume and mix of services provided by practitioners, the actual impact on total Medicare revenues will be different from those shown in Table 123 (CY 2022 PFS Estimated Impact on Total Allowed Charges by Specialty). The following is an explanation of the information represented in Table 123.
- Column A (Specialty): Identifies the specialty for which data are shown.
- Column B (Allowed Charges): The aggregate estimated PFS allowed charges for the specialty based on CY 2020 utilization and CY 2021 rates. That is, allowed charges are the PFS amounts for covered services and include coinsurance and deductibles (which are the financial responsibility of the beneficiary). These amounts have been summed across all services furnished by physicians, practitioners, and suppliers within a specialty to arrive at the total allowed charges for the specialty.
- Column C (Impact of Work RVU Changes): This column shows the estimated CY 2022 impact on total allowed charges of the changes in the work RVUs, including the impact of changes due to potentially misvalued codes.
- Column D (Impact of PE RVU Changes): This column shows the estimated CY 2022 impact on total allowed charges of the changes in the PE RVUs.
- Column E (Impact of MP RVU Changes): This column shows the estimated CY 2022 impact on total allowed charges of the changes in the MP RVUs.
- Column F (Combined Impact): This column shows the estimated CY 2022 combined impact on total allowed charges of all the changes in the previous columns. Column F may not equal the sum of columns C, D, and E due to rounding.
Start Printed Page 39531

Start Printed Page 39532

2. CY 2021 PFS Impact Discussion
a. Changes in RVUs
The most widespread specialty impacts of the RVU changes are generally related to the changes to RVUs for specific services resulting from the misvalued code initiative, including RVUs for new and revised codes. The estimated impacts for some specialties, including portable x-ray, family practice, hand surgery, general practice, and endocrinology, reflect increases relative to other physician specialties. These increases can be attributed largely to the proposed update to clinical labor pricing as the services that these specialties furnish rely primarily on clinical labor for their PE costs. These increases are also due to proposed increases in value for particular services in response to the recommendations from the American Medical Association (AMA)'s Relative Value Scale Update Committee (RUC), and CMS review and increased payments resulting from updates to supply and equipment pricing.
The estimated impacts for several specialties, including interventional radiology, vascular surgery, radiation oncology, and oral/maxillofacial surgery reflect decreases in payments relative to payment to other physician specialties which are largely the result of the redistributive effects of the proposed clinical labor pricing update. The services furnished by these specialties involve PE costs that rely primarily on supply or equipment items and therefore are affected negatively by the proposed updates to clinical labor pricing. Since PE is budget neutralized within itself, increased pricing for clinical labor holds a corresponding relative decrease for other components of PE such as supplies and equipment. These decreases are also due to the proposed revaluation of individual procedures based on reviews by the AMA RUC and CMS, as well as decreases resulting from the continued phase-in implementation of the previously finalized updates to supply and equipment pricing. The estimated impacts also reflect decreases due to continued implementation of previously finalized code-level reductions that are being phased in over several years. For independent laboratories, it is important to note that these entities receive approximately 83 percent of their Medicare revenues from services that are paid under the CLFS. As a result, the estimated 2 percent decrease for CY 2021 is only applicable to approximately 17 percent of the Medicare payment to these entities.
We often receive comments regarding the changes in RVUs displayed on the specialty impact table (Table 123), including comments received in response to the proposed valuations. We remind stakeholders that although the estimated impacts are displayed at the specialty level, typically the changes are driven by the valuation of a relatively small number of new and/or potentially misvalued codes. The percentage changes in Table 123 are based upon aggregate estimated PFS allowed charges summed across all services furnished by physicians, practitioners, and suppliers within a specialty to arrive at the total allowed charges for the specialty, and compared to the same summed total from the previous calendar year. Therefore, they are averages, and may not necessarily be representative of what is happening to the particular services furnished by a single practitioner within any given specialty. To illustrate how impacts can vary within specialties, we created a public use file that models the expected percentage change in total RVUs per practitioner. Using CY 2020 utilization data, Total RVUs change between -1 percent and 1 percent for more than 52 percent of practitioners, representing more than 48 percent of the changes in Total RVUs for all practitioners, with variation by specialty. Some specialties, such as chiropractic, clinical social worker, and emergency medicine, exhibit little variation in changes in total RVUs per practitioner. For these specialties, more than 90 percent of these practitioners would experience a change in Total RVUs between -1 percent and 1 percent. Other specialties exhibit more variation in changes in total RVUs per practitioner. For example, for diagnostic testing facilities, 32 percent of IDTFs would experience a 5 percent or more decrease in Total RVUs, but these suppliers represent only 28 percent of Total RVUs for this specialty. Meanwhile, one percent of IDTFs would experience 10 percent or more increases in Total RVUs and these suppliers account for 28 percent of Total RVUs for this specialty.
b. Impact
Column F of Table 123 displays the estimated CY 2022 impact on total allowed charges, by specialty, of all the RVU changes. A table showing the estimated impact of all of the changes on total payments for selected high volume procedures is available under "downloads" on the CY 2022 PFS proposed rule website at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/. We selected these procedures for sake of illustration from among the procedures most commonly furnished by a broad spectrum of specialties. The change in both facility rates and the nonfacility rates are shown. For an explanation of facility and nonfacility PE, we refer readers to Addendum A on the CMS website at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/.
D. Effect of Proposed Changes Related to Telehealth Services
Before the PHE for COVID-19, approximately 15,000 fee-for-service Medicare beneficiaries received a Medicare telemedicine service each Start Printed Page 39533 week. According to a report prepared by the Assistant Secretary for Planning and Evaluation (ASPE),[251] in the last week of April 2020, nearly 1.7 million beneficiaries received telehealth services. By April 2020, nearly half of all Medicare primary care visits were telehealth encounters, a level consistent with health care encounters more broadly. There are approximately 270 services currently included on the list of Medicare telehealth services, including more than 160 that were added on a temporary basis during the PHE for COVID-19 (including service categories such as emergency department visits, initial inpatient and nursing facility visits, and discharge day management services) that are covered through the end of the PHE. Preliminary data show that between mid-March and mid-October 2020, over 24.5 million out of 63 million Medicare beneficiaries and enrollees have received a Medicare telemedicine service during the PHE. It is important to note that preliminary data reflect that the largest increases in services furnished via telehealth communications systems, by beneficiary access/volume, were for services that were already on the Medicare telehealth services list before the PHE.
As discussed in section II.D. of this proposed rule, we are proposing to amend the regulatory definition of interactive telecommunications system for purposes of Medicare telehealth services to include audio-only communication technology under certain circumstances for mental health services furnished to established patients in their homes. We anticipate that this policy will increase utilization of Medicare telehealth mental health services relative to utilization that would occur without the change. The estimated cost impact on overall Medicare services is unclear, though these changes would largely maintain current policies and access to the specific mental health services that are available to beneficiaries during the PHE. By requiring that a modifier be appended to the claim to identify that the service was furnished via audio-only communication technology, we will be able to closely monitor utilization and address any potential concerns regarding overutilization through future rulemaking.
Section 123 of the CAA removed the geographic and site of service restrictions for telehealth services furnished for the purpose of diagnosis, evaluation, or treatment of a mental health disorder, and requires that a physician or practitioner furnish an in-person, non-telehealth service to a beneficiary within 6 months prior to the first time the physician or practitioner furnishes a telehealth service to the beneficiary, and thereafter, at intervals as specified by the Secretary. Section 125 of the CAA created a new Medicare provider type—the rural emergency hospital, effective beginning in CY 2023—and added rural emergency hospitals to the list of eligible telehealth originating sites at section 1834(m)(4)(C)(ii) of the Act. As discussed in section II.D. of this proposed rule, we are proposing to require that, as a condition of payment for a telehealth service described in section 1834(m)(7) of the Act, the billing physician or practitioner must have furnished an in-person, non-telehealth service to the beneficiary within the 6-month period before the date of service of a telehealth service as specified in section 1834(m)(7)(B)(i) of the Act.
We are also seeking comment on whether the required in-person, non-telehealth service could also be furnished by another physician or practitioner of the same specialty and same subspecialty within the same group as the physician or practitioner who furnishes the telehealth service. Given that the removal of the geographic and site of service restrictions for telehealth will expand the availability of mental health services, we anticipate that utilization of these mental health services will be comparable to observed utilization for mental health services during the COVID-19 PHE.
With regard to our proposal to retain all services added to the Medicare telehealth services list on a Category 3 basis until the end of CY 2023, we believe our proposals would provide clarity to the stakeholder community but will have a negligible impact on PFS expenditures. For example, services that have already been added to the permanent telehealth services list are furnished via telehealth, on average, less than 0.1 percent of the time they are reported. The statutory payment requirements for Medicare telehealth services under section 1834(m) of the Act, such as the originating site requirements related to geographic location and site of service, have limited increases in utilization outside of the COVID-19 PHE; however, we believe there is value in allowing physicians to furnish services added to the Medicare telehealth services list on a category 3 basis, and for patients to receive broader access to this care through telehealth. Additionally, for services added to the Medicare telehealth list on a Category 3 basis, outside of the circumstances of the PHE for COVID-19, all of the statutory restrictions under section 1834(m) of the Act will also apply to these services; therefore, we do not anticipate any significant increase in utilization.
E. Effect of Changes Related to Services Furnished in Whole or in Part by PTAs and OTAs
As discussed in section II.H., we are proposing revisions to the current de minimis policy for services furnished in whole or in part by PTAs/OTAs that we finalized in CY 2020 PFS final rule (84 FR 62702 through 62708) under which the CQ or CO modifier would apply when the PTA or OTA furnished more than 10 percent of a service or a 15-minute unit of service. Beginning January 1, 2022, CMS will apply a 15 percent reduction to the payment amount for a physical or occupational therapy service when the CQ/CO modifier is applied to the service. Our proposed revision to the policy would allow the PT/OT to bill without the CQ/CO modifier for the final 15-minute unit (in a multi-unit billing scenario) when the PT/OT meets the billing threshold of 8 minutes, which is when the minutes are greater than the midpoint (7.5 minutes) of the 15-minute unit, regardless of any minutes provided by the PTA/OTA for that final unit.
Under our proposal, the PT/OT services would not be inadvertently discounted as the result of any "left-over" minutes provided by the PTA/OTA when the therapist provides enough minutes on his or her own to meet the billing threshold amount. In these scenarios, the PTA's/OTA's minutes are considered immaterial for the purposes of billing. For example, if the PT/OT provided 23 minutes of a 15-minute service and the PTA/OTA provided another 20 minutes of the same service—three units of service can be billed for the 43 total minutes (38 minutes through 52 minutes). Here, one full 15-minute unit of service is billed without the CQ/CO modifier for the PT/OT service with 8 minutes remaining, and one full unit of service is billed with the CQ/CO modifier for the PTA/OTA service with 5 minutes remaining. Under the proposed policy, the third unit is billed without the CQ/CO modifier because the 8 minutes provided by the PT/OT meets the billing threshold amount. However, under our current de minimis policy, the 5 minutes provided by the PTA/OTA is more than 10 percent (it is 38 percent of the total service—PTA/OTA minutes divided by the total of PTA/OTA + PT/OT minutes: 5 divided by 13 = 38 Start Printed Page 39534 percent) meaning the CQ/CO modifier is applied to the third and final unit of service.
Under our current de minimis policy, under which the CQ/CO modifier is applied whenever the PTA/OTA provides more than 10 percent of a service whether or not the PT/OT furnishes enough of the service to bill for it without the portion furnished by the PTA/OTA, stakeholders have expressed concern that the PT/OT has a financial incentive not to have the PTA/OTA provide any additional minutes, regardless of the individual patient's needs, when those minutes of service would lead to a reduced payment for a unit of a service. There may be a cost implication to our proposal as fewer billing scenarios may result in application of the CQ/CO modifiers and consequent payment reduction. However, we believe that basing our proposed policy on a "midpoint rule" in which the PT/OT provides enough minutes on their own (8 or more minutes) to bill for the final unit of a billing scenario could eliminate the PT's/OT's financial incentive to not provide appropriate therapy to an individual patient when it is furnished by the PTA/OTA. On the other hand, if we were to continue with our de minimis standard to apply to all billing scenarios for PTA/OTA services that exceed the 10 percent standard, we are uncertain how to gauge the overall costs of this policy because of the possible altered PT/OT behavioral change that is due to the financial incentives built into that policy as discussed above.
F. Other Provisions of the Regulation
1. Rural Health Clinics (RHCs) and Federally Qualified Health Centers (FQHCs)
In section III.A. of this proposed rule, we make multiple proposals related to RHCs and FQHCs. In terms of estimated impacts to the Medicare program, Payment for Attending Physician Services Furnished by RHCs or FQHCs to Hospice Patients as required by section 132 of the CAA, 2021 and Concurrent Billing for CCM and Transitional Care Management TCM Services for RHCs and FQHCs will have negligible impact to Medicare spending.
Section 130 requires that all independent RHCs are now subject to the per-visit limit (which is also referred to as "cap") and phases in an increase in the statutory payment cap over an 8-year period. The cap in CY 2021, for services furnished after March 31, is set at $100 per visit, then at $126 per visit in 2022; at $139 per visit in 2023; at $152 per visit in 2024; at $152 per visit in 2025; at $165 per visit in 2026; at $178 per visit in 2027; and at $190 per visit in 2028. Beyond 2028 the limit is updated by the applicable Medicare Economic Index (MEI).
This provision also controls the annual rate of growth in payments to certain provider-based RHCs whose payments are currently higher than the payment limit. Each year, but for services provided after March 31 in 2021, the payment limit shall be set at the greater of: (1) The RHC per visit amount from the prior year, increased by the percentage increase in the applicable MEI; and (2) the cap limits applicable to each year as described above. In order to be eligible for this "grandfathering" policy, the RHC must have been based in a hospital with fewer than 50 beds and enrolled in Medicare as of December 31, 2019.
Section 2 of H.R. 1868, enacted on April 14, 2021, made technical corrections to section 130. First, for an RHC that is hospital-based and whose parent hospital has fewer than 50 beds, the date by which the RHC must be Medicare-certified, in order to be grandfathered, is changed from December 31, 2019 to December 31, 2020. Next, a clinic that is owned by a hospital with fewer than 50 beds and that submitted certain applications (received by Medicare) for certification as a Medicare RHC prior to the end of 2020 is to be grandfathered, and its clinic-specific cap is to be set based on its 2021 cost per visit. Lastly, a grandfathered RHC must continue to be owned by a hospital with fewer than 50 beds; if the parent hospital exceeds 50 beds, the RHC will lose its grandfathered status.
Table 124 are the FY estimates (in millions) for the impact of section 130, which improves payments to RHCs. These providers are currently paid an all-inclusive rate (AIR) for all medically necessary medical and mental health services, and qualified preventive health services furnished on the same day (with some exceptions). The AIR is subject to a payment limit, except for certain provider-based RHCs that have an exception to the payment limit. The RHC payment limit per visit for calendar year (CY) 2021 is $87.52, which is 1.4 percent higher than the CY 2020 payment limit of $86.31.

In section III.B. of this proposed rule, we discuss that we are proposing to revise the regulatory requirement that an RHC or FQHC mental health visit must be a face-to-face (that is, in person) encounter between an RHC or FQHC patient and an RHC or FQHC practitioner to also include encounters furnished through interactive, real-time telecommunications technology, but only when furnishing services for the purposes of diagnosis, evaluation, or treatment of a mental health disorder.
According to our analysis of Medicare Part B claims data for services furnished via Medicare telehealth under the PFS during the PHE, use of telehealth for many professional services spiked in utilization around April 2020 and diminished over time, but not to pre-pandemic levels. In contrast, Medicare claims data suggests that for mental health services both permanently and temporarily added to the Medicare Telehealth list, subsequent to April 2020, the trend is toward maintaining a steady state of usage over time. Given the expanded availability of mental health services at RHCs and FQHCs, we do anticipate that this policy will increase spending, however, we are not certain of the magnitude of this increase, since it is not clear at this time how or whether trends related to utilization of communication technology during the PHE will continue after such a time that the PHE were to end. While the estimated cost impact of this proposal is unclear, the proposed requirement that a modifier be appended to the claim to identify that the service was furnished via audio-only communication technology would allow us to closely monitor utilization and address any potential concerns Start Printed Page 39535 regarding overutilization through future rulemaking.
2. Requiring Certain Manufacturers To Report Drug Pricing Information for Part B (§§ 414.802 and 414.806)
This provision implements new statutory requirements under sections 1847A and 1927 of the Act, as amended by section 401 of the CAA (for the purposes of this section of this proposed rule, hereinafter is referred to as "section 401"). These new requirements will improve the accuracy of reported payment limits and limit the use of WAC-based pricing.
As described in section III.D.1. of this proposed rule, in considering whether to exclude repackagers from the reporting requirements at section 1847A(f)(2) of the Act, we conducted two analyses to estimate: (1) The proportion of repackaged products in our existing ASP data; (2) the number of new ASP submissions we can expect as a result of the new reporting requirements under section 401; and (3) the proportion of those (new) submissions that involve repackaged products.
Additionally, while we believe it will impact reporting volume and payment limits under section 1847A of the Act for many billing and payment codes, we are unable to estimate the magnitude of these effects for the following reasons. We estimate: (1) 361 non-reporting manufacturers (of either single source or multiple source drugs) will now be required to report ASP data under section 1847A(f)(2) of the Act; and (2) 6114 products payable under Part B that these non-reporting manufacturers sell. However, we do not know which Healthcare Common Procedure Coding System (HCPCS) code payment limits will be impacted by these 6114 products, nor do we know the sales volume of these 6114 products. Because this information is used to calculate volume-weighted ASP payment limits, we, are unable to quantitatively estimate the economic impacts of this provision (that is, the likely costs or savings) on beneficiaries, the government, and other stakeholders. (We note that the economic impacts on manufacturers, as a result of the information collection requirements of this provision is discussed in section V. of this proposed rule.)
For single source drugs, these changes may result in lower payment limits because, typically, the WAC plus 3 percent is higher than ASP plus 6 percent. This then translates to cost savings for both the government and beneficiaries, who will pay coinsurance on a lesser amount. However, for the reason stated previously, we are unable to predict the magnitude of this effect.
Similarly, payment limits for multiple source drugs could increase or decrease, and we are unable to predict the direction or magnitude of specific or aggregate effects at this time.
We do not anticipate that this provision of this proposed rule would necessitate the revision of existing Medicaid Drug Rebate Agreements.
We welcome comment on (1) the likely costs or savings to beneficiaries, the government, and other stakeholders and (2) other related impacts of this provision.
3. Determination of ASP for Certain Self-Administered Drug Products
a. Anticipated Effects
This provision implements new statutory requirements under section 1847A(g) of the Act, as amended by section 405 of the CAA 2021, (for the purposes of this section of this proposed rule, hereinafter is referred to as "section 405"). As identified by the OIG studies discussed in section III.D.2. of this proposed rule, the CMS payment-limit determination under section 1847A of the Act includes all versions of a product marketed under a single FDA approval, and consistent with section 1847A(b)(5) of the Act, the payment-limit determination does not exclude products based on packaging. Thus, the volume-weighted, average-ASP determination can include self-administered versions that may lead to increased program and beneficiary costs because of distorted ASP-based payment limits. In particular, the OIG studies identified two billing and payment codes that included self-administered NDCs. The OIG study determined that as a result of the inclusion of these NDCs in the calculation of the ASP payment limit, Medicare payment amounts remained inflated in 2017 and 2018, causing the program and its beneficiaries to pay an additional $497 million during this period. Since 2014, current payment methodology has resulted in an additional $173 million in Medicare beneficiary coinsurance for these two NDCs. (See OIG's July 2020 report titled, "Loophole in Drug Payment Rule Continues To Cost Medicare and Beneficiaries Hundreds of Millions of Dollars," available at https://oig.hhs.gov/oei/reports/OEI-BL-20-00100.asp.)
Implementation of the proposed regulatory changes has the potential to result in decreased payment limits for identified billing and payment codes that could, in turn, substantially reduce Medicare and beneficiary expenditures, as described in the OIG study. Since section 1847A(g)(3) of the Act requires CMS to implement the required payment changes beginning on July 1, 2021, these potential savings may be observed within the year.
By adding sections 1847A(g)(1) and (2) of the Act, section 405 also directs the OIG to conduct future studies with same or similar methodologies to those in the July 2020 report and directs CMS to apply the lesser of payment methodology to the applicable billing and payment codes. This has the potential to result in additional savings to the program and beneficiaries if additional products are identified by these periodic OIG studies.
b. Expected Benefits
Codifying the provisions set forth by section 405 would permit to CMS to apply the lesser of payment methodology at section 1847(g)(2) of the Act to billing and payment codes identified by future OIG studies (described in section III.D.2. of this proposed rule). This provision addresses distorted payment limits for these products and may result in payment amounts that are better aligned with versions of these products that are payable under Part B (for example, versions that are usually not self-administered). Although we are unable to quantify the total magnitude of the potential savings, these changes have the potential to substantially reduce program expenditures and beneficiary coinsurance.
4. Appropriate Use Criteria
Section 1834(q)(2) of the Act, as added by section 218(b) of the Protecting Access to Medicare Act (PAMA), established a program to promote the use of appropriate use criteria (AUC) for applicable imaging services furnished in an applicable setting.
In the CY 2019 PFS final rule (83 FR 59452), we performed a comprehensive regulatory impact analysis for this program. In this proposed rule, we are proposing to begin the payment penalty phase of the program on the later of January 1, 2023 or the January 1 of the year after the year in which the PHE for COVID-19 ends. Because, under our proposals, the payment penalty phase will be further delayed, we are updating the estimates for incremental changes from the regulatory impact analysis from the CY 2019 PFS final rule. Since we are not proposing new policy requirements nor do we have sufficient reason to change any of the assumptions made in the RIA finalized in the CY 2019 PFS (83 FR 60034 through 60044) Start Printed Page 39536 we are only updating the analysis to reflect 2019 Medicare claims data (updated from 2014). We identify four incremental changes from the CY 2019 PFS final rule estimates due to updated claims data: (1) Impact of required AUC consultations by ordering professionals; (2) impact to Medicare beneficiaries; (3) process efficiencies to potentially offset the estimated burden on Medicare beneficiaries; and (4) impact on transmitting orders for advanced diagnostic imaging services. Each of these incremental changes results in a lower estimate.
a. Impact of Required AUC Consultations by Ordering Professionals
As discussed in detail in the CY 2019 PFS final rule (83 FR 60035 through 60037), the annual impact estimate of consultations by ordering professionals was $70,001,700. In our estimates, we calculated the burden for auxiliary personnel to consult AUC under the direction of an ordering professional and the burden for ordering professionals to perform the consultation directly. We estimated that 90 percent of consultations would be performed by a medical assistant (occupation code 31-9092) and 10 percent of consultations would be performed by a general practitioner (occupation code 29-1062). We estimated that 43,181,818 2-minute consultations occur annually.
Using 2019 Medicare claims data as our basis for the analysis, we propose to change the methodology used to determine the volume of consultations and propose to use more granular data that will reduce potential double-counting of advanced diagnostic imaging services. For example, an imaging service furnished in an outpatient hospital department could have two claims associated with that service. There could be a claim from the facility and a claim from the physician that interprets that imaging service. In the CY 2019 RIA (83 FR 60034 through 60044) we were concerned that the estimate of 43,181,818 consultations may be an overestimate because it took into account total claims. For this CY 2022 RIA, we propose to change the method of counting the total number of advanced diagnostic imaging services that would be furnished under the AUC program which will correspond to the total number of consultations.
Using the Integrated Data Repository we identified Medicare claims using the following parameters: (1) 2019 Date of service; (2) claim lines containing one of the procedure codes identified in CR10481 and CR11268 at https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2018Downloads/R2040OTN.pdf and https://www.cms.gov/files/document/r2404otn.pdf, respectively; and (3) claims types of outpatient or practitioner. Claims were then separated based on the setting in which the imaging service was furnished and further by claim type. Using only services billed on the professional claim type, the total number of claim lines containing one of the identified procedure codes was included to total 30,359,901 advanced diagnostic imaging services estimated to be subject to the AUC program. By using this combination, we believe we can reduce the risk of double-counting services to obtain a more accurate estimate of total number of diagnostic imaging services subject to the AUC program. Therefore, this analysis will use the estimate of 30,359,901 AUC consultations.
Using the May 2020 BLS mean hourly wages, we update our estimates for a medical assistant (occupation code 31-9092) with mean hourly wage of $17.75 and 100 percent fringe benefits for 90 percent of consultations (910,797 hours) to be $30,511,701 (910,797 hours × $33.50/hour). The occupation for general practitioner is no longer listed on the BLS so, instead, we update our estimate using the occupation code for general internal medicine physician (29-1216) with mean hourly wage of $101.42 and 100 percent fringe benefits for 10 percent of consultations (101,200 hours) to be $20,527,408 (101,200 hours × $202.84/hour). The updated total annual estimated impact of consultations is $51,039,109, for an incremental change (reduction) of $18,962,591.
b. Impact to Medicare Beneficiaries
In the CY 2019 PFS final rule, we estimated that the additional 2-minute consultation would impact the Medicare beneficiary when their advanced diagnostic imaging service is ordered by the ordering professional by introducing additional time to their office visit. For this update, we used the updated number of consultations calculated above from claims data, as well as the May 2020 BLS mean hourly wage. To estimate this annual cost, we multiplied the annual burden of 1,011,997 hours by the BLS occupation code that represents all occupations in the BLS (00-0000) as mean hourly wage plus 100 percent fringe ($54.14/hr) for a total estimate of $54,789,518 per year for an incremental change (reduction) of $13,211, 482. We also estimated that, over time, process efficiencies may be implemented. We assumed that 50 percent of practices implemented an improvement process that streamlined AUC consultation so Medicare beneficiaries spent the same amount of time in the physician's office regardless of whether an advanced diagnostic imaging service was ordered. The updated estimate that such an improvement process could offset the estimated burden on Medicare beneficiaries by $27,394,759 annually for an incremental change (reduction) of $6,605,741.
c. Impact on Transmitting Orders for Advanced Diagnostic Imaging Services
In the CY 2019 PFS final rule, we estimated that including AUC consultation information on the order for an advanced diagnostic imaging service to the furnishing professional or facility is estimated as the additional 5 minutes spent by a medical secretary (occupation code 43-6013). To update this estimate, we use the May 2020 mean hourly wage of $18.75 plus 100 percent fringe benefits to transmit the order for the advanced diagnostic imaging service. In aggregate, we assumed in the CY 2019 PFS final rule that 40,000,000 advanced diagnostic imaging services are ordered annually. We propose to update that number to match the total number of AUC consultations proposed earlier in this RIA to 30,359,901, so the updated total annual burden to communicate additional information in the order is estimated as $94,495,192 ($18.75/hr × 2 × 0.083 hr × 30,359,901 orders) for an incremental change (reduction) of $20,044,808.
d. Impact on Furnishing Professionals and Facilities
As described in the CY 2019 PFS final rule, we identified an estimated 174,064 furnishing professionals (comprising radiologists, ASCs, IDTFs and hospitals) and assumed that every identified furnishing professional will choose to update their processes for the purposes of the AUC program in the same way by purchasing an automated solution to report AUC consultation information which was estimated to cost $10,000 for each furnishing professional. We update this cost to account for inflation and therefore the updated estimated cost is $10,636.07 ($10,000 adjusted for inflation to 2021 dollars) for a total estimated one-time update cost of $1,851,356,888.48 (174,064 × $10,636.07).
e. Appropriate Use Criteria for Advanced Diagnostic Imaging Services
As described in the CY 2019 PFS final rule, we assumed that there may be some savings to the Medicare program due to the AUC program requirements Start Printed Page 39537 and potential decreases in inappropriate utilization of advanced diagnostic imaging services. This assumption was based on literature describing prior experiences with clinical decision support in a pilot project conducted in Minnesota, a retrospective cohort study on evidence-based clinical decision support for lumbar MRI, brain MR and sinus CT and local implementation of clinical decision support, and we estimated that savings may account for $700,000,000 savings per year.
f. Summary of Delay-Attributable Changes and Discounted Rates
Table 125 summarizes the substantive changes from the CY 2019 PFS final rule to the CY 2022 PFS proposed rule impact estimates. The effect of a 3-year delay is approximated by applying 3 years' worth of discounting at 7 percent or 3 percent discount rates (Circular A-4, https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/assets/regulatory_matters_pdf/a-4.pdf).

5. Proposal To Remove Select National Coverage Determinations (NCDs)
We are proposing to remove two older NCDs that no longer contain clinically pertinent and current information or that involve items or services that are used infrequently by beneficiaries. Generally, proactively removing obsolete or unnecessary NCDs removes barriers to innovation and reduces burden for stakeholders and CMS. The two NCDs fall into two impact categories. First, eliminating an NCD for items and services that were previously covered means that the item or service will no longer be automatically covered by Medicare. Instead, the coverage determinations for those items and services will be made by Medicare Administrative Contractors (MACs). Second, if the previous national coverage determination barred coverage for an item or service under title XVIII, MACs would now be able to cover the item or service if the MAC determines that such action is appropriate under the statute. We believe that allowing local contractor flexibility in these cases better serves the needs of the Medicare program and its beneficiaries since we believe the future utilization for items and services within these policies will be limited, each affecting less than one percent of the Medicare FFS population.
For the one NCD where we are proposing to go from limited coverage to MAC discretion, claims data from 2019 shows that less than one percent of the Medicare population is affected. Specifically, we provide coverage with limitations for enteral nutrition and parenteral nutrition therapy under NCD 180.2, where in 2019 CMS paid 1,643,739 Medicare FFS claims for 83,551 unique beneficiaries totaling CMS payments of $356,228,606. While we have claims data available for 2020, the data shows a decrease in claims, unique beneficiaries and total amount paid by CMS. We believe this may be due in part to the COVID-19 pandemic; however, we do not have any information to be able to say that conclusively. The change could be due to other factors not examined here. We estimate there will be de minimis change to 2022 payments, compared to 2019 or 2020 because, as discussed in section III.F. of the proposed rule, local contractors have proposed LCDs that, if finalized, would provide parenteral and enteral nutrition coverage for certain Medicare beneficiaries. Therefore, we believe that removing this NCD would not result in significant changes to payments.
For the one non-covered NCD proposed to be eliminated, Positron Emission Tomography (PET) Scans under NCD 220.6, we would not expect to find historical claims data for the non-oncologic uses of PET at issue. We broadly noncover non-oncologic indications of PET, in other words, we required that every non-oncologic indication for PET must have its own NCD in order to receive coverage. Because this NCD provides for noncoverage on non-oncologic indications, we do not have accurate claims data to estimate total impact. However, based on the service, we expect future claims to affect less than one percent of Medicare FFS Start Printed Page 39538 beneficiaries. As discussed in section III.F. of this proposed rule, the NCD allows coverage for diagnostic PET imaging for oncologic uses not already determined by an NCD, to be made at the discretion of local MACs. We believe that extending local contractor discretion for non-oncologic indications of PET provides an immediate avenue to potential coverage in appropriate candidates and provides a framework that better serves the needs of the Medicare program and its beneficiaries. For clarity, we are not proposing to change any other subsections of 220.6. Thus, the NCDs listed at 220.6.1 through 220.6.20 would not be changed by this proposal.
6. Pulmonary Rehabilitation, Cardiac Rehabilitation and Intensive Cardiac Rehabilitation
As discussed in section III.H., Pulmonary Rehabilitation (PR), Cardiac Rehabilitation (CR) and Intensive Cardiac Rehabilitation (ICR), of this proposed rule, we are proposing largely conforming changes throughout §§ 410.47 (PR) and 410.49 (CR/ICR). These changes are intended to ensure consistency and accuracy in terminology, definitions and requirements where appropriate across PR and CR/ICR conditions of coverage. Specific to PR, we are proposing to remove the requirement for direct physician-patient contact related to the periodic review of the patient's treatment plan because such interaction within the PR program is not necessary for all patients and can be specified, as needed, in individualized treatment plans (ITPs). We are also proposing to add coverage of PR for beneficiaries who were hospitalized with a COVID-19 diagnosis and experience persistent symptoms, including respiratory dysfunction, for at least 4 weeks after hospital discharge.
In assessing the impact of these proposals, we note that the proposed expansion of PR coverage may increase utilization. Based on the low utilization rate discussed below, we do not believe the other proposed revisions will significantly impact utilization and the Medicare program.
To estimate the potential increase from the proposed expansion of coverage for PR, we searched the literature for articles that evaluated the utilization rate of PR for the currently eligible diagnosis of chronic obstructive pulmonary disease (COPD) in order to determine the historical utilization trends of this service.
Nishi et al. (2016) investigated the number of Medicare beneficiaries with COPD who received PR from January 1, 2003 to December 31, 2012. Their results included both individuals who had experienced hospitalizations for COPD and those who were outpatients only. The number of unique patients with COPD who initially participated in PR during the study period was 2.6 percent in 2003 (before conditions of coverage at § 410.47 were established) and 2.88 percent in 2012 (after conditions of coverage at § 410.47 were established).[252] In 2019, Spitzer, et al. published an article based on Medicare claims data from 2012, finding that 2.7 percent of eligible Medicare beneficiaries received PR within 12 months of hospitalization with COPD.[253] Using claims data from fee-for-service Medicare beneficiaries hospitalized for COPD in 2014, Lindenauer et al. (2020) reported that only 3 percent initiated PR within 1 year of their hospital discharge.[254] Taken together, this data informs us that utilization of PR in the Medicare population is very low, and that the majority of patients who avail themselves of this service do so, post hospitalization.
There are limitations to applying this data to identify the utilization rate of PR to the conditions of coverage specified at § 410.47. Most notably, some of these studies included patients whose services were billed with non-PR respiratory therapy codes (G0237, G0238 and G0239), instead of only patients whose services were billed with the PR code (G0424). But the authors also limited patient inclusion to those with a principal or secondary COPD diagnosis, so we believe this suggests that 3 percent is an upper bound for the utilization of PR currently in Medicare beneficiaries. Given that participation in PR has remained steady for many years, we do not expect this pattern to change. As such, for the purposes of this analysis, we assume that 3 percent of eligible beneficiaries under the proposed expansion of coverage (hospitalized with COVID-19 and experiencing persistent symptoms, including respiratory dysfunction, for at least 4 weeks after discharge) will participate in PR.
To identify the eligible beneficiaries under our proposal, we first identify the number of beneficiaries hospitalized with COVID-19 using the Preliminary Medicare COVID-19 Data Snapshot.[255] At the time of writing, the Snapshot included data from January 1, 2020 to March 20, 2021, and identified 1,141,592 total COVID-19 hospitalizations for Medicare beneficiaries. The Snapshot indicates that 18 percent of these patients expired so we reduce this number by 18 percent to 936,105 beneficiaries. A paper published by the Tony Blair Institute for Global Change[256] states that the Covid Symptom Study led by King's College London indicated that about 10 percent of survey participants reported symptoms (including shortness of breath and other symptoms like fatigue, headache and loss of smell) beyond a four-week recovery period. Using this information we estimate that the patient population we are proposing to expand PR coverage to, those who were hospitalized with a COVID-19 diagnosis and experienced persistent symptoms, including respiratory dysfunction, for at least 4 weeks after hospital discharge, to be 93,611 beneficiaries (936,105 × 0.10). Based on our assumption of utilization above, 3 percent, for the newly proposed covered patient population, we estimate 2,808 beneficiaries will receive PR (93,611 × 0.03).
Medicare covers PR for a maximum of 72 sessions. Using 2018 and 2019 Medicare claims data from the Chronic Conditions Data Warehouse (CCW), beneficiaries on average completed 14 sessions of PR. If we assume patients eligible based on our proposed expansion of coverage would participate, on average, in the same number of sessions, we estimate the proposed expansion of coverage will increase PR utilization by 39,312 sessions annually (2,808 beneficiaries × 14 average sessions completed per beneficiary).
Claims for PR are submitted using CPT code G0424. Our analysis of Medicare claims data indicates that 97.54 percent of PR sessions are billed under the Hospital OPPS at $55.66 (national average price) for an estimated total of $2,134,279 (39,312 PR sessions × 0.9754 × $55.66). The remaining 2.46 Start Printed Page 39539 percent of PR sessions are billed under the PFS, with 2.12 percent of PR sessions furnished in a physician's office which has a national average price of $30.36 and 0.34 percent billed by a physician when PR was furnished in a hospital outpatient department which has a national average price of $13.96. Taken together, the estimated total for this remaining 2.46 percent of PR sessions is $27,168 ((39,312 PR sessions × 0.0212 × $30.36) + (39,312 PR sessions × 0.0034 × $13.96)). We estimate the total added cost to the Medicare program of this proposed expansion of coverage to be $2,161,447 ($2,134,279 + $27,168) annually during and immediately following the public health emergency (PHE) for COVID-19. As hospitalizations and COVID-19 cases decline, we expect the annual impact to decrease because eligible patient populations will likely decrease, however we are unable to estimate the longer term impact of our proposals due to the unpredictable nature of the PHE and the lack of long term data on COVID-19.
7. Medical Nutrition Therapy
As discussed in section III.I., Medical Nutrition Therapy (MNT), of this proposed rule, we are proposing to remove the restriction that patients only be referred to MNT by the treating physician and update the glomerular filtration rate (GFR) eligibility for patients with chronic kidney disease. We do not anticipate any significant increase in utilization of MNT services resulting from our proposed revisions. Despite various policy changes that could have improved use, such as increasing payment via adding work RVUs to MNT codes in 2006, approving MNT for telehealth coverage in 2005 and including registered dieticians (RDs) and nutrition professionals as telehealth distant site providers, and waiving out-of-pocket costs to beneficiaries, MNT participation remains under 2 percent of eligible beneficiaries. Based on an analysis of Medicare claims data from 2018, 2019, 2020, we identify the utilization rate of MNT services among eligible beneficiaries to be between 1.5 and 1.8 percent.
Although MNT is covered by many state Medicaid programs and private insurers, use is low in the US.[257 258 259] The Academy of Nutrition and Dietetics recognizes that research specific to the underuse of MNT services is scant.[260] Anecdotal reports and related research on diabetes self-management training point to a multitude of reasons why utilization of the MNT services benefit have remained low. These potential barriers include lack of awareness of MNT by patients and clinicians, inconsistent coverage for MNT services by non-Medicare payers, patient travel and time issues to receive the services and lack of availability of services from RDs who may perceive the process of Medicare enrollment/insurance credentialing and billing as being burdensome and complex.[261] Of about 100,000 RDs in the US, only 1,589 submitted Medicare fee-for-service MNT claims in 2017. One study revealed that less than half of RDs providing MNT services in an ambulatory care setting indicated they were not Medicare providers due to reasons such as perceived low reimbursement rates, not providing MNT to Medicare eligible patients, not knowing how to become a Medicare provider, and providing MNT to Medicare patients for diagnoses not covered by Medicare.[262]
Our proposed revisions may increase beneficiary access to the MNT benefit and reduce primary care physician burden since we are proposing that referrals can come from other physicians and not only from the physician treating the patient for their diabetes or kidney disease; although, as discussed above, we do not expect the changes to make a significant impact on the Medicare program. We do not anticipate increased administrative burden as documentation in the medical record of any referred service is already a part of discharge planning in the hospital setting. The proposed changes to the GFR requirements are to conform our regulation to updated clinical standards and also do not pose a significant change.
8. Medicare Shared Savings Program
a. Modifications to the Shared Savings Program Quality Reporting Requirements Under the APP and the Quality Performance Standard
In section III.J.1.d. of this proposed rule, we are proposing to freeze the quality performance standard at the 30th percentile MIPS Quality Performance Category Score for performance year 2023, increasing to the 40th percentile MIPS Quality performance category score in performance year 2024. Without this proposed change, the quality standard would have increased to the 40th percentile in 2023. The quality performance standard is the minimum performance level ACOs must achieve in order to share in any savings earned, avoid maximum shared losses under certain payment tracks, and avoid quality-related compliance actions.
Our analysis of quality performance data reported by ACOs for performance years starting during 2019 indicates that about 20 percent of ACOs would have failed a quality performance standard defined as the 40th percentile across all MIPS Quality performance category scores as would apply for PY 2023 without the proposed 1-year delay. There is significant uncertainty whether PY 2023 would play out similarly to the baseline data. The fraction of ACOs ultimately failing to meet a higher standard in PY 2023 could change significantly if the universe of MIPS Quality performance category scores improves relative to ACOs' quality performance scores, or alternatively if ACOs, particularly ACOs at risk of failing, respond to the increased quality performance standard by boosting their performance. Utilizing a Monte Carlo approach, assuming that the simulated poor performing ACOs have a 50 percent chance of improving their quality performance beyond the 40th percentile, if CMS kept the quality performance standard at the 40th percentile, then the cost of reducing to the 30th percentile in 2023 would be $190 million (range $10 million to $370 million). There is a wide range because slight changes in quality scoring at the low end of the distribution could render the 40th percentile more or less of an effective point of discrimination among ACOs earning shared savings.
In addition, in section III.J.1.c. of this proposed rule, we are proposing an update to the quality reporting requirements under the APM Start Printed Page 39540 Performance Pathway or APP for performance years 2022 and 2023.
For performance year 2022: An ACO would be required to report on either
(a) The ten CMS Web Interface measures and administer a CAHPS for MIPS survey and CMS would calculate the two claims based measures included under the APP, or
(b) The three eCQM/MIPS CQM measures and administer a CAHPS for MIPS survey and CMS would calculate the two claims based measures included under the APP. If an ACO selects this option, meets the data completeness at § 414.1340 and the case minimum requirement at § 414.1380 for all three eCQM/MIPS CQM measures, and achieves a quality performance score equivalent to or higher than the 30th percentile on at least one measure in the APP measure set, the ACO would meet the quality performance standard used to determine eligibility for shared savings and to avoid maximum shared losses, if applicable, for that performance year. If an ACO chooses this option, its performance on all six measures in the APP measure set would be used for purposes of MIPS scoring under the APP. If an ACO decides to report both the ten CMS Web Interface measures and the three eCQM/MIPS CQM measures, it will receive the higher of the two quality scores for purposes of the MIPS Quality performance category.
If an ACO does not report any of the ten CMS Web Interface measures or any of the three eCQM/MIPS CQM measures and does not administer a CAHPS for MIPS survey under the APP, the ACO will not meet the quality performance standard.
For performance year 2023: An ACO would be required to report on either:
(a) The ten CMS Web Interface measures and at least one eCQM/MIPS CQM measure and administer a CAHPS for MIPS survey and CMS would calculate the two claims-based measures included under the APP or
(b) The three eCQM/MIPS CQM measures and administer a CAHPS for MIPS survey and CMS would calculate the two claims based measures included under the APP. If an ACO selects this option, meets the data completeness requirement at § 414.1340 and the case minimum requirement at § 414.1380 for all three eCQM/MIPS CQM measures, and achieves a quality performance score equivalent to or higher than the 30th percentile on one measure in the APP measure set, the ACO would meet the quality performance standard used to determine eligibility for shared savings and to avoid maximum shared losses, if applicable, for that performance year. If an ACO chooses this option, its performance on all six measures in the APP measure set would be used for purposes of MIPS scoring under the APP. If an ACO decides to report both the ten CMS Web Interface measures and the three eCQM/MIPS CQM measures, it will receive the higher of the two quality scores for purposes of the MIPS Quality performance category.
If an ACO does not report at least one eCQM/MIPS CQM measure the ACO would not meet the quality performance standard.
Absent the related proposal analyzed above to reduce the performance standard to the 30th percentile MIPS Quality performance category score, the proposed changes to the quality reporting requirements, including the accommodation to continue the availability of the CMS Web Interface as a reporting mechanism under the APP would have likely provided an easier path for a meaningful subset of ACOs that would have otherwise faced difficulty meeting the quality threshold previously established in rulemaking for PY 2023. However, we estimate that nearly all such ACOs would already meet the lower 30th percentile performance standard in PY 2023 without the additional reporting flexibility. Of the relatively few, remaining ACOs, that we estimate would fail to meet the proposed lower 30th percentile performance standard, we estimate that about half (on average) would meet the quality performance standard as a result of the proposed quality reporting flexibility, and thereby further increase shared savings payments to ACOs by about $20 million in PY 2023.
b. Modifications to Other Shared Savings Program Requirements
We do not anticipate a material aggregate impact for the other changes we are proposing related to the Shared Savings Program, specifically: revisions to the definition of primary care services used in the Shared Savings Program's beneficiary assignment methodology (section III.J.2. of this proposed rule); revisions to the repayment mechanism arrangement policy, including changes to the calculation and recalculation of repayment mechanism amounts (section III.J.3. of this proposed rule); revision of the requirements concerning disclosure of prior participation in the Shared Savings Program by the ACO, ACO participants, and ACO providers/suppliers, and revisions to Shared Savings Program requirements to reduce the frequency and circumstances under which ACOs submit sample ACO participant agreements and executed ACO participant agreements to CMS (section III.J.4. of this proposed rule); and revisions to the beneficiary notification requirement as it applies to ACOs under prospective assignment and ACOs under preliminary prospective assignment with retrospective reconciliation (section III.J.5. of this proposed rule).
However, as we note in section III.J.3. of this proposed rule, lower required repayment mechanism amounts could reduce costs for ACOs in fees charged by financial institutions for letters of credit and by insurance companies for surety bonds. We estimate that such relief, in total for all participating ACOs, could be worth $2 to $4 million annually under the proposed approach (assuming a reduction of approximately $196 million in repayment mechanism amounts, in aggregate) and $3 to $6 million annually under the second, alternative option (assuming a reduction of approximately $322 million in repayment mechanism amounts, in aggregate).
We also note that the proposed revisions to the definition of primary care services used in the assignment methodology may have differing effects on a subset of participating ACOs, for example by changing the competing ACO to which a beneficiary ultimately is assigned, for a small subset of beneficiaries. We do not anticipate such ACO-level changes would result in a net impact on program spending overall.
9. Medicare Ground Ambulance Data Collection System
In section III.K. of this proposed rule, we propose a series of changes to the Medicare Ground Ambulance Data Collection System including the proposed change to the data collection period and data reporting period for selected ground ambulance organizations in year 3, proposed revisions to the timeline for when the payment reduction for failure to report will begin and when the data will be publicly available, and proposed revisions to the Medicare Ground Ambulance Data Collection Instrument.
While we believe that these changes and clarifications will be well received by the ground ambulance stakeholders, we do not believe that these changes would have any substantive impact on the cost or time associated with completing the Medicare Ground Ambulance Data Collection Instrument. We note that the overall length of the Medicare Ground Ambulance Data Collection Instrument is the same as previously finalized (84 FR 62888) with these changes. Additionally, some of the Start Printed Page 39541 instructions which we propose to add are intended to improve clarity and may therefore reduce the time the ground ambulance organizations spend addressing the questions.
10. Medicare Diabetes Prevention Program Expanded
a. Effects of Proposals Relating to the Medicare Diabetes Prevention Program Expanded Model
(1) Effects on Beneficiaries
We propose to modify certain Medicare Diabetes Prevention Program (MDPP) expanded model policies to: (1) Allow CMS to remove the ongoing maintenance phase (months 13-24) of the MDPP set of services for those beneficiaries who started their first core session on or after January 1, 2022; (2) update the performance payments for the MDPP set of services in the core and core maintenance performance periods; and (3) waive the Medicare provider enrollment application fee for all organizations enrolling as MDPP suppliers on a prospective basis. These proposed changes will have a positive impact on beneficiaries' health by increasing the capacity of MDPP eligible organizations to enroll in Medicare as MDPP suppliers and increasing access to the MDPP set of services to beneficiaries. Eligible beneficiaries receive these services as preventive services, which require no copays or cost sharing. These proposed changes address MDPP supplier and beneficiary needs based upon all available monitoring and evaluation data. The proposed changes are also responsive respond to stakeholder comments.
(2) Effects on the Market
Currently, more than 1,000 organizations nationally are eligible to become MDPP suppliers based on their preliminary or full CDC Diabetes Prevention Recognition Program (DPRP) status. However, only 27 percent of eligible organizations are participating in MDPP. We anticipate that the removal of the second year of the MDPP set of services will make MDPP attractive and feasible to more MDPP eligible organizations. Not only does a 12-month MDPP services period align with that of the CDC's National DPP and the DPP model test, our data show that only 10 percent of enrolled MDPP participants continue with the Ongoing Maintenance phase sessions (Year 2), and the majority are reaching their weight loss milestone within the first 6 months of the set of MDPP services. Stakeholders report that the second year of MDPP, or the ongoing maintenance phase, is cost prohibitive due to the costs to retain beneficiaries in year 2 of the expanded model as well as the costs to deliver an additional year of the expanded model that is not supported by the CDC National DPP curriculum. The CDC's National DPP curriculum supports a 1-year program and suppliers have found it difficult to extrapolate the curriculum to a second year. Additionally, MDPP suppliers commented that they have an increasingly difficult time making the business case for MDPP given the costs associated with the ongoing maintenance phase and the low performance payments associated with the second year. Given the low volume of participants continuing in the second year of MDPP, delivering the MDPP ongoing maintenance period creates an undue burden to MDPP suppliers. The cost to offer and deliver the sessions to a small cohort of individuals outweigh the maximum payments available from Medicare.
Stakeholders have consistently commented that CMS should shorten the MDPP expanded model to 1 year, with payment levels at least equivalent to the levels provided in the DPP model test. For example, during the DPP model test, suppliers were paid an average of $462 per beneficiary for the 1-year model test. The second year has made delivering MDPP both financially unattractive and unstainable for many of the current and eligible MDPP suppliers. Suppliers have reported that it is very difficult to engage and retain beneficiaries in a second year, and the reimbursement levels for a second year are inadequate to cover supplier costs. Since a second year of the MDPP deviates from both the DPP model test and the National DPP clinical trial protocol, we propose to shorten the MDPP service period to 1 year and increase the performance payments in the first year. These changes respond to stakeholder feedback and may alleviate some of the difficulty retaining MDPP participants during the core maintenance phase of the program.
(3) Burden Related to Information Collection Requirements—No Impact
(a) Supplier Standards
MDPP suppliers may encounter the Medicare enrollment fee during the following Medicare provider enrollment transactions: Initial enrollment, revalidation (every 5 years for MDPP), or the addition of a new practice location. The provider/supplier enrollment fee for Calendar Year 2021 is $599. Although MDPP suppliers may submit a written request to CMS for a hardship exception to the application fee in accordance with § 424.514, many would not qualify and the hardship application process would simply add more burden on the organization. We have heard from the CDC as well as other stakeholders that the enrollment fee is a potential barrier to eligible MDPP suppliers who would not otherwise enroll in Medicare except for MDPP. Approximately 39 percent of our current suppliers are non-traditional suppliers that serve their local communities and play a critical role in enrolling more diverse, equitable, and inclusive cohorts of Medicare beneficiaries to MDPP. These non-traditional suppliers include, but are not limited to YMCAs, county health departments, community health centers, and non-profit organizations that focus on health education, and otherwise would neither enroll nor be able to enroll as a Medicare supplier at all if it were not for MDPP. They often serve as trusted sources of health information for their communities. However, they also represent a large number of eligible organizations who have not enrolled in Medicare as MDPP suppliers. We anticipate that waiving the enrollment fee along with the other programmatic adjustments are likely to result in more MDPP suppliers, increased beneficiary access to MDPP services, and an ongoing reduction of the incidence of diabetes in eligible Medicare beneficiaries, in both urban and rural communities.
In April 2020, CMS waived all provider enrollment application fees as part of the COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers. As a result, we saw an increase in MDPP supplier enrollment. We believe that granting a permanent waiver of the fee for MDPP suppliers to extend beyond the COVID-19 Emergency Declaration Blanket Waiver, along with the other proposed change to MDPP, may stimulate MDPP supplier enrollment and enhance the MDPP evaluation. We propose waiving the Medicare provider enrollment fee beyond COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers because the enrollment fee creates a potential barrier to MDPP supplier enrollment, beneficiary access to the program, and subsequently, our ability to evaluate MDPP. Specifically, we propose, to waive the enrollment fee as described in section 1866(j)(2)(C)(i) and (ii) of the Act during the MDPP expanded model test phase.
(b) Payment for MDPP Services
Our regulations at § 414.84 specify the payments MDPP suppliers may be eligible to receive, payments for Start Printed Page 39542 furnishing MDPP services, and meeting performance targets related to beneficiary weight loss and/or attendance. MDPP suppliers are paid by CMS by submitting claims for MDPP beneficiaries using claim form CMS-1500 (https://www.cms.gov/Medicare/CMS-Forms/CMS-Forms/Downloads/CMS1500.pdf). As a condition for payment, claims submitted by MDPP suppliers must be for services furnished to eligible beneficiaries in accordance with § 414.84(b) and (c). We have streamlined the proposed performance payments so that they are easier to understand and suppliers receive larger payments for participants reaching attendance and weight loss performance-based milestones. For example, the proposed attendance-based performance payments are based on a standardized per-session rate, paid after the 1st, 4th, and 9th sessions attended during the core sessions interval, and after attending the two (2) sessions during each of the core maintenance intervals. We have increased performance payments for beneficiary achievement of the 5 percent weight loss goal as well as continued attendance during the core maintenance intervals. Although the proposed maximum payment of $661 over a 1-year service period is less than the current maximum payment of $704 under the original 2-year payment structure, we believe eliminating the second year and its associated payments while increasing the first year payments will result in a financially sustainable expanded model.
Increasing the first year MDPP payment amounts should not negatively affect a supplier's performance (for example, participants' weight loss). The increase to the payment amounts are not applied until after the 4th core session and the largest payments to suppliers are still driven by weight loss achievement. Further, in order to maintain CDC Diabetes Prevention Recognition Program (DPRP) recognition status, which is required to be an MDPP supplier, certain levels of performance metrics (for example, weight loss) must be satisfied. There is no evidence that eliminating the second year maintenance sessions, shortening the MDPP services period to 1 year, will have any negative effects on performance.
(4) Effects on the Medicare Program
(a) Estimated 10-Year Impact of MDPP
Table 126 shows estimates (in millions) for the impact on Medicare spending of two proposed changes to the Medicare Diabetes Prevention Program (MDPP) to be implemented in 2022:
- Waiving the Medicare enrollment fee for all new MDPP suppliers; and
- Shortening the MDPP services period to 1 year and shifting some of the Ongoing Maintenance reimbursement amounts to year one.

These estimates by the CMS Office of the Actuary do not consider waiving the Medicare enrollment fee as a direct cost and assume there will be an additional 500 beneficiaries per year participating in MDPP. In the most recent year prior to the Public Health Emergency (PHE), 1,742 Medicare beneficiaries entered MDPP. Increasing the first year payment amounts to suppliers and waiving the Medicare enrollment fee should increase access to MDPP, resulting in more utilization of the MDPP set of services. Starting in 2022, we can assume that 2,000 beneficiaries would have entered the program each year without including the proposed changes. After including these changes, we will now assume 2,500 beneficiaries will enter the program each year. This assumption has a high level of uncertainty and we revisit it in the Sensitivity Analysis section.
(b) Sensitivity Analysis
Since the cost to suppliers for delivering the MDPP set of services is generally unknown, how utilization of the expanded model will be affected by the proposed changes is highly uncertain. Table 127 shows the 10-year impact estimates (in millions) for different levels of additional beneficiary participation as a result of the proposed changes:

Finally, higher projected savings are associated with increases in beneficiary participation, while no additional beneficiaries would result in an estimated cost.
b. Alternatives Considered
No alternatives were considered. The 2-year MDPP service period has depressed interest in MDPP among would-be MDPP suppliers. These proposed actions address stakeholder comments on the barriers to MDPP expanded model success. If we do not take action, we will not be able to scale MDPP as intended, impacting Medicare beneficiary access to this expanded model. Reducing the MDPP from a 24- to a 12-month services period, increasing the year 1 performance payments, and waiving the Medicare provider enrollment application fee not Start Printed Page 39543 only better aligns the expanded model with the evidence that helped certify the DPP model test initially, but it will encourage eligible organizations to enroll as MDPP suppliers.
c. Impact on Beneficiaries
This change will have a positive impact on eligible MDPP beneficiaries, as it better aligns with the CDC's National DPP, giving both the participants and the coaches similar messaging around this program, regardless of payer. MDPP suppliers often offer the MDPP set of services to mixed cohorts, or classes with participants who are not eligible for MDPP, but who are enrolled in a National DPP cohort. Since MDPP generally follows the CDC's National DPP and aligns its expanded model with the CDC's DPRP Standards, it is confusing to participants, coaches, and staff when talking about a 2-year set of services to its eligible Medicare participants when the non-Medicare participants have a 1-year program. Finally, reducing the MDPP service period from 2 years to 1 year allows more cohorts to start and finish MDPP during the expanded model initial period of performance, which ends in March 2023.
d. Estimating Regulatory Familiarization Costs
Given that we tried to align this rule as much as possible with the CDC DPRP Standards, there should be minimal regulatory familiarization costs. This rule impacts only enrolled MDPP suppliers and eligible beneficiaries who have started the MDPP expanded model or are interested in MDPP.
11. Medicare Provider and Supplier Enrollment Changes—Provider Enrollment
As explained in section III.N. of this proposed rule, we propose changes to three of our existing revocation reasons:
- We propose to expand § 424.535(a)(2) to permit revocation based on the OIG exclusion of an individual serving in an administrative or management services role for the provider/supplier, such as a billing specialist, accountant, or human resources specialist.
- We propose to expand § 424.535(a)(13) to permit revocation of a physician's or other eligible professional's enrollment if he or she surrenders his/her Drug Enforcement Administration (DEA) certificate of registration in response to an order to show cause.
- We propose to revise the factors in § 424.535(a)(8)(ii) (which permits revocation based on a pattern or practice of submitting non-compliant claims) to better enable CMS to target shorter periods of non-compliant billing.
We believe that all three of these changes would result in an increase in the number of revocations that CMS imposes. However, we believe this number will be rather small. We currently impose only a limited number of revocations under §§ 424.535(a)(2), (a)(13), and (a)(8). Accordingly, since our expansion of these three revocation reasons would be fairly modest, we do not foresee more than a very slight increase in revocations thereunder.
Table 128 outlines the number of revocations we estimate would ensue under our proposed revocation expansions. These numbers only account for additional revocations stemming from our changes:

Internal CMS data indicates that the average provider/supplier that would be affected by these regulatory expansions receives roughly $50,000 in Medicare payments each year. (We used a similar $50,000 annual payment estimate for our provider enrollment provisions in the CY 2020 PFS final rule) (84 FR 62568)). Providers/suppliers revoked under our proposed revocation expansions would thus not receive these payments. Hence, multiplying our $50,000 estimate by the revocation totals in Table 128 results in a projected transfer from these providers/suppliers to the federal government of $750,000 ($50,000 × 15 revocations).
12. Provider/Supplier Medical Review Requirements—Prepayment and Post-payment Reviews
In section III.N.2. of this proposed rule, we are proposing to: (1) Define key terms including "additional documentation," "additional documentation request," "post-payment medical review," and "prepayment medical review;" (2) codify contractors' authority to request additional documentation for prepayment and post-payment review within established timeframes; (3) codify timeframes for response to requests for documentation; and (4) codify result of a failure to comply with prepayment or post-payment documentation request(s) by a provider or supplier, specifically denial of payment. We do not believe these proposals involve any additional impact or burden on providers, suppliers, or states; however, we welcome feedback from stakeholders regarding the potential costs of these proposals.
The proposed regulations would incorporate already established key terms and definitions as well as processing requirements pertaining to prepayment and post-payment medical review into regulation. Although placing this information in regulation could improve provider and supplier understanding of the medical review process and their responsibilities in complying with our review contractor's requests, the proposed regulations represent no change to medical review requirements. As such, we do not anticipate any change in the number of prepayment medical reviews, post-payment medical reviews or the number of additional documentation requests made by contractors.
13. Effect of Proposed Modifications to Medicare Coverage for Opioid Use Disorder (OUD) Treatment Services Furnished by Opioid Treatment Programs (OTPs)
As discussed in section III.O of this proposed rule, we are proposing to allow OTPs to continue to furnish the therapy and counseling portions of the weekly bundles, as well as any additional counseling or therapy that is billed using the add-on code, using audio-only telephone calls rather than via two-way interactive audio-video communication technology in cases Start Printed Page 39544 where audio/video communication is not available to the beneficiary after the conclusion of the PHE for COVID-19, provided all other applicable requirements are met.
We believe the Part B cost impact of this proposal would be minimal, since payment for therapy and counseling is included in the bundled payment regardless of the modality used to deliver it and we do not expect that this proposal would increase the frequency at which medically necessary counseling and therapy services are billed using the counseling and therapy add-on code (HCPCS code G2080). However, we are also proposing to require that when these services are furnished using audio-only technology, practitioners certify that they had the capacity to furnish the services using two-way audio/video communication technology, but instead, used audio-only technology because audio/video communication technology was not available to the beneficiary. We believe this proposed change would facilitate broader access to these services for beneficiaries.
Additionally, as discussed in section III.O. of this proposed rule, the FDA recently announced the approval of a new, higher dose naloxone hydrochloride nasal spray product used to treat opioid overdose and that the newly approved product delivers 8mg of naloxone. In the CY 2021 PFS final rule (85 FR 84683 through 84685), we finalized payment for HCPCS code G2215 (Take-home supply of nasal naloxone (provision of the services by a Medicare-enrolled Opioid Treatment Program); List separately in addition to code for primary procedure). HCPCS code G2215 was priced based on an assumption of a typical case in which the beneficiary would be provided with a box of two 4mg nasal spray products. At the time of drafting this proposed rule, we do not yet have any available pricing information for this newly approved product. However, in order to be able to make payment to OTPs under Medicare for this product, we are proposing to create a new G-code describing a take-home supply of this higher dose naloxone hydrochloride nasal spray product. Since pricing information is unavailable for this new product at the time of drafting this proposed rule, the estimated cost impact is unclear at this time, however, we believe utilization would be low based on CY 2021 claims data received so far for HCPCS code G2215.
14. Physician Self-Referral Update
The physician self-referral law provisions are discussed in section III.P. of this proposed rule.
As discussed in section III.P. of this proposed rule, we are proposing to amend the provisions of § 411.354(c)(2) identifying unbroken chains of financial relationships that constitute "indirect compensation arrangements" to ensure that a long-standing prohibition on certain per-unit of service compensation formulas for determining charges for the rental of office space and equipment remains within the ambit of the law. This provision, which was inadvertently omitted when the definition of "indirect compensation arrangement" was revised in the December 2, 2020 final rule entitled "Modernizing and Clarifying the Physician Self-Referral Regulations" (85 FR 77492), is necessary to protect against potential abuses such as overutilization and anti-competitive behavior. We believe that most parties have continued to comply with the regulatory provisions on per-unit of service compensation formulas for the rental of office space and equipment as they have done since the requirements became effective on October 1, 2009. In response to stakeholder inquiries, we are also proposing to add provisions to assist stakeholders in identifying the individual unit to be analyzed under the provisions of § 411.354(c)(2)(ii)(A)(i) through (iii) and proposed § 411.354(c)(2)(ii)(A)(iv). We believe that the clarity provided by this proposal would facilitate compliance without adding burden.
As discussed in section III.P. of this proposed rule, we are proposing to permit the use of the exception for preventive screening tests, immunizations, and vaccines at § 411.355(h) for COVID-19 vaccines even when they are not subject to CMS-mandated frequency limits, provided that all other requirements of the exception are satisfied. We believe that this proposal would ensure that the physician self-referral law would not impede the availability of critically important COVID-19 vaccines for Medicare and other patients.
As discussed in section III.P. of this proposed rule, we are proposing regulatory updates related to the process for publication of the Code List for Certain Designated Health Services (the Code List). Specifically, we would update the Code List each calendar quarter. We would provide public notification on the CMS website in advance of each Code List update, followed by a 30-day public comment period. We would provide information on the CMS website regarding the process for submitting public comments through www.regulations.gov, and address all public comments on the Code List on the CMS website. In addition, instead of publishing in the PFS final rule, we would publish the Code List solely on the CMS website. Finally, we would revise the definition of "List of CPT/HCPCS Codes" at § 411.351 by updating the URL that indicates where the Code List is published on the CMS website. We believe that these proposals would make available the most recent updates in a timelier manner and allow easier access to the most up-to-date Code List.
15. Requirement for Electronic Prescribing for Controlled Substances for a Covered Part D Drug under a Prescription Drug Plan or an MA-PD Plan (section 2003 of the SUPPORT Act) In addition to the cost reflected in the Collection of Information section of this proposed rule, we expect that there would be an additional burden for CMS to award and work with a CMS contractor to develop a process for reviewing the PDE data to assess prescriber compliance with the regulatory provision and review and process prescriber attestations. Based on similar contracts, and conversations with the industry, we have estimated the costs of (A) development of operational strategy for the new program, (B) reviewing PDE data, and (C) prescriber case work. We solicit stakeholder feedback on this estimate and all our assumptions.
(A) Development of policy: We estimate that it would take our contractor a week of work, 40 hours, to develop the strategy for how the contractor will process the prescriber attestations. We estimate that it would an operations manager and compliance officer working together at a combined hourly wage of $193.60/hr ($120.90/hr + $72.70/hr) would need a full 40-hour work week to operationalize this aspect of it. Therefore, the aggregate cost would be $7,744 (40 hr * $193.60/hr).
(B) Since systems already exist to collect the appropriate PDE data, our contractor would only have to review the data for compliance with the EPCS mandate. We therefore estimate that it would take 2 computer systems analyst each working at $95.22/hr, a week and a half of work, 60 hours. Therefore, the aggregate cost is $5,713.20 (60 hr * $95.22/hr).
(C) We estimate that it would take 4 administrative support workers each working at $36.82/hr, 60 hours to generate the letters and disseminate them to the appropriate prescriber, Start Printed Page 39545 which means that it would cost our contractor $2,209.20/year (60 hr * $36.82/hr) in administrative support costs. We estimate that it would be the full-time job of a customer service representative working at $37.02/hr to field prescriber inquiries about the disseminated letters. Thus, we expect that our contractor will spend $77,001.60 ($37.02/hr * 40 * 52) on the salary of the customer service representative for this task.
The aggregate impact for our contractor is 200 hours at a cost of $92,668. We seek comment on the accuracy of this burden estimate and on any measures that CMS can take to decrease the impact of this provision, while maintaining its utility and implementing the statutory mandate
16. Open Payments
a. Payment Context Field for Teaching Hospitals
This proposal is for a mandatory freeform text context field. We have created this proposal at the request of stakeholders, particularly after conversations with teaching hospitals. The teaching hospitals confirmed that the majority of their disputes arise because of a lack of information within the record and an inability to attribute it to the correct area within their large organization, not the inaccuracy of the record itself. The benefit of this field is to give better context to the records attributed to teaching hospitals and thereby reduce the number of disputes. For this reason, we also believe it will also increase goodwill between the program's stakeholders. The cost is that reporting entities will need to collect an additional piece of information, which will increase burden. We do not believe this burden will be great because compared to physician covered recipient payments, the number of teaching hospital payments is much lower. In addition, we have given flexibility to this field so that the reporting entity can choose which piece of information is most appropriate and can be something that they already collect, such as a check number or name of the department in the hospital.
b. Optional Annual Recertification
The optional annual recertification is at the request of reporting entities and will increase the availability of communication to CMS. The burden associated with this action is low because it will be a low-effort process only completed by the entities who choose to do so.
c. Defining a Physician-Owned Distributorship
Since the program began in 2013, we have heard feedback that physician-owned distributorship (PODs) should be better represented in the data because the conflict of interest potentially created by PODs is at the heart of the program. We created this new definition due to the lack of an existing definition of a POD that would be appropriate for the program's needs. Though this is a new definition, it will only be a subset of the existing definitions of applicable manufacturer and applicable group purchasing organization. "Applicable manufacturer—POD" and "Group purchasing organization—POD" are already "business type" choices when registering in the Open Payments system. Therefore, this definition will not alter existing regulations beyond requiring PODs to identify themselves as such.
d. Disallowing Record Deletion Without Reason
We believe there is not currently language to prevent an applicable manufacturer or applicable group purchasing organization from submitting and attesting to records, then deleting the records to prevent publication. This action would be contrary to the spirit of transparency of the program. To help ensure compliance with this requirement, we are also adding a new field that will allow entities to communicate the reason for the deletion to CMS. Since the entities will have attested to the accuracy and completeness of these records, we believe it is appropriate to confirm the reason for the deletion. We have not perceived the behavior of inappropriate deletions within the data and do not believe it will increase burden beyond the additional field when deleting a record. We are preemptively closing a potential loophole.
e. Disallow Publication Delays of General Payments
The statute requires that delays are "made pursuant to product research or development agreements and clinical investigations" (1128G(c)(1)(E) of the Act). A small number of general payments are delayed annually, which we are unable to verify meet this requirement. Research payments contain the appropriate fields to ensure that the statutory provisions are being met. We do not believe that it will be a burden for the small number of general payments to either be reported as research payments or not delayed.
f. Short-Term Loans
Short-term loans are not required to be reported, but they must be shorter than 91 days to meet the exception. This proposal does not create burden because it only clarifies that those 90 days must be the cumulative total for a year, which is already outlined in subregulatory guidance. We do not anticipate that this will change reporting behavior but want to explain the exception more clearly within the text of the final rule.
g. Remove General Ownership Records
Ownership records have special rules for reporting outlined in the statute (section 1128G(a)(2) of the Act), which are not included in the format for general records. However, there is currently a general record for reporting ownership and investment interest (Nature of Payment = 11). We anticipate a small burden for the approximately 92 reporting entities who have previously used the general nature of payment category in order to fill out the different fields in the ownership record. This burden will allow the records to meet statutory mandates.
h. Updated Contact Information
Open Payments conducts regular compliance-related outreaches to reporting entities when it encounters data that may not meet program requirements. We have found that the two contacts provided by applicable manufacturers and group purchasing organizations often become obsolete, especially if a company has not updated its contact information during the recertification process. It is crucial for the integrity of the data that we have the ability to contact entities in the case of irregularities. Additionally, we believe that ensuring informal communications from CMS will reduce burden since it may prevent more formal compliance actions if the entity is unresponsive due to outdated contact information. However, we do not believe this is an issue for the majority of reporting entities, nor do we believe that keeping the contact information updated will create a large burden.
17. Updates to the Quality Payment Program
In section IV.A. of this proposed rule, we included our proposed policies for the Quality Payment Program. In this section, we first present the overall and incremental impacts to the number of expected QPs and associated APM Incentive Payments. In the following sections, we estimate the overall and incremental impacts to the total MIPS eligible population and the payment impacts by practice size for the 2022 Start Printed Page 39546 MIPS performance period based on various proposed policies, including to modify MIPS eligibility, the MIPS final score and the performance threshold and additional performance threshold as discussed in sections IV.A.3.a.(1) and IV.A.3.f. of this proposed rule.
This RIA uses the 2019 MIPS performance period submissions that were used for the CY 2021 PFS final rule RIA (85 FR 85011 through 85023). The submissions for the 2020 MIPS performance period were not available in time to incorporate into this model and to assess whether the data for 2020 can be used to effectively predict future performance. For the 2020 performance year, we applied the MIPS automatic extreme and uncontrollable circumstances policy to all individual MIPS eligible clinicians and allowed for extreme and uncontrollable applications due to the COVID-19 Public Health Emergency (PHE) (https://qpp.cms.gov/resources/covid19?py=2020). Due to these extreme and uncontrollable circumstances policy, not all clinicians or groups may have submitted performance category data for the 2020 MIPS performance period. We will evaluate for the final rule whether it is appropriate to use the 2020 performance period data and whether adjustments would need to be made if CY 2020 performance category submissions data are used.
We ran two RIA models: A baseline and proposed policies RIA model. The aim of the baseline model is to reflect the CY 2022 performance period/2024 MIPS payment year if this proposed rule did not take effect, and therefore, reflects previously finalized policies for the CY 2022 performance period/CY 2024 MIPS payment year. Select examples of the baseline policies scheduled to start in the CY 2022 MIPS performance period/2024 MIPS payment year include the removal of the Web Interface as a collection type and the change in the performance category weights. Our baseline model used the performance threshold and additional performance threshold of the CY 2021 MIPS performance period/2023 MIPS payment year since those values were not previously defined for the CY 2022 MIPS performance period/2024 MIPS payment year. The aim of the proposed policies model is to estimate the incremental effect of the proposed policies for the CY 2022 performance period/CY 2024 MIPS payment year on MIPS eligibility, MIPS final scores, and payment adjustments. Select examples of the proposed policies include, the inclusion of new MIPS eligible clinician types, the inclusion of the Web Interface as a collection type, the change in performance threshold and additional performance threshold, and the new complex patient bonus. Refer to section VII.F.17.d.(2) of this proposed rule for the detailed methods on how we integrated the policies into the baseline and proposed policies models.
a. Estimated APM Incentive Payments to QPs in Advanced APMs and Other Payer Advanced APMs
For payment years from 2019 through 2024, through the Medicare Option, eligible clinicians who have a sufficient percentage of their Medicare Part B payments for covered professional services or Medicare patients through Advanced APMs will be QPs in the applicable QP performance period for a year. These QPs will receive a lump-sum APM Incentive Payment equal to 5 percent of their estimated aggregate paid amounts for Medicare covered professional services furnished during the calendar year immediately preceding the payment year. Beginning in payment year 2021, in addition to the Medicare Option, eligible clinicians may become QPs through the All-Payer Combination Option. The All-Payer Combination Option allows eligible clinicians to become QPs by meeting the QP payment amount or patient count threshold through a pair of calculations that assess a combination of both Medicare Part B covered professional services furnished or patients through Advanced APMs and services furnished or patients through Other Payer Advanced APMs.
Eligible clinicians who become QPs for a year are not subject to MIPS reporting requirements and payment adjustments. Eligible clinicians who do not become QPs, but meet a lower threshold to become Partial QPs for the year, may elect to report to MIPS and, if they elect to report, would then be scored under MIPS and receive a MIPS payment adjustment. Partial QPs are not eligible to receive the APM Incentive Payment.
If an eligible clinician does not attain either QP or Partial QP status, and does not meet any another exemption category, the eligible clinician would be subject to MIPS, would report to MIPS, and would receive the corresponding MIPS payment adjustment.
Beginning in payment year 2026, the update to the PFS CF for services that are furnished by clinicians who achieve QP status for a year is 0.75 percent, while the update to the PFS CF for services that are furnished by clinicians who do not achieve QP status for a year is 0.25 percent. In addition, MIPS eligible clinicians would receive positive, neutral, or negative MIPS payment adjustments to payment for their Part B PFS services in a payment year based on performance during a prior performance period. Although the statute establishes overall payment rate and procedure parameters until 2026 and beyond, this impact analysis covers only the 2024 MIPS payment year of the Quality Payment Program.
Overall, we estimate that for the 2022 QP Performance Period between 225,000 and 290,000 eligible clinicians will become QPs, therefore be excluded from MIPS, and qualify for the lump sum APM incentive payment in Payment Year 2024 based on 5 percent of their Part B paid amounts for covered professional services in the preceding year. These paid amounts for QPs are estimated to be between approximately $12,000 million and $15,000 million in total for the 2022 performance year. The analysis for this proposed rule used the 2020 third snapshot participation file. We based APM Incentive Payment Amounts on paid amounts with service dates of January 1, through September 30, 2020. We multiplied the calculated amounts by 1.5 to approximate payment amounts for the full calendar year. We estimate that the total lump sum APM Incentive Payments will be approximately $600-750 million for the 2024 Quality Payment Program payment year.
In section VII.F.17.a. of this proposed rule, we projected the number of eligible clinicians that will be QPs, and thus excluded from MIPS, using several sources of information. First, the projections are anchored in the most recently available public information on Advanced APMs. The projections reflect Advanced APMs that will be operating during the 2022 QP Performance Period, as well as some Advanced APMs anticipated to be operational during the 2022 QP Performance Period. The projections also reflect an estimated number of eligible clinicians that would attain QP status through the All-Payer Combination Option. We note that the Kidney Care Choices Model and the Radiation Oncology model have been included in our analysis as we anticipate that the model will be Advanced APMs in 2022. The following APMs are expected to be Advanced APMs for the 2022 QP Performance Period:
- Bundled Payments for Care Improvement Advanced Model;
- Comprehensive Care for Joint Replacement Payment Model (CEHRT Track);
- Global and Professional Direct Contracting Model; Start Printed Page 39547
- Kidney Care Choices Model (Kidney Care First; Professional Option and Global Option);
- Maryland Total Cost of Care Model (Care Redesign Program; Maryland Primary Care Program);
- Medicare Shared Savings Program (Basic Track Level E, and the ENHANCED Track);
- Oncology Care Model (Two-Sided Risk Arrangements);
- Primary Care First (PCF) Model;
- Radiation Oncology model; and,
- Vermont All-Payer ACO Model (Vermont Medicare ACO Initiative).
We used the Participation Lists and Affiliated Practitioner Lists, as applicable, (see 81 FR 77444 through 77445 for information on the APM Participant Lists and QP determinations) on the 2020 third snapshot participation file to estimate the number of QPs, total Part B paid amounts for covered professional services, and the aggregate total of APM Incentive Payments for the 2022 QP Performance Period. We examined the extent to which Advanced APM participants would meet the QP Thresholds of having at least 50 percent of their Part B covered professional services or at least 35 percent of their Medicare beneficiaries furnished Part B covered professional services through the APM Entity.
b. Impact for the CY 2021 MIPS Performance Period/2023 MIPS Payment Year
In section IV.A.3.e.(2)(a)(ii) of this proposed rule, we proposed to double the complex patient bonus, and to increase its cap to 10 points. We expect this proposed policy to result in the median bonus to increase by 3 points, thus increasing MIPS final scores at the median by 3 points. We do not know the effects of the PHE for COVID-19 and its effect on MIPS performance in 2021, so we did not recreate the analysis and payment distributions with the updated bonus for the 2021 MIPS performance period (85 FR 85012 through 85019). Directionally, the increase in complex patient bonus points will result in smaller payment adjustments for three reasons. First, the resulting increase in final scores will reduce the budget neutral pool. Second, the increase in complex patient bonus points will increase the number of clinicians with scores above the performance threshold or additional performance threshold, meaning more clinicians will share in the budget neutral pool and additional $500 million for exceptional performance and potentially lowering the scaling factor that is applied to the MIPS payment adjustment and additional payment adjustment. Third, the average scores of those receiving a positive or additional adjustment will be higher, which means the adjustment rates for clinicians that have scores above the performance threshold or additional performance threshold will be lower.
c. Estimated Number of Clinicians Eligible for MIPS Eligibility for the 2022 MIPS Performance Period/2024 MIPS Payment Year
(1) Methodology To Assess MIPS Eligibility
(a) Clinicians Included in the Model Prior To Applying the Low-Volume Threshold Exclusion
To estimate the number of MIPS eligible clinicians for the 2022 MIPS performance period and the effect of the proposed policies in this proposed rule, we ran two models as described in section VII.F.17.: A baseline model and proposed policies model.
For the baseline and proposed policies models, we used the same eligibility files and approach as described in the CY 2021 PFS final rule (85 FR 85013), which resulted in the inclusion of 1.6 million clinicians who had PFS claims from October 1, 2018 to September 30, 2019 as well as additional clinicians associated with a group who had at least one PFS claim from October 1, 2019 through December 31, 2019. We used the same exclusion criteria to exclude clinicians from our MIPS eligibility assessment as described in the CY 2021 PFS final rule RIA (85 FR 85013), with the following model updates:
(1) In both the baseline and proposed policies models, we excluded practitioners in Next Generation ACOs because the Next Generation ACO model ends in the CY 2021 MIPS performance period.
(2) In both the baseline and proposed policies models, to determine which clinicians in the initial population of 1.6 million should be excluded as QPs, we used Advanced APM payment and patient percentages from the APM Participant List for the final snapshot date for the 2019 QP performance period. We elected to use this data source because the APM participant list for the 2019 final snapshot can reliably be used for RIA projections. From this data, we calculated the QP and Partial QP determinations as described in section of IV.A.4.c.(1)(b) of this proposed rule for the 2022 QP performance period for both models.
(3) In the proposed policies model, we included in our estimated MIPS eligible population for the CY 2022 performance period/2024 payment year clinical social workers and certified nurse-midwives as proposed in section IV.A.3.a.(1) of this proposed rule.
(4) In the proposed policies model, we are integrating the proposal that starting with the CY2022 MIPS performance period/2024 MIPS payment year, small practices, excluding virtual groups, must submit data as a group in any performance category to indicate that they wish to be scored as a group for Medicare Part B claims. This affects eligibility because previously a single Medicare Part B claims submission, without any other submission, started a group score. Once a group score is created, a clinician who was individually excluded from MIPS for being under the low-volume threshold, may now be eligible if the group exceeds the low-volume threshold. This proposed policy is described at section IV.A.3.a. of this rule.
(b) Assumptions Related To Applying the Low-Volume Threshold Exclusion
The low-volume threshold policy may be applied at the individual (TIN/NPI) or group (TIN) levels based on how data are submitted or at the APM Entity level if the clinician is part of an APM Entity in a MIPS APM (hereafter, a MIPS APM Entity) that elects to submit to MIPS. A clinician or group that exceeds at least one but not all three low-volume threshold criteria may become MIPS eligible by electing to opt-in and subsequently submitting data to MIPS, thereby getting measured on performance and receiving a MIPS payment adjustment.
For the proposed policies model, we describe below the estimated MIPS eligibility status and the associated PFS allowed charges of clinicians in the initial population of 1.6 million clinicians. We present in section VII.F.17.c.(1)(c) the incremental impact of the proposed policies from the baseline model for the CY 2022 performance period/2024 payment year on the MIPS eligible clinician population and their associated PFS allowed charges. We applied the same assumptions presented in the CY 2021 PFS final rule RIA to apply the low-volume threshold and to understand whether clinicians participate as a group, virtual group, APM entity, or as individuals (85 FR 85013 through 85016), except for three modifications. We assumed only individuals or APM TINs that exceeded the low-volume threshold would receive an APM Performance Pathway (APP) score consistent with the policy as finalized in the CY 2021 PFS final rule (85 FR Start Printed Page 39548 84897).[263] We assumed APM TINs that qualified for opt-in and submitted data as a TIN would also be eligible. Finally, we did not consider clinicians in groups as MIPS eligible clinicians and start a group score for clinicians in small practices with only Medicare Part B claims submissions to reflect the proposed policy at section IV.A.3.a. of this rule.
For the proposed policies model, we estimate approximately 212,000 clinicians[264] will be MIPS eligible because they exceed the low volume threshold as individuals and are not otherwise excluded. These clinicians may ultimately choose to participate in MIPS as an individual, group, virtual group or APM entity. We identify these clinicians as having "required eligibility" in Table 129. We estimate approximately 595,000 additional MIPS eligible clinicians will be eligible because they belong to an APM entity, group or virtual group that meets the low-volume threshold and submits to MIPS. These clinicians are identified as having "group eligibility" in Table 129. Finally, we estimate about 3,000 clinicians would be eligible through "opt-in eligibility" through the "opt-in" policy for a total MIPS eligible clinician population of approximately 810,000. This leads to an associated $67 billion allowed PFS charges estimated to be included in the 2022 performance period/2024 payment year.
Start Printed Page 39549

Furthermore, we estimate there will be approximately 412,000 clinicians who are not MIPS eligible, but could be if their practice decides to participate or they elect to opt-in. We describe this group as "Potentially MIPS eligible" in Table 129. These clinicians would be included as MIPS eligible in the unlikely scenario in which all group practices elect to submit data as a group, or clinicians in a group that does not submit are eligible to opt-into MIPS individually and choose to do so. This assumption is important because it quantifies the maximum number of MIPS eligible clinicians. When this unlikely scenario is modeled, we estimate the MIPS eligible clinician population could be as high as 1.2 million clinicians. Start Printed Page 39550
Finally, we estimate approximately 101,000 clinicians would not be MIPS eligible because they and their group are below the low-volume threshold on all three criteria and another approximately 304,000 would not be MIPS eligible because they are categorically excluded regardless of volume or submission activity.
Eligibility among many clinicians is contingent on submission to MIPS as a group, virtual group or election to opt-in, therefore we will not know the number of MIPS eligible clinicians who submit until the submission period for the 2022 MIPS performance period is closed. For this proposed policies model analysis, we use the estimated population of 809,625 MIPS eligible clinicians described above.
(c) Estimated Impact of the Proposed Policies on MIPS Eligibility and PFS Allowed Charges
We illustrate in Table 130 how the proposed policy to add clinical social workers and certified nurse-midwives as MIPS eligible clinician types as proposed in section IV.A.3.a.(1) of this proposed rule affects the estimated number of MIPS eligible clinicians. The first row presents the estimates from the RIA baseline model with the number of individuals that would be MIPS eligible clinicians for the 2022 MIPS performance period/2024 MIPS payment year if this proposed rule did not take effect. The second row presents estimates from the RIA proposed policies model with the incremental impact of adding the two new MIPS eligible clinician types on the number of MIPS eligible clinicians for the CY 2022 MIPS performance period/2024 MIPS payment year. As shown in Table 130, the proposed policy to add clinical social workers and certified nurse-midwives as MIPS eligible clinician types leads to a small increase in the number of MIPS eligible clinicians (1.1 percent increase) and a minimal increase in the PFS allowed charges (0.1 percent increase) for the CY 2022 performance period/2024 payment year.

d. Estimated Impacts on Payments to MIPS Eligible Clinicians for the CY 2022 Performance Period/2024 Payment Year
(1) Summary of Approach
In sections IV.A.3.d., IV.A.3.e. and IV.A.3.f. of this proposed rule, we present several provisions which impact the measures and activities that impact the performance category scores, final score calculation, and the MIPS payment adjustment. We discuss these changes in more detail in section VII.F.17.d. of this RIA as we describe our methodology to estimate MIPS payments for the CY 2022 performance period/2024 payment year. We then present the impact of the overall proposed policies on the CY 2022 performance period/2024 payment year and then compare select metrics to the baseline model, which only incorporates previously finalized policies for the CY 2022 performance period/2024 payment year. By comparing the baseline model to the proposed policies model, we are able to estimate the incremental impact of the proposed policies for the CY 2022 performance period/2024 payment year.
The payment impact for a MIPS eligible clinician is based on the clinician's final score, and MIPS eligible clinicians can participate as an individual, group, virtual group, or APM Entity in the four MIPS performance categories: Quality, cost, improvement activities, and Promoting Interoperability. As discussed in section VII.F.17. of this proposed rule, we generally used the most recently available submissions data from the Quality Payment Program which is data submitted for the 2019 MIPS performance period. For the cost performance category, we used the same data as the CY 2020 PFS final rule (84 FR 63169).
The estimated payment impacts presented in this proposed rule are averages by practice size weighted by Medicare utilization. The payment impact for a MIPS eligible clinician will vary from the average and would depend on the measure submissions, scores and their performance. The average percentage change in total revenues that clinicians earn would be less than the impact displayed here because MIPS eligible clinicians generally furnish services to both Medicare and non-Medicare patients; this program does not impact payment from non-Medicare patients. In addition, MIPS eligible clinicians may receive Medicare revenues for services under other Medicare payment systems, such as the Medicare FQHC PPS, that would not be affected by MIPS payment adjustment factors.
(2) Methodology To Assess Impact
To estimate participation in MIPS for the CY 2022 performance period/2024 MIPS payment year for this proposed rule, we generally used 2019 MIPS performance period data for both the baseline and proposed policies models. Our baseline and proposed policies scoring models included the 801,013 and 809,625 estimated MIPS eligible Start Printed Page 39551 clinicians, respectively, as described in section VII.F.17.c.(1) of this RIA.
To estimate the impact of MIPS policies on MIPS eligible clinicians, we generally used the 2019 MIPS performance period submissions data, including data submitted for the quality, improvement activities, and Promoting Interoperability performance categories. We supplemented this information with the most recent data available for CAHPS for MIPS and CAHPS for ACOs, testing data for the revised total per capita cost measure and Medicare Spending Per Beneficiary (MSPB) clinician measures which were finalized in the CY 2020 PFS final rule (84 FR 62969 through 62977), testing data for the new episode cost measures, administrative claims data for the new quality performance category measures, and other data sets.[265] We calculated a hypothetical final score for the 2022 MIPS performance period/2024 MIPS payment year for the baseline and proposed policies scoring models for each MIPS eligible clinician using score estimates for quality, cost, Promoting Interoperability, and improvement activities performance categories, where each are described in detail in the following subsections.
(a) Methodology To Estimate the Quality Performance Category Score
We estimated the quality performance category score using a methodology like the one described in the CY 2021 PFS final rule (85 FR 85016 through 85017) for baseline and proposed policies RIA models for the CY 2022 MIPS performance period/2024 payment year.
For the baseline policies RIA model, which does not reflect the proposed policies for CY 2022 MIPS performance period/2024 payment year, we made the following modifications to reflect the previously finalized policies for the CY 2022 performance period/2024 payment year for the quality performance category:
(1) As previously finalized in the CY 2021 PFS final rule, we removed the Web Interface as a collection type in MIPS and through the APP for the CY 2022 performance period/2024 MIPS payment year (85 FR 84870 and 85 FR 84843). Although the Web Interface is proposed to be reinstated for groups for the CY 2022 MIPS performance period/2024 MIPS payment year and ACOs for CY 2022 MIPS performance period/2024 MIPS payment year and CY 2023 MIPS performance period/2025 MIPS payment year as discussed in sections IV.A.3.d.(1)(d) and IV.A.3.c.(2)(a), respectively, the baseline model is attempting to capture the CY 2022 MIPS performance period/2024 MIPS payment year as if this proposal did not exist. Therefore, the baseline model does not incorporate the Web Interface as a collection type for groups and ACOs.
To estimate a quality performance category score for clinicians in groups who previously used the Web Interface as a collection type in 2019, we assumed these groups would use the other two other collection types (MIPS CQMs and eCQMs) available in the 2022 MIPS performance period/2024 MIPS payment year. We then applied the same methodology described in the CY 2021 PFS Proposed Rule when the removal of Web Interface as a collection type was previously proposed (85 FR 50387 through 50388) using 2019 MIPS submissions data. To estimate a quality performance category score for ACOs, we used the same methodology described in the CY 2021 PFS proposed rule when the Web Interface was not included in the APP (85 FR 50388).
(2) We used the published 2021 MIPS historical quality benchmarks file to identify measures subject to the topped out scoring cap that was finalized (82 FR 53721 through 53727).[266]
For the proposed policies model, we made the following modifications to the baseline model to reflect the newly proposed policies for the 2022 MIPS performance period/2024 MIPS payment year for the quality performance category:
(1) As discussed in section IV.A.3.d.(1)(e) of this proposed rule, we propose for this proposed rule two new administrative claims measures for those for whom it is applicable: (1) Risk-Standardized Acute Unplanned Cardiovascular-Related Admission Rates for Patients with Heart Failure for the Merit-based Incentive Payment System; and (2) Clinician and Clinician Group Risk-standardized Hospital Admission Rates for Patients with Multiple Chronic Conditions. To implement this policy in our proposed policies RIA model for the 2022 MIPS performance period/2024 MIPS payment year, we used testing data for these new administrative claims measures.
(2) As discussed in section IV.A.3.e.(1)(c) of this proposed rule, we proposed to use performance period benchmarks for the CY 2022 performance period in accordance with § 414.1380(b)(1)(ii) as opposed to a historical benchmark. Therefore, we used 2019 MIPS performance period benchmarks calculated from the CY 2019 MIPS submissions data because the performance data for this analysis came primarily from the 2019 MIPS performance period.
(3) As discussed in section IV.A.3.e.(1)(c)(iii)(A) of this proposed rule, we proposed to remove the 3-point floor for each measure that can be reliably scored against the benchmark and score the measure from 1 to 10 points. As described in section IV.A.3.e.(1)(c)(iii)(B) of this proposed rule, we also proposed to make the following changes: (1) Remove the special scoring policy of scoring 3 points for class 2 measures, except for clinicians in small practices; (2) introduce a 5-point floor for new measures for their first two performance periods that meet data completeness and can be reliably scored against a benchmark (class 4a measures); and (3) introduce 5 points for new measures in their first two performance periods that meet data completeness, but cannot be reliably scored against a benchmark because they lack a benchmark or do not meet case minimum in the program (class 4b measures). We incorporated these scoring changes into our proposed policies model. Because we are using 2019 MIPS performance period data, we assume that measures new to the 2018 and 2019 MIPS performance periods could qualify as class 4 measures.
(4) As discussed in sections IV.A.3.e.(1)(c)(vi) and IV.A.3.e.(1)(c)(vii) of this proposed rule, we also propose to end measure bonus points for reporting high priority measures and for submitting with end-to-end electronic reporting beginning in the 2022 MIPS performance period. We incorporated these scoring changes into our proposed rule model for all MIPS collection types.
(5) As discussed in section IV.A.3.d.(1)(d), we are proposing to extend Web Interface measures for the CY 2022 performance period/2024 MIPS payment year for groups and virtual groups using the existing 10 CMS Web Interface measures. To estimate the impact of this proposed policy, we used the same methodology described in the CY 2021 PFS final rule (85 FR 85016 through 85017) using 2019 MIPS submissions data.
(6) Finally, we are also proposing to extend the CMS Web Interface as a means of reporting quality under the APM Performance Pathway for Shared Savings Program ACOs for the CY 2022 and CY 2023 MIPS performance periods and 2024 and 2025 MIPS payment years as described in section IV.A.3.c.(2)(a) of Start Printed Page 39552 this proposed rule. Under the proposal, for the CY 2022 and CY 2023 MIPS performance periods and 2024 and 2025 MIPS payment years, Web Interface reporting would work in the same manner as for performance year 2021, where ACOs would have the option of reporting either the CMS Web Interface, the APP eCQM/MIPS CQM measure set, or both. To estimate the impact of this proposed policy, we used the same methodology described in the CY 2021 PFS final rule RIA (85 FR 85016 through 85017) when Web Interface was retained for the APP.
(b) Methodology To Estimate the Cost Performance Category Score
We estimated the cost performance category score using a similar methodology described in the CY 2020 PFS final rule (84 FR 63169) with the modifications to the baseline and the proposed policies RIA model described in this section.
In the baseline policies RIA model, we refined our methodology for developing benchmarks to better reflect the previously finalized policy in CY 2017 Quality Payment Program final rule (81 FR 77308 through 77309). We did not estimate cost improvement scoring that starts in the 2022 MIPS performance period/2024 MIPS payment year as previously finalized at § 414.1380(a)(1)(ii) and in the CY 2019 PFS final rule (83 FR 58956) since we did not have sufficient data to conduct improvement scoring, which requires 2 years of cost data to model.
In the proposed policies RIA model, we modified the baseline model to incorporate the proposal to add five new episode-based cost performance category measures in the CY 2022 MIPS performance period/2024 payment year as described in section IV.A.3.d.(2) of this proposed rule, by using claims data from January 1, 2019 to December 31, 2019. Cost measures were scored if the clinicians or groups met or exceeded the case volume: 10 Episodes for Melanoma Resection to align with the reporting case minimum for procedural cost measures currently in use in MIPS, 20 episodes for Sepsis to align with the reporting case minimum for acute inpatient condition cost measures currently in use in MIPS, 20 episodes for Diabetes and Asthma/COPD as used in field testing for these chronic measures, and 20 episodes for Colon Resection. These newly proposed cost episode-based measures were calculated for both the TIN/NPI and the TIN.
(c) Methodology To Estimate the Facility-Based Measurement Scoring
For the baseline model, we estimated the facility-based score using the scoring policies finalized in the CY2018 Quality Payment Program final rule (82 FR 53763) and the methodology described in the CY 2020 PFS final rule (84 FR 63169). For the proposed policies model, we used the methodology proposed for the CY 2022 MIPS performance period/2024 MIPS payment year as discussed in section IV.A.3.e.(2)(b)(v)(B) of this proposed rule. We propose at § 414.1380(e)(vi) that beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year, the MIPS quality and cost performance category scores will be based on the facility-based measurement scoring methodology unless a clinician or group receives a higher MIPS final score through another MIPS submission. Therefore, if a MIPS eligible clinician or a group is eligible for facility-based measurement, but they participate in MIPS as an individual or group, we used the higher final score between the facility-based scoring and MIPS submission-based scoring.
(d) Methodology To Estimate the Promoting Interoperability Performance Category Score
For the baseline RIA model, we used the CY 2019 MIPS Promoting Interoperability performance period data submissions data to estimate CY 2022 MIPS performance for the Promoting Interoperability performance category. We made the following two modifications to the 2019 performance period scoring to reflect the previously finalized policy changes between the CY 2019 and CY 2021 performance periods: (1) We doubled the bonus points for clinicians who submitted the PDMP measure as described in section IV.A.3.d.(4)(c)(i) of this proposed rule; and (2) we did not incorporate the Verify Opioid Treatment Agreement measure data, a measure that was finalized in the CY 2019 performance period (83 FR 59807) but removed in the CY 2020 performance period (84 FR 62994). We retained the PDMP bonus for the baseline model for continuity between the CY 2021 and 2022 performance periods and for consistency since bonuses for the quality performance category were retained for the baseline as well. Because we lacked data on who would adopt the finalized Health Information Exchange bi-directional exchange measure for the CY 2021 performance period and how these clinicians would score, we only used past reporting on the existing Health Information Exchange Objective measures to estimate CY 2022 Promoting Interoperability performance.
For the proposed rule model, we considered the following policy proposals as potential modifications to the baseline RIA model:
(1) In section IV.A.3.d.(4)(c)(i) of this proposed rule, we proposed for the PDMP measure to remain optional and at 10 points. Modifications were not made to reflect this proposed policy in the proposed policies model since the baseline model already incorporated this policy.
(2) In section IV.A.3.d.(4)(c)(iii) of this rule, we proposed to require two of the measures associated with the Public Health and Clinical Data Exchange Objective, beginning with the CY 2022 performance period: Immunization Registry Reporting; and Electronic Case Reporting. Because we lacked data, we did not integrate this requirement into our proposed rule model.
(3) In section IV.A.3.d.(4)(h)(i) of this rule, we proposed to automatically reweight the Promoting Interoperability performance category to another performance category and assigned a weight of zero only in the event a small practice did not submit any data for any of the measures specified for the Promoting Interoperability performance category. This policy was implemented in the proposed policies model.
(4) In section IV.A.3.d.(4)(h)(ii) of this proposed rule, we proposed to reweight the Promoting Interoperability performance category for clinical social workers. This policy was implemented in the proposed policies model.
(5) In section IV.A.3.d.(4)(d) of this proposed rule, we proposed the additional requirement that eligible clinicians must attest to conducting an annual assessment of the High Priority Guides of the SAFER Guides beginning with the 2022 MIPS performance period. This policy was not implemented in the proposed policies model as it does not affect eligibility or payment. We included this policy in our burden calculations in section V.B.8.g.(3) of this rule.
(e) Methodology To Estimate the Improvement Activities Performance Category Score
For the baseline model, we modeled the improvement activities performance category score based on CY 2019 MIPS performance period data and APM participation identified in section VII.F.17.c.(1) of this proposed rule. For clinicians and groups not participating in a MIPS APM, we used the CY 2019 submissions improvement activities Start Printed Page 39553 score. We did not model the policy finalized in the CY 2020 performance period (84 FR 62980) to require a minimum threshold of 50 percent of clinicians in a group to complete an improvement activity for the group to receive credit since we did not have data to determine the proportion of clinicians in a group that completed the improvement activity. We continued to apply the methodology described in the CY 2020 PFS final rule (84 FR 63170) to assign an improvement activities performance category score. For the APM participants identified in section VII.F.17.c.(1) of this proposed rule, we assigned an improvement activity performance category score of 100 percent.
For the proposed policies model, we did not make modifications to the baseline model to reflect the proposed policies in section IV.A.3.d.(3) of this proposed rule since we did not have the data to model those changes.
(f) Methodology To Estimate the Complex Patient Bonus Points
In section IV.A.3.e.(2)(a)(iii)(B) of this proposed rule, we proposed to continue the complex patient bonus, with updates, for the CY 2022 MIPS performance period/2024 payment year. For the baseline RIA model, we used the complex patient bonus information calculated for the 2019 performance period data for the 2022 MIPS performance period/2024 payment year, as was previously done in the CY 2021 PFS final rule (85 FR 85017).
For the proposed policies RIA model, we calculated the complex patient bonus using the calculation proposed in section IV.A.3.e.(2)(a)(iii)(B)(cc) of this proposed rule for the CY 2022 performance period/2024 payment year. In section IV.A.3.e.(2)(a)(iii)(B)(aa) of this proposed rule, we propose updates to the complex patient bonus for the CY 2022 performance period/2024 payment year and future MIPS performance periods/payment years to account for social and medical complexity, while still using our current established indicators of dual proportion and HCC risk scores, respectively. Consistent with the proposed policy for the 2022 performance period, our proposed policies RIA model calculated and applied the separate risk indicator complex patient bonus components methodology with a single overall cap described in section IV.A.3.e.(2)(a)(iii)(B)(dd) of this proposed rule.
(g) Methodology To Estimate the Final Score
We did not propose any changes for how we calculated the MIPS final score. Our baseline and proposed policies RIA models assigned a final score for each TIN/NPI by multiplying each estimated performance category score by the corresponding performance category weight, adding the products together, multiplying the sum by 100 points, adding the complex patient bonus, and capping at 100 points.
For the baseline policies RIA model, we applied the performance category weights and redistribution weights finalized in the CY 2021 PFS final rule (85 FR 84913 through 84916).
For the proposed policies RIA model, we proposed to modify the redistribution policy for small practices. Therefore, we applied redistribution weights proposed in section IV.A.3.e.(2)(b)(iii)(A) of this proposed rule.
For both models, after adding any applicable bonus for complex patients, we reset any final scores that exceeded 100 points to equal 100 points. For MIPS eligible clinicians who were assigned a weight of zero percent for any performance category, we redistributed the weights according to section IV.A.3.e.(2)(b)(ii) of this proposed rule.
(h) Methodology to Estimate the MIPS Payment Adjustment
For the baseline and proposed policies RIA models, we applied the hierarchy as finalized in the CY 2021 PFS final rule (85 FR 84917 through 84919) to determine which final score should be used for the payment adjustment for each MIPS eligible clinician when more than one final score is available. We then calculated the parameters of an exchange function in accordance with the statutory requirements related to the linear sliding scale, budget neutrality, minimum and maximum adjustment percentages, and additional payment adjustment for exceptional performance (as proposed under § 414.1405).
For the baseline policies model, we applied the performance threshold and additional performance thresholds finalized for the CY 2021 performance period/2023 payment year (85 FR 84923), of 60 and 85, respectively. For the proposed policies model, we used the performance threshold of 75 points as proposed in section IV.A.3.f. (2) and the additional performance threshold of 89 points as proposed in section IV.A.3.f.(3). We used these resulting parameters to estimate the positive or negative MIPS payment adjustment based on the estimated final score and the paid amount for covered professional services furnished by the MIPS eligible clinician. As discussed in the CY 2021 PFS final rule RIA (85 FR 85013), we adjusted the paid amount of non-engaged clinicians to equal their proportion of paid amount prior to the PHE for COVID-19 for the baseline and proposed policies RIA models.
(3) Impact of Payments by Practice Size
As we shift from previous MIPS transition policies by removing bonuses from the quality performance category and increasing the performance threshold and the additional performance threshold, we observe large changes between the baseline and proposed policies RIA models.
First, we observe an increase in the funds available for redistribution due to the increase in clinicians with final scores below the performance threshold. The baseline model estimates $428 million would be redistributed through budget neutrality and that $500 million would be distributed to MIPS eligible clinicians for exceptional performance. The mean and median final scores for the baseline model are 78.13 and 82.59, respectively. Our proposed policies model estimates that $587 million would be redistributed through budget neutrality. For clinicians who meet or exceed the additional performance threshold, an additional $425 million was estimated to be distributed. The mean and median final scores for the proposed policies model are 75.86 and 80.30, respectively.
In the proposed model, the estimated bonus for exceptional performance is less than the $500 million of available funding because the maximum additional payment adjustment for clinicians with exceptional performance reached 10 percent. As finalized in the 2017 QPP final rule (81 FR 77339 through 77340), we stated the maximum additional payment adjustment would be 10 percent, which is established by the statute, and that it would be multiplied by a scaling factor that cannot exceed 1.0. We reached the maximum additional payment adjustment allowed of 10 percent because the additional performance threshold is higher, and fewer clinicians performed above this higher additional performance threshold while a greater percentage of clinicians performed below the additional performance threshold. As a result, fewer clinicians were sharing the funds available through the additional bonus for exceptional performance. Start Printed Page 39554
Second, we observe an increase in the maximum positive payment adjustment. The baseline model estimates the maximum positive MIPS payment adjustment based on the budget neutral pool at 1.5 percent and the maximum positive MIPS additional payment adjustment for exceptional performance at 5.1 percent, for a combined maximum payment adjustment of 6.6 percent. The proposed policies model estimates the maximum MIPS positive payment adjustment based on the budget neutral pool at 4.0 percent and the maximum positive additional MIPS payment adjustment for exceptional performance bonus at 10.0 percent for a combined maximum payment adjustment of 14.0 percent.
Finally, we no longer observe large differences in performance across practice sizes due to the shift from MIPS transition policies. Table 131 shows the overall impact of the payment adjustments by practice size and based on whether clinicians are expected to submit data to MIPS for the proposed policies model. We estimate performance under the proposed policies will be similar across all practice sizes. The smallest proportion of clinicians receiving a positive or neutral payment adjustment is among clinicians in practices with 16 to 24 clinicians compared to other sized practices among those who submit data. Table 131 also shows that overall 67.5 percent of MIPS eligible clinicians that participate in MIPS are expected to receive positive or neutral payment adjustments. In Table 132, we present the overall impact of the baseline and the proposed policies models among clinicians who submit data to assess the incremental impact of the proposed policies. The overall proportion of clinicians receiving a positive or neutral payment adjustment decreases from 91.7 percent to 67.5 percent with the implementation of the proposed policies that shift away from MIPS transition policies. Among clinicians who receive a positive payment adjustment in the baseline model and receive a negative payment adjustment in the proposed policies model, only 30 percent receive a negative payment adjustment greater than 1 percent. In addition, we no longer observe a disproportionate number of clinicians in small practices receiving a negative payment adjustment when implementing the proposed policies.
For the CY 2022 performance period/2024 payment year, we have policies targeted towards small practices including special scoring policies to minimize burden and facilitate small practice participation in MIPS or APMs, which we describe in section VII.F.17.f.(2)(e) of this proposed rule. The intention of the proposed policies is to provide a more equitable participation process and reduce the disparity in performance between clinicians in large and small practices. These findings and proposed policies reflect movement away from the transition policies implemented during the early years of MIPS and how MIPS is focusing on value rather than primarily on engagement. However, non-engagement by not submitting data to MIPS among clinicians in small practices is still a concern. Among those who we estimate would not submit data to MIPS, 85 percent are in small practices (23,056 out of 27,108 clinicians who do not submit data). We intend to continue working with stakeholders to improve engagement in MIPS among clinicians in small practices.
We want to highlight we are using 2019 MIPS performance period submissions data to simulate a 2022 MIPS performance period final score, and it is likely that there will be changes that we cannot account for at this time, including services and payments disrupted by the PHE for COVID-19 or clinicians changing behavior in response to the performance thresholds increased for the 2022 performance period/2024 payment year to avoid a negative payment adjustment. It should also be noted that the estimated number of clinicians who do not submit data to MIPS may be an overestimate of non-engagement in MIPS for the CY 2022 MIPS performance period/2024 payment year. This is because the PHE for COVID-19 may have resulted in fewer clinicians submitting data to MIPS or more clinicians electing to apply for the extreme and uncontrollable circumstances policies due to the PHE for COVID-19 for the 2019 MIPS performance period. Therefore, engagement levels in MIPS for the CY 2022 performance period/2024 payment year may differ from these reported estimates. We also note this participation data is generally based off participation for the 2019 performance period, which is associated with the 2019 performance period/2021 payment year and had a performance threshold of 30 points, and that participation may change for the 2022 performance period/2024 payment year when the performance threshold is 75 points.
Finally, the combined impact of negative and positive adjustments and the additional positive adjustments for exceptional performance as a percent of paid amount among those that do not submit data to MIPS was not the maximum negative payment adjustment of 9 percent possible because some MIPS eligible clinicians that do not submit data to MIPS receive a non-zero score for the cost performance category, which utilizes administrative claims data and does not require separate data submission to MIPS.
Start Printed Page 39555

Start Printed Page 39556

e. Estimated Impacts on Payments to MIPS Eligible Clinicians for the 2023 MIPS Performance Period/2025 MIPS Payment Year
We are proposing for the CY 2023 MIPS Performance Period to begin transitioning to MIPS Value Pathways (MVPs) and introduce subgroup reporting in the CY 2023 MIPS performance period/2025 payment year. As described in section IV.A.3.b.(2)(d) of the proposed rule, the first step in the transition plan for MVPs and subgroup reporting is to be voluntary, where eventually MVPs and subgroups will become required. Additionally, subgroups, if applicable, will have the option to report an APP. Since MVP and subgroup reporting will only begin in the CY 2023 MIPS performance period/2025 payment year, we do not have the data to report who would select MVP and who would report through subgroup in the first year and how these clinicians will score. For this regulatory impact analysis, we assume clinicians who elect to use MVPs and subgroups for reporting to MIPS will perform similarly to how they performed through traditional MIPS because the scoring policies are similar. As discussed in section V.B.8.e.(7)(a) of this proposed rule, for the purposes of estimating burden associated with the proposal to implement MVP and subgroup reporting, we assume that 10 percent of MIPS eligible clinicians in the CY 2022 MIPS performance period/2024 payment year will report as MVP participants in the CY 2023 MIPS performance period/2025 payment year. In addition, we assume that there will be 20 subgroup reporters in the CY 2023 MIPS performance period/2025 payment year. We anticipate a per respondent reduction of 3 hours and $412 dollars per CQM/QCDR quality submission, 3 hours and $336 per eCQM quality submission, and 5 hours and $717 per claims quality submission. Overall, we estimate a net reduction in burden of $7,463,145 in the quality performance category ICRs due to the introduction of MVP and subgroup reporting in the CY 2023 MIPPS performance period/2025 payment year. We refer readers to section V.B.8.e.(7)(a)(iii) of the proposed rule for further discussion of burden associated with MVPs and subgroups.
f. Additional Impacts from Outside Payment Adjustments
(1) Burden Overall
In addition to policies affecting the payment adjustments, we are proposing several policies that have an impact on burden in the CY 2022 and CY 2023 MIPS performance periods/2024 and 2025 payment years. In section V.B.8 of this proposed rule, we outline estimates of the costs of data collection that includes both the effect of proposed policy updates and adjustments due to the use of updated data sources. For each proposal included in this regulation which impacts our estimate of collection burden, the incremental burden for each is summarized in Table 133. We also provide proposed additional burden discussions that we are not able to quantify.
Start Printed Page 39557

Start Printed Page 39558

(2) Additional Impacts to Clinicians
(a) Web Interface
As discussed in section IV.A.3.d.(1)(d) of this proposed rule, we are proposing to continue the use of the CMS Web Interface measures as a collection type for groups and virtual groups with 25 or more eligible clinicians for the CY 2022 MIPS performance period/2024 MIPS payment year. We are also proposing to sunset the CMS Web Interface measures as a collection type for groups and virtual groups with 25 or more eligible clinicians starting with the CY 2023 MIPS performance period/2025 MIPS payment year. We refer readers to sections V.B.8.e.(8) and V.B.8.e.(10) of this proposed rule for our discussion on the estimated burden associated with the extension of the CMS Web Interface collection type in CY 2022 MIPS performance period/2024 MIPS payment year and the sunset of the CMS Web Interface collection type in the CY 2023 MIPS performance period/2025 payment year. Additionally, we assume that the impacts associated with the sunset of CMS Web Interface measures as a collection type for groups and virtual groups with 25 or more eligible clinicians will remain the same as our discussion in the CY 2021 PFS final rule (85 FR 85020 through 85021).
As discussed in section IV.A.3.c.(2)(a), we are proposing to extend the CMS Web Interface as a means of reporting quality under the APP for Shared Savings Program ACOs for the CY 2022 MIPS performance period/2024 payment year and the CY 2023 MIPS performance period/2025 payment year.
(b) Administrative Claims Measure
As discussed in section IV.A.3.d.(1)(e), we are proposing to add the following two new administrative claims measures beginning in the 2022 MIPS performance period and for future performance periods: (1) Risk-Standardized Acute Unplanned Cardiovascular-Related Admission Rates for Patients with Heart Failure for the Merit-based Incentive Payment System; and (2) Clinician and Clinician Group Risk-standardized Hospital Admission Rates for Patients with Multiple Chronic Conditions. We acknowledge there are administrative burdens and related financial costs associated with each administrative claims measure that clinicians, groups, and organizations may choose to monitor. However, because these costs can vary significantly due to organizational size, number of administrative claims measures being reported, volume of clinicians reporting each measure, and the specific methods employed to improve performance, we are unable to provide an estimate of the financial impact each clinician, group, or organization may experience. In summary, we are acknowledging that while there are no data submission requirements per § 414.1325(a)(2)(i) for administrative claim measures, there may be associated costs for clinicians and group practices to monitor new administrative claim measures; however, we are unable to quantify that impact.
(c) Modifications to the Improvement Activities Inventory
As discussed in section IV.A.3.d.(3)(c)(ii) of this proposed rule, we are proposing the removal of 7 previously adopted improvement activities, modification of 15 existing improvement activities, and adoption of 5 new improvement activities. We refer readers to Appendix 2 of this proposed rule for further details. We do not believe these proposed changes to the inventory will impact time or financial burden on stakeholders because MIPS eligible clinicians are still required to submit the same number of activities and the per response time for each activity is uniform. We do not expect these proposed changes to the inventory to affect our currently approved information collection burden estimates in terms of neither the number of estimated respondents nor the burden per response. We anticipate most clinicians performing improvement activities, to comply with existing MIPS Start Printed Page 39559 policies, would continue to perform the same activities under the policies in this proposed rule because previously finalized improvement activities continue to apply for the current and future years unless otherwise modified per rulemaking (82 FR 54175). Most of the improvement activities in the Inventory remain unchanged for the CY 2022 MIPS performance period.
(d) Stakeholders Nominating Improvement Activities
In section IV.A.3.d.(3)(c)(i)(B) of this rule, we are proposing: (1) To revise the required criteria for improvement activity nominations received through the Annual Call for Activities; (2) changes to the timeline for improvement activities nomination during a public health emergency (PHE); and (3) to suspend activities that become obsolete or impacted by clinical practice guideline changes from the program when this occurrence happens outside of the rulemaking process.
With regard to the proposal to clarify the timeline for an improvement activity nominated during the PHE, we believe this proposal will not affect our currently approved burden estimates since we believe that the number of nominations will not change, but it would make an activity available for reporting to clinicians in the same performance year it was intended to be implemented. In section IV.A.3.d.(3)(c)(i)(B)(aa) of this rule, we are proposing that in order to implement a new improvement activity for a PHE during the same year as the nomination, the nomination would need to be received no later than January 5th of the nomination year to be included in a rule for notice-and-comment rulemaking during that fiscal or calendar year, a necessary precursor to implementation if it were to be finalized. As described in section V.B.8.j of this rule, we expect additional nominations may be received as a result of this proposal, but we do not have any data with which to estimate what the additional number may be. As a result, we are not making any proposed revisions to our currently approved burden estimate.
Regarding the proposal to suspend activities that become obsolete or impacted by clinical practice guideline changes from the program when this occurrence happens outside of the rulemaking process, we do not anticipate additional burden for stakeholders because of the proposal described above as the proposed policy does not change requirements for the nomination of improvement activities.
As described in section IV.A.3.d.(3)(c)(i)(B) of this rule, due to the proposals to add two new criteria and to increase the number of criteria stakeholders are required to meet when submitting an activity proposal from a minimum of 1 to all 8 criteria, which includes the two new proposed criteria, we propose to revise our estimated annual information collection burden for nomination of improvement activities to 136 hours (31 nominations × 4.4 hr/nomination) at a cost of $20,355 (31 × [(2.8 hr × $114.24/hr) + (1.6 hr × $210.44/hr)]).
(e) Impact on Small Practices
As described in section VII.F.17.d.(3) of this proposed rule RIA, we found 85 percent of clinicians who did not submit data to MIPS were in small practices. However, the estimated number of MIPS eligible clinicians who do not submit data, including those in small practices, may be smaller in the CY 2022 performance period/2024 payment year since more clinicians may choose to submit data after the PHE for COVID-19. CMS is committed to identifying flexibilities and options to help clinicians in small practices participate meaningfully and successfully in MIPS. Specifically, CMS made several proposals to support clinicians in small practices once they engage with MIPS in the quality, improvement activities and Promoting Interoperability performance categories for the CY 2022 performance period/2024 payment year. Based on our RIA model findings described in section VII.F.17.d.(3) of this proposed rule, the proposed policies for the CY 2022 performance period/2024 payment year led to clinicians in small practices no longer disproportionately receiving negative payment adjustments compared to clinicians in larger sized practices. Therefore, the combination of the special scoring policies for clinicians in small practices is expected to positively affect this group of clinicians and will hopefully encourage and improve future engagement in MIPS among clinicians in small practices.
(f) Impact on Third Party Intermediaries
In section IV.A.3.h. of this rule, we are proposing multiple changes to the third-party intermediary regulations at § 414.1400. Specifically, we are proposing: (1) Reorganization and consolidation of § 414.1400 generally; (2) expanding the general participation requirements of third-party intermediaries to third party intermediaries reporting to MIPS on behalf of APM Entities in order to align reporting requirements for all participants in MIPS; (3) beginning with the CY 2023 MIPS performance period/2025 payment year, QCDRs and qualified registries must support MVPs that are applicable to the MVP participants on whose behalf they submit MIPS data. Health IT vendors must support MVPs that are applicable to the MVP participants on whose behalf they submit MIPS data; (4) require QCDRs, qualified registries, health IT vendors, and CAHPS for MIPS survey vendors to support subgroup reporting, beginning with the CY 2023 MIPS performance period/2025 payment year; (5) require QCDRs and qualified registries that have never submitted data since the inception of MIPS (CY 2017 MIPS performance period/2019 payment year) through the 2020 MIPS performance period/2022 payment year, to submit a participation plan as part of their self-nomination for CY 2023; (6) beginning with the 2024 MIPS performance period/2026 payment year, a QCDR or qualified registry that was approved but did not submit any MIPS data for either of the 2 years preceding the applicable self-nomination period must submit a participation plan for CMS' approval; (7) beginning with the CY 2023 MIPS performance period/2025 payment year, the QCDR or qualified registry must submit a data validation plan annually, at the time of self-nomination, for CMS' approval, and may not change the plan once approved, without the prior approval of the agency; and (8) add a rejection criterion to state that a QCDR does not have permission to use a QCDR measure owned by another QCDR for the applicable performance period. Additionally, to provide further clarification of our current policy (84 FR 63070 through 63073), we are proposing to state, if a QCDR measure owner is not an approved active QCDR for a given self-nomination period, that QCDR measure will not be available for use. The inactive QCDR measure owner has the option to transfer ownership of the QCDR measure to an active QCDR or agree upon terms set forth with the active QCDR allowing co-ownership of the QCDR measure.
With regard to the reorganization and consolidation of § 414.1400 generally, we do not anticipate this to require any additional effort for affected entities as the proposal is to allow CMS to reorganize the existing information.
For the requirements related to expanding the general participation requirements of third-party intermediaries to third party intermediaries reporting to MIPS on behalf of APM Entities in order to align reporting requirements for all Start Printed Page 39560 participants in MIPS, we do not propose to revise our burden estimates as this requirement is not different from how third-party intermediaries currently submit data for the quality, improvement activities and Promoting Interoperability performance categories in MIPS on behalf of individual eligible clinicians and groups.
As previously discussed in section IV.A.3.h.(2)(b)(ii) of this rule, we are proposing to require QCDRs, qualified registries, health IT vendors, and CAHPS for MIPS survey vendors to support subgroup reporting, beginning with the CY 2023 MIPS performance period/2025 payment year. During the MVP Town Hall held in January 2021 (85 FR 74729), we heard from third-party intermediaries that they are confident that they can make the necessary updates to allow for subgroup reporting, if they have enough time. A few vendors suggested that we add subgroup reporting to the existing CEHRT requirements. Given our proposal described in section IV.A.3.b.(2)(d) of this rule to delay the implementation of subgroup reporting option to the CY 2023 MIPS performance period/2025 payment year, we assume that the proposed delay would give these entities adequate time to make the necessary updates. We assume that there will be no additional burden that third-party intermediaries will incur to implement the subgroup reporting option. We anticipate that there may be administrative burden associated with changes in workflows to their existing systems for submission of subgroup data for the CY 2023 MIPS performance period/2025 payment year. However, given that each of these entities and their information technology systems are unique, we are unable to quantify the burden for these entities to capture and submit data on behalf of clinicians who may choose to participate as subgroups.
We do not anticipate a significant impact to QCDRs and qualified registries resulting from the finalized proposal to require QCDRs and qualified registries to conduct an annual data validation audit and if one or more deficiencies or data errors are identified also conduct targeted audits. First, we are not revising our burden estimates because the finalized data validation requirements are similar to existing expectations which we have already accounted for the associated burden as stated in the CY 2017 Quality Payment Program final rule (81 FR 77383 through 77384) and the CY 2019 PFS final rule (83 FR 59998 through 59999). Second, we believe that the proposed requirements for conduct of the data validation audits are aligned with methods and procedures which stakeholders currently utilize.
As discussed in section IV.A.3.h.(3)(a)(i) of this rule, due to the proposal to require QCDRs and qualified registries that have never submitted data since the inception of MIPS (CY 2017 MIPS performance period/2019 payment year) through the CY 2020 MIPS performance period/2022 payment year to submit a participation plan as part of their self-nomination for CY 2023 MIPS performance period, we refer readers to section V.B.8.c.(2) of this rule for details on the adjusted burden.
As discussed in section V.B.8.c.(2) of this rule, due to the proposal related to new rejection criteria for QCDR measures, we estimate that the annual burden will range from 855 hours (90 QCDRs × 9.5 hr) to 1,035 hours (90 QCDRs × 11.5 hr) at a cost ranging from $81,413 (855 hr × $95.22/hr) and $98,553 (1,035 hr × $95.22/hr).
(g) Assumptions & Limitations
We note several limitations to our estimates of clinicians' MIPS eligibility and participation, negative MIPS payment adjustments, and positive payment adjustments for the CY 2022 performance year/2024 payment year. Due to the PHE for COVID-19, we are aware that there may be changes in health care delivery and billing patterns that will impact results for the 2022 performance year/2024 payment year that we are not able to model with our historic data sources. The scoring model results presented in this proposed rule assume that CY 2019 Quality Payment Program data submissions and performance are representative of CY 2022 Quality Payment Program data submissions and performance. The estimated performance for the CY 2022 performance year/2024 payment year using CY 2019 Quality Payment Program data may be underestimated because the performance threshold to avoid a negative payment adjustment for the 2019 MIPS performance period/2021 MIPS payment year was significantly lower (30 out of 100 points) than the performance threshold for the 2022 performance year/2024 payment year (75 out of 100). We anticipate clinicians may submit more performance categories to meet the higher performance threshold to avoid a negative payment adjustment.
In our MIPS eligible clinician assumptions, we assumed that clinicians who elected to opt-in in the CY 2019 Quality Payment Program and submitted data would continue to elect to opt-in in the CY 2022 performance year/2024 payment year. It is difficult to predict whether clinicians will elect to opt-in to participate in MIPS with the proposed policies.
There are additional limitations to our estimates in addition to the limitations described throughout the methodology sections: (1) To the extent that there are year-to-year changes in the data submission, volume and mix of services provided by MIPS eligible clinicians, the actual impact on total Medicare revenues will be different from those shown in Table 129; and (2) our cost data does not overlap with CY 2019 so we may not be capturing performance for all clinicians. Due to the limitations described, there is considerable uncertainty around our estimates that is difficult to quantify.
G. Alternatives Considered
This proposed rule contains a range of policies, including some provisions related to specific statutory provisions. The preceding preamble provides descriptions of the statutory provisions that are addressed, identifies those policies when discretion has been exercised, presents rationale for our policies and, where relevant, alternatives that were considered. For purposes of the payment impact on PFS services of the policies contained in this proposed rule, we presented the estimated impact on total allowed charges by specialty.
1. Alternatives Considered for Utilization Data in PFS Ratesetting
As discussed earlier in this section II.C.1 (Changes in Relative Value Unit (RVU) Impacts), our estimates of changes in Medicare expenditures for PFS services compared payment rates for CY 2021 with payment rates for CY 2022 using CY 2020 Medicare utilization. As an alternative to using CY 2020 data, we considered using CY 2019 utilization data for the purposes of determining the proposed CY 2022 RVUs, as well as in determining the proposed CY 2022 budget neutrality adjustment and conversion factor. We considered using CY 2019 data due to the PHE for COVID-19, which has impacted the delivery of health care services over the past 18 months. Increases in remote delivery of services to reduce risk of exposure to both practitioner and patients, as well as postponement of elective procedures have resulted in a change to service utilization patterns across Medicare FFS payment systems. Specific to the PFS, overall service utilization decreased by approximately 20 percent in CY 2020 compared to CY 2019, which caused us to question whether CY 2020 data is the Start Printed Page 39561 best available data to use for CY 2022 ratesetting.
In order to determine if lower overall utilization in CY 2020 would result in differential impacts on specialties and practitioners, we modeled the PFS ratesetting process using CY 2019 utilization data. We found that the use of CY 2020 as opposed to CY 2019 data in establishing payment rates had relatively little differential impacts on payment, despite the approximately 20 percent decrease in overall service utilization. Table 134 illustrates specialty-specific impacts using CY 2019 data.

Start Printed Page 39562

The majority of specialties experienced shifts of less than a percent when we used CY 2019 data, as opposed to CY 2020 data, as displayed in Table 123, as the basis for setting rates. Several specialties shifted by approximately one percent. We did not detect a pattern of specialties that were notably affected by the choice of claims data, either positively or negatively. While Pediatrics shifted from a 1 percent impact when we used CY 2020 claims data to a 5-payment impact when we used CY 2019 claims data, this shift is likely due to the smaller amount of allowed charges associated with the Pediatrics specialty.
We analyzed the percentage change in total RVUs per practitioner. Using CY 2019 utilization data, Total RVUs change between -1 percent and 1 percent for 53 percent of practitioners, representing more than 48 percent of the changes in Total RUs for all practitioners, similar to the results we found when using CY 2020 claims that we discussed in section II.C.1. Variations by specialty were also similar to the results we found using CY 2020 claims and are contained in the public use file that describes the percentage change in total RVUs per practitioner.
Similar to the process described in section II.C.1. of this proposed rule, we used CY 2019 claims data to estimate the CY 2021 PFS CF to be 33.6184 which reflects a budget neutrality adjustment under section 1848(c)(2)(B)(ii)(II) of the Act, which we estimated to be −0.04 using CY 2019 data, the 0.00 percent update adjustment factor specified under section 1848(d)(19) of the Act, and the expiration of the 3.75 percent fee schedule payment increase for CY 2021 provided by the CAA. The anesthesia CF, which reflects the same overall PFS adjustments with the addition of anesthesia-specific PE and MP adjustments, would shift by a similar magnitude as the PFS CF. Thus, the estimated PFS CF and anesthesia CF using CY 2019 data is slightly higher compared to using claims data for CY 2020 with an estimated difference of 0.0336 (a little less than three and half cents). We note that the budget neutrality adjustment will be recalculated for the CY 2022 PFS final rule and the use of CY 2019 claims may or may not be higher than the use of CY 2020 claims based on which policies are ultimately finalized.
2. Alternatives Considered for Clinical Labor Pricing Update
As discussed in the PE section of this rule (section II.B. of this proposed rule), we are proposing to update the clinical labor pricing for CY 2022, in conjunction with the final year of the supply and equipment pricing update. We believe it is important to update the clinical labor pricing to maintain relativity with the recent supply and equipment pricing updates. We noted in regard to the specialty impacts in Table 123 that the proposed clinical labor pricing update will have a significant effect on the valuation of many services. We have occasionally implemented significant updates based on new data through a phased transition across several calendar years.
For example, we utilized a 4-year transition for the market-based supply and equipment pricing update which will conclude in CY 2022. We considered, as an alternative, the use of a similar 4-year transition to implement the clinical labor pricing update that could smooth out the increases and decreases caused by the pricing update for affected stakeholders. In order to evaluate a phased approach to the clinical labor pricing update, we modeled the PFS ratesetting process using clinical labor prices which transitioned one quarter of the distance to their fully implemented updated price. Table 135 illustrates specialty-specific impacts using the first year of a potential 4-year phase-in for clinical labor pricing:
Start Printed Page 39563

Start Printed Page 39564

Table 135 reflects all other proposed policies for CY 2022 with the only difference from Table 123 being the phased approach to updating the clinical labor pricing. Due to the budget neutral nature of PE under the PFS, we do not project that there would be any changes to the conversion factor if we were to use a 4-year transition for clinical labor pricing. We note that a phased transition would delay the full implementation of updated pricing and continue to rely on outdated data for clinical labor pricing.
3. Alternatives Considered for Split (or Shared) Visits
In section II.F of this proposed rule, we proposed to continue our current policy allowing billing of certain "split" or "shared" evaluation and management (E/M) visits by a physician, when the visit is performed in part by both a physician and an NPP, who are in the same group and the physician performs a substantive portion of the visit. We proposed to codify in our regulations a definition of a split (or shared) visit as an E/M visit in the facility setting that is performed in part by both a physician and NPP who are in the same group, in accordance with applicable laws and regulations, such that the E/M visit could be billed by either the physician or the NPP if it were furnished independently by only one of them in the facility setting (rather than as a split (or shared) visit). We proposed that the physician or NPP who performed the substantive portion of the split (or shared) visit would bill for the visit. We proposed to define substantive portion as more than half of the total time spent by the physician and NPP.
We considered several alternative approaches. First, we considered the option of proposing to disallow split (or shared) visit billing beginning in CY 2022. Under this alternative, in settings where payment for "incident to" services is prohibited, physicians and NPPs would only be able to bill for visits they furnish in their entirety under their own NPI. Such a policy would be administratively simple, and reduce the likelihood of paying significantly more than the actual resource costs incurred. When physicians and practitioners furnish services in facility settings, they do not ordinarily incur the cost of clinical staff or other PE costs involved in furnishing the services. When the physician bills for an E/M visit, in accordance with section 1833(a)(1)(N) of the Act, the Medicare Part B payment is equal to 80 percent of the payment basis under the PFS which, under section 1848(a)(1) of the Act, is the lesser of the actual charge or the full fee schedule amount for the service. In contrast, if the NPP bills for it, in accordance with section 1833(a)(1)(O) of the Act, the Medicare Part B payment is equal to 80 percent of the lesser of the actual charge or 85 percent of the fee schedule rate. Because of this payment differential and the lower resource costs associated with E/M visits performed partly by a physician and partly by an NPP, it could be argued that the physician should not be able to bill for such a visit and be paid at the higher fee schedule amount. However, our understanding is that longstanding clinical practice relies substantially upon shared visits between physicians and NPPs in facility settings. To avoid the potential disruption in this common medical practice approach, we did not choose to propose to disallow billing for split (or shared) visits and we are instead seeking comment to confirm current practice patterns and whether there is a need for this kind of split billing, and how often.
We also considered, but did not propose, several alternatives for how to define the substantive portion of split (or shared) visit. We considered proposing to define "substantive portion" as any face-to-face portion of the split (or shared) visit, consistent with our current definition. We do not believe it would be appropriate to consider just any portion of the visit—with or without direct patient contact—as a substantive portion. For instance, we do not believe it would be appropriate to consider a brief or trivial interaction, with or without direct patient contact, such as where the physician merely "pokes their head" into the room, to be a substantive portion of the visit. We do not believe it would be appropriate to permit a physician to bill for a visit if they do not substantially participate in the visit, given that physicians are paid under the PFS at a higher rate than NPPs. Therefore, we are proposing to define "substantive portion" as more than half of the total time spent by the physician or NPP.
Another alternative we considered, but did not propose, was to utilize the medical decision making (MDM) to define substantive portion. We did not choose this approach because MDM is not easily attributed to a single physician or NPP when the work is shared, because MDM is not necessarily quantifiable and can depend on patient characteristics (for example, risk). We believe that time is a more precise factor than MDM to use as a basis for deciding which practitioner performs the substantive portion of the visit. We believe that using the time spent by each practitioner furnishing the split (or shared) visit would provide a more precise metric than potentially finding a way to parse MDM between the physician and the NPP.
We also considered defining substantive portion as performance of the history and/or physical exam, which are key components of certain E/M visits. Given recent changes in the CPT E/M Guidelines, history and physical exam are no longer necessarily included in all E/M visits, because for office/ Start Printed Page 39565 outpatient E/M visits, the visit level can now be selected based on either MDM or time, and history and exam are performed only as medically appropriate. Also, the CPT Editorial Panel is considering removing history and physical exam as key visit components for institutional visits, similar to the changes already made for office/outpatient E/M visits. Accordingly, defining "substantive portion" as any key component including history or exam is not a viable approach.
Lastly, we considered not defining substantive portion in this proposed rule and instead leaving determinations regarding the substantive portion to MAC and/or medical review discretion. However, this approach would impose a significant burden on MACs to assess individual cases and could lead to too much regional variation in payment. We seek public comment to help inform what we consider to be the "substantive portion" of a split (or shared) visit in institutional settings and assist us in consideration of our proposed definition of "substantive portion".
We considered disallowing split (or shared) billing in critical care, skilled nursing facility (SNF) and nursing facility (NF) visits, as well as new patient and initial patient visits. We require certain SNF/NF visits to be provided entirely by a physician, but we believe we should allow shared visit billing for other visits that can be shared in these settings. (We refer readers to our Conditions of Participation in 42 CFR 483.30 for information regarding the SNF/NF visits that are required to be performed in their entirety by a physician. That regulation requires that certain SNF/NF visits must be furnished directly and solely by a physician). However, we believe current clinical practice generally allows sharing of critical care visits by appropriately trained and qualified practitioners, and we are seeking comment on this belief and this alternative considered. We proposed to allow split (or shared) visit billing in critical care because we believe the practice of medicine has evolved towards a more team-based approach to care, and greater integration in the practice of physicians and NPPs, particularly when care is furnished by clinicians in the same group in the facility setting. Given this evolution in medical practice, the concerns that may have been present when we issued current policy may no longer be as relevant. We understand that there have been changes in the practice of medicine over the past several years, some facilitated by the advent of EHRs and other systems, toward a more team-based approach to care. There has also been an increase in alternative payment models that employ a more team-based approach to care.
We are proposing to allow split (or shared) visits for both new and established patients as well as initial and subsequent visits. After conducting an internal review, including consulting our medical officers, we believe that the practice of medicine has evolved toward a more team-based approach to care, and greater integration in the practice of physicians and NPPs, particularly when care is furnished by practitioners in the same group in the facility setting. Given this evolution in medical practice, the concerns that may have been present when we issued the manual instructions may no longer be as relevant. We understand that there have been changes in the practice of medicine over the past several years, some facilitated by the advent of electronic health records (EHRs) and other systems, toward a more team-based approach to care. There has also been an increase in alternative payment models that employ a more team-based approach to care. In considering and reevaluating our policy, we see no reason to preclude the physician or NPP from billing for split (or shared) visits for a new patient, in addition to an established patient, or for initial and subsequent split (or shared) visits. Therefore, we are proposing to permit the physician or NPP to bill for split (or shared) visits for both new and established patients, as well as for initial and subsequent visits. We believe this approach is also consistent with the CPT E/M Guidelines for split (or shared) visits, which does not exclude these types of visits from being billed when furnished as split (or shared) services.
4. Alternatives Considered for Requiring Certain Manufacturers To Report Drug Pricing Information for Part B (§§ 414.802, 414.806)
This provision implements new statutory requirements under sections 1847A and 1927 of the Act, as amended by section 401 of the CAA (for the purposes of this section of this proposed rule, hereinafter is referred to as "section 401"). These new requirements will improve the accuracy of reported prices and limit the use of WAC-based pricing.
As discussed in section III.D.1. of this proposed rule, section 1847A(c)(6)(A) of the Act incorporates the definition of manufacturer at section 1927(k)(5) of the Act, but permits the Secretary to exempt repackagers from the definition of manufacturer, as determined appropriate, for purposes of section 1847A(f)(2) of the Act.
We considered whether to implement the flexibility afforded by the statute. However, implementing the flexibility afforded by the statute could potentially lead to a gap in the ASP reporting requirements, meaning that ASPs could be distorted to the extent that certain sales are carved out of the reporting requirement through the use of repackagers.
As discussed previously in this regulatory impact analysis, we are unable to quantitatively estimate the impacts of this provision. We welcome comments on our approach, and on the alternative relative to: (1) The likely costs or savings (to manufacturers, beneficiaries, the government, and other stakeholders); and (2) any other related impacts of this provision.
5. Alternatives Considered for the MDPP Expanded Model Emergency Policy
For the MDPP Expanded Model Emergency Policy, no alternatives were considered. The 2-year MDPP service period has depressed interest in MDPP among would-be MDPP suppliers. These proposed actions address stakeholder comments on the barriers to MDPP expanded model success. If we do not take action, we will not be able to scale MDPP as intended, impacting Medicare beneficiary access to this program. Reducing the MDPP from a 24- to a 12-month services period, increasing the year 1 performance payments, and waiving the Medicare provider enrollment application fee not only better aligns the model with the evidence that helped certify the DPP model test initially, but it will encourage eligible organizations to enroll as MDPP suppliers.
6. Alternatives Considered for the Quality Payment Program
For purposes of the payment impact on the Quality Payment Program, we view the performance threshold as a critical factor affecting the distribution of payment adjustments. We ran a separate proposed policies RIA model based on the actual mean for the CY 2019 performance period/2021 payment year with a performance threshold of 86 and an additional performance threshold of 92 points, which are potential values that may be used for the CY 2022 performance period/2024 payment year. The model with a performance threshold of 86 and additional performance threshold of 92 has the same mean and median final score as our proposed policies RIA model since the performance threshold does not change the final score. We estimate that $ 885 million would be redistributed through budget neutrality. Start Printed Page 39566 For clinicians who meet or exceed the additional performance threshold, an additional $294 million was distributed. The maximum positive payment adjustment would be 15.5 percent prior to the maximum additional payment adjustment and 25.5 percent after considering the MIPS maximum positive payment adjustment and the additional MIPS payment adjustment for exceptional performance. In addition, 70.3 percent of MIPS eligible clinicians would receive a negative payment adjustment among those that submit data.
We report the findings for the baseline RIA model which describes the impact for the 2022 MIPS performance period/2024 MIPS payment year if this regulation did not exist. The baseline RIA model has a final score mean of 78.13 and median of 82.59. We estimate that $428 million would be redistributed through budget neutrality. There would be a maximum payment adjustment of 6.6 percent after considering the MIPS payment adjustment and the additional MIPS payment adjustment for exceptional performance. In addition, 8.3 percent of MIPS eligible clinicians would receive a negative payment adjustment among those that submit data.
H. Impact on Beneficiaries
We do not believe these provisions will have a negative impact on beneficiaries given overall PFS budget neutrality.
1. Requiring Certain Manufacturers To Report Drug Pricing Information for Part B (§§ 414.802, 414.806)
Section 1927(b)(3)(A)(iii) of the Act requires manufacturers with a Medicaid drug rebate agreement to report ASP data consistent with the information required for such reporting at section 1847A of the Act. Some manufacturers without Medicaid drug rebate agreements voluntarily submit ASP data for their single source drugs or biologicals that are payable under Part B, however other manufacturers without Medicaid drug rebate agreements do not voluntarily submit such data. Without manufacturer reported ASP data, CMS cannot calculate the ASP payment limit, and consequently, payment is typically based on Wholesale Acquisition Cost (WAC).
Consistent with section 1847A(c)(3) of the Act and our regulations at § 414.804(a)(2), the ASP is net of price concessions. However, consistent with the definition of WAC at section 1847A(c)(6)(B) of the Act, the WAC is not net of price concessions and is thus nearly always, and sometimes significantly, higher than ASP. Drugs with payment allowances based on WAC may have greater "spreads" between acquisition costs and payment than drugs for which there is an ASP-based payment allowance, which, in turn, may: (1) Incent the use of the drug based on its spread rather than on purely clinical considerations; (2) result in increased payments under Medicare Part B; and (3) result in increased beneficiary cost sharing. This provision implements new statutory requirements under sections 1847A and 1927 of the Act, as amended by section 401 of Division CC, Title IV of the CAA, 2021. These new requirements will improve the accuracy of reported payment limits and limit the use of WAC-based pricing.
For single source drugs, these changes may result in lower payment limits because, typically, the WAC plus 3 percent is higher than ASP plus 6 percent. This then translates to cost savings for both the government and beneficiaries, who will pay coinsurance on a lesser amount. However, for the reason stated earlier in this regulatory impact analysis (see section VII.G.4. of this proposed rule), we are unable to predict the magnitude of this effect.
Similarly, payment limits for multiple source drugs could increase or decrease, and we are unable to predict the direction or magnitude of specific or aggregate effects at this time.
We welcome comment on: (1) The likely costs or savings to beneficiaries; and (2) other related impacts of this provision.
2. Determination of ASP for Certain Self-Administered Drug Products
Although we are unable to quantify the total magnitude of the potential savings, these changes have the potential to substantially reduce program expenditures and beneficiary coinsurance. The OIG's July 2020 report (discussed in section III.D.2. of this proposed rule) determined that the inclusion of self-administered versions of certolizumab and abatacept in their respective volume-weighted, average ASPs, alone, has resulted in $173 million in additional Medicare beneficiary coinsurance between 2014 and 2018.
The proposed regulatory changes have the potential to result in decreased payment limits for identified billing and payment codes and could, in turn, substantially reduce beneficiary coinsurance. Since section 405 of Division CC, Title IV of the CAA, 2021 directs CMS to implement the statutory changes at section 1847A(g)(3) of the Act beginning on July 1, 2021, these potential savings may be observed within the year.
We welcome comment on: (1) The likely costs or savings to beneficiaries; and (2) other related impacts of this provision.
3. Medicare Diabetes Prevention Program Expanded Model Emergency Policy
This change will have a positive impact on eligible MDPP beneficiaries, as it better aligns with the CDC's National DPP, giving both the participants and the coaches similar messaging around this program, regardless of payer. MDPP suppliers often offer the MDPP set of services to mixed cohorts, or classes with participants who are not eligible for MDPP, but who are enrolled in a National DPP cohort. Since MDPP generally follows the CDC's National DPP and aligns its program with the CDC's DPRP Standards, it is confusing to participants, coaches, and staff when talking about a 2-year program to its eligible Medicare participants when the non-Medicare participants have a 1-year program. Finally, reducing the MDPP service period from 2 years to one (1) year allows more cohorts to start and finish MDPP during the expanded model initial period of performance, which ends in March 2023.
4. Quality Payment Program
There are several changes in this rule that are expected to have a positive effect on beneficiaries. In general, we believe that many of these changes, including the MVP and subgroup proposals if finalized will lead to meaningful feedback to beneficiaries on the type and scope of care provided by clinicians. Additionally, beneficiaries could use the publicly reported information on clinician performance in subgroups to identify and choose clinicians in multispecialty groups relevant to their care needs. Consequently, we anticipate this will improve the quality and value of care provided to Medicare beneficiaries. For example, several of the new measures include patient-reported outcome-based measures, which may be used to help patients make more informed decisions about treatment options. Patient-reported outcome-based measures provide information on a patient's health status from the patient's point of view and may also provide valuable insights on factors such as quality of life, functional status, and overall disease experience, which may not otherwise be available through routine clinical data collection. Patient-reported outcome-based measured are factors Start Printed Page 39567 frequently of interest to patients when making decisions about treatment.
K. Estimating Regulatory Familiarization Costs
If regulations impose administrative costs on private entities, such as the time needed to read and interpret this rule, we should estimate the cost associated with regulatory review. Due to the uncertainty involved with accurately quantifying the number of entities that will review the rule, we assumed that the total number of unique commenters on this year's proposed rule will be the number of reviewers of last year's rule. We acknowledge that this assumption may understate or overstate the costs of reviewing this rule. It is possible that not all commenters will review this year's rule in detail, and it is also possible that some reviewers will choose not to comment on the rule. For these reasons we thought that the number of commenters would be a fair estimate of the number of reviewers of last year's rule. We welcome any comments on the approach in estimating the number of entities which will review this rule.
We also recognized that different types of entities are in many cases affected by mutually exclusive sections of this rule, and therefore for the purposes of our estimate we assume that each reviewer reads approximately 50 percent of the rule. We are seeking comments on this assumption.
Using the wage information from the BLS for medical and health service managers (Code 11-9111), we estimate that the cost of reviewing this rule is $114.24 per hour, including overhead and fringe benefits https://www.bls.gov/oes/current/oes_nat.htm. Assuming an average reading speed, we estimate that it would take approximately 8.0 hours for the staff to review half of this rule. For each facility that reviews the rule, the estimated cost is $913.92 (8.0 hours × $114.24). Therefore, we estimated that the total cost of reviewing this regulation is $36,764,260 ($885.92 × 40,227 reviewers on last year's proposed rule).
J. Accounting Statement
As required by OMB Circular A-4 (available at http://www.whitehouse.gov/omb/circulars/a004/a-4.pdf), in Tables 132 and 133 (Accounting Statements), we have prepared an accounting statement. This estimate includes growth in incurred benefits from CY 2021 to CY 2022 based on the FY 2022 President's Budget baseline.


K. Conclusion
The analysis in the previous sections, together with the remainder of this preamble, provided an initial Regulatory Flexibility Analysis. The previous analysis, together with the preceding portion of this preamble, provides an RIA. In accordance with the provisions of Executive Order 12866, this regulation was reviewed by the Office of Management and Budget.
Start List of Subjects
List of Subjects
42 CFR Part 403
- Grant programs—health
- Health insurance
- Hospitals
- Intergovernmental relations
- Medicare
- Reporting and recordkeeping requirements
42 CFR Part 405
- Administrative practice and procedure
- Diseases
- Health facilities
- Health insurance
- Health professions
- Medical devices
- Medicare
- Reporting and recordkeeping requirements
- Rural areas
- X-rays
42 CFR Part 410
- Diseases
- Health facilities
- Health professions
- Laboratories
- Medicare
- Reporting and recordkeeping requirements
- Rural areas
- X-rays
42 CFR Part 411
- Diseases
- Medicare
- Reporting and recordkeeping requirements
42 CFR Part 414
- Administrative practice and procedure
- Biologics
- Diseases
- Drugs
- Health facilities
- Health professions
- Medicare
- Reporting and recordkeeping requirements
42 CFR Part 415
- Health facilities
- Health professions
- Medicare
- Reporting and recordkeeping requirements
42 CFR Part 423
- Administrative practice and procedure
- Emergency medical services
- Health facilities
- Health maintenance organizations (HMO)
- Health professionals
- Medicare
- Penalties
- Privacy
- Reporting and recordkeeping requirements
42 CFR Part 424
- Emergency medical services
- Health facilities
- Health professions
- Medicare Reporting and recordkeeping requirements
42 CFR Part 425
- Administrative practice and procedure
- Health facilities
- Health professions
- Medicare
- Reporting and recordkeeping requirements
End List of Subjects
For the reasons set forth in the preamble, the Centers for Medicare & Medicaid Services proposes to amend 42 CFR chapter IV as set forth below:
Start Part
PART 403—SPECIAL PROGRAMS AND PROJECTS
End Part Start Amendment Part
1. The authority citation for part 403 continues to read as follows:
End Amendment Part
42 U.S.C. 1302, and 1395hh.
End Authority Start Amendment Part
2. In § 403.902:
End Amendment Part Start Amendment Part
a. Amend the definition of "Ownership or investment interest" by adding paragraphs (3)(vi) and (vii);
End Amendment Part Start Amendment Part
b. Add the definition of "Physician-owned distributorship" in alphabetical order; and
End Amendment Part Start Amendment Part
c. Revise the definition of "Short term medical supply or device loan".
End Amendment Part
The additions and revision read as follows:
Definitions.
* * * * *
Ownership or investment interest * * *
(3) * * *:
(vi) A titular ownership or investment interest that excludes the ability or right to receive the financial benefits of ownership or investment, including, but not limited to, the distribution of profits, dividends, proceeds of sale, or similar returns on investment; or
(vii) An interest in an entity that arises from an employee stock ownership plan (ESOP) that is qualified under section 401(a) of the Internal Revenue Code of 1986.
* * * * *
Physician-owned distributorship, for the purposes of determining the existence of a reportable ownership or investment interest under this subpart, means an entity that:
(1) Meets the definition of an applicable manufacturer or applicable group purchasing organization as defined in this section, and
(2) Meets at least one of the following two conditions:
(i) Has a minimum of 5 percent direct or indirect ownership or investment interest in the applicable manufacturer or applicable group purchasing organization held by a physician or a physician's immediate family member, or
(ii) A physician or a physician's immediate family member receives compensation from the applicable manufacturer or group purchasing organization in the form of a commission, return on investment, profit sharing, profit distribution, or other remuneration directly or indirectly derived from the sale or distribution of devices by the applicable manufacturer or group purchasing organization in which the physician or physician's immediate family member has ownership.
(3) This physician owned distributor definition does not apply for purposes of any other laws or regulations, including, but not limited to, section 1877 of the Act, the regulations at 42 CFR part 411, subpart J, section 1128B of the Act, or the regulations at 42 CFR 1001.952.
* * * * *
Short term medical supply or device loan means the loan of a covered device or a device under development, or the provision of a limited quantity of medical supplies for a short-term trial period, not to exceed a loan period of 90 cumulative days per calendar year or a quantity of 90 cumulative days of average daily use per calendar year, to permit evaluation of the device or medical supply by the covered recipient.
* * * * *
Start Amendment Part
3. Amend § 403.904 by adding paragraph (a)(3) to read as follows:
End Amendment Part
Reports of payments or other transfers of value to covered recipients.
(a) * * *
(3) An applicable manufacturer or applicable group purchasing organization that has reported payments or transfers of value under the scope of this section may not remove, delete, or alter any record/(s) unless an error is discovered in the information that had been furnished, or the record is otherwise believed to meet exceptions for reporting.
* * * * *
Start Amendment Part
4. Amend § 403.908 by revising paragraph (c)(3) and adding paragraph (c)(4) to read as follows:
End Amendment Part
Procedures for electronic submission of reports.
* * * * *
(c) * * *
(3) During registration, applicable manufacturers and applicable group purchasing organizations must name two points of contact with appropriate contact information. These points of contact must be updated for 2 years following record submission.
(4) An applicable manufacturer or applicable group purchasing organization that meets the definition of physician-owned distributorship as defined in § 403.902 must identify its status as a physician-owned distributorship when registering or recertifying.
* * * * *
Start Part
PART 405—FEDERAL HEALTH INSURANCE FOR THE AGED AND DISABLED
End Part Start Amendment Part
5. The authority citation for part 405 continues to read as follows:
End Amendment Part Start Authority
42 U.S.C. 263a, 405(a), 1302, 1320b-12, 1395x, 1395y(a), 1395ff, 1395hh, 1395kk, 1395rr, and 1395ww(k).
End Authority Start Amendment Part
6. Amend § 405.902 by adding the definitions of "Additional documentation", "Additional documentation request (ADR)", "Post-payment medical review", and "Prepayment medical review" in alphabetical order to read as follows:
End Amendment Part
Definitions.
Additional documentation means any information requested by a contractor when conducting a prepayment review or post-payment review.
Additional documentation request (ADR) means a contractor's initial documentation request in reviewing claims selected for prepayment review or post-payment review.
* * * * *
Post-payment medical review (or post-payment review) means a review that occurs after payment is made on the selected claim to determine whether the initial determination for payment was appropriate.
Prepayment medical review (or prepayment review) means a review that occurs before an initial determination for payment is made on the selected claim to determine whether payment should be made.
* * * * *
Start Amendment Part
7. Add § 405.903 to read as follows:
End Amendment Part
Prepayment review.
(a) A contractor may select a claim(s) for prepayment review.
(b) In conducting a prepayment review, a contractor may issue additional documentation requests to a provider or supplier.
(1) A provider or supplier will be provided 45 calendar days to submit additional documentation in response to a contractor's request, except as stated in paragraph (b)(2) and (c) of this section.
(2) A contractor may accept documentation received after 45-calendar days for good cause. Good cause means situations such as natural disasters, interruptions in business practices, or other extenuating circumstances that the contractor deems Start Printed Page 39569 good cause in accepting the documentation.
(c) A provider or supplier will be provided 30 calendar days to submit additional documentation in response to a UPIC's request for additional documentation. A UPIC may accept documentation received after the 30 calendar days for good cause. Good cause means situations such as natural disasters, interruptions in business practices, or other extenuating circumstances that the UPIC deems good cause in accepting the documentation.
(d) A contractor's prepayment review will result in an initial determination under § 405.920.
Start Amendment Part
8. Add § 405.929 under the "Initial Determinations" heading in Subpart I to read as follows:
End Amendment Part
Post-payment review.
(a) A contractor may select a claim(s) for post-payment review, which is conducted under the reopening authority in § 405.980.
(b) In conducting a post-payment review, a contractor may issue an additional documentation request to a provider or supplier.
(1) A provider or supplier will be provided 45 calendar days to submit additional documentation in response to a contractor's request, except as stated in paragraph (b)(2) and (c) of this section.
(2) A contractor may accept documentation received after 45 calendar days for good cause. Good cause means situations such as natural disasters, interruptions in business practices, or other extenuating circumstances that the contractor deems good cause in accepting the documentation.
(c) A provider or supplier will be provided 30 calendar days to submit additional documentation in response to a UPIC's request for additional documentation. A UPIC may accept documentation received after 30 calendar days for good cause. Good cause means situations such as natural disasters, interruptions in business practices, or other extenuating circumstances that the UPIC deems good cause in accepting the documentation.
(d) The outcome of a contractor's review will result in either no change to the initial determination or a revised determination under § 405.984.
Start Amendment Part
9. Add § 405.930 under the "Initial Determinations" heading in Subpart I to read as follows:
End Amendment Part
Failure to respond to additional documentation request.
If a contractor gives a provider or supplier notice and time to respond to an additional documentation request and the provider or supplier does not provide the additional documentation in a timely manner, the contractor has authority to deny the claim.
Start Amendment Part
10. Amend § 405.986 by revising paragraph (a) introductory text to read as follows:
End Amendment Part
Good cause for reopening.
(a) Establishing good cause for reopening. Good cause may be established when—
* * * * *
Start Amendment Part
11. Amend § 405.2411 by—
End Amendment Part Start Amendment Part
a. Revising paragraph (b)(2);
End Amendment Part Start Amendment Part
b. Redesignating paragraph (b)(3) as (4); and
End Amendment Part Start Amendment Part
c. Adding a new paragraph (b)(3).
End Amendment Part
The revision and addition read as follows:
Scope of benefits.
* * * * *
(b) * * *
(2) Covered when furnished during a Part A stay in a skilled nursing facility only when provided by a physician, nurse practitioner, physician assistant, certified nurse midwife or clinical psychologist employed or under contract with the RHC or FQHC at the time the services are furnished;
(3) Inclusive of hospice attending physician services, and are covered when furnished during a patient's hospice election only when provided by an RHC/FQHC physician, nurse practitioner, or physician assistant designated by the patient at the time of hospice election as his or her attending physician and employed or under contract with the RHC or FQHC at the time the services are furnished; and
* * * * *
Start Amendment Part
12. Amend § 405.2446 by revising paragraph (c) to read as follows:
End Amendment Part
Scope of services.
* * * * *
(c) FQHC services are covered when provided in outpatient settings only, including a patient's place of residence, which may be a skilled nursing facility or a nursing facility, other institution used as a patient's home, or are hospice attending physician services furnished during a hospice election.
* * * * *
Start Amendment Part
13. Amend § 405.2462 by—
End Amendment Part Start Amendment Part
a. Redesignating paragraphs (c) through (g) as paragraphs (e) through (i), respectively;
End Amendment Part Start Amendment Part
b. Revising paragraphs (a) through (c)
End Amendment Part Start Amendment Part
c. Adding new paragraph (d); and
End Amendment Part Start Amendment Part
d. In newly redesignated paragraph (e) introductory text, by removing the reference to "paragraph (d)" and adding in its place the reference to "paragraph (f)".
End Amendment Part
The additions and revisions read as follows:
Payment for RHC and FQHC services.
(a) Payment to independent RHCs that are authorized to bill under the reasonable cost system. (1) RHCs that are authorized to bill under the reasonable cost system are paid on the basis of an all-inclusive rate, subject to a payment limit per visit determined in paragraph (b) of this section, for each beneficiary visit for covered services. This rate is determined by the Medicare Administration Contractor (MAC), in accordance with this subpart and general instructions issued by CMS.
(2) The amount payable by the MAC for a visit is determined in accordance with paragraphs (i)(1) and (2) of this section.
(b) RHC payment limit per visit. (1) In establishing limits on payment for rural health clinic services provided by rural health clinics the limit for services provided prior to April 1, 2021:
(i) In 1988, after March 31, at $46 per visit; and
(ii) In a subsequent year (before April 1, 2021), at the limit established for the previous year increased by the percentage increase in the Medicare Economic Index (MEI) (as defined in section 1842(i)(3) of the Act) applicable to primary care services (as defined in section 1842(i)(4) of the Act) furnished as of the first day of that year.
(2) In establishing limits on payment for rural health services furnished on or after April 1, 2021, by rural health clinics or any rural health clinic that is enrolled on or after January 1, 2021 under section 1866(j) of the Act), the limit for services provided:
(i) In 2021, after March 31, at $100 per visit;
(ii) In 2022, at $113 per visit;
(iii) In 2023, at $126 per visit;
(iv) In 2024, at $139 per visit;
(v) In 2025, at $152 per visit;
(vi) In 2026, at $165 per visit;
(vii) In 2027, at $178 per visit; and
(viii) In 2028, at $190 per visit.
(ix) In a subsequent year, at the limit established for the previous year increased by the percentage increase in MEI applicable to primary care services furnished as of the first day of such year.
(3) In establishing limits on payment for rural health services furnished on or after April 1, 2021, by provider-based rural health clinics as described in Start Printed Page 39570 section (c)(4) of this part, the limit for services provided:
(i) In 2021, after March 31, at an amount equal to the greater of:
(A) For rural health clinics that had an all-inclusive rate established for services furnished in 2020—
(1) The all-inclusive rate applicable to the rural health clinic for services furnished in 2020, increased by the percentage increase in the MEI applicable to primary care services furnished as of the first day of 2021, or
(2) The payment limit per visit applicable in paragraph (2) of this section.
(B) For rural health clinics that did not have an all-inclusive rate established for services furnished in 2020—
(1) The all-inclusive rate applicable to the rural health clinic for services furnished in 2021, or
(2) The payment limit per visit applicable in paragraph (2) of this section.
(ii) In a subsequent year, at an amount equal to the greater of:
(A) The amount established under paragraph (b)(3)(i)(A) or (B) of this section, as applicable for the previous year, increased by the percentage increase in MEI applicable to primary care services furnished as of the first day of such subsequent year, or
(B) The payment limit per visit applicable under paragraph (2) of this section for such subsequent year.
(c) Payment to provider-based RHCs that are authorized to bill under the reasonable cost system. (1) An RHC that is authorized to bill under the reasonable cost system is paid in accordance with parts 405 and 413 of this subchapter, as applicable, if the RHC is—
(i) An integral and subordinate part of a hospital, skilled nursing facility or home health agency participating in Medicare (that is, a provider of services); and
(ii) Operated with other departments of the provider under common licensure, governance and professional supervision.
(2) An RHC, described in paragraph (c)(1) of this section, is paid on the basis of an all-inclusive rate, subject to a payment limit per visit, described in paragraphs (b)(1) and (2) of this part, for each beneficiary visit for covered services when in a hospital with greater than 50 beds as determined in § 412.105(b) of this chapter. This all-inclusive rate is determined by the MAC, in accordance with this subpart and general instructions issued by CMS. The amount payable by the MAC for a visit is determined in accordance with paragraphs (i)(1) and (2) of this section.
(3) Prior to April 1, 2021, an RHC, described in paragraph (c)(1) of this section, is paid on the basis of an all-inclusive rate and is not subject to a payment limit per visit described in paragraphs (b)(1) and (2) of this part for each beneficiary visit for covered services when in a hospital with less than 50 beds as determined in § 412.105(b) of this chapter. This all-inclusive rate is determined by the MAC, in accordance with this subpart and general instructions issued by CMS. The amount payable by the MAC for a visit is determined in accordance with paragraphs (i)(1) and (2) of this section.
(4) On or after April 1, 2021, an RHC, described in paragraph (c)(1) of this section, is paid on the basis of an all-inclusive rate, subject to a payment limit per visit, described in paragraph (b)(3) of this part, for each beneficiary visit for covered services when it meets the specified qualifications in paragraph(d) of this part. This all-inclusive rate is determined by the MAC, in accordance with this subpart and general instructions issued by CMS. The amount payable by the MAC for a visit is determined in accordance with paragraphs (i)(1) and (2) of this section.
(d) Specified qualifications. A provider-based rural health clinic must meet the following qualifications to have a payment limit per visit established in accordance with section (b)(3) of this part.
(1) As of December 31, 2020, was in a hospital with less than 50 beds (as determined in § 412.105(b) of this chapter) and after December 31, 2020 in a hospital that continues to have less than 50 beds (not taking into account any increase in the number of beds pursuant to a waiver during the COVID-19 Public Health Emergency (PHE)); and one of the following circumstances:
(i) As of December 31, 2020, was enrolled under section 1866(j) of the Act (including temporary enrollment during the COVID-19 PHE); or
(ii) Submitted an application for enrollment under section 1866(j) of the Act (or a request for temporary enrollment during the COVID-19 PHE) that was received not later than December 31, 2020.
(2) [Reserved]
* * * * *
Start Amendment Part
14. Amend § 405.2463 by revising paragraphs (a)(1)(i) introductory text, and (b)(3) introductory text to read as follows:
End Amendment Part
What constitutes a visit.
(a) * * *
(1) * * *
(i) Face-to-face encounter (or, for mental health disorders only, an encounter that meets the requirements under paragraph (b)(3) of this section) between an RHC patient and one of the following:
* * * * *
(b) * * *
(3) Visit—Mental health. A mental health visit is a face-to-face encounter or an encounter furnished using interactive, real-time, audio and video telecommunications technology or audio-only interactions in cases where the patient is not capable of, or does not consent to, the use of video technology for the purposes of diagnosis, evaluation or treatment of a mental health disorder between an RHC or FQHC patient and one of the following:
* * * * *
Start Amendment Part
15. Amend § 405.2466 by revising paragraph (b)(1)(iv) to read as follows:
End Amendment Part
Annual reconciliation.
* * * * *
(b) * * *
(1) * * *
(iv) For RHCs and FQHCs, payment for pneumococcal, influenza, and COVID-19 vaccine and their administration is 100 percent of Medicare reasonable cost.
* * * * *
Start Amendment Part
16. Amend § 405.2469 by revising paragraph (d) to read as follows:
End Amendment Part
FQHC supplemental payments.
* * * * *
(d) Per visit supplemental payment. A supplemental payment required under this section is made to the FQHC when a covered face-to-face encounter or an encounter furnished using interactive, real-time, audio and video telecommunications technology or audio-only interactions in cases where beneficiaries do not wish to use or do not have access to devices that permit a two-way, audio/video interaction for the purposes of diagnosis, evaluation or treatment of a mental health disorder occurs between a MA enrollee and a practitioner as set forth in § 405.2463.
Start Part
PART 410—SUPPLEMENTARY MEDICAL INSURANCE (SMI) BENEFITS
End Part Start Amendment Part
17. The authority citation for part 410 continues to read as follows:
End Amendment Part Start Authority
42 U.S.C. 1302, 1395m, 1395hh, 1395rr, and 1395ddd.
End Authority Start Amendment Part
18. Amend § 410.33 by revising paragraphs (c), (g)(6)(i), (g)(6)(ii), (g)(8)(i), (g)(8)(ii), and (g)(9) to read as follows:
End Amendment Part
Start Printed Page 39571
Independent diagnostic testing facility.
* * * * *
(c) Nonphysician personnel. (1) Except as otherwise stated in paragraph (c)(2) of this section, any nonphysician personnel used by the IDTF to perform tests must demonstrate the basic qualifications to perform the tests in question and have training and proficiency as evidenced by licensure or certification by the appropriate State health or education department. In the absence of a State licensing board, the technician must be certified by an appropriate national credentialing body. The IDTF must maintain documentation available for review that these requirements are met.
(2) For services that do not require direct or in-person beneficiary interaction, treatment, or testing, any nonphysician personnel used by the IDTF to perform the tests must meet all applicable State licensure requirements for doing so. If there are any applicable State licensure requirements, the IDTF must maintain documentation available for review that these requirements are met.
* * * * *
(g) * * *
(6) * * *
(i) Except as otherwise stated in paragraph (g)(6)(ii) of this section, have a comprehensive liability insurance policy of at least $300,000 per location that covers both the place of business and all customers and employees of the IDTF. The policy must be carried by a nonrelative-owned company. Failure to maintain required insurance at all times will result in revocation of the IDTF's billing privileges retroactive to the date the insurance lapsed. IDTF suppliers are responsible for providing the contact information for the issuing insurance agent and the underwriter. In addition, the IDTF must—
(A) Ensure that the insurance policy must remain in force at all times and provide coverage of at least $300,000 per incident; and
(B) Notify the CMS designated contractor in writing of any policy changes or cancellations.
(ii) Paragraph (g)(6)(i) of this section does not apply to IDTFs that only perform services that do not require direct or in-person beneficiary interaction, treatment, or testing.
* * * * *
(8) * * *
(i) Except as otherwise stated in paragraph (g)(8)(ii) of this section, answer, document, and maintain documentation of a beneficiary's written clinical complaint at the physical site of the IDTF. (For mobile IDTFs, this documentation would be stored at their home office.) This includes, but is not limited to, the following:
(A) The name, address, telephone number, and health insurance claim number of the beneficiary.
(B) The date the complaint was received, the name of the person receiving the complaint, and a summary of actions taken to resolve the complaint.
(C) If an investigation was not conducted, the name of the person making the decision and the reason for the decision.
(ii) Paragraph (g)(8)(i) of this section does not apply to IDTFs that only perform services that do not require direct or in-person beneficiary interaction, treatment, or testing.
(9) Openly post these standards for review by patients and the public. (This requirement does not apply to IDTFs that only perform services that do not require direct or in-person beneficiary interaction, treatment, or testing.)
* * * * *
Start Amendment Part
19. Amend § 410.37 by adding paragraph (j) to read as follows:
End Amendment Part
Colorectal cancer screening tests: Conditions for and limitations on coverage.
* * * * *
(j) Effective January 1, 2022, colorectal cancer screening tests include a planned screening flexible sigmoidoscopy or screening colonoscopy that involves the removal of tissue or other matter or other procedure furnished in connection with, as a result of, and in the same clinical encounter as the screening test.
Start Amendment Part
20. Amend § 410.47 by—
End Amendment Part Start Amendment Part
a. Revising the section heading as set forth below;
End Amendment Part Start Amendment Part
b. In paragraph (a), revising the definitions of "Individualized treatment plan", "Medical director", Outcomes assessment", "Physician prescribed exercise", "Psychosocial assessment", and "Supervising physician";
End Amendment Part Start Amendment Part
c. Revising paragraphs (b), (c), (d), and (e);
End Amendment Part Start Amendment Part
d. Removing paragraph (f).
End Amendment Part Start Amendment Part
e. Redesignating paragraph (g) as new paragraph (f).
End Amendment Part
The revisions read as follows:
Pulmonary rehabilitation program: Conditions of coverage.
(a) * * *
Individualized treatment plan means a written plan tailored to each individual patient that includes all of the following:
(i) A description of the individual's diagnosis.
(ii) The type, amount, frequency, and duration of the items and services furnished under the plan.
(iii) The goals set for the individual under the plan.
Medical director means the physician who oversees the pulmonary rehabilitation program at a particular site.
Outcomes assessment means an evaluation of progress as it relates to the individual's rehabilitation which includes the following:
(i) Evaluations, based on patient-centered outcomes, which must be measured by the physician or program staff at the beginning and end of the program. Evaluations measured by program staff must be considered by the physician in developing and/or reviewing individualized treatment plans.
(ii) Objective clinical measures of exercise performance and self-reported measures of shortness of breath and behavior.
* * * * *
Physician-prescribed exercise means aerobic exercise, combined with other types of exercise (such as conditioning, breathing retraining, step, and strengthening) as determined to be appropriate for individual patients by a physician.
Psychosocial assessment means an evaluation of an individual's mental and emotional functioning as it relates to the individual's rehabilitation or respiratory condition which includes an assessment of those aspects of an individual's family and home situation that affects the individual's rehabilitation treatment, and psychosocial evaluation of the individual's response to and rate of progress under the treatment plan.
* * * * *
Supervising physician means a physician that is immediately available and accessible for medical consultations and medical emergencies at all times items and services are being furnished to individuals under pulmonary rehabilitation programs.
(b) General rule. (1) Covered conditions. Medicare Part B covers pulmonary rehabilitation for beneficiaries:
(i) With moderate to very severe COPD (defined as GOLD classification II, III and IV), when referred by the physician treating the chronic respiratory disease;
(ii) Who were hospitalized with a COVID-19 diagnosis and experience persistent symptoms, including respiratory dysfunction, for at least four weeks after hospital discharge;
(iii) Additional medical indications for coverage for pulmonary Start Printed Page 39572 rehabilitation may be established through a national coverage determination (NCD).
(2) Components. Pulmonary rehabilitation must include all of the following components:
(i) Physician-prescribed exercise during each pulmonary rehabilitation session.
(ii) Education or training that is closely and clearly related to the individual's care and treatment which is tailored to the individual's needs and assists in achievement of goals toward independence in activities of daily living, adaptation to limitations and improved quality of life. Education must include information on respiratory problem management and, if appropriate, brief smoking cessation counseling.
(iii) Psychosocial assessment.
(iv) Outcomes assessment.
(v) An individualized treatment plan detailing how components are utilized for each patient. The individualized treatment plan must be established, reviewed, and signed by a physician every 30 days.
(3) Settings. (i) Medicare Part B pays for pulmonary rehabilitation in the following settings:
(A) A physician's office.
(B) A hospital outpatient setting.
(ii) All settings must have the following:
(A) A physician immediately available and accessible for medical consultations and emergencies at all times when items and services are being furnished under the program. This provision is satisfied if the physician meets the requirements for direct supervision for physician office services at § 410.26 of this subpart; and for hospital outpatient services at § 410.27 of this subpart.
(B) The necessary cardio-pulmonary, emergency, diagnostic, and therapeutic life-saving equipment accepted by the medical community as medically necessary (for example, oxygen, cardiopulmonary resuscitation equipment, and defibrillator) to treat chronic respiratory disease.
(c) Medical director standards. The physician responsible for a pulmonary rehabilitation program is identified as the medical director. The medical director, in consultation with staff, is involved in directing the progress of individuals in the program and must possess all of the following:
(1) Expertise in the management of individuals with respiratory pathophysiology.
(2) Cardiopulmonary training in basic life support or advanced cardiac life support.
(3) Be licensed to practice medicine in the State in which the pulmonary rehabilitation program is offered.
(d) Supervising physician standards. Physicians acting as the supervising physician must possess all of the following:
(1) Expertise in the management of individuals with respiratory pathophysiology.
(2) Cardiopulmonary training in basic life support or advanced cardiac life support.
(3) Be licensed to practice medicine in the State in which the pulmonary rehabilitation program is offered.
(e) Limitations on coverage: The number of pulmonary rehabilitation sessions are limited to a maximum of 2 1-hour sessions per day for up to 36 sessions over up to 36 weeks with the option for an additional 36 sessions over an extended period of time if approved by the Medicare Administrative Contractor.
* * * * *
Start Amendment Part
21. Amend § 410.49 by—
End Amendment Part Start Amendment Part
a. In paragraph (a), revising the definition of "Medical director", revising paragraph (i) in the definition of "Outcomes assessment", and revising the definition of "Physician-prescribed exercise"; and
End Amendment Part Start Amendment Part
b. Revising paragraphs (b)(1) introductory text, (b)(2) paragraph heading and introductory text, (b)(2)(ii), (b)(3)(i) introductory text, (d) paragraph heading and introductory text, (e) paragraph heading and introductory text, and (f).
End Amendment Part
The revisions read as follows:
Cardiac rehabilitation program and intensive cardiac rehabilitation program: Conditions of coverage.
(a) * * *
Medical director means the physician who oversees the cardiac rehabilitation or intensive cardiac rehabilitation program at a particular site.
Outcomes assessment * * *
(i) Evaluations, based on patient-centered outcomes, which must be measured by the physician or program staff at the beginning and end of the program. Evaluations measured by program staff must be considered by the physician in developing and/or reviewing individualized treatment plans.
* * * * *
Physician-prescribed exercise means aerobic exercise combined with other types of exercise (such as strengthening and stretching) as determined to be appropriate for individual patients by a physician.
* * * * *
(b) * * *
(1) Covered conditions. Medicare Part B covers cardiac rehabilitation and intensive cardiac rehabilitation for beneficiaries who have experienced one or more of the following:
* * * * *
(2) Components. Cardiac rehabilitation and intensive cardiac rehabilitation must include all of the following:
* * * * *
(ii) Cardiac risk factor modification, including education, counseling, and behavioral intervention, tailored to the individual's needs.
* * * * *
(3) * * *
(i) Medicare Part B pays for cardiac rehabilitation and intensive cardiac rehabilitation in the following settings:
* * * * *
(d) Medical director standards. The physician responsible for a cardiac rehabilitation program or intensive cardiac rehabilitation program is identified as the medical director. The medical director, in consultation with staff, is involved in directing the progress of individuals in the program and must possess all of the following:
* * * * *
(e) Supervising physician standards. Physicians acting as the supervising physician must possess all of the following:
* * * * *
(f) Limitations on coverage. (1) Cardiac rehabilitation: The number of cardiac rehabilitation sessions are limited to a maximum of 2 1-hour sessions per day for up to 36 sessions over up to 36 weeks with the option for an additional 36 sessions over an extended period of time if approved by the Medicare Administrative Contractor.
(2) Intensive cardiac rehabilitation: Intensive cardiac rehabilitation sessions are limited to 72 1-hour sessions (as defined in section 1848(b)(5) of the Act), up to 6 sessions per day, over a period of up to 18 weeks.
Start Amendment Part
22. Amend § 410.59 by—
End Amendment Part Start Amendment Part
a. Revising paragraph (a)(4)(iii)(B); and
End Amendment Part Start Amendment Part
b. Adding paragraphs (a)(4)(iv) and (v).
End Amendment Part
The revision and additions read as follows:
Outpatient occupational therapy services: Conditions.
(a) * * *
(4) * * *
(iii) * * *
(B) Except as provided in paragraph (a)(4)(iv) of this section, furnishes a portion of a service, or in the case of a Start Printed Page 39573 15-minute timed code, a portion of a unit of service separately from the part furnished by the occupational therapist such that the minutes for that portion of a service (or unit of a service) furnished by the occupational therapist assistant exceed 10 percent of the total minutes for that service (or unit of a service).
(iv) Paragraph (a)(4)(iii)(B) of this section does not apply when determining whether the prescribed modifier applies to the last 15-minute unit of a service billed for a patient on a treatment day when the occupational therapist provides more than the midpoint of a 15-minute timed code, that is, 8 or more minutes, regardless of any minutes for the same service furnished by the occupational therapy assistant.
(v) Where there are two remaining 15-minute units to bill of the same service, and the occupational therapist and occupational therapy assistant each provided between 9 and 14 minutes of the service with a total time of at least 23 minutes and no more than 28 minutes, one unit of the service is billed with the prescribed modifier for the minutes furnished by the occupational therapy assistant and one unit is billed without the prescribed modifier for the service provided by the occupational therapist.
* * * * *
Start Amendment Part
23. Amend § 410.60 by—
End Amendment Part Start Amendment Part
a. Revising paragraph (a)(4)(iii)(B); and
End Amendment Part Start Amendment Part
b. Adding paragraphs (a)(4)(iv) and (v).
End Amendment Part
The revision and additions read as follows:
Outpatient physical therapy services: Conditions.
(a) * * *
(4) * * *
(iii) * * *
(B) Except as provided in paragraph (a)(4)(iv) of this section, furnishes a portion of a service, or in the case of a 15-minute timed code, a portion of a unit of service separately from the part furnished by the physical therapist such that the minutes for that portion of a service (or unit of a service) furnished by the physical therapist assistant exceed 10 percent of the total minutes for that service (or unit of a service).
(iv) Paragraph (a)(4)(iii)(B) of this section does not apply when determining whether the prescribed modifier applies to the last 15-minute unit of a service billed for a patient on a treatment day, when the physical therapist provides more than the midpoint of a 15-minute timed code, that is, 8 or more minutes, regardless of any minutes for the same service furnished by the physical therapist assistant.
(v) Where there are two remaining 15-minute units to bill of the same service, and the physical therapist and physical therapist assistant each provided between 9 and 14 minutes of the service with a total time of at least 23 minutes, one unit of the service is billed with the prescribed modifier for the minutes furnished by the physical therapist assistant and one unit is billed without the prescribed modifier for the service provided by the physical therapist.
* * * * *
Start Amendment Part
24. Amend § 410.67 by—
End Amendment Part Start Amendment Part
a. In paragraph (b), revising paragraphs (3) and (4) in the definition of "Opioid use disorder treatment service";
End Amendment Part Start Amendment Part
b. Revising paragraphs (d)(4)(ii) and (iii), and (d)(5);
End Amendment Part Start Amendment Part
c. Adding paragraph (d)(6).
End Amendment Part
The revisions and addition read as follows:
Medicare coverage and payment of Opioid use disorder treatment services furnished by Opioid treatment programs.
* * * * *
(b) * * *
Opioid use disorder treatment service * * *
(3) Substance use counseling by a professional to the extent authorized under State law to furnish such services including services furnished via two-way interactive audio-video communication technology, as clinically appropriate, and in compliance with all applicable requirements. During a Public Health Emergency, as defined in § 400.200 of this chapter, or for services furnished after the end of such emergency, in cases where audio/video communication technology is not available to the beneficiary, the counseling services may be furnished using audio-only telephone calls if all other applicable requirements are met.
(4) Individual and group therapy with a physician or psychologist (or other mental health professional to the extent authorized under State law), including services furnished via two-way interactive audio-video communication technology, as clinically appropriate, and in compliance with all applicable requirements. During a Public Health Emergency, as defined in § 400.200 of this chapter, or for services furnished after the end of such emergency, in cases where audio/video communication technology is not available to the beneficiary, the therapy services may be furnished using audio-only telephone calls if all other applicable requirements are met.
* * * * *
(d) * * *
(4) * * *
(ii) The payment amounts for the non-drug component of the bundled payment for an episode of care, the adjustments for counseling or therapy, intake activities, periodic assessments, and the non-drug component of the adjustment for take-home supplies of opioid antagonist medications will be geographically adjusted using the Geographic Adjustment Factor described in § 414.26 of this chapter.
(iii) The payment amounts for the non-drug component of the bundled payment for an episode of care, the adjustments for counseling or therapy, intake activities, periodic assessments, and the non-drug component of the adjustment for take-home supplies of opioid antagonist medications will be updated annually using the Medicare Economic Index described in § 405.504(d) of this chapter.
(5) Payment for medications delivered, administered or dispensed to a beneficiary as part of the bundled payment or an adjustment to the bundled payment under paragraph (d)(4)(i) of this section is considered a duplicative payment if a claim for delivery, administration or dispensing of the same medications for the same beneficiary on the same date of service was also separately paid under Medicare Part B or Part D. CMS will recoup the duplicative payment made to the opioid treatment program.
(6) When substance use counseling under paragraph (b)(3) of this section or therapy services under paragraph (b)(4) of this section are furnished using audio-only telephone calls after the end of the Public Health Emergency as defined in § 400.200 of this chapter, the practitioner must document in the beneficiary's medical record that the services were furnished using audio-only technology and the rationale for doing so. For purposes of the adjustment to the bundled payment under paragraph (d)(4)(i)(A) of this section, the practitioner must also certify, in a form and manner specified by CMS, that they had the capacity to furnish the services using two-way, audio/video communication technology, but used audio-only technology because audio/video communication technology was not available to the beneficiary.
* * * * *
Start Amendment Part
25. Add § 410.72 to read as follows:
End Amendment Part
Registered dietitians' and nutrition professionals' services.
(a) Definition: Registered dietitians and nutrition professionals. Meet the qualifications at § 410.134. Start Printed Page 39574
(b) Covered registered dietitian and nutrition professional services. Medicare Part B covers:
(1) Coverage condition. Medical nutrition therapy (MNT) services as defined at § 410.130 under the conditions of coverage at § 410.132.
(2) Other services. Registered dietitians and nutrition professionals may also provide diabetes self-management (DSMT) services if they are or represent an accredited DSMT entity and have an order from a physician or qualified nonphysician practitioner who is treating the patient's diabetic condition.
(3) Limits on MNT and DSMT. (i) DSMT and MNT cannot be furnished to a patient on the same date of service, and
(ii) MNT and DSMT services cannot be furnished incident to the professional services of a physician or nonphysician practitioner service.
(c) Limitations. The following services are not registered dietitian or nutrition professional services for purposes of billing Medicare Part B:
(1) Services furnished by a registered dietitian or nutrition professional to an inpatient of a Medicare-participating hospital.
(2) Services furnished by a registered dietitian or nutrition professional to an inpatient of a Medicare-participating SNF.
(3) Services furnished by a registered dietitian or nutrition professional to a patient in a Medicare-participating ESRD facility in accordance with the limitation on coverage of MNT service listed at § 410.132(b)(1).
(d) Professional services. Registered dietitians and nutrition professionals can be paid for professional services only when the services have been personally performed by them.
(e) Telehealth services. MNT and DMST services may be provided as telehealth service (meeting the requirements in § 410.78) when registered dietitians or nutrition professionals act as distant site practitioners.
(f) Restrictions. The services are provided on an assignment-related basis, and a registered dietitian or nutrition professional may not charge a beneficiary for a service not payable under this provision. If a beneficiary has made payment for a service, the registered dietitian or nutrition professional must make the appropriate refund to the beneficiary.
Start Amendment Part
26. Amend § 410.74 by revising paragraph (a)(2)(v) to read as follows:
End Amendment Part
Physician assistants' services.
(a) * * *
(2) * * *
(v) Prior to January 1, 2022, furnishes services that are billed by the employer of a physician assistant; and
* * * * *
Start Amendment Part
27. Amend 410.78 by—
End Amendment Part Start Amendment Part
a. Revising paragraph (a)(3); and
End Amendment Part Start Amendment Part
b. Adding paragraphs (b)(3)(xiii) and (xiv), and (b)(4)(iv)(D).
End Amendment Part
The revision and additions read as follows:
Telehealth services.
(a) * * *
(3) Interactive telecommunications system means, except as otherwise provided in this paragraph, multimedia communications equipment that includes, at a minimum, audio and video equipment permitting two-way, real-time interactive communication between the patient and distant site physician or practitioner. For services furnished for purposes of diagnosis, evaluation, or treatment of a mental health disorder to a patient in their home, interactive telecommunications may include two-way, real-time audio-only communication technology if the distant site physician or practitioner is technically capable to use an interactive telecommunications system as defined in the previous sentence, but the patient is not capable of, or does not consent to, the use of video technology. A modifier designated by CMS must be appended to the claim for services described in this paragraph to verify that these conditions have been met.
* * * * *
(b) * * *
(3) * * *
(xiii) A rural emergency hospital (as defined in section 1861(kkk)(2) of the Act), for services furnished on or after January 1, 2023.
(xiv) The home of a beneficiary for the purposes of diagnosis, evaluation, and/or treatment of a mental health disorder for services furnished on or after the first day after the end of the PHE as defined in our regulation at § 400.200. Payment will not be made for a telehealth service furnished under this paragraph unless the physician or practitioner has furnished an item or service in person, without the use of telehealth, for which Medicare payment was made (or would have been made if the patient were entitled to, or enrolled for, Medicare benefits at the time the item or service is furnished) within 6 months prior to the initial telehealth service and at least once within 6 months prior to the initial telehealth service and at least once within 6 months before any subsequent Medicare telehealth service.
(4) * * *
(iv) * * *
(D) Services furnished on or after the first day after the end of the PHE as defined in our regulation at § 400.200 for the purposes of diagnosis, evaluation, and/or treatment of a mental health disorder. Payment will not be made for a telehealth service furnished under this paragraph unless the physician or practitioner has furnished an item or service in person, without the use of telehealth, for which Medicare payment was made (or would have been made if the patient were entitled to, or enrolled for, Medicare benefits at the time the item or service is furnished) within 6 months prior to the initial telehealth service and within 6 months of any subsequent telehealth service.
* * * * *
Start Amendment Part
28. Amend § 410.79 by—
End Amendment Part Start Amendment Part
a. Revising paragraph (c)(1)(ii);
End Amendment Part Start Amendment Part
b. Removing the second duplicate paragraph (c)(3)(ii); and
End Amendment Part Start Amendment Part
c. Revising paragraph (e)(3)(v)(C).
End Amendment Part
The revisions read as follows:
Medicare Diabetes Prevention Program expanded model: Conditions of coverage.
* * * * *
(c) * * *
(1) * * *
(ii) An MDPP beneficiary is eligible for the first ongoing maintenance session interval only if the beneficiary:
(A) Starts his or her first core session on or before December 31, 2021;
(B) Attends at least one in-person core maintenance session during the final core maintenance session interval; and
(C) Achieves or maintains the required minimum weight loss at a minimum of one in-person core maintenance session during the final core maintenance session interval.
* * * * *
(e) * * *
(3) * * *
(v) * * *
(C) Beneficiaries who began the set of MDPP services between January 1, 2021 and December 31, 2021 and who are in the second year of the set of MDPP services as of the start of an applicable 1135 waiver event, whose in-person sessions are suspended due to the applicable 1135 waiver event, and who elect not to continue with MDPP services virtually, may restart the ongoing maintenance session interval in which they were participating at the start of the applicable 1135 waiver event or may resume with the most recent attendance session of record.
* * * * *
Start Amendment Part
29. Amend § 410.105 by— Start Printed Page 39575
End Amendment Part Start Amendment Part
a. Revising paragraph (d)(3)(ii); and
End Amendment Part Start Amendment Part
b. Adding paragraphs (d)(3)(iii) and (iv).
End Amendment Part
The revision and additions read as follows:
Requirements for coverage of CORF services.
* * * * *
(d) * * *
(3) * * *
(ii) Except as provided in paragraph (d)(3)(iii) of this section, furnishes a portion of a service, or in the case of a 15-minute timed code, a portion of a unit of service, separately from the part furnished by the physical or occupational therapist such that the minutes for that portion of a service (or unit of a service) exceed 10 percent of the total time for that service (or unit of a service).
(iii) Paragraph (d)(3)((ii) of this section does not apply when determining whether the prescribed modifier applies to the last 15-minute unit of a service billed for a patient on a treatment day when the physical or occupational therapist provides more than the midpoint of a 15-minute timed code, that is, 8 or more minutes, regardless of any minutes for the same service furnished by the physical therapist assistant or occupational therapy assistant.
(iv) Where there are two remaining 15-minute units to bill of the same service and the physical therapist and the physical therapist assistant or the occupational therapist and the occupational therapy assistant, as applicable, each provided between 9 and 14 minutes, with a total time of at least 23 minutes, one unit of the service is billed with the prescribed modifier for the minutes furnished by the physical therapist assistant or occupational therapy assistant and one unit is billed without the prescribed modifier for the service provided by the physical therapist or occupational therapist.
Start Amendment Part
30. Amend § 410.130 by—
End Amendment Part Start Amendment Part
a. Revising the definition of "Chronic renal insufficiency"; and
End Amendment Part Start Amendment Part
b. Removing the definition of "Treating physician".
End Amendment Part
The revision reads as follows:
Definitions.
* * * * *
Chronic renal insufficiency means the stage of renal disease associated with a reduction in renal function not severe enough to require dialysis or transplantation (glomerular filtration rate [GFR] 15-59 ml/min/1.73m2).
* * * * *
Start Amendment Part
31. Amend § 410.132 by revising paragraphs (a), (b)(5), and (c) to read as follows.
End Amendment Part
Medical nutrition therapy.
(a) Conditions for coverage of MNT services. Medicare Part B pays for MNT services provided by a registered dietitian or nutrition professional as defined in § 410.134 when the beneficiary is referred for the service by a physician.
(b) * * *
(5) An exception to the maximum number of hours in paragraphs (b)(2), (3), and (4) of this section may be made when a physician determines that there is a change of diagnosis, medical condition, or treatment regimen related to diabetes or renal disease that requires a change in MNT during an episode of care.
(c) Referrals. Referral may only be made by a physician when the beneficiary has been diagnosed with diabetes or renal disease as defined in this subpart with documentation noted by a referring physician in the beneficiary's medical record.
Start Amendment Part
32. Amend § 410.150 by revising paragraph (b)(15) to read as follows:
End Amendment Part
To whom payment is made.
* * * * *
(b) * * *
(15)(i) Prior to January 1, 2022, to the qualified employer of a physician assistant for professional services furnished by the physician assistant and for services and supplies provided incident to his or her services. Payment is made to the employer of a physician assistant regardless of whether the physician assistant furnishes services under a W-2, employer-employee employment relationship, or whether the physician assistant is an independent contractor who receives a 1099 reflecting the relationship. Both types of relationships must conform to the appropriate guidelines provided by the Internal Revenue Service. A qualified employer is not a group of physician assistants that incorporate to bill for their services. Payment is made only if no facility or other provider charges or is paid any amount for services furnished by a physician assistant.
(ii) Effective on or after January 1, 2022, payment is made to a physician assistant for professional services furnished by a physician assistant in all settings in both rural and nonrural areas and for services and supplies furnished incident to those services. Payment is made only if no facility or other provider charges, or is paid, any amount for the furnishing of professional services of the physician assistant.
* * * * *
Start Amendment Part
33. Amend § 410.152 by revising paragraphs (l) introductory text and (l)(5) to read as follows:
End Amendment Part
Amounts of payment.
* * * * *
(l) Amount of payment: Preventive services. Except as provided otherwise in this paragraph, Medicare Part B pays 100 percent of the Medicare payment amount established under the applicable payment methodology for the furnished by a provider or supplier for the following preventive services:
* * * * *
(5) Colorectal cancer screening tests (excluding barium enemas).
(i) For the colorectal cancer screening tests described in § 410.37(j) of this part, Medicare Part B pays at the specified percentage as follows:
(A) 80 percent for CY 2022.
(B) 85 percent for CY 2023 through 2026.
(C) 90 percent for 2027 through 2029.
(D) 100 percent beginning January 1, 2030.
(ii) [Reserved]
* * * * *
Start Part
PART 411—EXCLUSIONS FROM MEDICARE AND LIMITATIONS ON MEDICARE PAYMENT
End Part Start Amendment Part
34. The authority citation for part 411 continues to read as follows:
End Amendment Part Start Authority
42 U.S.C. 1302, 1395w-101 through 1395w-152, 1395hh, and 1395nn.
End Authority Start Amendment Part
35. Amend § 411.351 by revising the definition of "List of CPT/HCPCS Codes" to read as follows:
End Amendment Part
Definitions.
* * * * *
List of CPT/HCPCS Codes means the list of CPT and HCPCS codes that identifies those items and services that are designated health services under section 1877 of the Act or that may qualify for certain exceptions under section 1877 of the Act. It is updated each calendar quarter and posted on the CMS website at https://www.cms.gov/Medicare/Fraud-and-Abuse/PhysicianSelfReferral/List_of_Codes.
* * * * *
Start Amendment Part
36. Amend § 311.354 by—
End Amendment Part Start Amendment Part
a. Revising paragraphs (c)(2)(ii)(A)( 2) and (3);
End Amendment Part Start Amendment Part
b. Adding paragraph (c)(2)(ii)(A)( 4); and
End Amendment Part Start Amendment Part
c. Revising paragraph (c)(2)(ii)(B).
End Amendment Part
The revisions and addition read as follows:
Start Printed Page 39576
Financial relationship, compensation, and ownership or investment interest.
* * * * *
(c) * * *
(2) * * *
(ii) * * *
(A) * * *
(2) Is calculated using a formula that includes the physician's referrals to the entity furnishing designated health services as a variable, resulting in an increase or decrease in the amount of compensation that positively correlates with the number or value of the physician's referrals to the entity;
(3) Is calculated using a formula that includes other business generated by the physician for the entity furnishing designated health services as a variable, resulting in an increase or decrease in the amount of compensation per unit that positively correlates with the physician's generation of other business for the entity; or
(4) Is payment for anything other than services personally performed by the physician (or immediate family member).
(B) For purposes of applying paragraph (c)(2)(ii)(A) of this section:
(1) A positive correlation between two variables exists when one variable decreases as the other variable decreases, or one variable increases as the other variable increases.
(2) The individual unit is:
(i) Time, where the compensation paid to the physician (or immediate family member) is based solely on the period of time during which the services are provided.
(ii) Service, where the compensation paid to the physician (or immediate family member) is based solely on the service provided.
(iii) Time, where the compensation paid to the physician (or immediate family member) is not based solely on the period of time during which a service is provided or based solely on the service provided.
(3) Services that are personally performed by a physician (or immediate family member) do not include services that are performed by any person other than the physician (or immediate family member), including, but not limited to, the referring physician's (or immediate family member's) employees, independent contractors, group practice members, or persons supervised by the physician (or the immediate family member).
* * * * *
Start Amendment Part
37. Amend § 411.355 by revising paragraph (h) to read as follows:
End Amendment Part
General exceptions to the referral prohibition related to both ownership/investment and compensation.
* * * * *
(h) Preventive screening tests and vaccines. (1) Preventive screening tests and vaccines that meet the following conditions:
(i) The preventive screening test or vaccine is listed on the List of CPT/HCPCS Codes as a code to which the exception in this paragraph (h) is applicable.
(ii) The preventive screening test or vaccine is covered by Medicare.
(iii) The preventive screening test or vaccine is subject to a CMS-mandated frequency limit.
(2) During such period as the vaccine is not subject to a CMS-mandated frequency limit, paragraph (h)(1)(iii) of this section does not apply to a COVID-19 vaccine identified on the List of CPT/HCPCS Codes as a code to which the exception in this paragraph (h) is applicable.
* * * * *
Start Part
PART 414—PAYMENT FOR PART B MEDICAL AND OTHER HEALTH SERVICES
End Part Start Amendment Part
38. The authority citation for part 414 continues to read as follows:
End Amendment Part Start Authority
42 U.S.C. 1302, 1395hh, and 1395rr(b)(l).
End Authority Start Amendment Part
39. Amend § 414.64 by revising paragraph (a) to read as follows:
End Amendment Part
Payment for medical nutrition therapy.
(a) Payment under the physician fee schedule. Medicare payment for medical nutrition therapy is made under the physician fee schedule in accordance with subpart B of this part. Payment to nonphysician professionals, as specified in paragraph (b) of this section, is 80 percent (or 100 percent if such services are recommended with a grade of A or B by the United States Preventive Services Task Force for any indication or population and are appropriate for the individual) or the lesser of the actual charges or 85 percent of the physician fee schedule amount.
* * * * *
Start Amendment Part
40. Amend § 414.84 by—
End Amendment Part Start Amendment Part
a. Revising paragraphs (b)(1)(i)
End Amendment Part Start Amendment Part
b. In paragraph (b)(1)(ii) by removing the reference "CY 2018" and adding in its place the reference "CY 2022";
End Amendment Part Start Amendment Part
c. Revising paragraph (b)(2)(i)
End Amendment Part Start Amendment Part
d. In paragraph (b)(2)(ii) by removing the reference CY 2018" and adding in its place the reference "CY 2022";
End Amendment Part Start Amendment Part
e. Revising paragraph (b)(3)(i);
End Amendment Part Start Amendment Part
f. In paragraph (b)(3)(ii) by removing the reference "CY 2018" and adding in its place the reference "CY 2022";
End Amendment Part Start Amendment Part
g. Revising paragraph (b)(4)(i)(A);
End Amendment Part Start Amendment Part
h. In paragraphs (b)(4)(i)(B) and (b)(4)(ii)(B) by removing the reference "CY 2018" and adding in its place the reference "CY 2022"
End Amendment Part Start Amendment Part
i. Revising paragraph (b)(4)(ii)(A); and
End Amendment Part Start Amendment Part
j. Revising paragraphs (b)(5), (b)(6)(i), (b)(7)(i) and (ii), and (c).
End Amendment Part
The revisions read as follows:
Payment for MDPP Services.
* * * * *
(b) * * *
(1) * * *
(i) For a first core session furnished January 1, 2022 through December 31, 2022 the amount is $26.
* * * * *
(2) * * *
(i) For the fourth core session furnished January 1, 2022 through December 31, 2022 the amount is $78.
* * * * *
(3) * * *
(i) For the ninth core session furnished January 1, 2022 through December 31, 2022 the amount is $130.
* * * * *
(4) * * *
(i) * * *
(A) For a second core maintenance session January 1, 2022 through December 31, 2022 the amount is $106.
* * * * *
(ii) * * *
(A) For a second core maintenance session January 1, 2022 through December 31, 2022 the amount is $52.
* * * * *
(5) Performance Goal 5: Attends two ongoing maintenance sessions and maintains the required minimum weight loss during an ongoing maintenance session interval. For an MDPP beneficiary who attends his or her first core session on or before December 31, 2021, CMS makes a performance payment to an MDPP supplier if an MDPP beneficiary attends two ongoing maintenance sessions during an ongoing maintenance session interval, achieves attendance at that second ongoing maintenance session upon attendance at an ongoing maintenance session furnished by that supplier, and achieves or maintains the required minimum weight loss as measured in-person during an ongoing maintenance session furnished during the applicable ongoing maintenance session interval. CMS makes this performance payment to an MDPP supplier only once per MDPP beneficiary per ongoing maintenance session interval. The amount of this performance payment is determined as follows: Start Printed Page 39577
(i) For a second ongoing maintenance session furnished in interval 1 (months 13-15 of the MDPP services period), January 1, 2022 through December 31, 2022 the amount is $52.
(ii) For a second ongoing maintenance session furnished in interval 2 (months 16-18 of the MDPP services period), January 1, 2022 through December 31, 2022 the amount is $52.
(iii) For a second ongoing maintenance session furnished in interval 3 (months 19-21 of the MDPP services period), January 1, 2022 through December 31, 2022 the amount is $53.
(iv) For a second ongoing maintenance session furnished in interval 4 (months 22-24 of the MDPP services period), January 1, 2022 through December 31, 2022 the amount is $53.
(v) For a second ongoing maintenance session furnished during a subsequent year. The performance payment amount specified in this paragraph, adjusted as specified in paragraph (d) of this section.
(6) * * *
(i) For a core session or core maintenance session, as applicable, furnished January 1, 2022 through December 31, 2022 the amount is $189.
* * * * *
(7) * * *
(i) For a core session or core maintenance session, as applicable, furnished January 1, 2022 through December 31, 2022 the amount is $26.
(ii) For a core session or core maintenance session, as applicable, furnished during a calendar year subsequent to CY 2018. The performance payment amount specified in this paragraph, adjusted as specified in paragraph (d) of this section.
(c) Bridge payment. CMS makes a bridge payment to an MDPP supplier only for a core session or core maintenance session furnished to an MDPP beneficiary who has previously received MDPP services from a different MDPP supplier. An MDPP supplier that has previously been paid either a bridge payment or a performance payment for an MDPP beneficiary is not eligible to be paid a bridge payment for that beneficiary. A bridge payment is made only on an assignment-related basis in accordance with § 424.55 of this chapter, and MDPP suppliers must accept the Medicare allowed charge as payment in full and may not bill or collect from the beneficiary any amount. CMS will make a bridge payment only to an MDPP supplier that complies with all applicable enrollment and program requirements, and only for MDPP services furnished by an eligible coach, on or after his or her coach eligibility start date and, if applicable, before his or her coach eligibility end date. As a condition of payment, the MDPP supplier must report the NPI of the coach who furnished the session on the claim for the MDPP session. The amount of the bridge payment is determined as follows:
(1) For core session or core maintenance session, as applicable, furnished January 1, 2022 through December 31, 2022 the amount is $26.
(2) For core session and core maintenance session, as applicable, furnished during a calendar year subsequent to CY 2022. The bridge payment amount specified in this paragraph, adjusted as specified in paragraph (d) of this section.
* * * * *
Start Amendment Part
41. Amend § 414.626 by revising paragraphs (b)(1) and (f) to read as follows:
End Amendment Part
Data reporting by ground ambulance organizations.
* * * * *
(b) * * *
(1) Within 30 days of the date that CMS notifies a ground ambulance organization under paragraph (c)(3) of this section that it has selected the ground ambulance organization to report data under this section, the ground ambulance organization must select a data collection period that corresponds with its annual accounting period and provide the start date of that data collection period to CMS or its contractor.
* * * * *
(f) Public availability of data. Beginning in 2024, and at least once every 2 years thereafter, CMS will post on its website data that it collected under this section, including but not limited to summary statistics and ground ambulance organization characteristics.
* * * * *
Start Amendment Part
42. Amend § 414.802 by revising the definition of "Drug" to read as follows:
End Amendment Part
Definitions.
* * * * *
Drug means a drug or a biological, and for purposes of applying section 1847A(f) of the Act, includes an item, service, supply, or product that is payable under Medicare Part B as a drug or biological.
* * * * *
Start Amendment Part
43. Section 414.806 is revised to read as follows:
End Amendment Part
Penalties associated with misrepresentation and the failure to submit timely and accurate ASP data.
(a) Misrepresentation. Section 1847A(d)(4)(A) specifies the penalties associated with misrepresentations in the reporting of the manufacturer's average sales price for a drug as defined at § 414.802.
(b) Failure to provide timely information or the submission of false information. (1) For a manufacturer that has entered into and has in effect a rebate agreement under section 1927 of the Act, section 1927(b)(3)(C) of the Act specifies the penalties associated with a manufacturer's failure to submit timely information or the submission of false information.
(2) For a manufacturer that has not entered into and does not have in effect a rebate agreement under section 1927 of the Act, sections 1847A(d)(4)(B) and (C) of the Act specify the penalties associated with a manufacturer's failure to submit timely information or the submission of false information.
Start Amendment Part
44. Amend § 414.904 by adding paragraph (d)(4) to read as follows:
End Amendment Part
Average sales price as the basis for payment.
* * * * *
(d) * * *
(4) Payment adjustment for certain drugs for which there is a self-administered version.
(i) In general. Except as provided in paragraphs (d)(4)(ii) and (iii) of this section, if the Inspector General identifies a drug or biological product in a study described in section 1847A(g)(1) of the Act, the Secretary must apply the payment limit for the applicable billing and payment code as specified in paragraph (d)(4)(iv) of this section, beginning with the first day of the second quarter after such study is publicly available. The methodology described in this paragraph will be recalculated each quarter thereafter, except when conditions described in paragraph (d)(4)(ii) are met.
(ii) Exception. The adjustment described in paragraph (d)(4)(i) of this section does not apply to the payment limit for a billing and payment code for a quarter if, at the time that ASP calculations are finalized for such quarter, the drug in the dosage form described by the billing and payment code is included by the FDA on the drug shortage list in effect under section 506E of the Federal Food, Drug, and Cosmetic Act.
(iii) Special rule for certain billing and payment codes. Effective July 1, 2021, for a billing and payment code described under section 1847A(g)(3) of Start Printed Page 39578 the Act, the payment limit for the applicable billing and payment code must be determined as described in paragraph (d)(4)(iv) of this section, and the exception specified at paragraph (d)(4)(ii) of this section does not apply.
(iv) Lesser-of methodology. For purposes of this section, the payment limit is the lesser of:
(A) The payment limit determined under section 1847A of the Act for such billing and payment code if each National Drug Code for such product so identified under section 1847A(g)(1) of the Act were excluded from such determination; and
(B) The payment limit otherwise determined under section 1847A of the Act for such billing and payment code without application of section 1847A(g) of the Act.
(v) NDC changes. For an Inspector General-identified National Drug Code, as described under section 1847A(g)(1) or (3) of the Act, for which the manufacturer has redesignated the National Drug Code (without changes to the dosage form), the application of the lesser-of methodology described in this paragraph must use manufacturer-reported ASP data associated with the redesignated National Drug Code in the same manner as the one originally identified by the Inspector General.
* * * * *
Start Amendment Part
45. Amend § 414.1300 by revising paragraphs (a)(2) and (3) to read as follows:
End Amendment Part
Basis and scope.
(a) * * *
(2) Section 1848(k)—Quality Reporting System.
(3) Section 1848(m)—Incentive Payments for Quality Reporting.
* * * * *
Start Amendment Part
46. Amend § 414.1305 by—
End Amendment Part Start Amendment Part
a. Revising the definitions of "Collection type" and "Meaningful EHR user for MIPS";
End Amendment Part Start Amendment Part
b. In the definition of "MIPS determination period", revising paragraph (2).
End Amendment Part Start Amendment Part
c. In the definition of "MIPS eligible clinician", revising the introductory text, paragraph (2) introductory text, and adding paragraph (3);
End Amendment Part Start Amendment Part
d. Adding the definitions of "Multispecialty group", "MVP participant", "Population health measure", "QCDR measure", "Single specialty group", "Special status" and "Subgroup" in alphabetical order; and
End Amendment Part Start Amendment Part
e. Revising the definition of "Submission type".
End Amendment Part
The revision and additions read as follows:
Definitions.
* * * * *
Collection type means a set of quality measures with comparable specifications and data completeness criteria, as applicable, including, but not limited to: electronic clinical quality measures (eCQMs); MIPS clinical quality measures (MIPS CQMs); QCDR measures; Medicare Part B claims measures; for the CY 2017 through 2022 MIPS performance periods/2019 through 2024 MIPS payment years, CMS Web Interface measures; the CAHPS for MIPS survey; and administrative claims measures.
* * * * *
Meaningful EHR user for MIPS means a MIPS eligible clinician who possesses CEHRT, uses the functionality of CEHRT, reports on applicable objectives and measures specified for the Promoting Interoperability performance category for a performance period in the form and manner specified by CMS, does not knowingly and willfully take action (such as to disable functionality) to limit or restrict the compatibility or interoperability of CEHRT, and engages in activities related to supporting providers with the performance of CEHRT.
* * * * *
MIPS determination period means: * * *
(2) Subject to § 414.1310(b)(1)(iii), an individual eligible clinician, group, or APM Entity group that is identified as not exceeding the low-volume threshold or as having special status, as applicable, during the first segment of the MIPS determination period will be identified as such for the applicable MIPS payment year regardless of the results of the second segment of the MIPS determination period. An individual eligible clinician, group, or APM Entity group for which the unique billing TIN and NPI combination is established during the second segment of the MIPS determination period will be assessed based solely on the results of such segment.
MIPS eligible clinician as identified by a unique billing TIN and NPI combination used to assess performance, means any of the following (except as excluded under § 414.1310(b)):
* * * * *
(2) For the 2021 through 2023 MIPS payment years:
* * * * *
(3) For the 2024 MIPS payment year and future years:
(i) A clinician described in paragraph (2) of this definition;
(ii) A clinical social worker (as defined in section 1861(hh)(1) of the Act);
(ii) A certified nurse midwife (as defined in section 1861(gg)(2) of the Act); and
(vii) A group that includes such clinicians.
* * * * *
Multispecialty group means a group that consists of two or more specialty types as identified by eligible clinicians in the Medicare Provider Enrollment, Chain, and Ownership System (PECOS).
MVP participant means an individual MIPS eligible clinician, multispecialty group, single-specialty group, subgroup, or APM Entity that is assessed on an MVP in accordance with § 414.1365 for all MIPS performance categories. For the CY 2025 MIPS performance period/2027 MIPS payment year and future years, MVP Participant means an individual MIPS eligible clinician, single-specialty group, subgroup, or APM Entity that is assessed on an MVP in accordance with § 414.1365 for all MIPS performance categories.
* * * * *
Population health measure means a quality measure that indicates the quality of a population or cohort's overall health and well-being, such as access to care, clinical outcomes, coordination of care and community services, health behaviors, preventive care and screening, health equity, or utilization of health services.
* * * * *
QCDR measure means a quality measure that is submitted by a QCDR and approved by CMS under § 414.1400. QCDR measures consist of:
(1) Measures that are not included in the MIPS final list of quality measures described in § 414.1330(a)(1) for the applicable MIPS payment year; and
(2) Measures that are included in the MIPS final list of quality measures described in § 414.1330(a)(1) for the applicable MIPS payment year, but have undergone substantive changes, as determined by CMS.
* * * * *
Single-specialty group means a group that consists of one specialty type as identified by eligible clinicians in the PECOS.
* * * * *
Special status means that a MIPS eligible clinician:
(1) Meets the definition of an ASC-based MIPS eligible clinician, facility-based MIPS eligible clinician, hospital-based MIPS eligible clinician, non-patient facing MIPS eligible clinician, or is in a small practice; or Start Printed Page 39579
(2) Is located in an HPSA or rural area.
Subgroup means a subset of a group which contains at least one MIPS eligible clinician and is identified by a combination of the group TIN, subgroup identifier, and each eligible clinician's NPI.
Submission type means the mechanism by which the submitter type submits data to CMS, including, but not limited to:
(1) Direct;
(2) Log in and upload;
(3) Log in and attest;
(4) Medicare Part B claims; and
(5) For the CY 2017 through 2022 MIPS performance periods/2019 through 2024 MIPS payment years, the CMS Web Interface.
* * * * *
Start Amendment Part
47. Amend § 414.1310 by revising paragraph (e)(1) to read as follows:
End Amendment Part
Applicability.
* * * * *
(e) * * * (1) Except as provided under §§ 414.1315(a)(2), 414.1317(b), 414.1318(b), and 414.1370(f)(2) each MIPS eligible clinician in the group receives a final score based on the group's combined performance assessment.
* * * * *
Start Amendment Part
48. Amend § 414.1317 by revising paragraph (b)(2) to read as follows:
End Amendment Part
APM Entity groups.
* * * * *
(b) * * *
(2) Performance category weights. The cost performance category weight is zero percent of the final score for an APM Entity. The performance category reweighting scenarios under § 414.1380(c)(2) apply to an APM Entity.
* * * * *
Start Amendment Part
49. Section 414.1318 is added to subpart O to read as follows:
End Amendment Part
Subgroups.
(a) Eligibility and special status. (1) General. Except as provided under paragraph (a)(2) of this section, for a MIPS payment year, determinations of meeting the low-volume threshold criteria and special status for subgroups are determined at the group level in accordance with §§ 414.1305 and 414.1310.
(2) Exclusions. An individual eligible clinician or group that elects to participate in MIPS as a MIPS eligible clinician in accordance with § 414.1310(b)(iii)(A) or § 414.1310(b)(2) is not eligible to participate in a subgroup.
(b) Final score. Except as provided under § 414.1317(b), each MIPS eligible clinician in the subgroup receives a final score based on the subgroup's combined performance assessment.
(c) Subgroup reporting requirements. For individual eligible clinicians to participate in MIPS as a subgroup, all of the following requirements must be met:
(1) Individual eligible clinicians that elect to participate in MIPS as a subgroup must aggregate their quality and improvement activities performance data across the subgroup's identifier.
(2) Individual eligible clinicians that elect to participate in MIPS as a subgroup will have their performance assessed at the subgroup level across all the MIPS performance categories based on an MVP in accordance with § 414.1365 and on the APM Performance Pathway in accordance with § 414.1367, as applicable. Subgroups that are MVP Participants must adhere to an election process described in § 414.1365(b).
Start Amendment Part
50. Amend § 414.1320 by—
End Amendment Part Start Amendment Part
a. Redesignating paragraphs (d) through (g) as paragraphs (e) through (h).
End Amendment Part Start Amendment Part
b. Adding a new paragraph (d).
End Amendment Part
The addition reads as follows:
MIPS performance period.
* * * * *
(d) For purposes of the 2022 MIPS payment year, the performance period for:
(1) The quality and cost performance categories are the full calendar year (January 1 through December 31) that occurs 2 years prior to the applicable MIPS payment year.
(2) The improvement activities performance categories are a minimum of a continuous 90-day period within the calendar year that occurs 2 years prior to the applicable MIPS payment year, up to and including the full calendar year.
* * * * *
Start Amendment Part
51. Amend § 414.1325 by revising paragraph (c)(1) to read follows:
End Amendment Part
Data submission requirements.
* * * * *
(c) * * *
(1) For the quality performance category, the direct; login and upload; Medicare Part B claims (beginning with the CY 2019 MIPS performance period/2021 MIPS payment year, for small practices only); and, for the CY2017 through 2022 MIPS performance periods/2019 through 2024 MIPS payment years, CMS Web Interface (for groups consisting of 25 or more eligible clinicians or a third party intermediary submitting on behalf of a group) submission types.
* * * * *
Start Amendment Part
52. Amend § 414.1340 by—
End Amendment Part Start Amendment Part
a. Revising paragraph (a)(3);
End Amendment Part Start Amendment Part
b. Adding paragraph (a)(4);
End Amendment Part Start Amendment Part
c. Revising paragraph (b)(3); and
End Amendment Part Start Amendment Part
d. Adding paragraph (b)(4).
End Amendment Part
The revisions and additions read as follows:
Data completeness criteria for the quality performance category.
(a) * * *
(3) At least 70 percent of the MIPS eligible clinician or group's patients that meet the measure's denominator criteria, regardless of payer for MIPS payment years 2022, 2023, and 2024.
(4) At least 80 percent of the MIPS eligible clinician or group's patients that meet the measure's denominator criteria, regardless of payer for MIPS payment year 2025.
(b) * * *
(3) At least 70 percent of the applicable Medicare Part B patients seen during the performance period to which the measure applies for MIPS payment years 2022, 2023, and 2024.
(4) At least 80 percent of the applicable Medicare Part B patients seen during the performance period to which the measure applies for MIPS payment year 2025.
* * * * *
Start Amendment Part
53. Amend § 414.1350 by revising paragraph (c)(4) and adding paragraph (c)(6) to read as follows:
End Amendment Part
Cost performance category.
* * * * *
(c) * * *
(4) For the procedural episode-based measures specified beginning with the 2019 performance period, the case minimum is 10, unless otherwise specified for individual measures. Beginning with the 2022 performance period, the case minimum for Colon and Rectal Resection procedural episode-based measure is 20 episodes.
* * * * *
(6) For the chronic condition episode-based measures specified beginning with the 2022 performance period, the case minimum is 20.
* * * * *
Start Amendment Part
54. Amend § 414.1360 by revising paragraph (a)(2) to read as follows:
End Amendment Part
Data submission criteria for the improvement activities performance category.
(a) * * *
(2) Groups and virtual groups. Beginning with the 2022 performance year, each improvement activity for which groups and virtual groups submit a yes response in accordance with paragraph (a)(1) of this section must be performed by at least 50 percent of the Start Printed Page 39580 NPIs that are billing under the group's TIN or virtual group's TINs or that are part of the subgroup, as applicable; and the NPIs must perform the same activity during any continuous 90-day period within the same performance year.
* * * * *
Start Amendment Part
55. Section 414.1365 is added to subpart O to read as follows:
End Amendment Part
MIPS Value Pathways.
(a) General. (1) Beginning with the CY 2023 MIPS performance period/2025 MIPS payment year, CMS uses MVPs included in the MIPS final inventory of MVPs established by CMS through rulemaking to assess performance for the quality, cost, improvement activities, and Promoting Interoperability performance categories.
(2) [Reserved]
(b) MVP/Subgroup registration. (1) To report an MVP, an MVP Participant must register for the MVP, and if applicable, as a subgroup during a period that begins on April 1 and ends on November 30 of the applicable CY performance period or a later date specified by CMS. To report the CAHPS for MIPS survey associated with an MVP, a group, subgroup or APM Entity must complete their registration by June 30 of such performance period or a later date specified by CMS.
(2) At the time of registration, the MVP Participant must submit the following information, as applicable:
(i) Each MVP Participant must select an MVP, 1 population health measure included in the MVP, and any outcomes-based administrative claims measure on which the MVP Participant intends to be scored.
(ii) Each subgroup must submit a list of each TIN/NPI associated with the subgroup and a plain language name for the subgroup.
(c) MVP reporting requirements. (1) Quality. Except as provided in paragraph (c)(1)(i) of this section, an MVP Participant must select and report, if applicable, 4 quality measures, including 1 outcome measure (or, if an outcome measure is not available, 1 high priority measure), included in the MVP, excluding the population health measure required under paragraph (c)(4)(ii) of this section.
(i) Paragraph (c)(1) of this section does not apply to a small practice that reports on an MVP that includes fewer than 4 Medicare Part B claims measures, provided that the small practice reports each such measure that is applicable.
(ii) [Reserved]
(2) Cost. An MVP Participant is scored on the cost measures included in the MVP that they select and report.
(3) Improvement activities. An MVP Participant who reports an MVP, must report one of the following:
(i) Two medium-weighted improvement activities;
(ii) One high-weighted improvement activity;
(iii) Participation in a certified or recognized patient-centered medical home (PCMH) or comparable specialty practice, as described at § 414.1380(b)(3)(ii).
(4) Foundational layer—(i) Promoting interoperability. An MVP Participant is required to meet the Promoting Interoperability performance category reporting requirements described at § 414.1375(b).
(A) For the CY 2023 and 2024 performance periods/2025 and 2026 MIPS payment years, an MVP Participant that is a subgroup is required to submit its affiliated group's data for the Promoting Interoperability performance category.
(B) [Reserved]
(ii) Population health measures. Each MVP Participant is scored on 1 population health measure in accordance with paragraph (d)(1) of this section.
(d) MVP scoring—(1) General. An MVP Participant that is not an APM Entity is scored on measures and activities included in the MVP in accordance with paragraphs (d)(1) through (3) of this section. An MVP Participant that is an APM Entity is scored on measures and activities included in the MVP in accordance with § 414.1317(b).
(2) Performance standards. Unless otherwise indicated in this paragraph (d), the performance standards described at § 414.1380(a)(1)(i) through (iv) apply to the measures and activities included in the MVP.
(3) Performance categories. An MVP Participant is scored under MIPS in four performance categories.
(i) Quality performance category. Except as provided in paragraphs (d)(3)(i)(A)(1) and (B) of this section, the quality performance category score for MVP Participants is calculated in accordance with § 414.1380(b)(1) based on measures included in the MVP.
(A) Population health measures. Except as provided in paragraph (d)(3)(i)(A)(1) of this section, each selected population health measure that does not have a benchmark or meet the case minimum requirement is excluded from the MVP participant's total measure achievement points and total available measure achievement points.
(1) Subgroups are scored on each selected population health measure that does not have a benchmark or meet the case minimum requirement based on their affiliated group score, if available. If the subgroup's affiliated group score is not available, each such measure is excluded from the subgroup's total measure achievement points and total available measure achievement points.
(2) [Reserved]
(B) Outcomes-based administrative claims measures. MVP Participants receive zero measure achievement points for each selected outcomes-based administrative claims measure that does not have a benchmark or meet the case minimum requirement.
(ii) Cost performance category. The cost performance category score is calculated for an MVP Participant using the methodology at § 414.1380(b)(2)(i) through (v) and the cost measures included in the MVP that they select and report.
(iii) Improvement activities performance category. The improvement activities performance category score is calculated based on the submission of high- and medium-weighted improvement activities. MVP Participants will receive 20 points for each medium-weighted improvement activity and 40 points for each high-weighted improvement activity required under § 414.1360 on which data is submitted in accordance with § 414.1325 or for participation in a certified or recognized patient-centered medical home (PCMH) or comparable specialty practice, as described at § 414.1380(b)(3)(ii).
(iv) Promoting interoperability performance category. The Promoting Interoperability performance category score is calculated for an MVP Participant using the methodology at § 414.1380(b)(4), except as provided in paragraph (d)(3)(iv)(A) of this section.
(A) If a subgroup does not submit its affiliated group's data for the Promoting Interoperability performance category, the subgroup will receive a score of zero for the Promoting Interoperability performance category.
(B) [Reserved]
(e) Final score calculation. The final score is calculated for an MVP Participant using the methodology at § 414.1380(c), unless otherwise indicated in this paragraph (e).
(1) MVP performance category weights. For an MVP Participant that is not an APM Entity, the final score is calculated using the performance category weights described at § 414.1380(c)(1). For an MVP Participant that is an APM Entity, the final score is calculated using the performance category weights described at § 414.1317(b). Start Printed Page 39581
(2) Reweighting MVP performance categories—(i) General reweighting. For an MVP Participant that is not an APM Entity, in accordance with paragraph (e)(2)(iii) of this section, a scoring weight different from the weights described at § 414.1380(c)(1) will be assigned to a performance category, and its weight as described at § 414.1380(c)(1) will be redistributed to another performance category or categories, in the circumstances described at § 414.1380(c)(2)(i)(A)(2) through (9) and § 414.1380(c)(2)(i)(C). For an MVP Participant that is an APM Entity, the performance category weights will be redistributed in accordance with § 414.1317(b).
(ii) Subgroups. For an MVP Participant that is a subgroup, any reweighting applied to its affiliated group will also be applied to the subgroup. In addition, if reweighting is not applied to the affiliated group, the subgroup may receive reweighting in the following circumstances independent of the affiliated group:
(A) A subgroup may submit an application to CMS demonstrating that it was subject to extreme and uncontrollable circumstances and receive reweighting in accordance with § 414.1380(c)(2)(i)(A)(6) and § 414.1380(c)(2)(i)(C)(2). In the event that a subgroup submits data for a performance category, the scoring weight described at § 414.1380(c)(1) would be applied and its weight would not be redistributed.
(B) A subgroup will receive reweighting if CMS determines, based on information known to the agency prior to the beginning of the relevant MIPS payment year, that data for the subgroup are inaccurate, unusable or otherwise compromised due to circumstances outside of the control of the subgroup and its agents, in accordance with § 414.1380(c)(2)(i)(A)(9) and § 414.1380(c)(2)(i)(C)(10).
(iii) Reweighting scenarios. For an MVP Participant that is not an APM Entity, a scoring weight different from the weights described at § 414.1380(c)(1) will be assigned to a performance category, and its weight as described at § 414.1380(c)(1) will be redistributed to another performance category or categories, in accordance with § 414.1380(c)(2)(ii). For an MVP Participant that is an APM Entity, the performance category weights will be redistributed in accordance with § 414.1317(b).
(3) Facility-based scoring. If an MVP Participant, that is not an APM Entity, is eligible for facility-based scoring, a facility-based score also will be calculated in accordance with § 414.1380(e).
(4) Complex patient bonus. A complex patient bonus will be added to the final score for an MVP Participant in accordance with § 414.1380(c)(3).
Start Amendment Part
56. Amend § 414.1375 by—
End Amendment Part Start Amendment Part
a. Revising paragraphs (b)(2)(ii);
End Amendment Part Start Amendment Part
b. Revising paragraphs (b)(3) paragraph heading and (b)(3)(ii) introductory text; and
End Amendment Part Start Amendment Part
c. Adding paragraph (b)(3)(iii).
End Amendment Part
The revisions and additions read as follows:
Promoting Interoperability (PI) performance category.
* * * * *
(b) * * *
(2) * * *
(ii) Beginning with the 2021 MIPS payment year:
(A) Report that the MIPS eligible clinician completed the actions included in the Security Risk Analysis measure during the year in which the performance period occurs;
(B) For each required measure, as applicable, report the numerator (of at least one) and denominator, or yes/no statement, or an exclusion for each measure that includes an option for an exclusion; and
(C) Beginning with the 2024 MIPS payment year, report that the MIPS eligible clinician completed the actions included in the SAFER Guides measure during the year in which the performance period occurs.
(3) Engaging in activities related to supporting providers with the performance of CEHRT; support for health information exchange and the prevention of information blocking; actions to limit or restrict the compatibility or interoperability of CEHRT. * * *
* * * * *
(ii) Support for health information exchange and the prevention of information blocking. For the 2019, 2020, 2021, 2022, and 2023 MIPS payment years, the MIPS eligible clinician must attest to CMS that he or she—
* * * * *
(iii) Actions to limit or restrict the compatibility or interoperability of CEHRT. Beginning with the 2024 MIPS payment year, the MIPS eligible clinician must attest to CMS that he or she—
(A) Did not knowingly and willfully take action (such as to disable functionality) to limit or restrict the compatibility or interoperability of certified EHR technology.
(B) [Reserved]
Start Amendment Part
57. Amend § 414.1380 by—
End Amendment Part Start Amendment Part
a. Revising paragraphs (b)(1)(i) introductory text, and(b)(1)(i)(A)( 1);
End Amendment Part Start Amendment Part
b. Adding paragraphs (b)(1)(i)(A)( 3) and (b)(1)(i)(C);
End Amendment Part Start Amendment Part
c. Revising paragraphs (b)(1)(iii) and (b)(1)(iv)(B);
End Amendment Part Start Amendment Part
d. Revising paragraphs (b)(1)(v)(A) introductory text and (b)(1)(v)(B) introductory text;
End Amendment Part Start Amendment Part
e. Adding paragraph (b)(1)(v)(B)( 1)(iii);
End Amendment Part Start Amendment Part
f. Revising paragraphs (b)(1)(vi)(C) introductory text, (b)(1)(vi)(C)( 4), (b)(1)(vi)(E), (b)(1)(vii) introductory text, (b)(1)(vii)(A), (b)(2)(iii) introductory text and (b)(2)(v) introductory text;
End Amendment Part Start Amendment Part
g. Adding paragraphs (b)(2)(v)(A) and (B);
End Amendment Part Start Amendment Part
h. Revising paragraph (b)(4)(ii) introductory text and (b)(4)(ii)(C);
End Amendment Part Start Amendment Part
i. Revising the table in paragraph (c) introductory text;
End Amendment Part Start Amendment Part
j. Revising paragraph (c)(2)(i)(A)( 4);
End Amendment Part Start Amendment Part
k. Removing and reserving paragraph (c)(2)(i)(A)( 5);
End Amendment Part Start Amendment Part
l. Revising paragraphs (c)(2)(i)(C)( 9), (c)(2)(ii)(A) and (c)(2)(ii)(F);
End Amendment Part Start Amendment Part
m. Adding paragraph (c)(2)(ii)(G);
End Amendment Part Start Amendment Part
n. Revising paragraph (c)(3);
End Amendment Part Start Amendment Part
o. Revising paragraph (e)(6)(iv) through (vi).
End Amendment Part
The revisions and additions read as follows:
Scoring.
* * * * *
(b) * * *
(1) * * *
(i) Measure achievement points. For the CY 2017 through 2021 performance periods/2019 through 2023 MIPS payment years, MIPS eligible clinicians receive between 3 and 10 measure achievement points (including partial points) for each measure required under § 414.1335 on which data is submitted in accordance with § 414.1325 that has a benchmark at paragraph (b)(1)(ii) of this section, meets the case minimum requirement at paragraph (b)(1)(iii) of this section, and meets the data completeness requirement at § 414.1340 and for each administrative claims-based measure that has a benchmark at paragraph (b)(1)(ii) of this section and meets the case minimum requirement at paragraph (b)(1)(iii) of this section. Except as provided under paragraph (b)(1)(i)(C) of this section, beginning with the CY 2022 performance period/2024 MIPS payment year, MIPS eligible clinicians receive between 1 and 10 measure achievement points (including partial points) for each such measure. The number of measure achievement points received for each such measure is determined based on the applicable benchmark decile category and the percentile distribution. MIPS eligible Start Printed Page 39582 clinicians receive zero measure achievement points for each measure required under § 414.1335 on which no data is submitted in accordance with § 414.1325. MIPS eligible clinicians that submit data in accordance with § 414.1325 on a greater number of measures than required under § 414.1335 are scored only on the required measures with the greatest number of measure achievement points. Beginning with the CY 2019 performance period/2021 MIPS payment year, MIPS eligible clinicians that submit data in accordance with § 414.1325 on a single measure via multiple collection types are scored only on the data submission with the greatest number of measure achievement points.
(A) * * *
(1) Except as provided in paragraphs (b)(1)(i)(A)(2) and (3) of this section, for the CY 2017 through 2021 MIPS performance periods/2019 through 2023 MIPS payment years, MIPS eligible clinicians receive 3 measure achievement points for each submitted measure that meets the data completeness requirement, but does not have a benchmark or meet the case minimum requirement. Beginning with the CY 2022 performance period/2024 MIPS payment year, MIPS eligible clinicians other than small practices receive 0 measure achievement points for each such measure, and small practices receive 3 measure achievement points for each such measure.
* * * * *
(3) Beginning with the CY 2022 performance period/2024 MIPS payment year, MIPS eligible clinicians receive 5 measure achievement points for each submitted new measure that meets the data completeness requirement, but does not have a benchmark or meet the case minimum requirement.
* * * * *
(C) New measures. (1) Beginning with the CY 2022 performance period/2024 MIPS payment year, MIPS eligible clinicians receive between 5 and 10 measure achievement points (including partial points) for each measure required under § 414.1335 on which data is submitted in accordance with § 414.1325 that has a benchmark at paragraph (b)(1)(ii) of this section, meets the case minimum requirement at paragraph (b)(1)(iii) of this section, and meets the data completeness requirement at § 414.1340.
(2) For purposes of this section, "new measure" means a quality measure that is in its first 2 years in the program.
* * * * *
(iii) Minimum case requirements. Except as otherwise specified in the MIPS final list of quality measures described in § 414.1330(a)(1), the minimum case requirement is 20 cases.
(iv) * * *
(B) Except as provided in paragraph (b)(1)(iv)(B)(1) of this section, beginning with the 2021 MIPS payment year, each measure (except for measures in the CMS Web Interface) for which the benchmark for the applicable collection type is identified as topped out for 2 or more consecutive years receives no more than 7 measure achievement points in the second consecutive year it is identified as topped out, and beyond.
(1) For the CY 2022 MIPS performance period/2024 MIPS payment year, MIPS eligible clinicians receive no more than 7 measure achievement points for each measure (except for measures in the CMS Web Interface) for which the applicable benchmark is identified as topped out for 2 or more consecutive years based on the historical benchmarks published for the CY 2021 MIPS performance period and continues to be identified as topped out based on the performance period benchmarks published for the CY 2022 MIPS performance period.
(2) [Reserved]
* * * * *
(v) * * *
(A) High priority measures. Subject to paragraph (b)(1)(v)(A)(1) of this section, for the CY 2017 through 2021 MIPS performance periods/2019 through 2023 MIPS payment years, MIPS eligible clinicians receive 2 measure bonus points for each outcome and patient experience measure and 1 measure bonus point for each other high priority measure. Beginning with the 2021 MIPS payment year, MIPS eligible clinicians do not receive such measure bonus points for CMS Web Interface measures.
* * * * *
(B) End-to-end electronic reporting. Subject to paragraph (b)(1)(v)(B)(1) of this section, for the CY 2017 through 2021 MIPS performance periods/2019 through 2023 MIPS payment years, MIPS eligible clinicians receive 1 measure bonus point for each measure (except claims-based measures) submitted with end-to-end electronic reporting for a quality measure under certain criteria determined by the Secretary.
(1) * * *
(iii) Beginning in the 2024 MIPS payment year, MIPS eligible clinicians will no longer receive measure bonus for submitting using end-to-end electronic reporting.
* * * * *
(vi) * * *
(C) The improvement percent score is assessed at the performance category level for the quality performance category and included in the calculation of the quality performance category score as described in paragraph (b)(1)(vii) of this section.
* * * * *
(4) Beginning with the CY 2018 performance period/2020 MIPS payment year, we will assume a quality performance category achievement percent score of 30 percent if a MIPS eligible clinician earned a quality performance category score less than or equal to 30 percent in the previous year.
* * * * *
(E) For the purpose of improvement scoring methodology, the term "improvement percent score" means the score that represents improvement for the purposes of calculating the quality performance category score as described in paragraph (b)(1)(vii) of this section.
* * * * *
(vii) Quality performance category score. A MIPS eligible clinician's quality performance category score is the sum of all the measure achievement points assigned for the measures required for the quality performance category criteria plus the measure bonus points in paragraph (b)(1)(v) of this section. The sum is divided by the sum of total available measure achievement points. The improvement percent score in paragraph (b)(1)(vi) of this section is added to that result. The quality performance category score cannot exceed 100 percentage points.
(A) For each measure that is submitted, if applicable, and impacted by significant changes or errors prior to the applicable data submission deadline at § 414.1325(e), performance is based on data for 9 consecutive months of the applicable CY performance period. If such data are not available or CMS determines that they may result in patient harm or misleading results, the measure is excluded from a MIPS eligible clinician's total measure achievement points and total available measure achievement points. For purposes of this paragraph (b)(1)(vii)(A), "significant changes or errors" means changes to or errors in a measure that are outside the control of the clinician and its agents and that CMS determines may result in patient harm or misleading results. Significant changes or errors include, but are not limited to, changes to codes (such as ICD-10, CPT, Start Printed Page 39583 or HCPCS codes) or the active status of codes, the inadvertent omission of codes or inclusion of inactive or inaccurate codes, or changes to clinical guidelines or measure specifications. CMS will publish on the CMS website a list of all measures scored under this paragraph (b)(1)(vii)(A) as soon as technically feasible, but by no later than the data submission deadline at § 414.1325(e)(1).
* * * * *
(2) * * *
(iii) The cost performance category score is the sum of the following, not to exceed 100 percent:
* * * * *
(v) A cost performance category score is not calculated if a MIPS eligible clinician or group is not attributed any cost measures for the performance period because the clinician or group has not met the minimum case volume specified by CMS for any of the cost measures or a benchmark has not been created for any of the cost measures that would otherwise be attributed to the clinician or group.
(A) Beginning with the 2024 MIPS payment year, if data used to calculate a score for a cost measure are impacted by significant changes during the performance period, such that calculating the cost measure score would lead to misleading or inaccurate results, then the affected cost measure is excluded from the MIPS eligible clinician's or group's cost performance category score. For purposes of this paragraph (b)(2)(v)(A), "significant changes" are changes external to the care provided, and that CMS determines may lead to misleading or inaccurate results. Significant changes include, but are not limited to, rapid or unprecedented changes to service utilization, and will be empirically assessed by CMS to determine the extent to which the changes impact the calculation of a cost measure score that reflects clinician performance.
(B) [Reserved]
* * * * *
(4) * * *
(ii) Beginning with the 2019 performance period/2021 MIPS payment year, a MIPS eligible clinician's Promoting Interoperability performance category score equals the sum of the scores for each of the required measures and any applicable bonus scores, not to exceed 100 points.
* * * * *
(C) Each optional measure is worth five or ten bonus points, as specified by CMS.
* * * * *
(c) * * *

* * * * *
(2) * * *
(i) * * *
(A) * * *
(4) For the Promoting Interoperability performance category: (i) For the 2021 through 2024 MIPS payment years, the MIPS eligible clinician is a physical therapist, occupational therapist, clinical psychologist, qualified audiologist, qualified speech-language pathologist, or a registered dietitian or nutrition professional. In the event that a MIPS eligible clinician submits data for the Promoting Interoperability performance category, the scoring weight specified in paragraph (c)(1) of this section will be applied and its weight will not be redistributed.
(ii) For the 2019 through 2024 MIPS payment years, the MIPS eligible clinician is a nurse practitioner, physician assistant, clinical nurse specialist, or certified registered nurse anesthetist. In the event that a MIPS eligible clinician submits data for the Promoting Interoperability performance category, the scoring weight specified in paragraph (c)(1) of this section will be applied and its weight will not be redistributed.
(iii) For the 2024 MIPS payment year, the MIPS eligible clinician is a clinical social worker. In the event that a MIPS eligible clinician submits data for the Promoting Interoperability performance Start Printed Page 39584 category, the scoring weight specified in paragraph (c)(1) of this section will be applied and its weight will not be redistributed.
* * * * *
(C) * * *
(9) For the 2020 MIPS payment year through the 2023 MIPS payment year the MIPS eligible clinician demonstrates through an application submitted to CMS that they are in a small practice as defined in § 414.1305, and overwhelming barriers prevent them from complying with the requirements for the Promoting Interoperability performance category. Beginning with the 2024 MIPS payment year the MIPS eligible clinician is in a small practice as defined in § 414.1305.
* * * * *
(ii) * * *
(A) For the 2019 MIPS payment year:

* * * * *
(F) Except as provided in paragraph (c)(2)(ii)(G) of this section, beginning with the 2024 MIPS payment year:

(G) For small practices beginning with the 2024 MIPS payment year:
Start Printed Page 39585

* * * * *
(3) Complex patient bonus. For the CY 2020, 2021, 2022, and 2023 MIPS payment years and associated performance periods, provided that a MIPS eligible clinician, group, virtual group or APM Entity submits data for at least one MIPS performance category for the applicable performance period for the MIPS payment year, a complex patient bonus will be added to the final score for the MIPS payment year, as stated in paragraphs (c)(3)(i) through (c)(3)(iv) of this section. Beginning with the CY 2022 MIPS performance period/CY 2024 MIPS payment year, provided that a MIPS eligible clinician, group, subgroup, virtual group or APM Entity submits data for at least one MIPS performance category for the applicable performance period for the MIPS payment year, a complex patient bonus will be added to the final score for the MIPS payment year, if applicable, as described in paragraphs (c)(3)(v) through (c)(3)(viii) of this section.
(i) For the CY 2020, 2021, 2022, and 2023 MIPS payment years and associated performance periods, for MIPS eligible clinicians and groups, the complex patient bonus is calculated as follows: [The average HCC risk score assigned to beneficiaries (pursuant to the HCC risk adjustment model established by CMS pursuant to section 1853(a)(1) of the Act) seen by the MIPS eligible clinician or seen by clinicians in a group] + [the dual eligible ratio × 5].
(ii) For the CY 2020, 2021, 2022, and 2023 MIPS payment years and associated performance periods, for APM Entities and virtual groups, the complex patient bonus is calculated as follows: [The beneficiary weighted average HCC risk score for all MIPS eligible clinicians, and if technically feasible, TINs for models and virtual groups which rely on complete TIN participation within the APM Entity or virtual group, respectively] + [the average dual eligible ratio for all MIPS eligible clinicians, and if technically feasible, TINs for models and virtual groups which rely on complete TIN participation, within the APM Entity or virtual group, respectively, × 5].
(iii) For the 2020, 2021, 2022, and 2023 MIPS payment years and associated performance periods, the complex patient bonus cannot exceed 5.0 except as provided in paragraph (c)(3)(iv) of this section.
(iv) For the 2022 and 2023 MIPS payment years and associated performance periods, the complex patient bonus is calculated pursuant to paragraphs (c)(3)(i) and (ii) of this section, and the resulting numerical value is then multiplied by 2.0. The complex patient bonus cannot exceed 10.0.
(v) Beginning with the CY 2022 MIPS performance period/CY2024 MIPS payment year, the complex patient bonus is limited to MIPS eligible clinicians, groups, subgroups, APM Entities, and virtual groups with a risk indicator at or above the risk indicator calculated median.
(vi) Beginning with the CY2022 MIPS performance period/CY2024 MIPS payment year, for MIPS eligible clinicians, groups, and subgroups, the complex patient bonus components are calculated as follows for the specific risk indicators: Medical complex patient bonus component = 1.5+4* associated HCC standardized score calculated with the average HCC risk score assigned to beneficiaries (pursuant to the HCC risk adjustment model established by CMS pursuant to section 1853(a)(1) of the Act) seen by the MIPS eligible clinician or seen by clinicians in a group or subgroup; social complex patient bonus component = 1.5+4* associated dual proportion standardized score. The components are added together to calculate one overall complex patient bonus. A standardized score for each risk indicator is determined based on the mean and standard deviation of the raw risk indicator score and provides a standardized measurement of how far each risk score is from the mean: (raw risk indicator score−risk indicator mean)/risk indicator standard deviation.
(vii) Beginning with the CY2022 MIPS performance period/CY2024 MIPS payment year, for APM Entities and Start Printed Page 39586 virtual groups, the complex patient bonus components are calculated as follows for the specific risk indicators: Medical complex patient bonus component = 1.5+4* the beneficiary weighted average HCC risk standardized score for all MIPS eligible clinicians, and if technically feasible, TINs for models and virtual groups which rely on complete TIN participation within the APM Entity or virtual group, respectively; social complex patient bonus component = 1.5+4* the average dual proportion standardized score for all MIPS eligible clinicians, and if technically feasible, TINs for models and virtual groups which rely on complete TIN participation, within the APM Entity or virtual group, respectively. The components are added together to calculate one overall complex patient bonus. A standardized score for each risk indicator is determined based on the mean and standard deviation of the raw risk indicator score and provides a standardized measurement of how far each risk score is from the mean: (raw risk indicator score−risk indicator mean)/risk indicator standard deviation.
(viii) Beginning with the CY2022 MIPS performance period/CY2024 MIPS payment year, the complex patient bonus cannot exceed 10.0 and cannot be below 0.0.
* * * * *
(e) * * *
(6) * * *
(iv) Quality. The quality performance category score is established by determining the percentile performance of the facility in the value-based purchasing program for the specified year as described in paragraph (e)(1) of this section and awarding a score associated with that same percentile performance in the MIPS quality performance category score for those MIPS-eligible clinicians who are not eligible to be scored using facility-based measurement for the MIPS payment year. A clinician or group receiving a facility-based performance score will not earn improvement points based on prior performance in the MIPS quality performance category
(v) Cost. The cost performance category score is established by determining the percentile performance of the facility in the value-based purchasing program for the specified year as described in paragraph (e)(1) of this section and awarding a score associated with that same percentile performance in the MIPS cost performance category score for those MIPS eligible clinicians who are not eligible to be scored using facility-based measurement for the MIPS payment year. A clinician or group receiving a facility-based performance score will not earn improvement points based on prior performance in the MIPS cost performance category.
(A) Other cost measures. MIPS eligible clinicians who are scored under facility-based measurement are not scored on cost measures described in paragraph (b)(2) of this section.
(B) [Reserved]
(vi) Use of score from facility-based measurement. The MIPS quality and cost performance category scores will be based on the facility-based measurement scoring methodology described in paragraph (e)(6) of this section unless:
(A) For the CY 2019 MIPS performance period/2021 MIPS payment year, through the CY 2021 MIPS performance period/2023 MIPS payment year, a clinician or group receives a higher combined MIPS quality and cost performance category score through another MIPS submission.
(B) Beginning with the CY 2022 MIPS performance period/2024 MIPS payment year, a clinician or group receives a higher MIPS final score through another MIPS submission.
Start Amendment Part
58. Amend § 414.1395 by revising paragraph (c) to read as follows:
End Amendment Part
Public reporting.
* * * * *
(c) New measures and activities. (1) CMS does not publicly report any data on new quality or cost measure for the first 2 years in which it is in the program, after which CMS evaluates the measure to determine whether it is suitable for public reporting under paragraph (b) of this section.
(2) CMS does not publicly report any MVP data on new improvement activity or Promoting Interoperability measure, objective, or activity included in an MVP for the first year in which it is included in the MVP.
* * * * *
Start Amendment Part
59. Revise § 414.1400 to read as follows:
End Amendment Part
Third party intermediaries.
(a) General. (1) MIPS data may be submitted on behalf of a MIPS eligible clinician, group, virtual group, subgroup, or APM Entity by any of the following third party intermediaries:
(i) QCDR;
(ii) Qualified registry;
(iii) Health IT vendor; or
(iv) CMS-approved survey vendor.
(2) Third party intermediary approval criteria—
(i) To be approved as a third party intermediary, an entity must agree to meet the applicable requirements of this section, including, but not limited to, the following:
(A) A third party intermediary's principle place of business and retention of any data must be based in the U.S.
(B) If the data is derived from CEHRT, a QCDR, qualified registry, or health IT vendor must be able to indicate its data source.
(C) All data must be submitted in the form and manner specified by CMS.
(D) If the clinician chooses to opt-in in accordance with § 414.1310, the third party intermediary must be able to transmit that decision to CMS.
(E) The third party intermediary must provide services throughout the entire performance period and applicable data submission period.
(F) Prior to discontinuing services to any MIPS eligible clinician, group, virtual group, subgroup, or APM Entity during a performance period, the third party intermediary must support the transition of such MIPS eligible clinician, group, virtual group, subgroup, or APM Entity to an alternate third party intermediary, submitter type, or, for any measure on which data has been collected, collection type according to a CMS approved a transition plan.
(ii) The determination of whether to approve an entity as a third party intermediary for a MIPS payment year may take into account:
(A) Whether the entity failed to comply with the requirements of this section for any prior MIPS payment year for which it was approved as third party intermediary; and
(B) Whether the entity provided inaccurate information regarding the requirements of this subpart to any eligible clinician.
(iii) Beginning with the 2023 MIPS payment year, third party intermediaries must attend and complete training and support sessions in the form and manner, and at the times, specified by CMS.
(3) All data submitted to CMS by a third party intermediary on behalf of a MIPS eligible clinician, group, virtual group, subgroup, or APM Entity must be certified by the third party intermediary as true, accurate, and complete to the best of its knowledge. Such certification must be made in a form and manner and at such time as specified by CMS.
(b) Additional requirements for QCDRs and qualified registries—(1) General. (i) Beginning with the CY 2021 MIPS performance period/2023 MIPS payment year, QCDRs and qualified registries must be able to submit data for all of the following MIPS performance categories: Start Printed Page 39587
(A) Quality, except:
(1) The CAHPS for MIPS survey; and
(2) For qualified registries, QCDR measures;
(B) Improvement activities; and
(C) Promoting Interoperability, if the eligible clinician, group, virtual group, or subgroup is using CEHRT, unless:
(1) The third party intermediary's MIPS eligible clinicians, groups, virtual groups, or subgroups fall under the reweighting policies at § 414.1380(c)(2)(i)(A)(4)(i) through (iii) or (c)(2)(i)(C)(1) through (7) or (c)(2)(i)(C)(9).
(2) [Reserved]
(ii) Beginning with the 2023 MIPS performance period/2025 MIPS payment year, QCDRs and qualified registries must support MVPs that are applicable to the MVP participant on whose behalf they submit MIPS data. QCDRs and qualified registries may also support the APP.
(2) Self-nomination. For the 2018 and 2019 MIPS performance periods/2020 and 2021 MIPS payment years, entities seeking to qualify as a QCDR or qualified registry must self-nominate September 1 until November 1 of the CY preceding the applicable performance period. For the 2020 MIPS performance period/2022 MIPS payment year and future years, entities seeking to qualify as a QCDR or qualified registry must self-nominate during a 60-day period during the CY preceding the applicable performance period (beginning no earlier than July 1 and ending no later than September 1). Entities seeking to qualify as a QCDR or qualified registry for a performance period must provide all information required by CMS at the time of self-nomination and must provide any additional information requested by CMS during the review process. For the 2019 MIPS performance period/2021 MIPS payment year and future years, existing QCDRs and qualified registries that are in good standing may attest that certain aspects of their previous year's approved self-nomination have not changed and will be used for the applicable performance period.
(3) Conditions for approval. (i) Beginning with the 2020 MIPS performance period/2022 MIPS payment year, the QCDR or qualified registry must have at least 25 participants by January 1 of the year prior to the applicable performance period.
(ii) If an entity seeking to qualify as a QCDR or qualified registry uses an external organization for purposes of data collection, calculation, or transmission, it must have a signed, written agreement with the external organization that specifically details the responsibilities of the entity and the external organization. The written agreement must be effective as of September 1 of the year preceding the applicable performance period.
(iii) Beginning with the 2021 MIPS performance period/2023 MIPS payment year, the QCDR or qualified registry must provide performance feedback to their clinicians and groups at least 4 times a year, and provide specific feedback to their clinicians and groups on how they compare to other clinicians who have submitted data on a given measure within the QCDR or qualified registry. Exceptions to this requirement may occur if the QCDR or qualified registry submits notification to CMS within the performance period promptly within the month of realization of the impending deficiency and provides sufficient rationale as to why they do not believe they would be able to meet this requirement (for example, if the QCDR does not receive the data from their clinician until the end of the performance period).
(iv) Beginning with the 2023 MIPS performance period/2025 MIPS payment year, the QCDR or qualified registry must submit a data validation plan annually, at the time of self-nomination for CMS's approval and may not change the plan once approved without the prior approval of the agency.
(v) Beginning with the 2021 MIPS performance period/2023 MIPS payment year, the QCDR or qualified registry must conduct annual data validation audits in accordance with this paragraph (b)(3)(v) of this section.
(A) The QCDR or qualified registry must conduct data validation for the payment year prior to submitting any data for that payment year to CMS for purposes of the MIPS program.
(B) The QCDR or qualified registry must conduct data validation on data for each performance category for which it will submit data, including if applicable the Quality, Improvement Activities, and Promoting Interoperability performance categories.
(C) The QCDR or qualified registry must conduct data validation on data for each submitter type for which it will submit data, including MIPS eligible clinicians, groups, virtual groups, subgroups, APM entities, voluntary participants, and opt-in participants, if applicable.
(D) The QCDR or qualified registry must use clinical documentation (provided by the clinicians they are submitting data for) to validate that the action or outcome measured actually occurred or was performed.
(E) The QCDR or qualified registry must conduct each data validation audit using a sampling methodology that meets the following requirements:
(1) Uses a sample size of at least 3 percent of the TIN/NPIs for which the QCDR or qualified registry will submit data to CMS, except that if a 3 percent sample size would result in fewer than 10 TIN/NPIs, the QCDR or qualified registry must use a sample size of at least 10 TIN/NPIs, and if a 3 percent sample size would result in more than 50 TIN/NPIs, the QCDR or qualified registry may use a sample size of 50 TIN/NPIs.
(2) Uses a sample that includes at least 25 percent of the patients of each TIN/NPI in the sample, except that the sample for each TIN/NPI must include a minimum of 5 patients and does not need to include more than 50 patients.
(F) Each QCDR or qualified registry data validation audit must include the following:
(1) Verification of the eligibility status of each eligible clinician, group, virtual group, subgroup, opt-in participant, and voluntary participant.
(2) Verification of the accuracy of TINs and NPIs.
(3) Calculation of reporting and performance rates.
(4) Verification that only the MIPS quality measures and QCDR measures, as applicable, that are relevant to the performance period will be used for MIPS submission.
(G) In a form and manner and by a deadline specified by CMS, the QCDR or qualified registry must report the results of each data validation audit, including the overall data deficiencies or data error rate, the types of deficiencies or data errors discovered, the percentage of clinicians impacted by any deficiency or error, and, how and when each deficiency or data error type was corrected.
(1) QCDRs and qualified registries must conduct validation on the data they intend to submit for the MIPS performance period and provide the results of the executed data validation plan by May 31st of the year following the performance period.
(2) [Reserved]
(vi) Beginning with the 2021 MIPS performance period/2023 MIPS payment year, the QCDR or qualified registry must conduct targeted audits in accordance with this paragraph (b)(3)(vi).
(A) If a data validation audit under paragraph (b)(3)(v) of this section identifies one or more deficiency or data error, the QCDR or qualified registry must conduct a targeted audit into the impact and root cause of each such Start Printed Page 39588 deficiency or data error for that MIPS payment year.
(B) The QCDR or qualified registry must conduct any required targeted audits for the MIPS payment year and correct any deficiencies or data errors identified through such audit prior to the submission of data for that MIPS payment year.
(C) The QCDR or qualified registry must conduct the targeted audit using the sampling methodology that meets the requirements described in paragraph (b)(3)(iv)(E) of this section. The sample for the targeted audit must not include data from the sample used for the data validation audit in which the deficiency or data error was identified.
(D) In a form and manner and by a deadline specified by CMS, the QCDR or qualified registry must report the results of each targeted audit, including the overall deficiency or data error rate, the types of deficiencies or data errors discovered, the percentage of clinicians impacted by each deficiency or data error, and how and when each deficiency or data error type was corrected.
(vii) For the 2023 MIPS performance period/2025 MIPS payment year, a QCDR or qualified registry that was approved but did not submit any MIPS data for any of the 2019 through 2023 MIPS payment years must submit a participation plan for CMS's approval. The participation plan must include the QCDR and/or qualified registry's detailed plans about how the QCDR or qualified registry intends to encourage clinicians to submit MIPS data to CMS through the QCDR or qualified registry.
(viii) Beginning with the 2024 MIPS performance period/2026 MIPS payment year, a QCDR or qualified registry that was approved but did not submit any MIPS data for either of the 2 years preceding the applicable self-nomination period must submit a participation plan for CMS's approval. This participation plan must include the QCDR's and/or qualified registry's detailed plans about how the QCDR or qualified registry intends to encourage clinicians to submit MIPS data to CMS through the QCDR or qualified registry.
(4) QCDR measures for the quality performance category—(i) QCDR measure self-nomination requirements. For the 2018 MIPS performance period/2020 MIPS payment year and future years, at the time of self-nomination an entity seeking to become a QCDR must submit the following information for any measure it intends to submit for the payment year.
(A) For MIPS quality measures, the entity must submit specifications including the MIPS measure IDs and specialty-specific measure sets, as applicable.
(B) For QCDR measures, the entity must submit for CMS-approval measure specifications including: Name/title of measures, NQF number (if NQF- endorsed), descriptions of the denominator, numerator, and when applicable, denominator exceptions, denominator exclusions, risk adjustment variables, and risk adjustment algorithms. In addition, no later than 15 calendar days following CMS approval of any QCDR measure specifications, the entity must publicly post the measure specifications for that QCDR measure (including the CMS- assigned QCDR measure ID) and provide CMS with a link to where this information is posted.
(ii) QCDR measure submission requirements. A QCDR must include the CMS-assigned QCDR measure ID when submitting data on any QCDR measure to CMS.
(iii) QCDR measure approval criteria. (A) QCDR measure requirements for approval are:
(1) QCDR measures that are beyond the measure concept phase of development.
(2) QCDR measures that address significant variation in performance.
(3) Beginning with the 2022 MIPS performance period/2024 MIPS payment year, all QCDR measures must meet face validity. To be approved for the 2023 MIPS performance period/2025 MIPS payment year, all QCDR measures must meet face validity for the initial MIPS payment year for which it is approved. For subsequent years, all QCDR measures must be fully developed and tested, with complete testing results at the clinician level, prior to submitting the QCDR measure at the time of self-nomination.
(i) To be included in an MVP for the 2022 MIPS performance period/2024 MIPS payment year and future years, a QCDR measure must be fully tested.
(ii) [Reserved]
(4) Beginning with the 2022 MIPS performance period/2023 MIPS payment year, QCDRs are required to collect data on a QCDR measure, appropriate to the measure type, prior to submitting the QCDR measure for CMS consideration during the self-nomination period.
(5) Beginning with the 2020 MIPS performance period/2022 MIPS payment year, CMS may provisionally approve the individual QCDR measures for 1 year with the condition that QCDRs address certain areas of duplication with other approved QCDR measures or MIPS quality measures in order to be considered for the program in subsequent years. If such areas of duplication are not addressed, CMS may reject the duplicative QCDR measure.
(B) QCDR measure considerations for approval include, but are not limited to:
(1) Measures that are outcome-based rather than clinical process measures.
(2) Measures that address patient safety and adverse events.
(3) Measures that identify appropriate use of diagnosis and therapeutics.
(4) Measures that address the domain of care coordination.
(5) Measures that address the domain for patient and caregiver experience.
(6) Measures that address efficiency, cost, and resource use.
(7) Beginning with the 2021 performance period—
(i) That QCDRs link their QCDR measures as feasible to at least one cost measure, improvement activity, or an MVP at the time of self-nomination.
(ii) In cases where a QCDR measure does not have a clear link to a cost measure, improvement activity, or an MVP, we would consider exceptions if the potential QCDR measure otherwise meets the QCDR measure requirements and considerations.
(8) Beginning with the 2020 MIPS performance period/2022 MIPS payment year CMS may consider the extent to which a QCDR measure is available to MIPS eligible clinicians reporting through QCDRs other than the QCDR measure owner for purposes of MIPS. If CMS determines that a QCDR measure is not available to MIPS eligible clinicians, groups, and virtual groups reporting through other QCDRs, CMS may not approve the measure.
(9) Greater consideration is given to measures for which QCDRs:
(i) Conducted an environmental scan of existing QCDR measures; MIPS quality measures; quality measures retired from the legacy Physician Quality Reporting System (PQRS) program; and
(ii) Utilized the CMS Quality Measure Development Plan Annual Report and the Blueprint in the CMS Measures Management System to identify measurement gaps prior to measure development.
(10) Beginning with the 2020 MIPS performance period/2022 MIPS payment year, we place greater preference on QCDR measures that meet case minimum and reporting volumes required for benchmarking after being in the program for 2 consecutive CY performance periods. Those that do not, may not continue to be approved.
(i) Beginning with the 2020 MIPS performance period/2022 MIPS payment year, in instances where a QCDR believes the low-reported QCDR Start Printed Page 39589 measure that did not meet benchmarking thresholds is still important and relevant to a specialist's practice, that the QCDR may develop and submit a QCDR measure participation plan for our consideration. This QCDR measure participation plan must include the QCDR's detailed plans and changes to encourage eligible clinicians and groups to submit data on the low-reported QCDR measure for purposes of the MIPS program.
(ii) [Reserved]
(C) Beginning with the 2021 MIPS performance period/2023 MIPS payment year, QCDR measures may be approved for 2 years, at CMS discretion by attaining approval status by meeting QCDR measure considerations and requirements. Upon annual review, CMS may revoke a QCDR measure's second year approval, if the QCDR measure is found to be: Topped out; duplicative of a more robust measure; reflects an outdated clinical guideline; or if the QCDR self-nominating the QCDR measure is no longer in good standing.
(iv) QCDR measure rejection criteria. Beginning with the 2020 MIPS performance period/2022 MIPS payment year, QCDR measure rejection considerations include, but are not limited to:
(A) QCDR measures that are duplicative or identical to other QCDR measures or MIPS quality measures that are currently in the program.
(B) QCDR measures that are duplicative or identical to MIPS quality measures that have been removed from MIPS through rulemaking.
(C) QCDR measures that are duplicative or identical to quality measures used under the legacy Physician Quality Reporting System (PQRS) program, which have been retired.
(D) QCDR measures that meet the topped out definition as described at § 414.1305.
(E) QCDR measures that are process-based, with consideration to whether the removal of the process measure impacts the number of measures available for a specific specialty.
(F) Whether the QCDR measure has potential unintended consequences to a patient's care.
(G) Considerations and evaluation of the measure's performance data, to determine whether performance variance exists.
(H) QCDR measures that split a single clinical practice or action into several QCDR measures.
(I) QCDR measures that are "check-box" with no actionable quality action.
(J) QCDR measures that do not meet the case minimum and reporting volumes required for benchmarking after being in the program for 2 consecutive years.
(K) QCDR measures with clinician attribution issues, where the quality action is not under the direct control of the reporting clinician.
(L) QCDR measures that focus on rare events or "never events" in the measurement period.
(M) QCDR does not have permission to use a QCDR measure owned by another QCDR for the applicable performance period.
(N) If a QCDR measure owner is not approved or is not in good standing, any associated QCDR measures will not be approved.
(c) Additional requirements for Health IT vendors. (1) Beginning with the CY 2021 MIPS performance period/2023 MIPS payment year, health IT vendors must be able to submit data for the MIPS performance categories as follows:
(i) Health IT vendors that support MVPs must be able to submit data for all of the MIPS performance categories:
(A) Quality, except:
(1) The CAHPS for MIPS survey; and
(2) QCDR measures;
(B) Improvement activities; and
(C) Promoting Interoperability, if the eligible clinician, group, virtual group, or subgroup is using CEHRT, unless:
(1) The third party intermediary's MIPS eligible clinicians, groups, virtual groups, or subgroups fall under the reweighting policies at § 414.1380(c)(2)(i)(A)(4)(i) through (iii) or (c)(2)(i)(C)(1) through (7) or (c)(2)(i)(C)(9).
(2) [Reserved]
(ii) Health IT vendors that do not support MVPs must be able to submit data for at least one of the MIPS performance categories described in paragraphs (c)(1)(i) of this section.
(iii) Beginning with the 2023 MIPS performance period/2025 MIPS payment year, Health IT vendors must support MVPs that are applicable to the MVP participant on whose behalf they submit MIPS data. Health IT vendors may also support the APP.
(2) [Reserved]
(d) Additional requirements for CMS-approved survey vendors. (1) CMS-approved survey vendors may submit data on the CAHPS for MIPS survey for the MIPS quality performance category.
(2) Entities seeking to be a CMS-approved survey vendor for any MIPS performance period must submit a survey vendor application to CMS in a form and manner specified by CMS for each MIPS performance period for which it wishes to transmit such data. The application and any supplemental information requested by CMS must be submitted by deadlines specified by CMS. For an entity to be a CMS-approved survey vendor, it must meet the following criteria:
(3) The entity must have sufficient experience, capability, and capacity to accurately report CAHPS data, including:
(i) At least 3 years of experience administering mixed-mode surveys (that is, surveys that employ multiple modes to collect date), including mail survey administration followed by survey administration via Computer Assisted Telephone Interview (CATI);
(ii) At least 3 years of experience administering surveys to a Medicare population;
(iii) At least 3 years of experience administering CAHPS surveys within the past 5 years;
(iv) Experience administering surveys in English and at least one other language for which a translation of the CAHPS for MIPS survey is available;
(v) Use equipment, software, computer programs, systems, and facilities that can verify addresses and phone numbers of sampled beneficiaries, monitor interviewers, collect data via CATI, electronically administer the survey and schedule call-backs to beneficiaries at varying times of the day and week, track fielded surveys, assign final disposition codes to reflect the outcome of data collection of each sampled case, and track cases from mail surveys through telephone follow-up activities; and
(vi) Employment of a program manager, information systems specialist, call center supervisor and mail center supervisor to administer the survey.
(4) The entity has certified that it has the ability to maintain and transmit quality data in a manner that preserves the security and integrity of the data.
(5) The entity has successfully completed, and has required its subcontractors to successfully complete, vendor training(s) administered by CMS or its contractors.
(6) The entity has submitted a quality assurance plan and other materials relevant to survey administration, as determined by CMS, including cover letters, questionnaires and telephone scripts.
(7) The entity has agreed to participate and cooperate, and has required its subcontractors to participate and cooperate, in all oversight activities related to survey administration conducted by CMS or its contractors.
(8) The entity has sent an interim survey data file to CMS that establishes Start Printed Page 39590 the entity's ability to accurately report CAHPS data.
(e) Remedial action and termination of third party intermediaries. (1) If CMS determines that a third party intermediary has ceased to meet one or more of the applicable criteria for approval, has submitted a false certification under paragraph (a)(3) of this section, or has submitted data that are inaccurate, unusable, or otherwise compromised, CMS may take one or more of the following remedial actions after providing written notice to the third party intermediary:
(i) Require the third party intermediary to submit a corrective action plan (CAP) by a date specified by CMS. The CAP must address the following issues, unless different or additional information is specified by CMS:
(A) The issues that contributed to the non-compliance.
(B) The impact to individual clinicians, groups, or virtual groups, regardless of whether they are participating in the program because they are MIPS eligible, voluntary participating, or opting in to participating in the MIPS program.
(C) The corrective actions to be implemented by the third party intermediary to ensure that the non-compliance has been resolved and will not recur in the future.
(D) The detailed timeline for achieving compliance with the applicable requirements.
(ii) Publicly disclose the entity's data error rate on the CMS website until the data error rate falls below 3 percent.
(2) CMS may immediately or with advance notice terminate the ability of a third party intermediary to submit MIPS data on behalf of a MIPS eligible clinician, group, or virtual group for one or more of the following reasons:
(i) CMS has grounds to impose remedial action;
(ii) CMS has not received a CAP within the specified time-period or the CAP is not accepted by CMS; or
(iii) The third party intermediary fails to correct the deficiencies or data errors by the date specified by CMS.
(3) Contains data inaccuracies affecting the third party intermediary's total clinicians may lead to remedial action/termination of the third party intermediary for future program year(s) based on CMS discretion.
(4) For purposes of paragraph (e) of this section, CMS may determine that submitted data are inaccurate, unusable, or otherwise compromised, including but not limited to, if the submitted data:
(i) Includes, without limitation, TIN/NPI mismatches, formatting issues, calculation errors, or data audit discrepancies.
(ii) [Reserved]
(f) Auditing of entities submitting MIPS data. Any third party intermediary must comply with the following procedures as a condition of its qualification and approval to participate in MIPS as a third party intermediary.
(1) The entity must make available to CMS the contact information of each MIPS eligible clinician or group on behalf of whom it submits data. The contact information must include, at a minimum, the MIPS eligible clinician or group's practice phone number, address, and, if available, email.
(2) The entity must retain all data submitted to CMS for purposes of MIPS for 6 years from the end of the MIPS performance period.
(3) For the purposes of auditing, CMS may request any records or data retained for the purposes of MIPS for up to 6 years from the end of the MIPS performance period.
Start Amendment Part
60. Amend § 414.1405 by adding paragraphs (b)(9), (d)(7) and (g) to read as follows:
End Amendment Part
Payment.
* * * * *
(b) * * *
(9) Pursuant to the methodology established at paragraph (g) of this section, the performance threshold for the 2024 MIPS payment year is 75 points. The prior period used to determine the performance threshold is the 2019 MIPS payment year.
* * * * *
(d) * * *
(7) The additional performance threshold for the 2024 MIPS payment year is 89 points.
* * * * *
(g) Performance threshold methodology. For each of the 2024, 2025, and 2026 MIPS payment years, the performance threshold is the mean of the final scores for all MIPS eligible clinicians from a prior period as specified under paragraph (b) of this section.
Start Amendment Part
61. Amend § 414.1430 by—
End Amendment Part Start Amendment Part
a. Revising paragraph (a)(1)(iii); and
End Amendment Part Start Amendment Part
b. Adding paragraph (a)(1)(iv).
End Amendment Part
The revision and addition read as follows:
Qualifying APM participant determination: QP and partial QP thresholds.
(a) * * *
(1) * * *
(iii) 2023 and 2024: 50 percent
(iv) 2025 and later: 75 percent
* * * * *
Start Amendment Part
62. Amend § 414.1450 by revising paragraph (c) introductory text to read as follows:
End Amendment Part
APM incentive payment.
* * * * *
(c) APM Incentive Payment recipient. CMS will pay the APM Incentive Payment amount for a payment year to a solvent TIN or TINs associated with the QP, identified based on Medicare Part B claims submitted for covered professional services during the base period or payment year, according to this section. If no TIN or TINs with which the QP has an association can be identified at a step, CMS will move to the next and successive steps listed in paragraphs (c)(1) through (8) of this section until CMS identifies a TIN or TINs with which the QP is associated, and to which CMS will make the APM Incentive Payment. If more than one TIN is identified at a step, the payment will be proportionately divided among the TINs according to the relative total paid amounts for Part B covered professional services paid to each TIN for services provided during the base year.
* * * * *
Start Part
PART 415—SERVICES FURNISHED BY PHYSICIANS IN PROVIDERS, SUPERVISING PHYSICIANS IN TEACHING SETTINGS, AND RESIDENTS IN CERTAIN SETTINGS
End Part Start Amendment Part
63. The authority citation for part 415 continues to read as follows:
End Amendment Part Start Authority
42 U.S.C. 1302 and 1395hh.
End Authority Start Amendment Part
64. Section 415.140 is added to subpart D to read as follows:
End Amendment Part
Conditions for payment: Split (or shared) visits.
(a) Definitions. For purposes of this section, the following definitions apply:
(1) Split (or shared) visit means an evaluation and management (E/M) visit in the facility setting that is performed in part by both a physician and a nonphysician practitioner who are in the same group, in accordance with applicable law and regulations such that the service could be could be billed by either the physician or nonphysician practitioner if furnished independently by only one of them.
(2) Facility setting for purposes of this section means institutional settings in which payment for services and supplies furnished incident to a physician or practitioner's professional services is prohibited under § 410.26(b)(1).
(3) Substantive portion means more than half of the total time spent by the physician and nonphysician Start Printed Page 39591 practitioner performing the split (or shared) visit.
(b) Conditions of payment. For purposes of this section, the following conditions of payment apply: (1) Substantive portion of split (or shared) visit. In general, payment is made to the physician or nonphysician practitioner who performs the substantive portion of the split (or shared) visit.
(2) Medical record documentation. Documentation in the medical record must identify the physician and nonphysician practitioner who performed the visit. The individual who performed the substantive portion of the visit (and therefore bills for the visit) must sign and date the medical record.
(3) Claim modifier. The designated modifier must be included on the claim to identify that the service was a split (or shared) visit.
Start Part
PART 423—VOLUNTARY MEDICARE PRESCRIPTION DRUG BENEFIT
End Part Start Amendment Part
65. The authority citation for part 423 continues to read as follows:
End Amendment Part Start Authority
42 U.S.C. 1302, 1306, 1395w-101 through 1395w-152, and 1395hh.
End Authority Start Amendment Part
66. Amend § 423.160 by revising paragraph (a)(5) to read as follows:
End Amendment Part
Standards for electronic prescribing.
(a) * * *
(5) Beginning on January 1, 2021, prescribers must, except in the circumstances described in paragraphs (a)(5)(i) through (iv) of this section, conduct prescribing for at least 70 percent of their Schedule II, III, IV, and V controlled substances that are Part D drugs electronically using the applicable standards in paragraph (b) of this section. Compliance actions against prescribers writing prescriptions for beneficiaries in long-term care facilities, and who do not meet the compliance threshold, will commence on or after January 1, 2025. Compliance actions against other prescribers who do not meet the compliance threshold will commence on or after January 1, 2023. Prescribers will be exempt from this requirement in the following situations:
(i) Prescriber and dispensing pharmacy are the same entity.
(ii) Prescriber who issue 100 or fewer controlled substance prescriptions for Part D drugs per calendar year as determined using CMS claims data as of December 31st of the preceding year.
(iii) Prescriber with an NCPDP database address in the geographic area of an emergency or disaster declared by a federal, state or local government entity.
(iv) Prescriber has received a CMS-approved waiver because the prescriber is unable to conduct electronic prescribing of controlled substances (EPCS) due to circumstances beyond the prescriber's control.
* * * * *
Start Part
PART 424—CONDITIONS FOR MEDICARE PAYMENT
End Part Start Amendment Part
67. The authority for part 424 continues to read as follows:
End Amendment Part Start Authority
42 U.S.C. 1302 and 1395hh.
End Authority Start Amendment Part
68. Amend § 424.205 by—
End Amendment Part Start Amendment Part
a. Redesignating paragraphs (b)(5) and (6) as paragraphs (b)(6) and (7), respectively; and
End Amendment Part Start Amendment Part
b. Adding new paragraph (b)(5);
End Amendment Part
The addition reads as follows:
Requirements for Medicare Diabetes Prevention Program suppliers.
* * * * *
(b) * * *
(5) The Medicare provider enrollment application fee does not apply to all Medicare Diabetes Prevention Program (MDPP) suppliers that submit an enrollment application on or after January 1, 2022.
* * * * *
Start Amendment Part
69. Amend § 424.502 by revising the definition of "Institutional provider" to read as follows:
End Amendment Part
Definitions.
* * * * *
Institutional provider means any provider or supplier that submits a paper Medicare enrollment application using the CMS-855A, CMS-855B (not including physician and nonphysician practitioner organizations), CMS-855S, or an associated internet-based PECOS enrollment application.
* * * * *
Start Amendment Part
70. Amend § 424.530 by revising paragraphs (a)(2) introductory text and (a)(11)(i) to read as follows:
End Amendment Part
Denial of enrollment in the Medicare program.
(a) * * *
(2) Provider or supplier conduct. The provider or supplier, or any owner, managing employee, authorized or delegated official, medical director, supervising physician, or other health care or administrative or management services personnel furnishing services payable by a federal health care program, of the provider or supplier is—
* * * * *
(11) * * * (i) A physician or other eligible professional's Drug Enforcement Administration (DEA) Certificate of Registration to dispense a controlled substance is currently suspended or revoked or is surrendered in response to an order to show cause;
* * * * *
Start Amendment Part
71. Amend § 424.535 by revising paragraphs (a)(2) introductory text, (a)(8)(ii), (a)(13)(i), and (e) to read as follows:
End Amendment Part
Revocation of enrollment in the Medicare program.
(a) * * *
(2) Provider or supplier conduct. The provider or supplier, or any owner, managing employee, authorized or delegated official, medical director, supervising physician, or other health care or administrative or management services personnel furnishing services payable by a federal health care program, of the provider or supplier is—
* * * * *
(8) * * *
(ii) CMS determines that the provider or supplier has a pattern or practice of submitting claims that fail to meet Medicare requirements. In making this determination, CMS considers, as appropriate or applicable, the following:
(A) The percentage of submitted claims that were denied during the period under consideration.
(B) Whether the provider or supplier has any history of final adverse actions and the nature of any such actions.
(C) The type of billing non-compliance and the specific facts surrounding said non-compliance (to the extent this can be determined).
(D) Any other information regarding the provider or supplier's specific circumstances that CMS deems relevant to its determination.
* * * * *
(13) * * *
(i) A physician or other eligible professional's Drug Enforcement Administration (DEA) Certificate of Registration to dispense a controlled substance is currently suspended or revoked or is surrendered in response to an order to show cause;
* * * * *
(e) Reversal of revocation. If the revocation was due to adverse activity (sanction, exclusion, or felony) against the provider's or supplier's owner, managing employee, authorized or delegated official, medical director, supervising physician, or other health care or administrative or management services personnel furnishing services payable by a federal health care program, the revocation may be reversed if the provider or supplier terminates and submits proof that it has terminated its business relationship with that individual within 30 days of the revocation notification.
* * * * *
Start Printed Page 39592
[Amended]
Start Amendment Part
72. Amend § 424.545 in paragraph (b) by removing the reference "§ 405.374" and adding in its place the reference "§ 424.546."
End Amendment Part Start Amendment Part
73. Add § 424.546 to read as follows:
End Amendment Part
Deactivation rebuttals.
(a) Rebuttal submittal period. (1) If a provider or supplier receives written notice from CMS or its contractor that the provider's or supplier's billing privileges are to be or have been deactivated under § 424.540, the provider or supplier has 15 calendar days from the date of the written notice to submit a rebuttal to CMS as permitted under § 424.545(b).
(2) CMS may, at its discretion, extend the 15-day time-period referenced in paragraph (a)(1) of this section.
(b) Rebuttal requirements. A rebuttal submitted pursuant to this section and § 424.545(b) must:
(1) Be in writing.
(2) Specify the facts or issues about which the provider or supplier disagrees with the deactivation's imposition and/or the effective date, and the reasons for disagreement.
(3) Submit all documentation the provider or supplier wants CMS to consider in its review of the deactivation.
(4) Be submitted in the form of a letter that is signed and dated by the individual supplier (if enrolled as an individual physician or nonphysician practitioner), the authorized official or delegated official (as those terms are defined in 42 CFR 424.502), or a legal representative (as defined in 42 CFR 498.10). If the legal representative is an attorney, the attorney must include a statement that he or she has the authority to represent the provider or supplier; this statement is sufficient to constitute notice of such authority. If the legal representative is not an attorney, the provider or supplier must file with CMS written notice of the appointment of a representative; this notice of appointment must be signed and dated by, as applicable, the individual supplier, the authorized official or delegated official, or a legal representative.
(c) Waiver of rebuttal rights. The provider's or supplier's failure to submit a rebuttal that is both timely under paragraph (a) of this section and fully compliant with all of the requirements of paragraph (b) of this section constitutes a waiver of all rebuttal rights under this section and § 424.545(b).
(d) CMS review. Upon receipt of a timely and compliant deactivation rebuttal, CMS reviews the rebuttal to determine whether the imposition of the deactivation and/or the designated effective date are correct.
(e) Imposition. Nothing in this section or in § 424.545(b) requires CMS to delay the imposition of a deactivation pending the completion of the review described in paragraph (d) of this section.
(f) Initial determination. A determination made under this section is not an initial determination under § 498.3(b) of this chapter and therefore not appealable.
Start Part
PART 425—MEDICARE SHARED SAVINGS PROGRAM
End Part Start Amendment Part
74. The authority citation for part 425 continues to read as follows:
End Amendment Part Start Authority
42 U.S.C. 1302, 1306, 1395hh, and 1395jjj.
End Authority Start Amendment Part
75. Amend § 425.116 by revising paragraph (c) to read as follows:
End Amendment Part
Agreements with ACO participants and ACO providers/suppliers.
* * * * *
(c) Submission of agreements. The ACO must submit an executed ACO participant agreement for each ACO participant that it requests to add to its list of ACO participants in accordance with § 425.118. The agreements may be submitted in the form and manner set forth in § 425.204(c)(6) or as otherwise specified by CMS.
Start Amendment Part
76. Amend § 425.204 by—
End Amendment Part Start Amendment Part
a. Revising paragraphs (b), (c)(6), (f)(4)(ii)(A) and (B), (f)(4)(iii) introductory text, and (f)(4)(iii)(A); and
End Amendment Part Start Amendment Part
b. Adding paragraph (f)(4)(v).
End Amendment Part
The revisions and addition read as follows:
Content of the application.
* * * * *
(b) Prior participation. Upon request by CMS during the application cycle, the ACO must submit information regarding prior participation in the Medicare Shared Savings Program by the ACO, its ACO participants, or its ACO providers/suppliers, including such information as may be necessary for CMS to determine whether to approve an ACO's application in accordance with § 425.224(b).
(c) * * *
(6) Upon request by CMS during the application cycle or at any point during an agreement period, the ACO must submit documents demonstrating that its ACO participants, ACO providers/suppliers, and other individuals or entities performing functions or services related to ACO activities are required to comply with the requirements of the Shared Savings Program. Upon such a request, the evidence to be submitted must include, without limitation, sample or form agreements and, in the case of ACO participant agreements, the first and signature page(s) of each executed ACO participant agreement. CMS may request all pages of an executed ACO participant agreement to confirm that it conforms to the sample form agreement submitted by the ACO. The ACO must certify that all of its ACO participant agreements comply with the requirements of this part.
* * * * *
(f) * * *
(4) * * *
(ii) * * *
(A) One-half percent of the total per capita Medicare Parts A and B fee-for-service expenditures for the ACO's assigned beneficiaries, based on expenditures and the number of assigned beneficiaries for the most recent calendar year for which 12 months of data are available.
(B) One percent of the total Medicare Parts A and B fee-for-service revenue of its ACO participants, based on revenue for the most recent calendar year for which 12 months of data are available, and based on the ACO's number of assigned beneficiaries for the most recent calendar year for which 12 months of data are available.
(iii) CMS recalculates the ACO's repayment mechanism amount for the second and each subsequent performance year in the agreement period in accordance with paragraph (f)(4)(ii) of this section based on the certified ACO participant list for the relevant performance year, except that the number of assigned beneficiaries used in the calculations is the number of beneficiaries assigned to the ACO at the beginning of the relevant performance year under § 425.400(a)(2)(i) (for ACOs under preliminary prospective assignment with retrospective reconciliation) or § 425.400(a)(3)(i) (for ACOs under prospective assignment).
(A) If the recalculated repayment mechanism amount exceeds the existing repayment mechanism amount by at least $1,000,000, CMS notifies the ACO in writing that the amount of its repayment mechanism must be increased to the recalculated repayment mechanism amount.
* * * * *
(v)(A) An ACO that established a repayment mechanism to support its participation in a two-sided model beginning on July 1, 2019, January 1, 2020 or January 1, 2021, may elect to decrease the amount of its repayment mechanism if the repayment mechanism amount for performance year 2022, as recalculated pursuant to paragraph Start Printed Page 39593 (f)(4)(iii) of this section, is less than the existing repayment mechanism amount.
(B) CMS will notify the ACO in writing if the ACO may elect to decrease the amount of its repayment mechanism pursuant to this paragraph (f)(4)(v). The ACO must submit such election, and revised repayment mechanism documentation, in a form and manner and by a deadline specified by CMS. CMS will review the revised repayment mechanism documentation and may reject the election if the repayment mechanism documentation does not comply with the requirements of paragraph (f) of this section.
* * * * *
Start Amendment Part
77. Amend § 425.312 by—
End Amendment Part Start Amendment Part
a. Revising paragraph (a)(2)(ii); and
End Amendment Part Start Amendment Part
b. Adding paragraph (a)(2)(iii).
End Amendment Part
The revision and addition read as follows:
Beneficiary notifications.
(a) * * *
(2) * * *
(ii) In the case of an ACO that has selected preliminary prospective assignment with retrospective reconciliation, by the ACO or ACO participant providing each fee-for-service beneficiary with a standardized written notice prior to or at the first primary care visit of the performance year in the form and manner specified by CMS.
(iii) In the case of an ACO that has selected prospective assignment, by the ACO or ACO participant providing each prospectively assigned beneficiary with a standardized written notice prior to or at the first primary care visit of the performance year in the form and manner specified by CMS.
* * * * *
Start Amendment Part
78. Amend § 425.400 by—
End Amendment Part Start Amendment Part
a. Revising paragraph (c)(1)(v) introductory text;
End Amendment Part Start Amendment Part
b. Adding paragraph (c)(1)(vi); and
End Amendment Part Start Amendment Part
c. Revising paragraphs (c)(2)(i) introductory text, (c)(2)(i)(A)(2), and (c)(2)(ii).
End Amendment Part
The addition and revisions read as follows:
General.
* * * * *
(c) * * *
(1) * * *
(v) For the performance year starting on January 1, 2021:
* * * * *
(vi) For the performance year starting on January 1, 2022, and subsequent performance years as follows:
(A) CPT codes:
(1) 96160 and 96161 (codes for administration of health risk assessment).
(2) 99201 through 99215 (codes for office or other outpatient visit for the evaluation and management of a patient).
(3) 99304 through 99318 (codes for professional services furnished in a nursing facility; professional services or services reported on an FQHC or RHC claim identified by these codes are excluded when furnished in a SNF).
(4) 99319 through 99340 (codes for patient domiciliary, rest home, or custodial care visit).
(5) 99341 through 99350 (codes for evaluation and management services furnished in a patient's home for claims identified by place of service modifier 12).
(6) 99354 and 99355 (add-on codes, for prolonged evaluation and management or psychotherapy services beyond the typical service time of the primary procedure; when the base code is also a primary care service code under this paragraph (c)(1)(vi)).
(7) 99421, 99422, and 99423 (codes for online digital evaluation and management).
(8) 99439 (code for non-complex chronic care management).
(9) 99483 (code for assessment of and care planning for patients with cognitive impairment).
(10) 99484, 99492, 99493 and 99494 (codes for behavioral health integration services).
(11) 99X21, 99487, 99489, 99490 and 99491 (codes for chronic care management).
(12) 99495 and 99496 (codes for transitional care management services).
(13) 99497 and 99498 (codes for advance care planning; services identified by these codes furnished in an inpatient setting are excluded).
(14) 99X22, 99X23, 99X24, and 99X25 (codes for principal care management services).
(B) HCPCS codes:
(1) G0402 (code for the Welcome to Medicare visit).
(2) G0438 and G0439 (codes for the annual wellness visits).
(3) G0442 (code for alcohol misuse screening service).
(4) G0443 (code for alcohol misuse counseling service).
(5) G0444 (code for annual depression screening service).
(6) G0463 (code for services furnished in ETA hospitals).
(7) G0506 (code for chronic care management).
(8) G2010 (code for the remote evaluation of patient video/images).
(9) G2012 and G2252 (codes for virtual check-in).
(10) G2058 (code for non-complex chronic care management).
(11) G2064 and G2065 (codes for principal care management services).
(12) G2212 (code for prolonged office or other outpatient visit for the evaluation and management of a patient).
(13) G2214 (code for psychiatric collaborative care model).
(C) Primary care service codes include any CPT code identified by CMS that directly replaces a CPT code specified in paragraph (c)(1)(vi)(A) of this section or a HCPCS code specified in paragraph (c)(1)(vi)(B) of this section, when the assignment window (as defined in § 425.20) for a benchmark or performance year includes any day on or after the effective date of the replacement code for payment purposes under FFS Medicare.
(2)(i) Except as otherwise specified in paragraph (c)(2)(i)(A)(2) of this section, when the assignment window (as defined in § 425.20) for a benchmark or performance year includes any month(s) during the COVID-19 Public Health Emergency defined in § 400.200 of this chapter, in determining beneficiary assignment, we use the primary care service codes identified in paragraph (c)(1) of this section, and additional primary care service codes as follows:
(A) * * *
(2) 99441, 99442, and 99443 (codes for telephone evaluation and management services). These codes are used in determining beneficiary assignment as specified in paragraphs (c)(2)(i) and (ii) of this section and until they are no longer payable under Medicare fee-for-service payment policies as specified under section 1834(m) of the Act and §§ 410.78 and 414.65 of this chapter.
* * * * *
(ii) Except as otherwise specified in paragraph (c)(2)(i)(A)(2) of this section, the additional primary care service codes specified in paragraph (c)(2)(i) of this section are applicable to all months of the assignment window (as defined in § 425.20), when the assignment window includes any month(s) during the COVID-19 Public Health Emergency defined in § 400.200 of this chapter.
Start Amendment Part
79. Amend § 425.512 by—
End Amendment Part Start Amendment Part
a. Revising paragraphs (a)(2) and (3);
End Amendment Part Start Amendment Part
b. Redesignating paragraph (a)(4) as paragraph (a)(5);
End Amendment Part Start Amendment Part
c. Adding a new paragraph (a)(4);
End Amendment Part Start Amendment Part
d. Revising newly redesignated paragraph (a)(5);
End Amendment Part Start Amendment Part
e. Adding paragraph (a)(6);
End Amendment Part Start Amendment Part
f. Revising paragraphs (b)(2)(i) and (ii) and (b)(3)(i);
End Amendment Part Start Amendment Part
g. Redesignating paragraph (b)(3)(ii) as paragraph (b)(3)(iii);
End Amendment Part Start Amendment Part
h. Adding a new paragraph (b)(3)(ii); and Start Printed Page 39594
End Amendment Part Start Amendment Part
i. Revising newly redesignating paragraph (b)(3)(iii).
End Amendment Part
Determining the ACO quality performance standard for performance years beginning on or after January 1, 2021.
(a) * * *
(2) For the first performance year of an ACO's first agreement period under the Shared Savings Program. If the ACO reports data via the APP and meets the data completeness requirement at § 414.1340 of this chapter and the case minimum requirement at § 414.1380 of this chapter on the measures specified in this paragraph (a)(2) for the applicable performance year, the ACO will meet the quality performance standard.
(i) For performance year 2022. The ten CMS Web Interface measures or the three eCQM/MIPS CQM measures, and the CAHPS for MIPS survey.
(ii) For performance year 2023. The ten CMS Web Interface measures and at least one eCQM/MIPS CQM measure or the three eCQM/MIPS CQM measures, and the CAHPS for MIPS survey.
(iii) For performance year 2024 and subsequent performance years. The three eCQM/MIPS CQM measures and the CAHPS for MIPS survey.
(3) For performance year 2021—(i) Except as specified in paragraph (a)(2) of this section, CMS designates the quality performance standard as the ACO reporting quality data via the APP established under § 414.1367 of this chapter, according to the method of submission established by CMS and achieving a quality performance score that is equivalent to or higher than the 30th percentile across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring.
(ii) If an ACO does not report any of the ten CMS Web Interface measures or any of the three eCQM/MIPS CQM measures and does not administer a CAHPS for MIPS survey under the APP, the ACO will not meet the quality performance standard.
(4) For performance year 2022—(i) Except as specified in paragraph (a)(2) of this section, CMS designates the quality performance standard as the ACO reporting via the APP established under § 414.1367 of this chapter either:
(A) According to the method of submission established by CMS and achieving a quality performance score that is equivalent to or higher than the 30th percentile across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring, or
(B) The three eCQM/MIPS CQM measures in the APP measure set, meeting the data completeness requirement at § 414.1340 of this chapter and the case minimum requirement at § 414.1380 of this chapter for all three eCQM/MIPS CQM measures, and achieving a quality performance score equivalent to or higher than the 30th percentile of the performance benchmark on at least one measure in the APP measure set.
(ii) If an ACO does not report any of the ten CMS Web Interface measures or any of the three eCQM/MIPS CQM measures and does not administer a CAHPS for MIPS survey under the APP, the ACO will not meet the quality performance standard.
(5) For performance year 2023—(i) Except as specified in paragraph (a)(2) of this section, CMS designates the quality performance standard as the ACO reporting via the APP established under § 414.1367 of this chapter either:
(A) According to the method of submission established by CMS, including at least one eCQM/MIPS CQM measure, and achieving a quality performance score that is equivalent to or higher than the 30th percentile across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring, or
(B) The three eCQM/MIPS CQM measures in the APP measure set, meeting the data completeness requirement at § 414.1340 of this chapter and the case minimum requirement at § 414.1380 of this chapter for all three eCQM/MIPS CQM measures, and achieving a quality performance score equivalent to or higher than the 30th percentile of the performance benchmark on at least one measure in the APP measure set.
(ii) If the ACO does not report at least one eCQM/MIPS CQM in the APP measure set, the ACO will not meet the quality performance standard.
(6) For performance years 2024 and subsequent performance years. (i) Except as specified in paragraph (a)(2) of this section, CMS designates the quality performance standard as the ACO reporting quality data via the APP established under § 414.1367 of this chapter, according to the method of submission established by CMS and achieving a quality performance score that is equivalent to or higher than the 40th percentile across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring.
(ii) If an ACO does not report any of the three eCQM/MIPS CQM measures and does not administer a CAHPS for MIPS survey under the APP, the ACO will not meet the quality performance standard.
(b) * * *
(2) * * *
(i) For performance years 2021 through 2023, the ACO's minimum quality performance score is set to the equivalent of the 30th percentile MIPS Quality performance category score across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring for the relevant performance year.
(ii) For performance year 2024 and subsequent performance years, the ACO's minimum quality performance score is set to the equivalent of the 40th percentile MIPS Quality performance category score across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring, for the relevant performance year.
(3) * * *
(i) For performance years 2021 and 2022, if the ACO reports quality data via the APP and meets data completeness and case minimum requirements, CMS will use the higher of the ACO's quality performance score or the equivalent of the 30th percentile MIPS Quality performance category score across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring, for the relevant performance year.
(ii) For performance year 2023, if the ACO reports quality data via the APP, including at least one eCQM/MIPS CQM measure, and meets data completeness and case minimum requirements, CMS will use the higher of the ACO's quality performance score or the equivalent of the 30th percentile MIPS Quality performance category score across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring, for the performance year.
(iii) For performance year 2024 and subsequent performance years, if the ACO reports quality data via the APP and meets data completeness and case minimum requirements, CMS will use the higher of the ACO's quality performance score or the equivalent of the 40th percentile MIPS Quality performance category score across all MIPS Quality performance category scores, excluding entities/providers eligible for facility-based scoring, for the relevant performance year.
* * * * *
Start Signature
Dated: July 9, 2021.
Xavier Becerra,
Secretary, Department of Health and Human Services.
End Signature
Note:
The following appendices will not appear in the Code of Federal Regulations.
Start Printed Page 39595
Appendix 1: MIPS Quality Measures
Note: Except as otherwise noted in this proposed rule, previously finalized measures and specialty measure sets will continue to apply for the 2022 MIPS performance period/2024 MIPS payment year and future years. In addition, electronic clinical quality measures (eCQMs) that are National Quality Forum (NQF) endorsed are shown in Table A as follows: NQF #/eCQM NQF #.

Start Printed Page 39596

Start Printed Page 39597

Start Printed Page 39598

Start Printed Page 39599

Start Printed Page 39600

Start Printed Page 39601

Start Printed Page 39602

Start Printed Page 39603

Start Printed Page 39604

Start Printed Page 39605

Start Printed Page 39606

Start Printed Page 39607

Start Printed Page 39608

Start Printed Page 39609

Start Printed Page 39610

Start Printed Page 39611


Start Printed Page 39612

Start Printed Page 39613

Start Printed Page 39614

Start Printed Page 39615

Start Printed Page 39616

Start Printed Page 39617


Start Printed Page 39618

Start Printed Page 39619

Start Printed Page 39620

Start Printed Page 39621

Start Printed Page 39622

Start Printed Page 39623

Start Printed Page 39624

Start Printed Page 39625

Start Printed Page 39626

Start Printed Page 39627

Start Printed Page 39628

Start Printed Page 39629

Start Printed Page 39630


Start Printed Page 39631

Start Printed Page 39632

Start Printed Page 39633

Start Printed Page 39634

Start Printed Page 39635

Start Printed Page 39636


Start Printed Page 39637

Start Printed Page 39638

Start Printed Page 39639

Start Printed Page 39640

Start Printed Page 39641

Start Printed Page 39642

Start Printed Page 39643

Start Printed Page 39644

Start Printed Page 39645

Start Printed Page 39646

Start Printed Page 39647

Start Printed Page 39648

Start Printed Page 39649

Start Printed Page 39650

Start Printed Page 39651

Start Printed Page 39652

Start Printed Page 39653

Start Printed Page 39654

Start Printed Page 39655

Start Printed Page 39656

Start Printed Page 39657

Start Printed Page 39658


Start Printed Page 39659

Start Printed Page 39660

Start Printed Page 39661

Start Printed Page 39662

Start Printed Page 39663

Start Printed Page 39664


Start Printed Page 39665

Start Printed Page 39666

Start Printed Page 39667

Start Printed Page 39668

Start Printed Page 39669

Start Printed Page 39670

Start Printed Page 39671

Start Printed Page 39672

Start Printed Page 39673

Start Printed Page 39674

Start Printed Page 39675

Start Printed Page 39676

Start Printed Page 39677

Start Printed Page 39678


Start Printed Page 39679

Start Printed Page 39680

Start Printed Page 39681

Start Printed Page 39682

Start Printed Page 39683

Start Printed Page 39684

Start Printed Page 39685

Start Printed Page 39686

Start Printed Page 39687

Start Printed Page 39688

Start Printed Page 39689


Start Printed Page 39690

Start Printed Page 39691

Start Printed Page 39692

Start Printed Page 39693

Start Printed Page 39694


Start Printed Page 39695

Start Printed Page 39696

Start Printed Page 39697


Start Printed Page 39698

Start Printed Page 39699


Start Printed Page 39700

Start Printed Page 39701

Start Printed Page 39702

Start Printed Page 39703

Start Printed Page 39704

Start Printed Page 39705

Start Printed Page 39706

Start Printed Page 39707

Start Printed Page 39708

Start Printed Page 39709

Start Printed Page 39710

Start Printed Page 39711

Start Printed Page 39712


Start Printed Page 39713


Start Printed Page 39714

Start Printed Page 39715

Start Printed Page 39716

Start Printed Page 39717

Start Printed Page 39718

Start Printed Page 39719

Start Printed Page 39720

Start Printed Page 39721

Start Printed Page 39722

Start Printed Page 39723

Start Printed Page 39724

Start Printed Page 39725

Start Printed Page 39726

Start Printed Page 39727

Start Printed Page 39728

Start Printed Page 39729

Start Printed Page 39730

Start Printed Page 39731

Start Printed Page 39732

Start Printed Page 39733

Start Printed Page 39734

Start Printed Page 39735

Start Printed Page 39736

Start Printed Page 39737

Start Printed Page 39738

Start Printed Page 39739

Start Printed Page 39740

Start Printed Page 39741

Start Printed Page 39742

Start Printed Page 39743

Start Printed Page 39744

Start Printed Page 39745

Start Printed Page 39746

Start Printed Page 39747

Start Printed Page 39748


Start Printed Page 39749

Start Printed Page 39750

Start Printed Page 39751


Start Printed Page 39752

Start Printed Page 39753

Start Printed Page 39754

Start Printed Page 39755

Start Printed Page 39756


Start Printed Page 39757

Start Printed Page 39758

Start Printed Page 39759


Start Printed Page 39760

Start Printed Page 39761

Start Printed Page 39762

Start Printed Page 39763

Start Printed Page 39764

Start Printed Page 39765

Start Printed Page 39766

Start Printed Page 39767

Start Printed Page 39768

Start Printed Page 39769

Start Printed Page 39770

Start Printed Page 39771

Start Printed Page 39772

Start Printed Page 39773

Start Printed Page 39774


Start Printed Page 39775

Start Printed Page 39776

Start Printed Page 39777

Start Printed Page 39778

Start Printed Page 39779

Start Printed Page 39780

Start Printed Page 39781

Start Printed Page 39782

Start Printed Page 39783


Start Printed Page 39784

Start Printed Page 39785

Start Printed Page 39786

Start Printed Page 39787

Start Printed Page 39788

Start Printed Page 39789

Start Printed Page 39790

Start Printed Page 39791


Start Printed Page 39792

Start Printed Page 39793


Start Printed Page 39794


Start Printed Page 39795

Start Printed Page 39796


Start Printed Page 39797


Start Printed Page 39798

Start Printed Page 39799


Start Printed Page 39800

Start Printed Page 39801

Start Printed Page 39802

Start Printed Page 39803


Start Printed Page 39804

Start Printed Page 39805

Start Printed Page 39806

Start Printed Page 39807

Start Printed Page 39808

Start Printed Page 39809



Start Printed Page 39810


Start Printed Page 39811


Start Printed Page 39812

Start Printed Page 39813

Start Printed Page 39814

Start Printed Page 39815

Start Printed Page 39816

Start Printed Page 39817

Start Printed Page 39818

Start Printed Page 39819

Start Printed Page 39820

Start Printed Page 39821


Start Printed Page 39822

Start Printed Page 39823



Start Printed Page 39824

Start Printed Page 39825

Start Printed Page 39826

Start Printed Page 39827


Start Printed Page 39828


Start Printed Page 39829

Start Printed Page 39830

Start Printed Page 39831


Start Printed Page 39832


Start Printed Page 39833


Start Printed Page 39834


Start Printed Page 39835


Start Printed Page 39836


Start Printed Page 39837



Start Printed Page 39838


Start Printed Page 39839

Start Printed Page 39840

Start Printed Page 39841

Start Printed Page 39842



Start Printed Page 39843



Start Printed Page 39844

Start Printed Page 39845

Start Printed Page 39846

Start Printed Page 39847


Start Printed Page 39848



Start Printed Page 39849



Start Printed Page 39850

Start Printed Page 39851

Start Printed Page 39852

Start Printed Page 39853


Start Printed Page 39854


Start Printed Page 39855

Appendix 2: Improvement Activities
Note: In this proposed rule, for the 2022 MIPS performance/2024 MIPS payment year and future years, we are proposing to add 7 new improvement activities, modify 15 previously adopted improvement activities, and remove 6 previously adopted improvement activities. These proposals are discussed in detail below. We request comments on our proposals.
Start Printed Page 39856

Start Printed Page 39857

Start Printed Page 39858

Start Printed Page 39859

Start Printed Page 39860

Start Printed Page 39861

Start Printed Page 39862

Start Printed Page 39863

Start Printed Page 39864

Start Printed Page 39865

Start Printed Page 39866

Start Printed Page 39867

Start Printed Page 39868

Start Printed Page 39869

Start Printed Page 39870

Start Printed Page 39871

Start Printed Page 39872

Start Printed Page 39873

Start Printed Page 39874

Start Printed Page 39875

Start Printed Page 39876

Start Printed Page 39877

Start Printed Page 39878

Appendix 3: Mvp Inventory
Start Printed Page 39879

Start Printed Page 39880

Start Printed Page 39881

Start Printed Page 39882

Start Printed Page 39883

Start Printed Page 39884

Start Printed Page 39885

Start Printed Page 39886

Start Printed Page 39887

Start Printed Page 39888

Start Printed Page 39889

Start Printed Page 39890

Start Printed Page 39891

Start Printed Page 39892

Start Printed Page 39893

Start Printed Page 39894

Start Printed Page 39895

Start Printed Page 39896

Start Printed Page 39897

Start Printed Page 39898

Start Printed Page 39899

Start Printed Page 39900

Start Printed Page 39901

Start Printed Page 39902

Start Printed Page 39903

Start Printed Page 39904

Start Printed Page 39905

Start Printed Page 39906

Start Printed Page 39907

BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-C
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
[FR Doc. 2021-14973 Filed 7-13-21; 4:15 pm]
BILLING CODE 4120-01-C
Source: https://www.federalregister.gov/documents/2021/07/23/2021-14973/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
Posted by: xeniadubache0193167.blogspot.com
Post a Comment for "Download July Through June Calendar 2019-2020 Ascending"